Abstract
Herpesviruses, like most DNA viruses, replicate and package their genomes into capsids in the host cell nucleus. Capsids then transit to the cytoplasm in a fascinating process called nuclear egress, which includes several unusual steps: Movement of capsids from the nuclear interior to the periphery, disruption of the nuclear lamina, capsid budding through the inner nuclear membrane, and fusion of enveloped particles with the outer nuclear membrane. Here, we review recent advances and emerging questions relating to herpesvirus nuclear egress, emphasizing controversies regarding mechanisms for capsid trafficking to the nuclear periphery, and implications of recent structures of the two-subunit, viral nuclear egress complex for the process, particularly at the step of budding through the inner nuclear membrane.
Graphical Abstract

Introduction
Herpesviruses are double-stranded DNA viruses that infect a broad spectrum of vertebrates and some invertebrates [1]. The Herpesviridae family includes α-, β-, and γ-herpesvirus subfamilies, which are exemplified by human pathogens such as herpes simplex viruses (HSV) 1 and 2 (α), human cytomegalovirus (HCMV; β), and Epstein-Barr virus (EBV; γ). After entry into cells, herpesvirus capsids migrate to the nuclear envelope and release their DNA into the nucleus through nuclear pores. Following viral DNA synthesis and packaging into newly assembled capsids, capsids transit from the nucleus to the cytoplasm in a process known as nuclear egress. Advances in our understanding of nuclear egress reveal a complex interplay between herpesviruses and the host cell nucleus.
Nuclear egress entails four major steps (Figure 1). First, capsids move from their assembly sites in the nuclear interior to the nuclear rim. Second, the virus directs localized dissolution of the nuclear lamina by viral and/or cellular kinases so that capsids can gain access to the inner nuclear membrane (INM). Then, as herpesvirus capsids are too large (~125nm) to exit through the nuclear pore, they bud through the INM (primary envelopment; step 3). Finally, these primary enveloped particles become de-enveloped at the outer nuclear membrane and released into the cytoplasm, which entails a mechanism akin to that used during entry of virions into cells (step 4). While nuclear egress was long thought to be a unique feature of herpesvirus infection, a similar process has been described for certain ribonucleoprotein complexes in Drosophila muscle cells [2,3]. Thus, the process by which herpesvirus capsids exit the nucleus may represent a general cellular mechanism by which large macromolecules transit across the nuclear envelope. However, there are several important differences between the Drosophila and viral processes, including an apparent requirement for nuclear lamins in the Drosophila process [2], while lamins represent a barrier to herpesvirus nuclear egress [4,5].
Fig 1. Overview of nuclear egress.
1) Newly assembled herpesvirus capsids migrate from the nuclear interior to the nuclear rim. 2) The NEC recruits viral and/or cellular kinases for phosphorylation and disruption of the nuclear lamina. 3) Capsids engage the NEC and bud through the inner nuclear membrane (primary envelopment). 4) The perinuclear capsid-containing vesicle (primary enveloped particle) fuses with the outer nuclear membrane and releases capsid into the cytoplasm (de-envelopment). The inset shows the structure of an NEC (that of HCMV) and summarizes four distinctive structural features: 1) The N-terminal heterodimerization domain on the nucleoplasmic NEC subunit (in blue) composed of two helices angled to form a V shape, 2) A vise-like heterodimerization mechanism on the INM-anchored NEC subunit whereby a C-terminal helix (light purple) acts as a moveable jaw and swings out (red arrow) to accommodate the V-shaped heterodimerization domain of the nucleoplasmic subunit and clamp it to the rest of the INM-anchored globular body. The form of the INM-anchored subunit not bound to the nucleoplasmic subunit is shown in gray (from the structure of MCMV M50) and superimposed onto the bound form shown in light purple. The C-terminal helix which swings out in the unbound versus NEC-bound form of the transmembrane subunit is indicated with a red star. 3) The nucleoplasmic subunit includes a zinc finger, and 4) Both subunits have a Bergerat fold shown in wheat. The INM-anchored subunit attaches to the inner nuclear membrane through a C-terminal extension that is predicted to be unstructured (light purple dashed lines), and to make a single pass through the INM with a very short segment in the perinuclear space.
