Abstract
Background
The pancreatic- and brain-derived hormone amylin promotes negative energy balance and is receiving increasing attention as a promising obesity therapeutic. However, the neurobiological substrates mediating amylin’s effects are not fully characterized. We postulated that amylin acts in the lateral dorsal tegmental nucleus (LDTg), an understudied neural-processing hub for reward and homeostatic feeding signals.
Methods
We used immunohistochemical (IHC) and quantitative PCR analyses to examine expression of the amylin receptor complex in rat LDTg tissue. Behavioral experiments were performed to examine the mechanisms underlying the hypophagic effects of amylin receptor activation in the LDTg.
Results
IHC and quantitative PCR analyses show expression of the amylin receptor complex in the LDTg. Activation of LDTg amylin receptors by the agonist salmon calcitonin dose-dependently reduces body weight, food intake, and motivated feeding behaviors. Acute pharmacological studies and longer-term adeno-associated viral (AAV)-knockdown experiments indicate that LDTg amylin receptor signaling is physiologically and potentially pre-clinically relevant for energy balance control. Finally, IHC data indicate that LDTg amylin receptors are expressed on gamma-aminobutyric acid (GABA)-ergic neurons and behavioral results suggest that local GABA receptor signaling mediates the hypophagia following LDTg amylin receptor activation.
Conclusions
These findings identify the LDTg as a novel nucleus with therapeutic potential in mediating amylin’s effects on energy balance through GABA receptor signaling.
Keywords: food intake, motivated behavior, obesity, reward, calcitonin, IAPP
Introduction
In the search for effective pharmacological treatments for obesity, much attention has focused on neuroanatomical targets in the central nervous system (CNS) (1) such as the hypothalamus and caudal brainstem, each historically linked with the homeostatic regulation of energy balance (2–7). While these studies have informed the field about cellular and molecular mechanisms mediating the metabolic effects of many gastrointestinal- and adipose tissue-derived hormones, the chronic hyperphagia underlying human obesity is not related to disproportionate homeostatic feeding, but rather is more likely based on excessive appetitive and motivational processes directed towards the consumption of highly palatable/rewarding food (8–11). Indeed, targeting non-homeostatic/reward-based systems may provide a unique opportunity to treat obesity and metabolic diseases (12, 13). Urgently needed, however, is a deeper understanding of the relevant CNS reward circuitry and how it responds to and integrates energy balance signals to control food intake and body weight.
The lateral dorsal tegmental nucleus (LDTg) (14) is a nucleus in the caudal midbrain that is uniquely positioned as a processing hub for the integration of reward-based and homeostatic energy balance signaling (14–16), yet has been understudied for its role in feeding and other motivational processes. Indeed, the LDTg has reciprocal projections with many feeding-relevant nuclei throughout the neuraxis, including but not limited to, the nucleus tractus solitarius (NTS) (17), the ventral tegmental area (VTA) (16), the lateral hypothalamus (LH) (16), and the parabrachial nucleus (PBN) (14). Given that the LDTg expresses receptors for a variety of feeding peptides (e.g. amylin, ghrelin, glucagon-like peptide-1, peptide YY) (16, 18–20), we hypothesize that energy balance-relevant neuroendocrine signals may act directly in the LDTg to modulate the neural processing of feeding-relevant information and affect motivational aspects of food reward.
Following initiation of a meal, a cascade of endocrine events occurs, including secretion of the peptide hormone amylin from the pancreatic β cells. Amylin activates its receptors within the CNS to suppress ongoing feeding during the meal and increase satiation (21, 22). Historically, the contribution of central amylin signaling to food intake control has centered on its action in homeostatic feeding centers, primarily the area postrema (AP) of the caudal brainstem (23–30), and secondarily in hypothalamic subnuclei including the arcuate nucleus (ARH) and ventromedial hypothalamus (VMH) (31–33). However, recent work has also established the VTA and nucleus accumbens (NAc) as relevant nuclei for amylin’s energy balance effects, particularly for reward-based feeding (34–36). While this growing body of literature highlights a more distributed CNS system mediating amylin’s energy balance effects than originally thought, the action of amylin in these aforementioned nuclei cannot wholly explain the energy balance and food reward effects of amylin signaling (23, 37, 38). In fact, as the neural control of energy balance is distributed across the CNS (2, 39) and in vitro radiography studies show that amylin binds to sites throughout the brain (40, 41), the ability of amylin receptor signaling in other CNS nuclei to produce hypophagia requires more extensive evaluation.
Given that amylin is being considered as an anti-obesity therapeutic, it is critical to more fully understand the neural substrates mediating amylin’s effects on reward-based feeding in addition to its impact on homeostatic intake (21, 42–45). That the LDTg binds amylin (41) and is widely connected with a variety of energy balance-relevant nuclei (14), collectively supports our hypothesis that amylin receptor signaling in the LDTg may control food intake, body weight, and motivated behaviors directed towards food reward. Thus, data presented here lend greater insight into amylin receptor singling through the CNS by identifying the LDTg as a novel nucleus mediating the anorexigenic effects of amylin, while underscoring the LDTg-Gamma Aminobutyric acid (GABA)-ergic system as a potential target for amylin-based therapies for the treatment of obesity.
Materials and Methods
Details regarding all drugs used, stereotaxic surgery, quantitative PCR, immunohistochemical analyses, colchicine treatment, co-localization of CTR and GABAergic markers, all behavioral experiments, and detailed statistical analyses are available in the Supplement.
Animals
Male Sprague-Dawley rats (310–325g upon arrival; Charles River, Wilmington, MA, USA) individually housed in hanging wire cages (12h light/dark cycle) had ad libitum access to chow (Purina LabDiet 5001) and water unless otherwise noted. For experiments labeling GABAergic neurons, male Sprague-Dawley rats (250g upon arrival; Envigo Labs) individually housed in hanging wire cages (12h light/dark cycle) had ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at University of Pennsylvania or University of Southern California and were performed according to the National Institutes of Health guidelines.
Behavioral testing
General procedures
Drug injections were made prior to the onset of the dark cycle unless otherwise specified. For experiments measuring ad libitum food intake, weights of food hoppers were recorded to the nearest 0.1g and food spillage is accounted for in cumulative food intake measurements. Food intake was recorded at 1, 3, 6, and 24h after injection, while body weight was measured at 0 and 24h after injection, except where noted. Injections were administered using a within-subjects counterbalanced design and were separated by at least 72h.
Statistical Analyses
All data are represented as mean ± SEM. The α level was set to p≤0.050 for all studies. Statistical analyses were performed using Statistica (StatSoft).
