Abstract
Introduction
Late revision nerve surgery for incomplete motor recovery due to partial reinnervation would improve muscle function only if all muscle fibers were protected from developing denervation atrophy.
Methods
Sixty immature Sprague-Dawley rats underwent the following tibial nerve manipulations (n=15/group): Group A, partial denervation (2/3 of nerve resected and remaining 1/3 crushed), revision repair at 8 months; Group B, partial denervation; Group C, complete denervation, immediate reconstruction; Group D, complete denervation, reconstruction at 8 months; Group E, control group. Final testing at 11 months included muscle force, weight, and histology.
Results
Muscle weight was significantly (P < 0.05) different among all groups (highest to lowest: E>B>C>A>D), and force was significantly lower in A and D compared to E. Muscle fiber cross-sectional area was statistically smaller in A than in B, C, or E.
Discussion
Partial reinnervation still allowed substantial muscle recovery, but it did not preserve the noninnervated muscle fibers.
Keywords: partial nerve injury, denervation, revision nerve repair, rodent, atrophy, muscle histology
Introduction
Suboptimal muscle recovery following peripheral nerve injury is not uncommon. Although some patients obtain satisfactory strength, many only achieve weak muscle contraction 1–3. The latter level of recovery is not functionally useful, yet indicates that some, though not enough, axons have reached the muscle for some re-innervation. In such situations, salvage options include tendon transfers, innervated local or free tissue transfers, accepting the deficit and encouraging the patient to “live with it”, or doing a delayed or re-do nerve reconstruction. Usually, by the time clinical recovery has been assessed and found to be inadequate, conventional teaching suggests that there is not enough regeneration time to do a “new” nerve repair. Conversely, the presence of partial reinnervation has been suggested to have a muscle preserving effect, in which case a “new” repair attempt may be worthwhile.
Medinaceli and Seaber, compared nerve repair techniques in a rat model and noted that re-operation on a nerve repair with initial unsatisfactory recovery failed to improve functional results. They felt that an unsuccessful repair resulted in loss of the nerve “blueprint”, and subsequent neurite regrowth was random and therefore, not satisfactory 4. Similarly, a study by Yoshimura et al., looked at 2 episodes of denervation/reinnervation (versus 1) and found a significant adverse effect on final motor strength 5. Yuksel et al. reported their experience in re-exploring 28 wrist injuries that involved the severance of at least three structures, one of which was the median or ulnar nerve (spaghetti wrists). With an average time since injury of 10 months, the authors noted that sensory improvement was gratifying, but motor recovery was unimpressive 6.
The idea that temporary early reinnervation could have a muscle preserving or protective effect was popularized initially by Terzis and referred to as the “baby sitter” concept7. Basically, when a prolonged reinnervation period is expected (i.e. long regeneration length), an axon source closer to the denervated muscle is provisionally substituted (via a preliminary and deliberately temporary nerve transfer), while the more desirable axons regenerate. When the preferred axon source has had time to regenerate toward the target muscle, a second surgery takes down the earlier “temporary” repair, and a second nerve coaptation is performed between the more desirable proximal stump and the target distal stump 7. Though the muscle undergoes 2 denervation processes, the total amount of time that the muscle cells are denervated is theoretically less. Animal data support this concept 8, though it is not clear what percentage of the muscle is being temporarily innervated.
Inadequate recovery following nerve repair or spontaneous recovery may be related to multiple factors, though insufficient numbers of regenerating axons making contact with appropriately matched muscle cells likely have a large role in most cases 9,10. Despite physiologic compensatory mechanisms, such as motor unit enlargement (each axon innervates increased numbers of muscle fibers compared with pre-injury levels) 11,12, a net decrease in the number of reinnervated muscle cells results in net muscle force reduction 9,13,14. Cederna et al. proposed that denervated muscle cells exist among the reinnervated muscle cells, adding mass but not function 14. After a certain period of time, denervated cells permanently lose the ability to regain meaningful function and suffer irreversible denervation atrophy 15,16. We hypothesize that in a partial but inadequate reinnervation scenario, those muscle cells not initially reinnervated will continue to degenerate. Taking down intact or regenerated axons to redo the repair will leave a mixed population of muscle cells. However, once a crucial time period has passed (from initial injury to final reinnervation) the percentage of “preserved” muscle cells will be irreversibly lowered, and even with marked increases in regenerating axons, the percentage of functional muscle recovery can only go down. Therefore, a delayed initial repair (or revision of an existing repair) despite partial spontaneous recovery will not significantly improve final motor recovery. Therefore the purpose of this study was to assess delayed initial repair and delayed re-do repair scenarios in various rodent models.