Nuclear egress is orchestrated by a virally encoded two-subunit protein complex known as the nuclear egress complex (NEC), which is conserved across α-, β-, and γ-herpesviruses. The NEC is comprised of an INM-anchored subunit and a nucleoplasmic subunit (Figure 1). The INM anchored subunit is termed UL34 for α-herpesviruses HSV and pseudorabies virus (PRV), UL50 for HCMV and M50 for murine cytomegalovirus (MCMV) (β-herpesviruses), and BFRF1 for EBV and ORF67 for Kaposi’s Sarcoma-associated herpesvirus (KSHV) (γ-herpesviruses). The nucleoplasmic subunit is termed UL31 (HSV-1 and PRV), UL53 (HCMV), M53 (MCMV), BFLF2 (EBV), or ORF69 (KSHV). Accumulation of herpesvirus capsids in the cytoplasm is severely impaired during infections with viral mutants that lack one or both NEC subunits or fail to form the NEC, indicating a crucial role for the NEC in nuclear egress and viral replication [6,7••,8•,9,10,11,12,13,14,15].
This review will highlight recent advances in nuclear egress with a particular emphasis on two steps of nuclear egress: 1) How herpesvirus capsids migrate from their assembly sites to the nuclear periphery (step 1), and 2) how recently solved, high resolution NEC structures, together with in vitro vesiculation assays and electron microscopy studies in vitro and in infected cells, have led to a model for primary envelopment (step 3).
Capsid movement to the nuclear periphery
How newly packaged capsids migrate through the dense nuclear environment toward the nuclear periphery remains poorly understood and controversial. It was initially reported using live cell imaging and single particle tracking (SPT) analysis that HSV-1 capsids undergo movement toward the nuclear periphery in a manner that was antagonized by inhibitors of myosin ATPase and actin filament (F-actin) polymerization (with latrunculin A; LatA), suggesting that capsids use the nuclear actomyosin system for directed movement [16]. In support of this notion, it was subsequently found that HSV-1 and PRV infection induces nuclear F-actin in certain cells, and that capsids colocalize with F-actin and myosin V [17].
More recently, however, whether herpesvirus infection induces nuclear F-actin, and whether capsids undergo actin-dependent, directed movement toward the nuclear periphery have become controversial [18, 19••, 20, 21 22••]. One report found that infections with several herpesviruses did not induce nuclear F-actin in murine fibroblasts stably expressing the actin-binding peptide, LifeAct-GFP [18]. Instead, these authors found that treatment with LatA induced nuclear F-actin rods, potentially by increasing the nuclear concentration of monomeric actin due to the depolymerization of cytoplasmic F-actin, and that these rods frequently associated with capsids. It was concluded that LatA stalls movement by inducing actin structures that trap capsids. In a separate study, the same group used a novel live cell imaging platform and SPT analysis and did not find evidence of directed intranuclear movement of PRV or HSV-1 capsids [19••]. Instead, their results suggested that α-herpesvirus infection reorganizes chromatin so that capsids (and microinjected beads) can diffuse more freely. Consistent with this proposal, another group found that HSV-1 infection induced channels through marginalized chromatin near the nuclear periphery in which capsids could be found [21].
In contrast to some of these findings, it was shown that HCMV, but not HSV-1 infection, induces nuclear F-actin in human fibroblasts stably expressing a nuclear-localized version of LifeAct-GFP [22••], and that LatA could fully depolymerize these filaments. Furthermore, electron microscopy revealed that LatA treatment caused a significant decrease in nuclear egress (number of cytoplasmic capsids) and in the percentage of capsids that had moved out of the nuclear interior towards the periphery. Thus, at least one herpesvirus induces nuclear F-actin, and this induction appears to be important for nuclear egress, particularly at the step of capsid migration to the nuclear rim. Whether this occurs by promoting actomyosin-dependent capsid motility [16] or by involvement of F-actin in remodeling nuclear architecture [19••] remains to be determined.
Are NEC subunits involved in capsid movement?
It has been suggested that nucleoplasmic NEC subunits participate in capsid movement to the nuclear periphery, as well as earlier events such as DNA packaging [23,24,25,26]. Recently, it was shown that an HSV-1 mutant in which two basic patches of the N-terminal segment of UL31 were made less basic is defective for nuclear egress, but was not affected for colocalization between UL31 and capsids in the nucleoplasm [27]. This mutant, and other N-terminal mutants, displayed less UL31 at the nuclear rim, which was interpreted to mean that the N-terminal segment is important for HSV-1 capsid migration to the nuclear periphery. Although other interpretations involving effects on UL31-UL34 interactions might be invoked, the results raise the intriguing possibility of an interaction of UL31 with some other factor(s) to facilitate capsid movement to the nuclear periphery, and that the nucleoplasmic NEC unit serves as a bridge between processes in the nuclear interior and those at the nuclear rim.