Results
The components of the amylin receptor complex are expressed in the LDTg
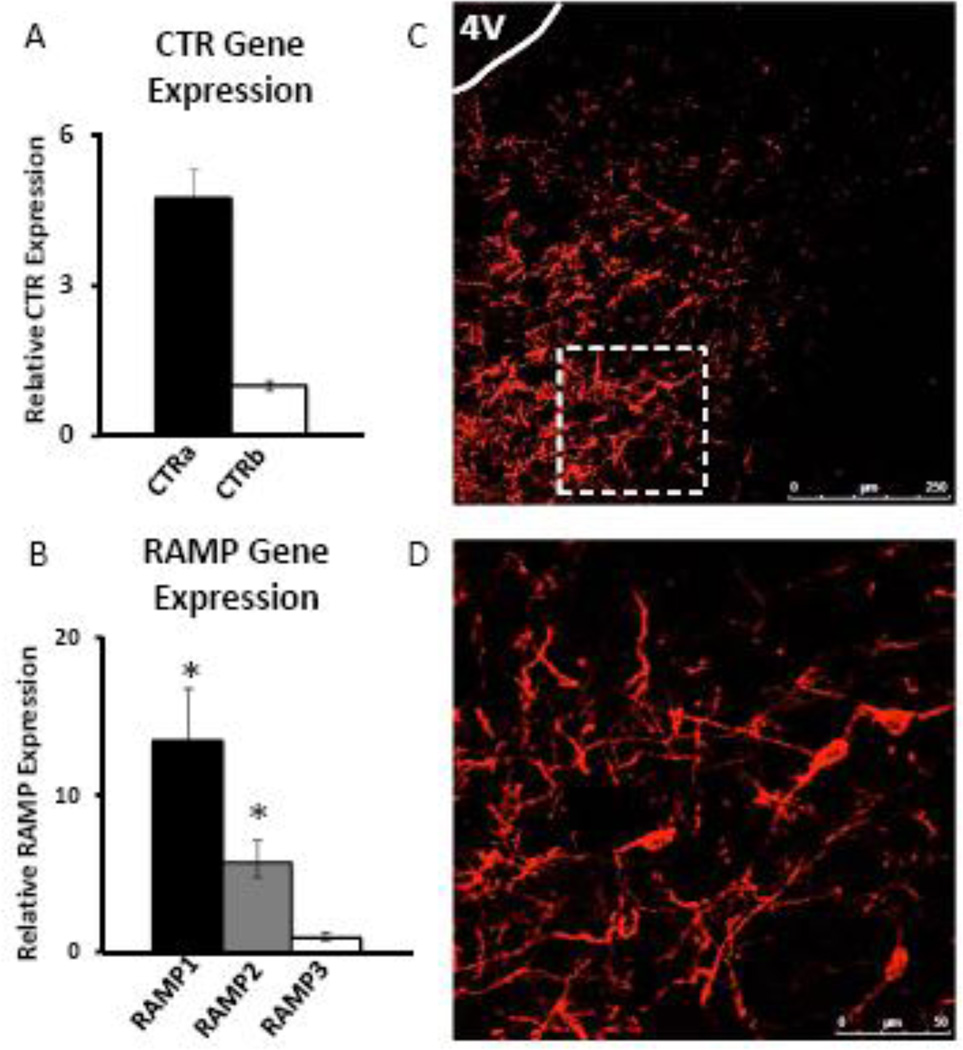
Amylin receptors are formed by heteromeric interaction between one of two Gs/Gq-coupled calcitonin receptors (CTRa or CTRb) and one of three receptor activity-modifying proteins (RAMP1–3) (56, 57). Although the LDTg binds amylin (41), no studies to date have examined expression of the amylin receptor complex within this nucleus. Therefore, we used quantitative real-time PCR to determine expression of the components of the amylin receptor (CTRa/b, RAMP1–3) in the LDTg, and found that both CTRs and all three RAMPs are indeed expressed in this nucleus (n=6). CTRa gene expression is approximately 5-fold greater than expression of CTRb, although this does not reach statistical significance (F1,3=4.04, p=0.14; Figure 1A). Gene expression of RAMP1 is approximately 2-fold greater than the expression of RAMP2 (F2,6=13.04, p<0.01; posthoc test, p<0.05) and approximately 13-fold greater than the expression of RAMP3 (posthoc test, p<0.01; Figure 1B). These findings are consistent with data from the AP and VTA, which also show higher expression of CTRa compared to CTRb and abundant RAMP1 expression (21, 35).
Figure 1. The components of the amylin receptor complex are expressed in the LDTg.
Micropunches of LDTg-enriched tissue (n=6) show expression that gene expression of CTRa is ~5 fold higher than CTRb (A), and gene expression of RAMP1 is ~2-fold higher than RAMP2 and ~13-fold higher than RAMP3 (B). Immunohistochemical data using CTR to label amylin receptor-expressing cells (n=6) show dense labeling of cell bodies and projections in the caudal LDTg (C, D). The dotted box in C (20×) represents the field of view in D (20× with a 2× optical zoom). * indicates significance by repeated measures ANOVA (p<0.05).
Next, we performed immunohistochemical (IHC) analyses to label cells that express CTR (n=6). Data show labeling throughout the rostral-caudal axis of the LDTg, with particularly dense labeling in the caudal LDTg (8.6–9.1mm posterior to bregma), providing evidence of amylin receptor expression at the protein level. Representative images from the caudal LDTg are shown in Figure 1C–D. Together, data in Figure 1 show that components of the amylin receptor complex are expressed in the LDTg at the gene and protein levels. Due to the dense CTR expression observed in the caudal LDTg, we targeted this subregion in our behavioral experiments.
LDTg amylin suppresses cumulative chow intake and body weight
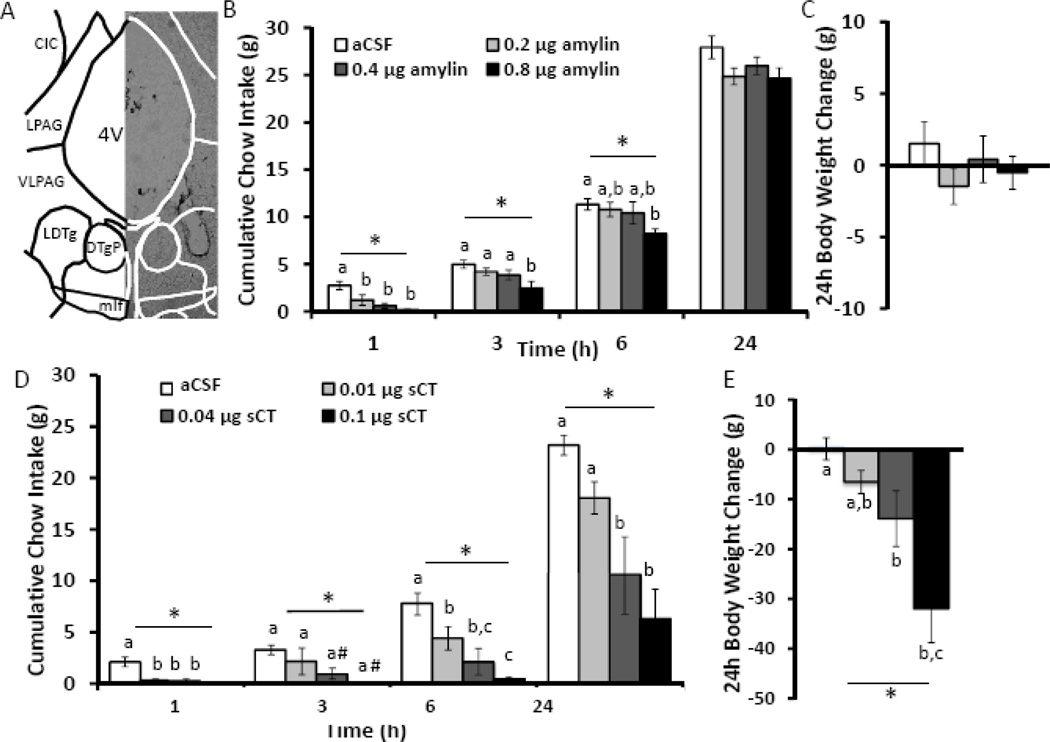
To test whether activation of amylin receptors in the LDTg by the native amylin peptide is sufficient to decrease food intake, rats (n=10) were unilaterally injected in the LDTg with amylin (0, 0.2, 0.4, and 0.8µg; 100 nl aCSF; see Figure 2A for representative injection placement) and subsequent chow intake and body weight change were recorded over a 24h period. Injection of amylin in the LDTg dose-dependently decreases food intake over 6h (F3,27≥3.00, p<0.05; Figure 2B) but not 24h food intake or body weight change (F3,27≤2.32, p>0.05; Figure 2C). Consistent with previous reports of amylin-induced hypophagia at early time points (49, 58), all doses of amylin administered to the LDTg suppress chow intake at 1h (p<0.01), but only the highest dose of amylin (0.8µg) suppresses chow intake at 3 and 6h after injection (p<0.05) compared to aCSF treatment.
Figure 2. Intra-LDTg amylin receptor activation dose dependently suppresses chow intake and body weight.
Amylin was unilaterally injected into the LDTg in a counterbalanced within-subjects design at the onset of the dark cycle using the following doses: 0 (aCSF), 0.2, 0.4, and 0.8 µg (n=10). A representative image of the LDTg injection site from a 35 µm thick section is shown (A). These doses of amylin dose-dependently decrease chow intake over 6h but have no effect on 24h chow intake (B) or body weight change (C). The key in B also applies to C. In a separate cohort of rats, the amylin receptor agonist sCT was unilaterally injected into the LDTg in a counterbalanced within subjects design at the onset of the dark cycle using the following doses: 0 (aCSF), 0.01, 0.04, and 0.1 µg (n=6). These doses of sCT suppress chow intake at every time point tested over 24h (D) and decrease 24h body weight gain (E). * indicates significance by repeated measures ANOVA (p<0.05), # indicates a trend for significance by post-hoc Neuman-Keuls (p<0.1). Different letters are significantly different from each other (p<0.05) according to post-hoc tests. The key in D also applies to E. Atlas image is −8.7 mm from bregma, based on Paxinos & Watson, 2007. 4V = 4th ventricle, CIC = central nucleus inferior colliculus, DTgP = Dorsal tegmental nucleus pericent, LDTg= lateral dorsal tegmental nucleus, LPAG = lateral periaqueductal gray, mlf = medial longitudinal fasciculus, VLPAG = ventral lateral periaqueductal gray.