Materials and Methods
Sixty immature (3 month old) female Sprague-Dawley rats were utilized in this study after obtaining necessary approval from our institution’s animal review board in accordance with national guidelines. All animals were housed in a temperature and humidity controlled environment with 12:12 day-night cycle and were provided food and water ad libitum. Anesthesia for surgical procedures and terminal testing was induced with 5% isoflurane in a closed chamber and maintained using 2–3% isoflurane via nose cone inhalation. Post-operative analgesia was accomplished with subcutaneous administration of buprenorphine 0.5 mg/kg and was augmented by oral consumption of acetaminophen 272mg/100cc added to the drinking water.
Surgical Procedures
Four surgical groups (Groups A, B, C, and D) and 1 control group (Group E- the contralateral limb from group B) were utilized. Nerve exposure for each surgical cohort was performed with a standard biceps femoris-semitendinosis muscle splitting approach using proper sterile technique.
Partial Denervation Cohorts
Groups A (n=15) and B (n=15) served as the partial denervation cohorts. Initially, the left tibial nerve was isolated 15mm above the knee and divided longitudinally into thirds, leaving only the third closest to the fibular nerve intact. The 2/3 nerve segment furthest from the fibular nerve was split 5mm in either direction so that the cut nerve ends could be separated and buried in opposite muscles to prevent inadvertent regeneration. The intact 1/3 of the nerve then underwent a 5-second crush using micro forceps. This partial denervation technique has previously been reported in detail and has been shown to consistently result in weakened gastrocnemius muscles after adequate time for reinnervation17. Eight months after initial surgery, Group A underwent a second surgery in which the 1 centimeter (cm) of “altered” nerve was transected and repaired with a 1 cm autograft taken from the contralateral tibial nerve. Group B did not undergo a subsequent nerve repair, and the partially denervated nerve was allowed to recover without revision.
Full Denervation Cohorts
Groups C (n=15) and D (n=15) served as the full denervation cohorts. The left tibial nerve was isolated 15mm above the knee, and 1 cm of the entire nerve was excised. Group C underwent immediate reconstruction with 1 cm tibial nerve autograft from the contralateral leg. Eight months after initial surgery, Group D underwent a second surgery in which the 1 cm defect was repaired with a 1 cm autograft taken from the contralateral tibial nerve. All nerve manipulations were done under microscope magnification with 4 epineural stitches using 10-0 nylon suture where applicable. Group E was the contralateral limb from Group B and was utilized as a non-surgical control group.
Muscle Function Testing
Terminal muscle strength testing was performed 11 months after the initial surgery for all groups. Testing consisted of exposure of the tibial nerve and isolation of the medial gastrocnemius muscle and tendon (for all groups). The hind limb was secured to a platform via placement of Kirschner wires through the femoral condyle and the distal tibia, and the medial gastrocnemius tendon was transected and coupled to a force transducer using 4-0 silk suture. Strength testing was performed with a Grass stimulator (Model SD9, Astro-Med Inc., West Warwick, RI) and platinum electrodes. Stimulation was performed using 2 ms duration and 2 ms delay at varying voltages. Stimulus intensity and muscle fiber length were optimized as previously described17 and as recommended by Shin et al18. After optimization of muscle parameters, 3 supramaximal stimulations (5V, 1 Hz) were delivered to the sciatic nerve with 2-minute rest intervals between stimulations17. Contraction strength was converted to digital data using ADI Instruments Power Lab system (ADInstruments, Inc., Colorado Springs, CO) and recorded using a Sony VAIO laptop computer (Sony Corporation, Tokyo, Japan).