Structural features of the NEC
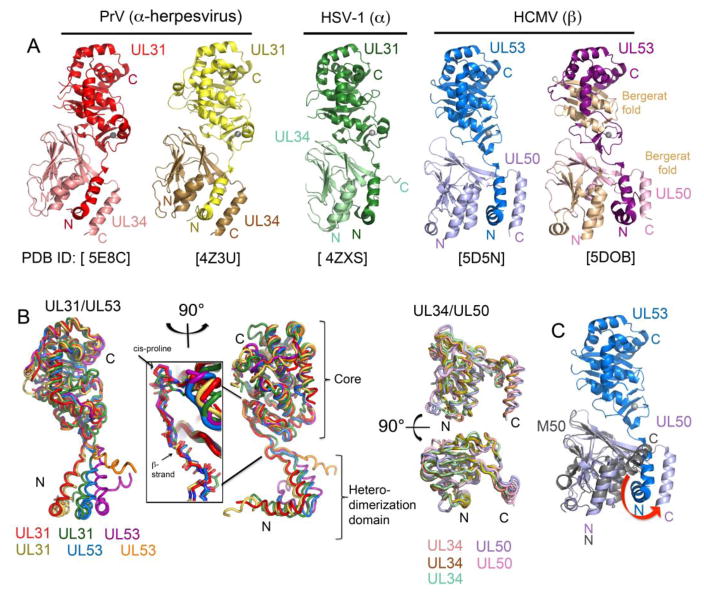
In 2015, five papers described structures of NECs from HCMV, HSV-1 and PRV, and monomeric HCMV UL53 and MCMV M50 [7••,8•,28•,29••,30••] (Figure 2). Despite low to moderate sequence identities among the homologs, the general structures of the NECs are strikingly conserved (Figure 2A). Several features are conserved structural hallmarks of these proteins (Figure 1 and Figure 2): 1) A heterodimerization domain composed of two N-terminal helices on the nucleoplasmic subunit, 2) a C-terminal helix on the transmembrane subunit mediating a vise-like heterodimerization mechanism, 3) a zinc finger (ZnF) on the nucleoplasmic subunit, and 4) a Bergerat fold on both subunits.
Fig 2. Structures of the nuclear egress complex (NEC) and its subunits.
A. NEC structures from (left to right) Pseudorabies virus (PrV; two crystal forms shown in shades of red [29] and yellow [30] respectively), herpes simplex virus-1 (HSV-1; shades of green [30]), and human cytomegalovirus (HCMV; two crystal forms shown in shades of blue [28] and purple [7] respectively). The PDB accession codes are listed under each structure. In the right-most structure, the Bergerat fold found in both subunits is shown in wheat. All structures display a zinc ion represented as a gray sphere. The nucleoplasmic (UL31/UL53) and INM-anchored (UL34/UL50) subunits are labeled in the same color code as in the structures. B. (Left) Structural alignment of the nucleoplasmic (UL31/UL53) subunit from PrV (red and yellow), HSV-1 (green), and HCMV (blue and purple). The UL53 monomeric subunit [7] is also superimposed, and shown in orange. View from a 90 degree rotation from the left is also shown, highlighting the cis-proline and β-strand (insert) preceding the diverging V-shaped heterodimerization domains of the nucleoplasmic (UL31/UL53) subunit structures. The stable core and heterodimerization domain that flank the linker region of the nucleoplasmic (UL31/UL53) subunit are labeled. (Right) Structural alignment of INM-anchored (UL34/UL50) subunit structures in the bound state, but shown without the nucleoplasmic subunit. C. HCMV NEC (PDB ID: 5D5N) [28] shown with the NMR structure of the unbound state of the MCMV INM-anchored subunit M50 (PDB ID: 5A3G) [8]. M50 is superimposed onto UL50 in the bound state. The red arrow indicates the movement of the C-terminal helix of UL50 that is required for binding to UL53.
The nucleoplasmic subunit’s N-terminal region contains two helices in a V-shape that form a major part of the NEC interaction interface (Figure 1 and Figure 2B). New mutational data [7••,8•] corroborate and extend previous evidence [12,31,32,33,34,35,36,37] for the essentiality of residues in this region for NEC formation and viral viability. Notably, the orientation of this N-terminal heterodimerization domain to the core among the different structures is highly diverse, indicating a flexible linker region mediating the orientation of the INM-anchored subunit relative to the nucleoplasmic subunit. (Figure 2B). This linker region is preceded by a fully conserved cis-proline and leucine residue and a β-strand, after which the heterodimerization domain splays out at or close to a lysine residue. While crystal contacts may contribute to differences in orientation, the flexibility of this linker region may be important for budding (see below).