LDTg amylin receptor activation suppresses cumulative chow intake and body weight
To determine whether pharmacological LDTg amylin receptor activation with the long-acting amylin receptor agonist sCT produces more durable and more potent hypophagic effects, sCT (0, 0.01, 0.04, or 0.1µg), was injected unilaterally into the LDTg and subsequent chow intake and body weight change were recorded over a 24h period (n=6). Notably, the two lower doses of sCT, 0.01 and 0.04 µg, are subthreshold for prolonged effects on food intake and body weight when applied to the 3rd ventricle (35). Results of this study show that intra-LDTg amylin receptor activation with sCT dose-dependently suppresses chow intake at 1, 3, 6, and 24h after injection (F3,12≥3.66, p<0.05; Figure 2B). Post-hoc analyses reveal that all 3 doses of sCT produce a significant suppression of chow intake, compared to aCSF vehicle treatment, at 1h (p<0.01) and 6h (p<0.05) after injection. Additionally, the two highest sCT doses (0.04 and 0.1 µg) decrease food intake at 24h after injection (p<0.05). Body weight gain over the 24h post-injection is also significantly reduced by intra-LDTg administration of 0.04 or 0.1 µg sCT (F3,12=11.00, p<0.01; compared to aCSF, p<0.05; Figure 2C). These data indicate that LDTg amylin receptor activation dose-dependently suppresses chow intake and body weight over 24h. Taken together and consistent with previous literature (51, 59), LDTg amylin receptor activation with sCT results in more potent and longer-lasting hypophagic effects than LDTg administration of native peptide amylin.
LDTg amylin receptor activation suppresses meal size
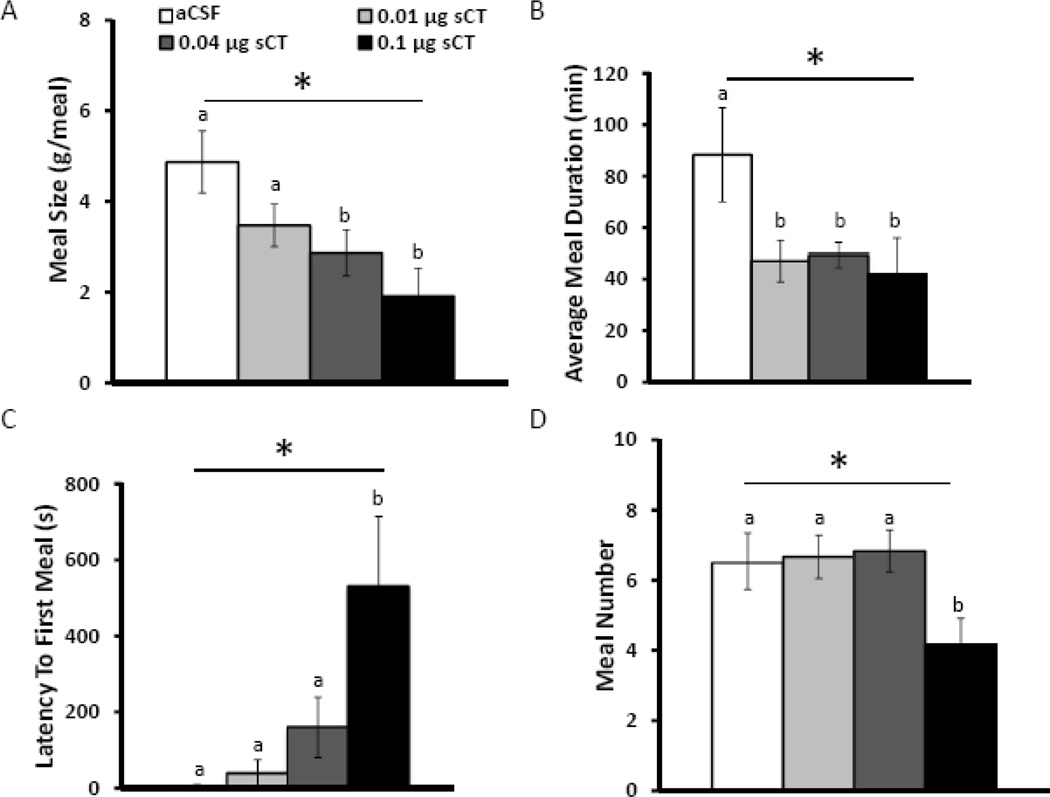
To evaluate the behavioral mechanisms driving the hypophagia following LDTg amylin receptor activation, meal patterns were analyzed (n=5). Unilateral injection of sCT in the LDTg at doses effective for reducing overall intake (0, 0.01, 0.04, or 0.1 µg) significantly suppresses meal size 24h after injection (F3,15=5.18, p<0.01; Figure 3A). Post-hoc analyses reveal that administration of the two highest doses, 0.04 and 0.1 µg sCT, significantly decreases meal size compared to aCSF treatment (p<0.05), consistent with the established role of amylin as a satiation signal (21, 34, 55). Along with this suppression in meal size, intra-LDTg administration of sCT also reduces meal duration at all doses tested (F3,15=5.51, p<0.01; p<0.05, compared to aCSF treatment; Figure 3B). LDTg amylin receptor activation increases latency to first meal (F3,15=4.90, p<0.05; Figure 3C) at the highest dose (p<0.05, compared to aCSF treatment), which indicates a decreased motivation to initiate feeding. Intra-LDTg administration of sCT decreases meal number over 24h after injection (F3,15≥3.77, p<0.05: Figure 3D), but only with the highest dose, 0.1 µg sCT (p<0.05, compared to aCSF treatment). These data show that LDTg amylin receptor activation reduces food intake predominately via suppression of meal size rather than meal number. Importantly, this reduction in meal size is concomitant with a decrease in meal duration, which may be a consequence of reduced within-meal motivation to continue to feed and/or reflect the normal physiological characteristics of amylin’s effects on the behavioral satiation sequence.
Figure 3. Intra-LDTg amylin receptor activation predominately suppresses meal size rather than meal frequency.
To determine the behavioral mechanism driving intake suppression, animals were housed in a custom-made automated feedometer to analyze meal patterns. The amylin receptor agonist, sCT, was unilaterally injected into the LDTg in a counterbalanced within subjects design at the onset of the dark cycle using the following doses: 0 (aCSF), 0.01, 0.04, and 0.1 µg (n=5). Intra-LDTg sCT suppresses meal size over 24h at the two higher doses (A), but all 3 doses suppress average meal duration over 24h (B). Only the highest dose of sCT increases latency to first meal (C) and suppresses meal frequency over 24h (D). The key applies to all graphs. * indicates significance by repeated measures ANOVA (p<0.05), different letters are significantly different from each other according to post-hoc tests (p<0.05).