Average Muscle Fiber Cross-Sectional Area (CSA) Measurements
The medial gastrocnemius muscles were harvested and weighed prior to embedding in tissue freezing medium, flash frozen in 2-Methylbutane using liquid nitrogen, and stored at -80 degrees Celsius. Ten-micron sections were taken from the muscle 1 cm distal to its origin using a Thermoscientific Microm HM550 and stained with hematoxylin and eosin. Five equally spaced images (10X) were digitally captured per muscle sample (Olympus BX41), and the CSA of 25 muscle fibers (arranged in a 5×5 array) per image were manually measured (using MediaCybernetics software Image-Pro Plus v. 7.0). Samples and images were blinded for this analysis to remove bias by the analyzer.
At the conclusion of testing all animals were euthanized with an intraperitoneal injection of 150mg Euthasol and were disposed of according to our institutional policy.
Statistical Analysis
An a priori sample size estimation based on previously published data evaluating reductions in muscle force associated with decreased axonal regeneration 14 determined that 15 rats per group would be sufficient to find statistically significant differences at an alpha level of 0.05 and power (1-β) of 0.9.
In this study, surgical cohorts and the control group served as independent variables. Main outcome measures included developed force [Newtons (N)], muscle weight [grams (gm)], muscle fiber cross-sectional area [square microns (um2)], and muscle fiber cross-sectional area variability [standard deviation ((um2)]. One-way analysis of variance with post-hoc Tukey tests were performed to compare groups with an a priori level of significance set P<0.05. Data were analyzed using Statistical Package for Social Sciences (SPSS) version 20.0 (SPSS, Inc, Chicago, IL).
Results
Prior to the end-point of the experiment, some animals were euthanized due to their health post-surgery: 1 rat from Group A, 4 rats from Group C, and 5 rats from Group D.
Developed force (N)
Group means for developed force were as follows: Group A=0.656±0.279N, Group B=0.859±0.260N, Group C=0.854±0.613N, Group D=0.665±0.478N, and Group E=1.212±0.387N (Figure 1A). Delayed repair after 8 months in both the partial denervation (Group A, P=0.004) and complete denervation groups (Group D, P=0.012) resulted in significantly less force generation when compared to the control group (Group E).
Figure 1.
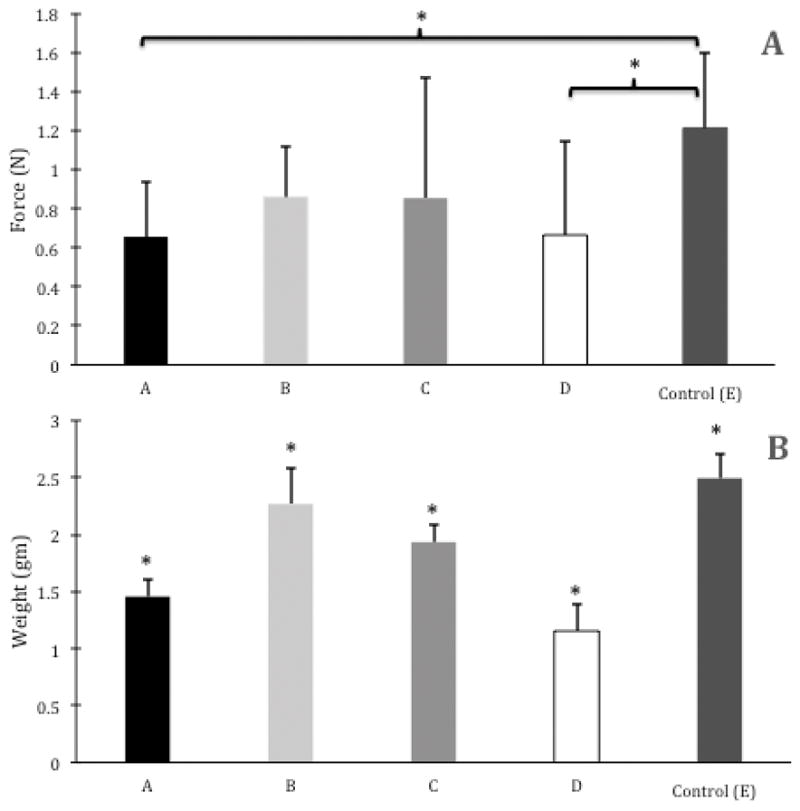
Group means ± 1 SD for (A) Developed Force (N) and (B) Muscle Weight (gm). Brackets and asterisks indicate significant differences between groups (P<.05). In B, no brackets are shown, because all pairwise comparisons were significant between groups (P<.05).