Comparison of INM-anchored subunits with that of monomeric MCMV M50 [8•] reveals a second hallmark feature, a mechanism of NEC formation in which a C-terminal helix acts like the moveable jaw of a vise [7••] that swings away from the rest of the subunit, and clamps the nucleoplasmic subunit’s heterodimerization domain into a groove (Figure 1 and Figure 2C). It has been proposed that NEC heterodimerization, and this mechanism in particular, might serve as a target for new antiherpesvirus drugs [7••,8•,32].
The globular core formed by the C-terminal region of the nucleoplasmic subunit includes a zinc finger composed of three cysteines and one histidine (C3H) (Figure 1 and Figure 2A). Mutational analyses of the C3H motif have found decreased interactions between the two subunits [7••,29••], which may relate to the proximity of the ZnF to the interface with the INM-anchored subunit or may be an indirect effect. Additionally, decreased vesiculation activity [29••], and decreased ability to support viral replication [7••,29••] have been observed. Some mutations give rise to only partial effects in certain assays [7••,29••,31]. The ZnF may also play a role in inter-heterodimer interactions (see below). It will be interesting to determine if the ZnF is particularly important for a specific NEC function, or whether the ZnF mutations might simply result in misfolding, negatively impacting all NEC functions.
A fourth structural hallmark is that each subunit includes a domain known as a Bergerat fold (Figure 1 and Figure 2A), possibly due to gene duplication. Usually, Bergerat folds contain ATP-binding loops and are found in nucleotide binding proteins including certain bacterial protein kinases. However, in the NEC subunits, the ATP-binding loop is replaced with a β-strand on which the histidine of the ZnF lies. It is tempting to speculate that one or both subunits initially had a nucleotide binding activity but evolved to use other proteins to provide this activity. Nevertheless, Bergerat folds mediate intra-hexameric contacts in NEC structures, as discussed below. Additionally, in the INM-anchored subunit, the two helices of the fold interact with the heterodimerization domain of the nucleoplasmic subunit [7••]. Whether the fold has additional functions remains to be determined.
How does the NEC induce budding at the INM?
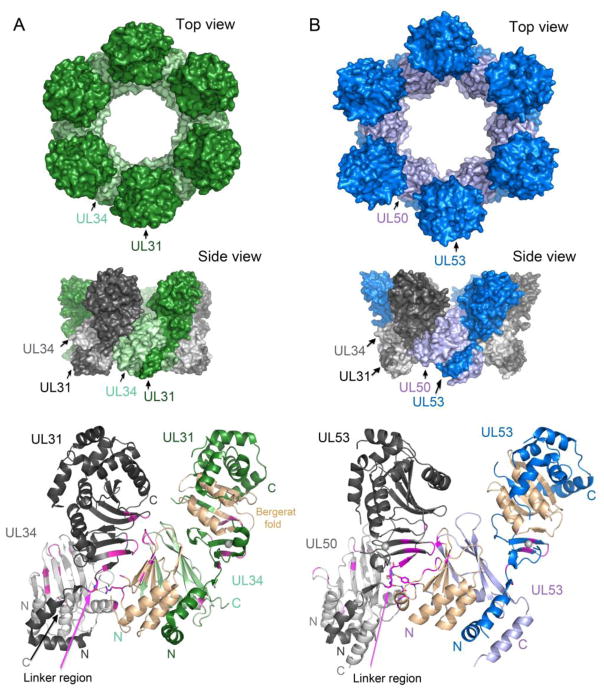
Multiple studies utilizing a variety of techniques have come together leading to a model of how the NEC mediates budding at the INM (Figure 1). In this model, the NEC initially forms a planar patch on the INM, as visualized by electron cryo-microscopy and tomography (cryoEM/T) [38••]. During budding, this patch curves and expands to form a complete coat around the capsid as it buds through the INM [38••]. Strikingly, the two NEC subunits, expressed in cells in the absence of any other viral proteins, can induce vesicle formation at the INM [39]. A major recent advance, first reported for the HSV-1 NEC [40••], is that purified NEC can induce the formation of vesicles in a cell-free system. In both the cell-based and cell-free systems, hexagonal arrays can be visualized on the membranes of the spherical vesicles [30••,38••,40••]. Clues to how these arrays form come from hexameric crystal forms of the HSV-1 and HCMV NECs (Figure 3A), and from modeling of the PRV NEC into cryoEM/T structures of the arrays [28•,29••,30••,38••,40••]. In these, residues on the loops and β-strands of the Bergerat fold in the INM-anchored subunit of one heterodimer interact with the globular core of the nucleoplasmic subunit of a neighboring NEC (Figure 3). The linker region and the ZnF also participate in these inter-heterodimeric interactions. Substitutions of residues that interact in the HSV-1 hexameric crystals reduce vesicle formation in the cell-free system [30••,40••]. One such substitution in the INM-anchored subunit impairs budding in virus-infected cells, and this phenotype can be suppressed by a substitution in the nucleoplasmic subunit [41]. This second substitution also acts as a suppressor in the cell-free system, evidently by strengthening hexameric contacts [30••]. Thus, an appealing model is that these inter-heterodimer interactions drive vesicle formation and budding.