LDTg amylin receptor activation attenuates motivation for a palatable food
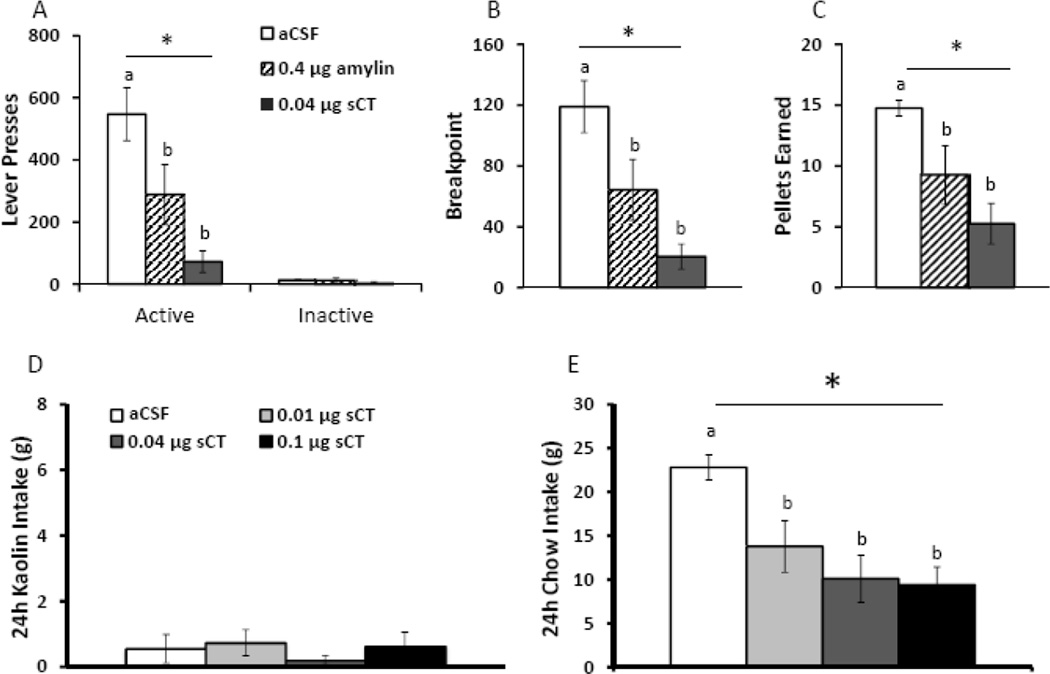
As the LDTg is a reward-relevant nucleus (15, 16), we tested the hypothesis that LDTg amylin receptor activation attenuates motivated feeding as measured by sucrose self-administration on a progressive ratio (PR) schedule of reinforcement (n=8). Unilateral injection of either the native peptide amylin (0.4µg) or amylin receptor agonist sCT (0.04 µg) into the LDTg significantly suppresses active lever responses for sucrose (F2,14=11.52, p<0.01: Figure 4A), breakpoint (F2,14=11.26, p<0.01: Figure 4B), and sucrose pellets earned (F2,14=7.72, p<0.01; Figure 4C) compared to aCSF treatment. Notably, there is no difference between treatments on inactive lever responding (F2,14=1.13, p=0.35; Figure 4A). These data indicate that LDTg amylin receptor activation, both with the potent amylin receptor agonist, sCT, and the native ligand, amylin, reduces motivation to self-administer a palatable food.
Figure 4. Intra-LDTg amylin receptor activation suppresses motivated feeding but does not produce malaise.
The ability of LDTg amylin receptor activation to reduce sucrose self-administration on a PR schedule of reinforcement was assessed (n=8). Intra-LDTg amylin receptor activation with amylin (0.4µg) or sCT (0.04µg) suppresses active lever presses (A), breakpoint (B), and pellets earned (C). To determine if LDTg amylin receptor activation produces nausea/malaise, pica (ingestion of non-nutritive substances in response to a noxious stimulus) was measured. Animals received access to both chow and kaolin clay for one week prior to the beginning of the experiment. The amylin receptor agonist, sCT, was unilaterally injected into the LDTg using the following doses: 0 (aCSF), 0.01, 0.04, and 0.1 µg (n=6). Intra-LDTg amylin receptor activation does not increase kaolin clay intake (D) but suppresses chow intake at 24h (E). Key in A applies to A, B and C; key in D applies to D and E. * indicates significance by repeated measures ANOVA (p<0.01); different letters are significantly different from each other according to post-hoc tests (p<0.05).
LDTg amylin receptor activation does not produce malaise
To determine if nausea/malaise contributes to the intake suppression following central amylin receptor activation, pica was measured after intra-LDTg sCT administration. Pica is the ingestion of non-nutritive substances, such as kaolin clay, and is a well-established model for nausea/malaise in non-vomiting species such as the rat (60–64). The same doses of sCT used in the previous behavioral studies (0, 0.01, 0.04, or 0.1 µg) were injected unilaterally in the LDTg, and intakes of chow and kaolin clay were measured 24h after injection (n=6). Intra-LDTg sCT does not increase kaolin intake at any dose (F3,15=0.98, p=0.45; Figure 4D), but all three doses significantly suppress chow intake at 24h compared to aCSF treatment (F3,15=7.93, p<0.01; post-hoc test, p<0.05, Figure 4E). These data suggest that the hypophagia and decreased motivation to feed following intra-LDTg amylin receptor activation are likely not due to induction of nausea/malaise.
LDTg amylin receptor blockade attenuates the intake suppressive effects of peripheral amylin receptor activation
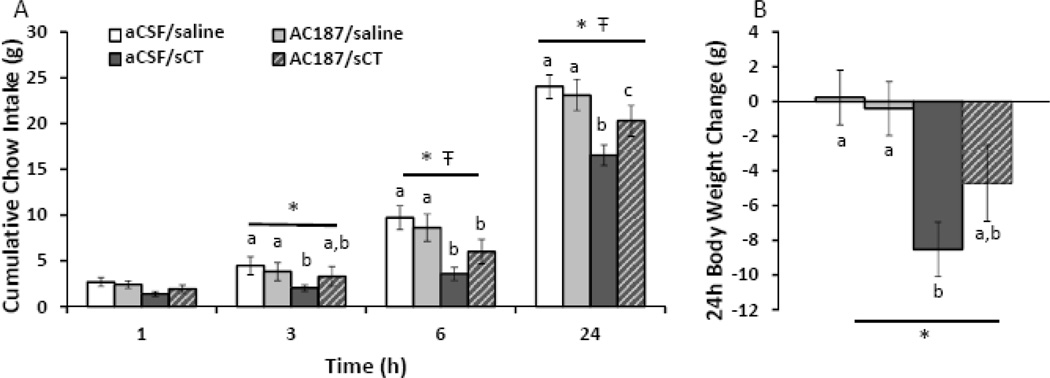
Given that pharmacological activation of amylin receptors directly in the LDTg suppresses food intake, the ability of peripherally administered amylin or amylin receptor agonists to access the CNS, and act specifically within the LDTg, is a key consideration in the development of amylin-based anti-obesity pharmaceuticals and denotes potential pre-clinical relevance in animal models and clinical relevance in humans. Thus, to begin to address this critical question using a pre-clinical rodent model, we evaluated whether the intake- and body weight-suppressive effects of systemic sCT (5 µg/kg, IP) would be attenuated by acute LDTg amylin receptor blockade. We intentionally chose a dose of the amylin receptor antagonist AC187 that is subthreshold for an effect on feeding when delivered bilaterally within the LDTg (0.8 µg/hemisphere; n=11) so as not to have competing orexigenic and anorexic behavioral responses. As expected, systemic administration of sCT significantly suppresses cumulative chow intake at 3, 6, and 24h after injection (main effects of sCT, F1,10≥11.31, p<0.01; planned comparisons of aCSF/sCT versus aCSF/saline or AC187/saline at 3, 6, and 24h, p<0.05; Figure 5A). A significant interaction between sCT and AC187 occurs at 6h and 24h after injection (F1,10≥5.20, p<0.05); post hoc analyses reveal that pre-treatment with intra-LDTg AC187 significantly attenuates the intake-suppressive effects of peripheral sCT at 24h (p<0.05). Systemic administration of sCT also decreases 24h body weight gain (F1,10=20.30, p<0.01, main effect of sCT; Figure 5B). Treatment with aCSF/sCT suppresses 24h body weight gain compared to aCSF/saline and AC187/saline conditions (planned comparisons, p<0.05). Importantly, amylin receptor blockade alone (AC187/saline) does not significantly increase chow intake at any time point (no main effects of AC187, F1,10<1.61, p>0.2) or body weight (no main effect of AC187, F1,10=1.56, p>0.2). These data show that intra-LDTg amylin receptor blockade attenuates the intake-suppressive effects of a systemically delivered amylin receptor agonist, suggesting the potential pre-clinical relevance of LDTg amylin receptor signaling.
Figure 5. LDTg amylin receptor blockade attenuates the intake suppressive effects of an amylin receptor agonist.