Muscle weight (gm)
All pairwise comparisons were significant at P <0.05 with the following sequence of largest to smallest muscle weights: Group E=2.494±0.211gm > Group B=2.268±0.313gm > Group C=1.934±0.151gm > Group A=1.456±0.143gm > Group D=1.154±0.227gm (Figure 1B).
Muscle fiber CSA (um2)
Muscle fiber CSA means were as follows: Group A=8886.0±0.002um2, Group B=15776.5±1780.6um2, Group C=14545.9±1567.5um2, Group D=12138.6±2172.3um2, and Group E=20293.1±4715.8um2 (Figure 2A). Group A had the smallest CSA and it was significantly smaller than Group B (P<0.001), Group C (P<0.001), and Group E (P<0.001). Group D had the second smallest CSA, and it was significantly smaller than Group B (P<0.049) and Group E (P<0.001). Lastly, Group B (P=0.002) and Group C (P<0.001) both had significantly smaller CSAs than the control group (Group E).
Figure 2.
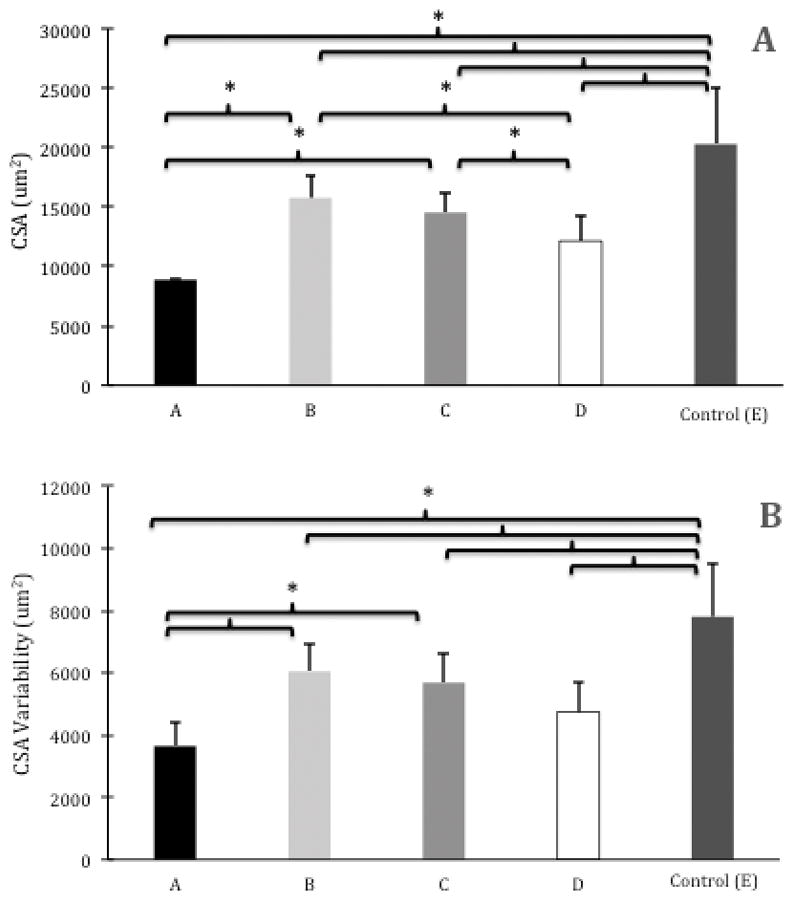
Group means ± 1 SD for (A) Muscle Fiber Cross-Sectional Area (CSA) (um2) and (B) Muscle Fiber CSA Variability [as measured by average standard deviation in muscle fiber CSA (um2)]. Brackets and asterisks indicate significant differences between groups (P<.05).