Fig 3. The NEC hexameric interface.
(A). Hexameric arrangement of the HSV-1 NEC shown from the top (top panel) and side (middle panel) [30]. A single heterodimer is shown in dark and light green for the HSV nucleoplasmic (UL31) and INM-anchored (UL34) subunits, respectively. For clarity when comparing interactions between neighboring heterodimers (middle and bottom panels), the alternating subunits are shown in dark and light gray for the UL31 and UL34 subunits respectively. The linker region in UL31 is labeled with a pink arrow, and selected interacting residues at the hexameric interface shown, specifically focusing on interactions with the linker region. The Bergerat fold in both subunits is shown in wheat (B). Hexameric arrangement of the HCMV with the nucleoplasmic (UL53) and INM-anchored (UL50) subunits in dark and light blue respectively [28]. Representations of the structure follow the same format as in A.
However, the hexameric arrays seen in HSV-1 and HCMV crystal structures are planar. Perhaps, they represent the planar patches seen in infected cells at the INM [38••], but they cannot form spheres without the introduction of either pentamers [42], or, as proposed by Bigalke et. al. [30••], irregularities. (Although neither pentamers nor irregularities have been observed in the EM studies of the cell-free or cell-based system, that might be due to their being infrequent and/or missed because of averaging.) Therefore, the existence of crystal forms of the NEC that do not form hexagonal arrays [7••,29••,30••] may be germane, as these represent low energy conformations with different inter-heterodimer interactions. These alternative conformations along with the flexible linker of the nucleoplasmic subunit (Figure 2B) and the apparently disordered C-terminal segment of the INM-anchored subunit might abet formation of pentamers or irregularities.
Two caveats must be mentioned: 1) The PRV nucleoplasmic subunit, artificially tethered to a membrane, and thus lacking any of the inter-heterodimeric interactions, is reportedly sufficient for vesiculation in the cell-free system [43•]. 2) Although the HSV-1 and PRV NEC are sufficient for vesiculation in the cell-free system, they and the NECs of other herpesviruses may not be sufficient in cells. In particular, host proteins may be important. For example, it has been reported that the ESCRT system is important for EBV nuclear egress [44,45]. Regardless, a consensus has emerged that the hexagonal arrays are key to budding at the INM.
Conclusions
The molecular mechanisms underlying the remarkable process of herpesvirus nuclear egress are emerging. The first step, capsid movement to the nuclear periphery has recently become controversial, with some studies suggesting roles for the nuclear actomyosin system in capsid motility [16,17,22••], and others not [18,19••]. Additional studies are needed to rigorously test the capacity of different herpesvirus capsids to undergo directed movement in the nucleus.
In contrast to these controversies, there is a growing consensus regarding budding at the INM, albeit with important caveats and questions. New structural information on NECs together with new cryo-EM structures of capsids [46] should permit structure-guided mutational studies that should glean insight into parallels and differences between herpesvirus nucleocapsid and cellular nuclear egress processes [2,47].
Highlights.
Egress of herpesvirus capsids from the nucleus in a fascinating, multi-step process.
How herpesvirus capsids move from nuclear interior to nuclear rim is controversial.
The structurally conserved nuclear egress complex (NEC) exhibits unusual features.