To determine if LDTg amylin receptor signaling is pre-clinically relevant, the amylin receptor antagonist, AC187, was bilaterally injected in the LDTg (0.8µg/hemisphere) followed 45 minutes later by a systemic injection of sCT (5µg/kg, IP) shortly before the onset of the dark cycle (n=11). Pre-treatment of AC187 alone has no significant effect on chow intake or body weight at any time point. Administration of sCT significantly suppresses intake at 3, 6, and 24h (A) as well as 24h body weight gain (B). Pre-treatment of AC187 with sCT significantly attenuates the intake suppressive effects of systemically-delivered sCT. Legend applies to both graphs. * indicates a significant main effect of sCT by repeated measures ANOVA (p<0.01), Ŧ indicates a significant main interaction between sCT and AC187 by repeated measures ANOVA (p<0.05), and different letters are significantly different from each other according to post-hoc planned comparisons (p<0.05).
Knockdown of calcitonin receptors in the LDTg increases chow intake and body weight
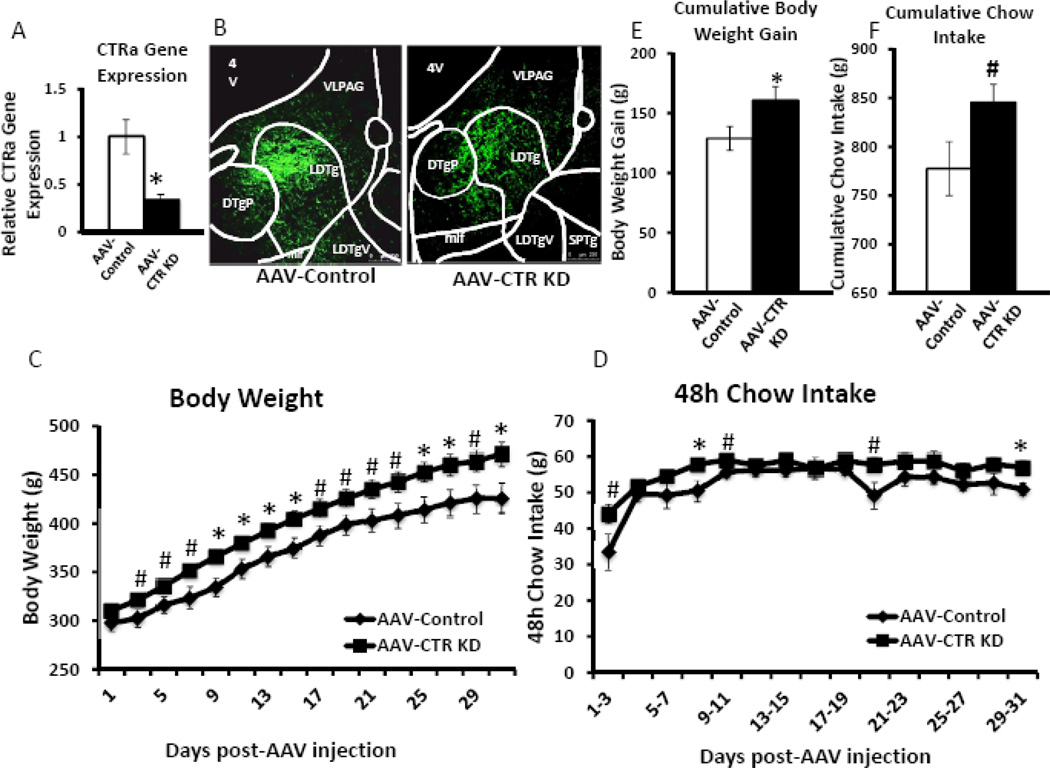
In order to determine if endogenous LDTg amylin receptor signaling is physiologically required for the normal day-to-day control of energy balance, an adeno-associated virus of serotype 1 (AAV1) that encodes a short hairpin RNA (shRNA) to knockdown CTR, the core component of the amylin receptor (AAV-CTR KD), or an empty vector control (AAV-Control) (34) was injected bilaterally into the LDTg (200nl/hemisphere). Compared to AAV-Control animals, AAV-CTR KD decreases LDTg CTRa expression by approximately 67% (Figure 6A, F1,10=5.43, p<0.05). Representative GFP visualization of viral targeting and spread from a separate cohort of animals sacrificed two weeks after bilateral LDTg viral injection (n=3/viral condition) is shown in Figure 6B.
Figure 6. CTR knockdown in the LDTg produces sustained increases in body weight and chow intake.
To determine if LDTg amylin receptor signaling is physiologically relevant for the long-term control of food intake and body weight regulation, an AAV that knocks down the core component of the amylin receptor, the CTR (AAV-CTR KD), or an empty vector AAV (AAV-Control) was injected bilaterally in the LDTg (200nl/hemisphere). Food intake and body weight was measured every 48h for 31 days following viral injection (n=7/viral condition). (A) Compared to AAV-Control, the AAV-CTR KD produces a statistically significant 67% decrease of CTRa. A separate cohort of animals received either virus (n=3/viral condition), were sacrificed two weeks later, and the brains were processed for GFP visualization. Representative images show GFP labeling of viral expression in AAV-Control (left) and AAV-CTR KD (right) (B). In behavioral studies, AAV-CTR KD produces an increase in body weight that was sustained over the behavioral test period (C, E). Chow intake is transiently increased in AAV-CTR KD animals compared to AAV-Control animals when graphed in 48 bins (D), and trending for significance when graphed cumulatively over the entire behavioral test period (F, p<0.1). * indicates significance by ANOVA (p≤0.050), # indicates a trend for significance by ANOVA (p<0.1). 4V = 4th ventricle, DTPg = Dorsal tegmental nucleus pericent, LDTg= lateral dorsal tegmental nucleus, LDTgV = lateral dorsal tegmental nucleus ventral, mlf = medial longitudinal fasciculus, SPTg = subpeducuncular tegmental nucleus, VLPAG = ventral lateral periaqueductal gray.
Animals with LDTg amylin receptor knockdown show a sustained elevation in body weight compared to the AAV-Control rats (Figure 6C). ANOVAs show that AAV-CTR KD animals weigh more than AAV-Control animals, either approaching (F1,12≥3.30, p<0.1) or reaching (F1,12≥4.75, p<0.05) statistical significance on any given experimental test day, beginning 3 days after viral injection. When analyzed as cumulative body weight gain from D0 to D31, AAV-CTR KD produces a significant increase in body weight gain compared to AAV-Control (F1,12=4.73, p=0.050; Figure 6E).
AAV-CTR KD treatment causes small increases in 48h binned food intake (Figure 6D). ANOVAs show that AAV-CTR KD animals eat significantly more in 48h bins than AAV-Control animals on days 7–9 and 29–31 (F1,12≥5.78, p<0.05), with a trend for significance (F1,12≥3.50, p<0.1) on days 1–3, 9–11, and 19–21. When graphed cumulatively from day 0 to day 31, AAV-CTR KD rats have a trend for increased cumulative intake compared to AAV-Control rats (F1,12=3.92, p<0.1; Figure 6F). Together, these data show that endogenous amylin accesses the LDTg and establish a physiological role for LDTg amylin receptor signaling in the normal control of food intake and body weight regulation.