Muscle fiber CSA variability (standard deviation)
Muscle fiber CSA standard deviation means were as follows: Group A=3651.4±756.8, Group B=6051.4±893.9, Group C=5688.1±933.9, Group D=4746.3±945.7, and Group E=7808.0±1727.7 (Figure 2B). Group A had the least variability in muscle fiber CSA and significantly less variability than Group B (P<0.001), Group C (P=0.001), and Group E (P<0.001). Group E had the most muscle fiber CSA variability and had significantly more variability than all other groups (P<0.004 for all comparisons).
Discussion
Partial muscle recovery is an important functional problem often neglected by nerve researchers. Clinically, an injured nerve that seems to be regenerating is often “observed” with the expectation of continued muscle recovery. When this recovery is not realized and the muscle remains in a weakened state, there may be few options other than delayed repair or distal nerve transfer (which is not always possible). Prolonged denervation, however, leads to gradually more profound yet irreversible degradation in muscle fiber size and contractile protein composition19. The question of whether or not partial reinnervation blunts the development of denervation atrophy in muscle has not been answered.
The concept of purposeful partial “babysitting” innervation requires a donor nerve close to the target muscle that can be repaired to the distal nerve stump while the more distant but more desirable donor nerve is given time to regenerate. The early nerve transfer quickly innervates the target muscle and prevents denervation atrophy20 and is “taken down” when the percussion sign (indicating progressive axonal elongation) reaches the distal nerve stump, and the more desirable donor nerve is repaired to the distal stump. Since theoretically only the muscle fibers that have been innervated by the “babysitting” axons will be protected, the more complete the early innervation, the better the muscle preservation21.
Due to the remarkable regenerative capacity of the rodent nervous system, this clinical scenario is actually quite difficult to replicate. We previously established a technically reproducible model of partial innervation by transecting two-thirds of a rat tibial nerve over a 1 cm length and crushing the remaining third closest to the fibular nerve to produce temporary complete denervation. After allowing enough time for reinnervation to occur, several tibial innervated muscles were evaluated. The reinnervated medial gastrocnemius was consistently weakened compared to normal muscle, indicating that functionally significant partial denervation of this particular muscle had been achieved via this manipulation17. Though we used the same model in this study, the developed force between the partial denervation group (B) was not significantly different from the unmanipulated control group (E). During development of the partial denervation model, 2 months were allowed to elapse between nerve damage and final testing compared to 11 months in this study, and thus our current results pose a question about whether this added time could have allowed either progressive strengthening of the partially denervated medial gastrocnemius muscle or inadvertent regeneration of the transected portion of the tibial nerve. The ends of the transected tibial nerve were separated and sutured into opposite muscle bellies, but nerve histology to confirm consistently low distal axon counts was not performed. Furthermore, previous studies by Kalliainen et al.22 and van der Meulen et al.23 have demonstrated that both innervated and denervated rodent muscle fibers experience atrophy after partial denervation through 2-week and 4-month follow-ups, respectively. When comparing the developed force data after 2 months of denervation from our previous pilot study and after 11 total months of denervation in the current study, there was only a 4.1% difference.