Budding through the inner nuclear membrane entails hexameric NEC lattice formation.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers R01 AI026077 to D.M.C. and J.M.H. and F31 AI20651 to A.R.W.), and that support is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pellett PE, Roizman B. Herpesviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 2013. pp. 1802–1822. [Google Scholar]
- 2.Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang Y-T, Li Q, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jokhi V, Ashley J, Nunnari J, Noma A, Ito N, Wakabayashi-Ito N, Moore MJ, Budnik V. Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope. Cell Rep. 2013;3:988–995. doi: 10.1016/j.celrep.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma M, Bender BJ, Kamil JP, Lye MF, Pesola JM, Reim NI, Hogle JM, Coen DM. Human cytomegalovirus UL97 phosphorylates the viral nuclear egress complex. J Virol. 2015;89:523–534. doi: 10.1128/JVI.02426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mou F, Wills EG, Park R, Baines JD. Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane. J Virol. 2008;82:8094–8104. doi: 10.1128/JVI.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma M, Kamil JP, Coughlin M, Reim NI, Coen DM. Human cytomegalovirus UL50 and UL53 recruit viral protein kinase UL97, not protein kinase C, for disruption of nuclear lamina and nuclear egress in infected cells. J Virol. 2014;88:249–262. doi: 10.1128/JVI.02358-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Lye MF, Sharma M, Omari El K, Filman DJ, Schuermann JP, Hogle JM, Coen DM. Unexpected features and mechanism of heterodimer formation of a herpesvirus nuclear egress complex. EMBO J. 2015;34:2937–2952. doi: 10.15252/embj.201592651. These authors solved crystal structures of the monomeric HCMV nucleoplasmic subunit as well as the NEC (which was not in a hexameric arrangement in the crystal), and analyzed the effects of mutational substitutions in the zinc finger and heterodimerization interface on co-localization of the subunits and on viral replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Leigh KE, Sharma M, Mansueto MS, Boeszoermenyi A, Filman DJ, Hogle JM, Wagner G, Coen DM, Arthanari H. Structure of a herpesvirus nuclear egress complex subunit reveals an interaction groove that is essential for viral replication. Proc Natl Acad Sci USA. 2015;112:9010–9015. doi: 10.1073/pnas.1511140112. The first structure available of any of the subunits of the NEC, this NMR structure of the MCMV INM-anchored subunit revealed a compact, overall unique fold with a binding groove for interaction with the nucleoplasmic subunit. The effects of substituting certain residues in this groove were assessed using isothermal titration calorimetry, co-localization, and viral replication studies, revealing that single substitutions that eliminated heterodimerization also eliminated viral replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol. 2001;75:8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farina A, Feederle R, Raffa S, Gonnella R, Santarelli R, Frati L, Angeloni A, Torrisi MR, Faggioni A, Delecluse H-J. BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress. J Virol. 2005;79:3703–3712. doi: 10.1128/JVI.79.6.3703-3712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klupp BG, Granzow H, Mettenleiter TC. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol. 2000;74:10063–10073. doi: 10.1128/jvi.74.21.10063-10073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bubeck A, Wagner M, Ruzsics Z, Lötzerich M, Iglesias M, Singh IR, Koszinowski UH. Comprehensive mutational analysis of a herpesvirus gene in the viral genome context reveals a region essential for virus replication. J Virol. 2004;78:8026–8035. doi: 10.1128/JVI.78.15.8026-8035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J Virol. 2002;76:364–378. doi: 10.1128/JVI.76.1.364-378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang YE, Roizman B. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J Virol. 1993;67:6348–6356. doi: 10.1128/jvi.67.11.6348-6356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J Virol. 2000;74:117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forest T, Barnard S, Baines JD. Active intranuclear movement of herpesvirus capsids. Nat Cell Biol. 2005;7:429–431. doi: 10.1038/ncb1243. [DOI] [PubMed] [Google Scholar]