LDTg amylin receptors are expressed on GABAergic neurons
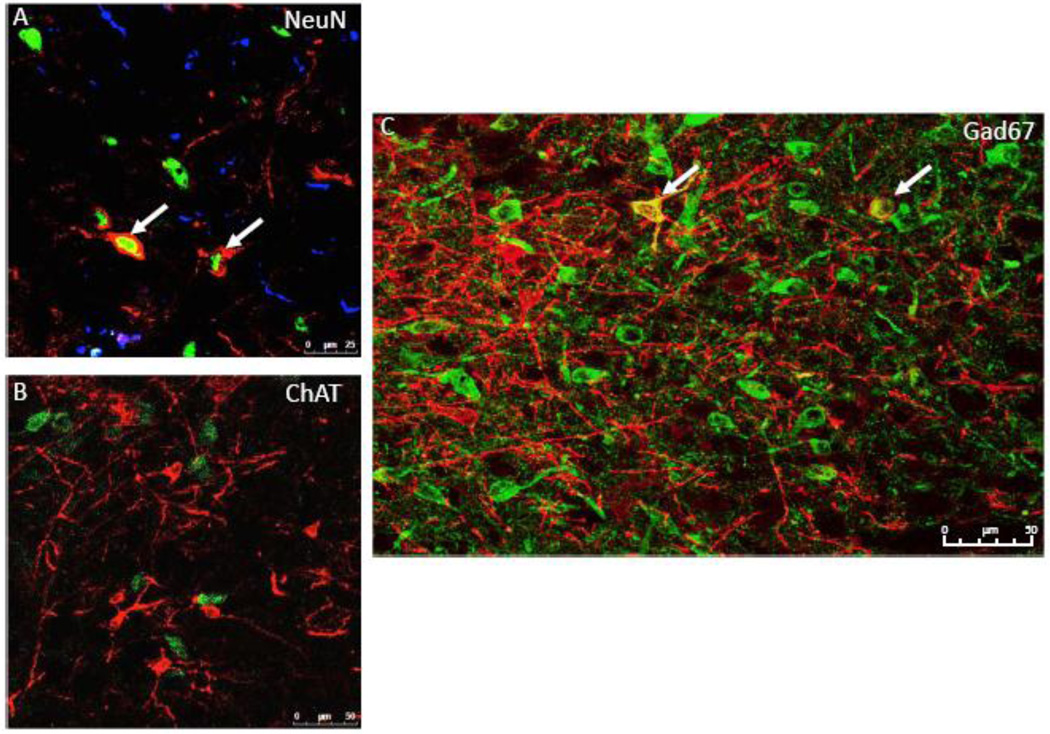
Next, we performed immunohistochemistry to determine the phenotype of amylin receptor-expressing cells in the LDTg. Sections were labeled for the amylin receptor (CTR), NeuN (a neuronal marker), and GFAP (a glia/astrocyte marker). CTR expressing cells in the LDTg co-localize exclusively with NeuN (Figure 7A), suggesting that amylin receptor-expressing cells in the LDTg are primarily, if not exclusively, neuronal (n=3).
Figure 7. CTR-expressing cells in the LDTg are GABAergic.
IHC analyses show that CTR-expressing cells in the LDTg co-localize with the neuronal marker NeuN and not with the glial cell marker GFAP (A; 20× with a 2× optical zoom; n=3). CTR-expressing cells in the LDTg do not co-localize with the cholinergic marker ChAT (B; 40×; n=6) but co-localize with the GABAergic marker Gad67 (C; 20×; n=1). Red = CTR-positive cells; Blue = GFAP-positive cells; Green = cellular marker of interest: NeuN (A), ChAT (B), Gad67 (C). White arrows indicate co-localization.
To begin to evaluate the phenotype of LDTg CTR-expressing neurons, further IHC experiments tested if the CTR-positive neurons within the LDTg are cholinergic or GABAergic, as these represent classic LDTg neurotransmitter phenotypes (65). Results indicate that CTR in the LDTg does not co-localize with ChAT, a marker for cholinergic neurons (Figure 7B; n=6). After colchicine treatment (47), 13.7% of CTR neurons in the LDTg, specifically in the caudal LDTg (−8.6mm to −9.1mm from bregma) co-localize with the GABAergic neuronal marker, Gad67 (Figure 7C; n=1). These data suggest that at least a portion of amylin receptor-expressing cells in the LDTg are GABAergic neurons, though we cannot rule out the possibility that colchicine treatment did not result in labeling of all Gad67 cells.
Intra-LDTg GABA-A/B receptor blockade reverses the intake-suppressive effects of LDTg amylin receptor activation
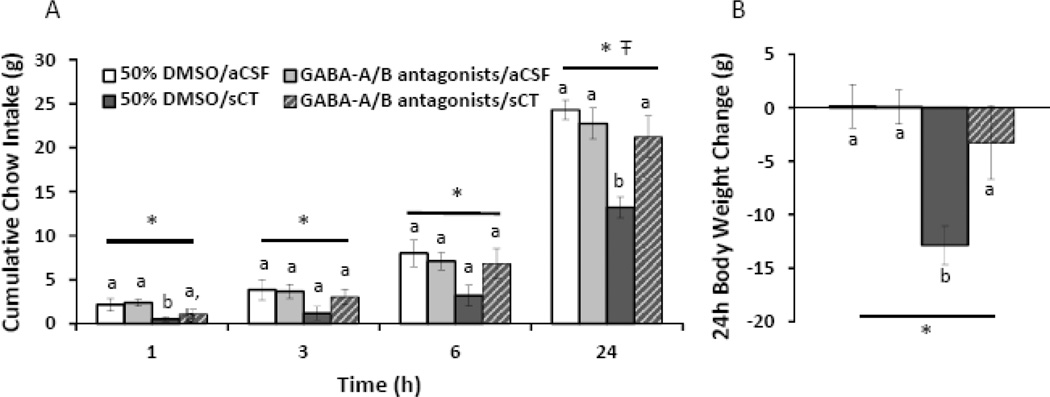
As our data show that LDTg amylin receptor signaling regulates food intake and body weight, and that a portion of LDTg amylin receptor-expressing cells are GABAergic, we next evaluated the hypothesis that LDTg GABA receptor signaling is downstream of LDTg amylin receptor activation and mediates LDTg amylin-induced hypophagia. To test this hypothesis, a cocktail composed of the GABA-A receptor antagonist bicuculline (100 ng) and the GABA-B receptor antagonist saclofen (500 ng) was unilaterally injected in the LDTg at doses subthrehold for an effect on feeding (100 nl, 50% DMSO in aCSF) followed by a unilateral injection of sCT (0.04µg; 100 nl, aCSF vehicle) in the ipsilateral LDTg; subsequent chow intake and body weight change were measured (n=8).
For cumulative chow intake (Figure 8A), repeated measures ANOVAs show a significant main effect of sCT at all timepoints (F1,7≥8.30, p<0.05) and a significant main interaction between sCT and GABA receptor blockade at 24h after injection (F1,7=7.47, p<0.05). Specifically, chow intake following 50% DMSO/sCT is significantly suppressed at 1 and 24h after injection, compared to all other conditions (planned comparisons, p<0.05). Importantly, intra-LDTg GABA-A/B receptor blockade does not affect feeding on its own (F1,7<4.70, p>0.1), but reverses the intake-suppressive effects of intra-LDTg sCT at 24h after injection (GABA-A/B receptor antagonists/sCT versus 50% DMSO/sCT, p<0.05; versus 50% DMSO/aCSF, p>0.4). Based on the feeding data, we analyzed the body weight change (Figure 8B) as a one-way repeated measures ANOVA by treatment (F3,21=7.77, p<0.01). Body weight gain following 50% DMSO/sCT treatment is significantly suppressed compared to all other treatments (p<0.01). Importantly, GABA receptor blockade alone (GABA-A/B receptor antagonists/aCSF) does not significantly alter body weight (p>0.9) compared to 50% DMSO/aCSF treatment. These data show that intra-LDTg GABA-A/B receptor blockade attenuates the anorexia produced by an intra-LDTg amylin receptor agonist.
Figure 8. Intra-LDTg GABA receptor blockade reverses the intake suppressive effects of intra-LDTg amylin receptor activation.
To determine the role of GABA receptor signaling in the intake suppressive effects of LDTg amylin receptor activation, a cocktail of a GABA-A receptor antagonist (bicuculline, 100 ng) and a GABA-B receptor antagonist (saclofen, 500 ng) was administered unilaterally in the LDTg followed by sCT (0.04µg; 100 nl; n=8). GABA receptor blockade reverses the intake (A) and body weight-suppressive effects (B). Key applies to both graphs. * indicates a significant main effect of sCT (A) or treatment (B) by repeated measures ANOVA (p<0.05), Ŧ indicates a significant main interaction between sCT and the GABA-receptor antagonists by repeated measures ANOVA (p<0.05), different letters are significantly different from each other according to post-hoc planned comparisons (p<0.05).