The ability of rodent muscle to compensate for decreased axon counts has been reported9,24 but few studies follow rodents for so long post injury. Mersa et al. reported no adverse effect from prolonged denervation on the “babysitter” procedure but considered 3 months “prolonged denervation.”7 Indeed, the long follow up was felt necessary to ensure that denervation atrophy was established enough that some residual deficit would be present even with reinnervation. Previous studies suggest that in rodent models this occurs at detectable levels by one month16,25. After 8 months, the significant reduction in developed force in our prolonged denervation group (D) when compared to the control group demonstrated that denervation atrophy was successfully established in our study. However, we had slightly lower than anticipated sample sizes in groups A (n=14), C (n=11), and D (n=10) due to high rodent mortality prior to final data collection and thus we may have been underpowered to detect differences in muscle force between partial denervation with delayed repair (Group A), partial denervation without repair (Group B), and prolonged denervation (Group D) groups even though the final rodent numbers in this study were similar to others in the literature. Of particular interest is the head-to-head comparison of partial denervation with delayed repair (Group A) versus partial denervation without revision repair (Group B). Force generation was reduced 26.8% in Group A. However, statistical significance was only detected in this group when individually compared to the control group (Group E). Collectively, these results suggest that the subsequent re-do repair after partial re-innervation either has no added benefit or potentially degrades muscle function.
Muscle weight has been suggested as a more sensitive test in detecting differences in muscle atrophy16,23, and when focusing on this particular outcome parameter, our hypothesis is supported. The more established the denervation atrophy (i.e. the longer the period of denervation), the smaller the muscle fiber; and the more widespread the denervation, the greater the percentage of small muscle fibers. Even with complete denervation, immediate repair results in only minimal final deficit, and other studies have demonstrated similar high levels of return as seen in our rodents subjected to this manipulation26 (Group C). Prolonged complete denervation of 8 months, on the other hand, would be expected to result in all affected muscle fibers developing some level of permanent denervation atrophy limiting the final recovery size (Group D)9,27. For our hypothesis, the most important datum was the greater muscle weight in group B compared with group A. Though group A achieved some functional recovery (as evidence by the developed force for that group), the lower final muscle weight suggests greater overall atrophy. Muscle fibers not preserved over the 8 months between nerve damage and revision surgery would have a limited capacity for hypertrophy if reinnervated. All of the muscle fibers protected for the 8 months might not achieve re-innervation following the re-do repair, and even those that did would have been adversely affected by the second episode of denervation. Though partial innervation might seem a better option than immediate repair when based on the muscle weight data, this obviously would depend of the degree of partial innervation. Also considering the challenges of the rodent model, translation of this observation to human clinical scenarios should be done with caution.
Muscle histology was processed to support our findings based on muscle weight. We reasoned that in group B, there should be some larger (preserved) muscle fibers and some very small (atrophic) muscle fibers, and group E should have many large (and few small) muscle fibers. This was an over-simplification, since the normal muscle had the most variability. From a physiological standpoint, a spectrum of different muscle fiber types is normal, and a shift toward homogenicity would be expected after multiple episodes of denervation/reinnervation28, perhaps explaining the smallest variability of fiber CSAs in group A. Further interpretation of the variability noted in the muscle fibers across groups would be conjecture, and more sophisticated histologic staining will have to be used in the future to better understand this process. The mean muscle fiber size data, and in particular the smaller fiber CSA in group A compared to group B, support the conclusion that partial innervation failed to protect the muscle body as a whole. The lack of difference in CSA of the muscle fibers in group A versus group D was surprising, since we had expected many of the muscle fibers to have been preserved in group A. The added stress of 2 denervation episodes in Group A may have been more detrimental than the previous work on “babysitting” nerve transfers may have indicated.
We did not analyze nerve regeneration and may be missing alternative explanations for our findings. Fu and Gordon demonstrated a reduction in axonal regeneration into a distal nerve stump following prolonged denervation9. At least for Groups A and B in our study, the distal nerve stumps maintained some traversing axons, though the implications of this are not known.
In conclusion, though our study may have pushed the limits of the rodent animal model for nerve regeneration, the data in general support the hypothesis that once enough time has passed, reconstruction of a partially reinnervated nerve injury will not improve the final results and indeed, may be harmful. Exactly how much partial innervation has occurred and the exact amount of time of muscle denervation are important variables that cannot be easily translated from this small animal model to clinical practice. Further study would be necessary to better delineate treatment options for similar clinical scenarios and to better understand the potential detrimental effects of multiple episodes of denervation.