- 17.Feierbach B, Piccinotti S, Bisher M, Denk W, Enquist LW. Alpha-herpesvirus infection induces the formation of nuclear actin filaments. PLoS Pathog. 2006;2:e85. doi: 10.1371/journal.ppat.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosse JB, Virding S, Thiberge SY, Scherer J, Wodrich H, Ruzsics Z, Koszinowski UH, Enquist LW. Nuclear herpesvirus capsid motility is not dependent on F-actin. MBio. 2014;5:e01909-14–e01909–14. doi: 10.1128/mBio.01909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Bosse JB, Hogue IB, Feric M, Thiberge SY, Sodeik B, Brangwynne CP, Enquist LW. Remodeling nuclear architecture allows efficient transport of herpesvirus capsids by diffusion. Proc Natl Acad Sci USA. 2015;112:E5725–E5733. doi: 10.1073/pnas.1513876112. These authors used “ring-sheet microscopy” and particle tracking analysis and did not find evidence of directed movements of PRV or HSV-1 capsids. Their work suggests that α-herpesvirus infection enlarges interchromatin domains to enable capsid diffusion to the nuclear periphery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosse JB, Enquist LW. The diffusive way out: Herpesviruses remodel the host nucleus, enabling capsids to access the inner nuclear membrane. Nucleus. 2016;7:13–19. doi: 10.1080/19491034.2016.1149665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myllys M, Ruokolainen V, Aho V, Smith EA, Hakanen S, Peri P, Salvetti A, Timonen J, Hukkanen V, Larabell CA, et al. Herpes simplex virus 1 induces egress channels through marginalized host chromatin. Scientific Reports. 2016;6:28844. doi: 10.1038/srep28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Wilkie AR, Lawler JL, Coen DM. A role for nuclear F-actin induction in human cytomegalovirus nuclear egress. MBio. 2016;7:e01254–16. doi: 10.1128/mBio.01254-16. These authors found that HCMV, but not HSV-1 infection, induces nuclear F-actin in human fibroblasts. Depolymerization of filaments caused defects in capsid localization toward the nuclear rim and nuclear egress, suggesting a role for nuclear F-actin in HCMV capsid movement to the nuclear periphery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogoda M, Bosse JB, Wagner FM, Schauflinger M, Walther P, Koszinowski UH, Ruzsics Z. Characterization of conserved region 2-deficient mutants of the cytomegalovirus egress protein pM53. J Virol. 2012;86:12512–12524. doi: 10.1128/JVI.00471-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popa M, Ruzsics Z, Lötzerich M, Dölken L, Buser C, Walther P, Koszinowski UH. Dominant negative mutants of the murine cytomegalovirus M53 gene block nuclear egress and inhibit capsid maturation. J Virol. 2010;84:9035–9046. doi: 10.1128/JVI.00681-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granato M, Feederle R, Farina A, Gonnella R, Santarelli R, Hub B, Faggioni A, Delecluse H-J. Deletion of Epstein-Barr virus BFLF2 leads to impaired viral DNA packaging and primary egress as well as to the production of defective viral particles. J Virol. 2008;82:4042–4051. doi: 10.1128/JVI.02436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang YE, Van Sant C, Krug PW, Sears AE, Roizman B. The null mutant of the U(L)31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funk C, Ott M, Raschbichler V, Nagel C-H, Binz A, Sodeik B, Bauerfeind R, Bailer SM. The herpes simplex virus protein pUL31 escorts nucleocapsids to sites of nuclear egress, a process coordinated by its N-terminal domain. PLoS Pathog. 2015;11:e1004957–31. doi: 10.1371/journal.ppat.1004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Walzer SA, Egerer-Sieber C, Sticht H, Sevvana M, Hohl K, Milbradt J, Muller YA, Marschall M. Crystal structure of the human cytomegalovirus pUL50-pUL53 core nuclear egress complex provides insight into a unique assembly scaffold for virus-host protein interactions. J Biol Chem. 2015;290:27452–27458. doi: 10.1074/jbc.C115.686527. These authors solved a crystal structure of the HCMV NEC. In this crystal, the NEC is in a hexameric arrangement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Zeev-Ben-Mordehai T, Weberruß M, Lorenz M, Cheleski J, Hellberg T, Whittle C, Omari El K, Vasishtan D, Dent KC, Harlos K, et al. Crystal structure of the herpesvirus nuclear egress complex provides insights into inner nuclear membrane remodeling. Cell Rep. 2015;13:2645–2652. doi: 10.1016/j.celrep.2015.11.008. These authors solved a crystal structure of the NEC from PRV, and analyzed the effects of substitutions in the zinc finger for co-localization, vesiculation in giant unilamellar vesicles, and viral replication. Although the NEC was not in a hexameric arrangement in the crystal, it could be fit into the cryo-EM map of the hexagonal lattices observed in NEC-induced vesicles in cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Bigalke JM, Heldwein EE. Structural basis of membrane budding by the nuclear egress complex of herpesviruses. EMBO J. 2015;34:2921–2936. doi: 10.15252/embj.201592359. These authors solved the structure of the PrV NEC (not in a hexameric arrangement) and, notably, the HSV-1 NEC, which was in a hexameric arrangement that could be modeled into the hexagonal NEC coat observed in their previous cryo-EM studies of vesicles formed by the NEC in giant unilamellar vesicles. The effects of substitutions of selected residues at the hexameric interfaces on vesicle formation support the importance of this hexameric arrangement for budding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luitweiler EM, Henson BW, Pryce EN, Patel V, Coombs G, McCaffery JM, Desai PJ. Interactions of the Kaposi’s Sarcoma-associated herpesvirus nuclear egress complex: ORF69 is a potent factor for remodeling cellular membranes. J Virol. 2013;87:3915–3929. doi: 10.1128/JVI.03418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sam MD, Evans BT, Coen DM, Hogle JM. Biochemical, biophysical, and mutational analyses of subunit interactions of the human cytomegalovirus nuclear egress complex. J Virol. 