Discussion
The current obesity epidemic (66) highlights the urgent need to understand the neuroendocrine signals and neurobiological substrates that regulate energy balance, which in turn will inform the identification of novel opportunities for obesity pharmacotherapies. Recent attention has focused on targeting the amylin system for treating obesity, as the amylin analogue pramlintide is FDA-approved for diabetes and also decreases food intake and body weight in obese patients (11, 67). Although research on amylin’s effects on energy balance has predominately focused on hindbrain and hypothalamic structures [see (21, 43, 68) for review], in vitro radiography data show amylin binding sites are found throughout the brain (41), suggesting the likelihood of more distributed effects. The LDTg of the caudal midbrain represents one such amylin binding site; this nucleus receives information from and projects to several hindbrain, midbrain, and forebrain structures important for food intake, body weight regulation, and reward (14). Our experiments here show that the components of the amylin receptor complex are expressed in the LDTg and that amylin receptor signaling in the LDTg is important for the control of food intake and body weight regulation. Additionally, our data identify a portion of LDTg amylin receptor-expressing cells as GABAergic neurons that we speculate may be interneurons. These findings highlight the LDTg as a potential energy balance hub and show that this nucleus is of potential pre-clinical relevance as a neural substrate that can be targeted for future amylin-based pharmacotherapies for obesity.
Despite the fact that the LDTg receives information from and projects to a number of feeding- and reward-relevant nuclei throughout the brain (14) and expresses receptors for a variety of feeding peptides (e.g. amylin, ghrelin, glucagon-like peptide-1, peptide YY) (16, 18–20), little attention has been paid to this nucleus for its role in energy balance control. The current data showing that LDTg amylin receptor activation suppresses food intake and body weight is highly novel and consistent with the satiating properties following systemic or intracerebroventricular administration of amylin [see (21, 69) for review]. Importantly, the suppression in food intake by LDTg amylin receptor activation is not likely due to nausea/malaise as LDTg amylin receptor activation does not produce pica, suggesting the specificity of the energy balance effects. Two additional explanations underlying the body weight changes following LDTg amylin receptor activation are decreases in intestinal food weight and/or reductions in prandial drinking. The aforementioned experiments utilize the amylin receptor agonist sCT, which binds irreversibly with high affinity to amylin receptors but also with low affinity to calcitonin receptors (51, 70, 71). In contrast, amylin itself binds with moderate affinity to amylin receptors and with very low affinity to calcitonin receptors (57, 70). Furthermore, while we show evidence of gene expression of the amylin receptor complex in the LDTg, it is important to point out that the qPCR micropunch data cannot establish whether both components of the amylin receptor are expressed in the same cell. However, given that intra-LDTg amylin administration suppresses food intake and body weight in a dose-dependent manner, the current collective data suggest that complete amylin receptors are likely expressed in the LDTg and amylin receptor signaling is likely mediating the observed hypophagic response.
The within-meal intake inhibitory effects of LDTg amylin signaling may be explained by a reduction in the rewarding value of the ongoing meal. Indeed, LDTg amylin receptor activation not only suppresses the size of the meal, but also produces a concomitant decrease in meal duration, as well as a decrease in motivation to work for a palatable sucrose reward. The LDTg is reciprocally connected to both the NTS and VTA (14). Given the role of the NTS in meal size control [see (2, 6) for review] and the VTA in reward processing [see (12, 13) for review], the suppression of meal size observed after LDTg amylin receptor activation likely involves amylinergic modulation of NTS-LDTg-VTA neural processing. However, future systematic neuroanatomical studies are needed to confirm that amylin receptor-expressing LDTg neurons impinge on this proposed NTS-LDTg-VTA circuitry through putative LDTg GABAergic inhibition of the NTS-LDTg-VTA polysynaptic communication. Alternatively, the decreased PR responding may be a secondary response to LDTg amylin receptor signaling inducing satiation signaling more generally and potentially independent of reward signaling.
Previous studies established a role for the LDTg in reward processing for drugs of abuse and natural rewards (e.g. food, sex) through modulation of VTA dopaminergic cell firing (15, 16, 72–74). Our findings extend this literature on the role of the LDTg in modulating feeding behavior and energy balance, and provide novel evidence that LDTg signaling modulates the rewarding value of the ongoing meal. Given that activation of a LDTg-VTA pathway and its downstream targets can promote feeding and reward-associated behaviors, such as conditioned place preference and cocaine seeking (15, 16, 75–77), amylin receptor activation of inhibitory GABA neurons in the LDTg may decrease VTA dopaminergic cell firing, ultimately leading to hypophagia and a reduction in motivated feeding. Our IHC and behavioral data provide converging evidence in support of this hypothesis. We speculate that LDTg amylin receptors may be expressed on putative GABAergic interneurons, suggesting that LDTg amylin receptor activation could result in local inhibition of a variety of output neural pathways, including those projecting to the VTA. Future studies should therefore examine whether LDTg amylin receptor activation suppresses VTA activity in response to a food reward, and whether this outcome is in fact LDTg-GABA mediated.
Arguably one of the most important findings from the current data set is that acute blockade of LDTg amylin receptors attenuates the intake-suppressive effects of a systemic amylin receptor agonist. Though a significant attenuation of the intake-suppressive effects is not observed until the 24h timepoint, these data are comparable to a similar experiment performed in the VTA (35) in which effects were also only observed at 24h. In contrast to the VTA and LDTg, previous reports have shown that systemically delivered amylin agonists are able to activate AP amylin receptors more rapidly (22, 43). Thus, there appears to be a temporal difference in systemic amylin agonists’ action in distributed nuclei throughout the neuraxis that requires further investigation. Nevertheless, the data suggest that amylin receptor agonists administered systemically can access the LDTg, and thus LDTg amylin receptors may represent a pre-clinically relevant CNS population that can be targeted by peripherally-administered amylin receptor ligands for the treatment of obesity. Importantly, the dose of AC187 utilized here was selected to be subthreshold for an effect on food intake when administered in the LDTg. However, future experiments should conduct dose-dependent analyses of LDTg AC187 on food intake. Furthermore, the longer-term physiological role of LDTg amylin receptor signaling for energy balance control is supported by our study examining the effects of LDTg amylin receptor knockdown. Virogenetic knockdown of LDTg CTR increased body weight and food intake, suggesting that endogenous amylin can access the LDTg and that LDTg amylin receptors exert chronic control over energy balance. Interestingly, binned increases in food intake were modest compared to binned increases in body weight, suggesting an unexplored contribution of decreased energy expenditure following LDTg amylin receptor knockdown.
The novel findings here support the hypothesis that amylin receptor signaling in the LDTg is important for food intake and body weight regulation. These data highlight the importance of focusing further attention on this understudied nucleus in the field of obesity research. We have identified a subset of amylin receptor expressing cells in the LDTg are GABAergic neurons, which allows for future dissection of the downstream neurons and nuclei that are presumably inhibited by LDTg amylin receptor activation. As the LDTg also expresses receptors for other energy balance-relevant hormones (16, 18–20), future studies should explore how amylin signaling in the LDTg potentially interacts with other feeding-related signals to exert integrated control of energy balance and food reward.
Supplementary Material
Acknowledgments
This research was supported by MH014654 (DJR), DK105858 (DJR), DK103804 (EGM-B), DA037897 and DA039393 (HDS), DK104897 (SEK), DK096139 and DK105155 (MRH) and the University of Pennsylvania Research Foundation (MRH). The authors thank Misgana Ghidewon, Marissa Kamarck, Vaibhav Konanur, Rinzin Lhamo, John Maurer, Chan Nguyen, Tram Pham, and Evan Shaulson for valuable technical assistance. A portion of these data were presented at the 2014 annual meeting for the Society for the Study of Ingestive Behavior.