Acknowledgments
Microscopy was performed at the VCU – Dept. of Anatomy & Neurobiology Microscopy Facility, supported, in part, by funding from NIH-NINDS Center Core Grant 5 P30 NS047463 and, in part, by funding from the NIH-NCI Cancer Center Support Grant P30 CA016059.
Abbreviations
- cm
Centimeter
- um2
Square micron
- N
Newton
- gm
Gram
- CSA
Cross-sectional area
Footnotes
Financial Disclosure and Conflicts of Interest
Jonathan Isaacs has received speaking fees and is involved in contracted clinical research sponsored by AxoGen, Inc. The study was performed in a VCU lab that has received partial research support from AxoGen Inc., including partial funding for Jonathan Isaacs, Satya Mallu, and Gaurangkumar Patel. None of the financial relationships influenced the results of this study. There are no other financial disclosures or conflicts of interest for any other authors.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Sakallarides H. A follow-up study of 172 peripheral nerve injuries in the upper extremity in civilians. J Bone and Joint Surg (AM) 1962;44:140–148. [PubMed] [Google Scholar]
- 2.Jongen SJ, Van Twisk R. Results of primary repair of ulnar and median nerve injuries at the wrist: an evaluation of sensibility and motor recovery. Neth J Surg. 1988 Jun;40(3):86–9. [PubMed] [Google Scholar]
- 3.Millesi H, Meissl G, Berger A. Further experience with interfascicular grafting of the median, ulnar, and radial nerves. J Bone Joint Surg Am. 1976 Mar;58(2):209–18. [PubMed] [Google Scholar]
- 4.de Medinaceli L, Seaber AV. Experimental nerve reconnection: importance of initial repair. Microsurgery. 1989;10(1):56–70. doi: 10.1002/micr.1920100111. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura K, Asato H, Jejurikar SS, Cederna PS, Urbanchek MG, Kuzon WM., Jr The effect of two episodes of denervation and reinnervation on skeletal muscle contractile function. Plast Reconstr Surg. 2002 Jan;109(1):212–9. doi: 10.1097/00006534-200201000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Yuksel F, Peker F, Acikel C, CelIkoz B. Secondhand management of “spaghetti wrist”: do not hesitate to explore. Ann Plast Surg. 2002 Nov;49(5):500–4. doi: 10.1097/01.SAP.0000020131.54883.EE. discussion 504–5. [DOI] [PubMed] [Google Scholar]
- 7.Mersa B, Tiangco DA, Terzis JK. Efficacy of the “baby-sitter” procedure after prolonged denervation. J Reconstr Microsurg. 2000 Jan;16(1):27–35. doi: 10.1055/s-2000-7538. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Zhang W, Zhu W, Wu S. Experimental study on the improving effect of motor nerve babysitting on delayed nerve anastomosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008 Sep;22(9):1064–7. [PubMed] [Google Scholar]
- 9.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995 May;15(5 Pt 2):3886–95. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundborg G, Rosen B. Hand function after nerve repair. Acta Physiol (Oxf) 2007 Feb;189(2):207–17. doi: 10.1111/j.1748-1716.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 11.Luff AR, Hatcher DD, Torkko K. Enlarged motor units resulting from partial denervation of cat hindlimb muscles. J Neurophysiol. 1988 May;59(5):1377–94. doi: 10.1152/jn.1988.59.5.1377. [DOI] [PubMed] [Google Scholar]
- 12.Gordon T, Yang JF, Ayer K, Stein RB, Tyreman N. Recovery potential of muscle after partial denervation: a comparison between rats and humans. Brain Res Bull. 1993;30(3–4):477–82. doi: 10.1016/0361-9230(93)90281-f. [pii] [DOI] [PubMed] [Google Scholar]