2009;83:2996–3006. doi: 10.1128/JVI.02441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lötzerich M, Ruzsics Z, Koszinowski UH. Functional domains of murine cytomegalovirus nuclear egress protein M53/p38. J Virol. 2006;80:73–84. doi: 10.1128/JVI.80.1.73-84.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milbradt J, Auerochs S, Sevvana M, Muller YA, Sticht H, Marschall M. Specific residues of a conserved domain in the N terminus of the human cytomegalovirus pUL50 protein determine its intranuclear interaction with pUL53. J Biol Chem. 2012;287:24004–24016. doi: 10.1074/jbc.M111.331207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milbradt J, Kraut A, Hutterer C, Sonntag E, Schmeiser C, Ferro M, Wagner S, Lenac T, Claus C, Pinkert S, et al. Proteomic analysis of the multimeric nuclear egress complex of human cytomegalovirus. Mol Cell Proteomics. 2014;13:2132–2146. doi: 10.1074/mcp.M113.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paßvogel L, Klupp BG, Granzow H, Fuchs W, Mettenleiter TC. Functional characterization of nuclear trafficking signals in pseudorabies virus pUL31. J Virol. 2015;89:2002–2012. doi: 10.1128/JVI.03143-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang L, Baines JD. Identification of an essential domain in the herpes simplex virus 1 UL34 protein that is necessary and sufficient to interact with UL31 protein. J Virol. 2005;79:3797–3806. doi: 10.1128/JVI.79.6.3797-3806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Hagen C, Dent KC, Zeev-Ben-Mordehai T, Grange M, Bosse JB, Whittle C, Klupp BG, Siebert CA, Vasishtan D, Bäuerlein FJB, et al. Structural basis of vesicle formation at the inner nuclear membrane. Cell. 2015;163:1692–1701. doi: 10.1016/j.cell.2015.11.029. In this study, electron cryo-microscopy and tomography of PrV-infected cells and of cellular vesicles induced by the PrV NEC revealed a distinct hexagonal lattice into which the NEC could be modeled in a hexameric arrangement, supporting the role of this arrangement in the mechanism of budding through the inner nuclear membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klupp BG, Granzow H, Fuchs W, Keil GM, Finke S, Mettenleiter TC. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc Natl Acad Sci USA. 2007;104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Bigalke JM, Heuser T, Nicastro D, Heldwein EE. Membrane deformation and scission by the HSV-1 nuclear egress complex. Nat Commun. 2014;5:4131. doi: 10.1038/ncomms5131. This study showed for the first time that a purified NEC (from HSV-1) could induce the formation of intraluminal vesicles within giant unilamellar vesicles, indicating that the NEC alone is sufficient to drive vesicle formation, at least in this cell-free system. Cryo-EM studies of these vesicles revealed ordered NEC coats on the membrane that suggested a possible mechanism for NEC-mediated budding and scission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roller RJ, Bjerke SL, Haugo AC, Hanson S. Analysis of a charge cluster mutation of herpes simplex virus type 1 UL34 and its extragenic suppressor suggests a novel interaction between pUL34 and pUL31 that is necessary for membrane curvature around capsids. J Virol. 2010;84:3921–3934. doi: 10.1128/JVI.01638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caspar DL, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 43•.Lorenz M, Vollmer B, Unsay JD, Klupp BG, García-Sáez AJ, Mettenleiter TC, Antonin W. A single herpesvirus protein can mediate vesicle formation in the nuclear envelope. J Biol Chem. 2015;290:6962–6974. doi: 10.1074/jbc.M114.627521. This study showed that purified PrV NEC and, interestingly, the nucleoplasmic protein alone artificially tethered onto membranes, could induce vesicle formation in giant unilamellar vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee C-P, Liu P-T, Kung H-N, Su M-T, Chua H-H, Chang Y-H, Chang C-W, Tsai C-H, Liu F-T, Chen M-R. The ESCRT machinery is recruited by the viral BFRF1 protein to the nucleus-associated membrane for the maturation of Epstein-Barr Virus. PLoS Pathog. 2012;8:e1002904. doi: 10.1371/journal.ppat.1002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee C-P, Liu G-T, Kung H-N, Liu P-T, Liao Y-T, Chow L-P, Chang L-S, Chang Y-H, Chang C-W, Shu W-C, et al. The ubiquitin ligase itch and ubiquitination regulate BFRF1-mediated nuclear envelope modification for Epstein-Barr virus maturation. J Virol. 2016;90:8994–9007. doi: 10.1128/JVI.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huet A, Makhov AM, Huffman JB, Vos M, Homa FL, Conway JF. Extensive subunit contacts underpin herpesvirus capsid stability and interior-to-exterior allostery. Nat Struct Mol Biol. 2016;23:531–539. doi: 10.1038/nsmb.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fradkin LG, Budnik V. This bud’s for you: mechanisms of cellular nucleocytoplasmic trafficking via nuclear envelope budding. Curr Opin Cell Biol. 2016;41:125–131. doi: 10.1016/j.ceb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]