- 13.Lien SC, Cederna PS, Kuzon WM., Jr Optimizing skeletal muscle reinnervation with nerve transfer. Hand Clin. 2008 Nov;24(4):445–54. vii. doi: 10.1016/j.hcl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Cederna PS, Youssef MK, Asato H, Urbanchek MG, Kuzon WM., Jr Skeletal muscle reinnervation by reduced axonal numbers results in whole muscle force deficits. Plast Reconstr Surg. 2000 May;105(6):2003–9. doi: 10.1097/00006534-200005000-00014. discussion 2010–1. [DOI] [PubMed] [Google Scholar]
- 15.Anzil AP, Wernig A. Muscle fibre loss and reinnervation after long-term denervation. J Neurocytol. 1989 Dec;18(6):833–45. doi: 10.1007/BF01187235. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, et al. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997 Jul;20(7):858–66. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs J, Mallu S, Wo Y, Shah S. A rodent model of partial muscle re-innervation. J Neurosci Methods. 2013 Sep 30;219(1):183–7. doi: 10.1016/j.jneumeth.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Shin RH, Vathana T, Giessler GA, Friedrich PF, Bishop AT, Shin AY. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery. 2008;28(6):452–7. doi: 10.1002/micr.20520. [DOI] [PubMed] [Google Scholar]
- 19.Gulati AK. Restoration of denervated skeletal muscle transplants after reinnervation in rats. Restor Neurol Neurosci. 1990 Jan 1;2(1):23–9. doi: 10.3233/RNN-1990-2103. [DOI] [PubMed] [Google Scholar]
- 20.Terzis JK, Tzafetta K. The “babysitter” procedure: minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast Reconstr Surg. 2009 Mar;123(3):865–76. doi: 10.1097/PRS.0b013e31819ba4bb. [DOI] [PubMed] [Google Scholar]
- 21.Kalantarian B, Rice DC, Tiangco DA, Terzis JK. Gains and losses of the XII-VII component of the “baby-sitter” procedure: a morphometric analysis. J Reconstr Microsurg. 1998 Oct;14(7):459–71. doi: 10.1055/s-2007-1000208. [DOI] [PubMed] [Google Scholar]
- 22.Kalliainen LK, Jejurikar SS, Liang LW, Urbanchek MG, Kuzon WM., Jr A specific force deficit exists in skeletal muscle after partial denervation. Muscle Nerve. 2002 Jan;25(1):31–8. doi: 10.1002/mus.1216. [DOI] [PubMed] [Google Scholar]
- 23.Malushte TS, Kerns JM, Huang CC, Shott S, Safanda J, Gonzalez M. Assessment of recovery following a novel partial nerve lesion in a rat model. Muscle Nerve. 2004 Nov;30(5):609–17. doi: 10.1002/mus.20152. [DOI] [PubMed] [Google Scholar]
- 24.Tam SL, Archibald V, Jassar B, Tyreman N, Gordon T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001 Jan 15;21(2):654–67. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aydin MA, Mackinnon SE, Gu XM, Kobayashi J, Kuzon WM., Jr Force deficits in skeletal muscle after delayed reinnervation. Plast Reconstr Surg. 2004 May;113(6):1712–8. doi: 10.1097/01.prs.0000118049.93654.ca. [DOI] [PubMed] [Google Scholar]
- 26.Isaacs J, Adams S, Mallu S, Loveland K, Sandbulte Z. Comparison of the performance of chronically versus freshly denervated autograft in nerve repair. J Hand Surg Am. 2010 Dec;35(12):2001–7. doi: 10.1016/j.jhsa.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 27.Carlson BM. The Denervated Muscle: 45 years later. Neurol Res. 2008 Mar;30(2):119–22. doi: 10.1179/174313208X281127. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura K, Asato H, Cederna PS, Urbanchek MG, Kuzon WM. The effect of reinnervation on force production and power output in skeletal muscle. J Surg Res. 1999 Feb;81(2):201–8. doi: 10.1006/jsre.1998.5498. [DOI] [PubMed] [Google Scholar]