MRH receives research support from Novo Nordisk and MRH and EGM-B receive research support from Zealand Pharma that was not used in support of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 2.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell metabolism. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity. 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 4.Merlino DJ, Blomain ES, Aing AS, Waldman SA. Gut-Brain Endocrine Axes in Weight Regulation and Obesity Pharmacotherapy. Journal of clinical medicine. 2014;3:763–794. doi: 10.3390/jcm3030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz TA. Amylinergic control of food intake. Physiology & behavior. 2006;89:465–471. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Moran TH. Gut peptides in the control of food intake. International journal of obesity. 2009;33(Suppl 1):S7–S10. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 7.Moran TH, Ladenheim EE. Adiposity signaling and meal size control. Physiology & behavior. 2011;103:21–24. doi: 10.1016/j.physbeh.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoud HR. The neurobiology of food intake in an obesogenic environment. The Proceedings of the Nutrition Society. 2012;71:478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. The Journal of nutrition. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nature reviews Endocrinology. 2010;6:255–269. doi: 10.1038/nrendo.2010.19. [DOI] [PubMed] [Google Scholar]
- 12.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nature neuroscience. 2012;15:1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Frontiers in neuroendocrinology. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain research bulletin. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. The European journal of neuroscience. 2009;30:1358–1369. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Molecular and cellular endocrinology. 2011;340:80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacology & therapeutics. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Cabral A, Fernandez G, Perello M. Analysis of brain nuclei accessible to ghrelin present in the cerebrospinal fluid. Neuroscience. 2013;253:406–415. doi: 10.1016/j.neuroscience.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. The European journal of neuroscience. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 21.Lutz TA. Control of energy homeostasis by amylin. Cellular and molecular life sciences : CMLS. 2012;69:1947–1965. doi: 10.1007/s00018-011-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth JD, Maier H, Chen S, Roland BL. Implications of amylin receptor agonism: integrated neurohormonal mechanisms and therapeutic applications. Archives of neurology. 2009;66:306–310. doi: 10.1001/archneurol.2008.581. [DOI] [PubMed] [Google Scholar]
- 23.Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides. 1998;19:309–317. doi: 10.1016/s0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- 24.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25:1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 25.Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1475–R1484. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- 26.Lutz TA. Roles of amylin in satiation, adiposity and brain development. Forum Nutr. 2010;63:64–74. doi: 10.1159/000264394. [DOI] [PubMed] [Google Scholar]
- 27.Potes CS, Lutz TA, Riediger T. Identification of central projections from amylin-activated neurons to the lateral hypothalamus. Brain Res. 2010;1334:31–44. doi: 10.1016/j.brainres.2010.03.114. [DOI] [PubMed] [Google Scholar]
- 28.Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiol Behav. 2010;100:511–518. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Potes CS, Boyle CN, Wookey PJ, Riediger T, Lutz TA. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin’s eating inhibitory effect. Am J Physiol Regul Integr Comp Physiol. 2012;302:R340–R351. doi: 10.1152/ajpregu.00380.2011. [DOI] [PubMed] [Google Scholar]
- 30.Roth JD, Erickson MR, Chen S, Parkes DG. GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Br J Pharmacol. 2012;166:121–136. doi: 10.1111/j.1476-5381.2011.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turek VF, Trevaskis JL, Levin BE, Dunn-Meynell AA, Irani B, Gu G, et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151:143–152. doi: 10.1210/en.2009-0546. [DOI] [PubMed] [Google Scholar]
- 32.Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol Metab. 2010;21:473–479. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Dunn-Meynell AA, Le Foll C, Johnson MD, Lutz TA, Hayes MR, Levin BE. Endogenous VMH amylin signaling is required for full leptin signaling and protection from diet-induced obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310:R355–R365. doi: 10.1152/ajpregu.00462.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, et al. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:372–385. doi: 10.1038/npp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, et al. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1685–1697. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baisley SK, Baldo BA. Amylin receptor signaling in the nucleus accumbens negatively modulates mu-opioid-driven feeding. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:3009–3017. doi: 10.1038/npp.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, et al. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293:R1855–R1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- 38.Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL, et al. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. International journal of obesity. 2010;34:385–395. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- 39.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Frontiers in neuroendocrinology. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 40.Becskei C, Riediger T, Zund D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain research. 2004;1030:221–233. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 42.Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacological reviews. 2015;67:564–600. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- 43.Mietlicki-Baase EG, Hayes MR. Amylin activates distributed CNS nuclei to control energy balance. Physiology & behavior. 2014;136:39–46. doi: 10.1016/j.physbeh.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth JD. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Current opinion in endocrinology, diabetes, and obesity. 2013;20:8–13. doi: 10.1097/MED.0b013e32835b896f. [DOI] [PubMed] [Google Scholar]
- 45.Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nature reviews Endocrinology. 2013;9:425–433. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- 46.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Gao F, Zhu J, Guo E, Song X, Wang S, et al. Immunofluorescently labeling glutamic acid decarboxylase 65 coupled with confocal imaging for identifying GABAergic somata in the rat dentate gyrus-A comparison with labeling glutamic acid decarboxylase 67. Journal of chemical neuroanatomy. 2014;61–62:51–63. doi: 10.1016/j.jchemneu.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Halasy K, Hajszan T, Kovacs EG, Lam TT, Leranth C. Distribution and origin of vesicular glutamate transporter 2-immunoreactive fibers in the rat hippocampus. Hippocampus. 2004;14:908–918. doi: 10.1002/hipo.20006. [DOI] [PubMed] [Google Scholar]
- 49.Mietlicki-Baase EG, Olivos DR, Jeffrey BA, Hayes MR. Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. American journal of physiology Endocrinology and metabolism. 2015;308:E1116–E1122. doi: 10.1152/ajpendo.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000;141:850–853. doi: 10.1210/endo.141.2.7378. [DOI] [PubMed] [Google Scholar]
- 51.Lutz TA, Tschudy S, Rushing PA, Scharrer E. Amylin receptors mediate the anorectic action of salmon calcitonin (sCT) Peptides. 2000;21:233–238. doi: 10.1016/s0196-9781(99)00208-9. [DOI] [PubMed] [Google Scholar]
- 52.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell metabolism. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. American journal of physiology Endocrinology and metabolism. 2012;303:E496–E503. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2310–2321. doi: 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American journal of physiology Endocrinology and metabolism. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacological reviews. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 57.Morfis M, Tilakaratne N, Furness SG, Christopoulos G, Werry TD, Christopoulos A, et al. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149:5423–5431. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- 58.Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides. 1998;19:1533–1540. doi: 10.1016/s0196-9781(98)00114-4. [DOI] [PubMed] [Google Scholar]
- 59.Reidelberger RD, Kelsey L, Heimann D. Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2002;282:R1395–R1404. doi: 10.1152/ajpregu.00597.2001. [DOI] [PubMed] [Google Scholar]
- 60.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62:1916–1927. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacology, biochemistry, and behavior. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods and findings in experimental and clinical pharmacology. 2002;24:135–138. doi: 10.1358/mf.2002.24.3.802297. [DOI] [PubMed] [Google Scholar]
- 63.Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Autonomic neuroscience : basic & clinical. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell D, Krusemark ML, Hafner D. Pica: a species relevant behavioral assay of motion sickness in the rat. Physiology & behavior. 1977;18:125–130. doi: 10.1016/0031-9384(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 65.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. The European journal of neuroscience. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 67.Edelman S, Maier H, Wilhelm K. Pramlintide in the treatment of diabetes mellitus. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2008;22:375–386. doi: 10.2165/0063030-200822060-00004. [DOI] [PubMed] [Google Scholar]
- 68.Rushing PA. Central amylin signaling and the regulation of energy homeostasis. Current pharmaceutical design. 2003;9:819–825. doi: 10.2174/1381612033455387. [DOI] [PubMed] [Google Scholar]
- 69.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annual review of nutrition. 2014;34:237–260. doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Molecular pharmacology. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 71.Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. The Journal of pharmacology and experimental therapeutics. 2000;294:61–72. [PubMed] [Google Scholar]
- 72.Steidl S, Cardiff KM, Wise RA. Increased latencies to initiate cocaine self-administration following laterodorsal tegmental nucleus lesions. Behavioural brain research. 2015;287:82–88. doi: 10.1016/j.bbr.2015.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Floody OR, Cramer RR. Effects of tegmental lesions on lordosis and body weight in female hamsters. Brain research bulletin. 1986;17:59–66. doi: 10.1016/0361-9230(86)90161-9. [DOI] [PubMed] [Google Scholar]
- 74.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dickson SL, Hrabovszky E, Hansson C, Jerlhag E, Alvarez-Crespo M, Skibicka KP, et al. Blockade of central nicotine acetylcholine receptor signaling attenuate ghrelin-induced food intake in rodents. Neuroscience. 2010;171:1180–1186. doi: 10.1016/j.neuroscience.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PloS one. 2012;7:e49557. doi: 10.1371/journal.pone.0049557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life sciences. 1995;57:1993–2001. doi: 10.1016/0024-3205(95)02197-q. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Kelly L, Heiman M, Greengard P, Friedman JM. Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell metabolism. 2015;22:1059–1067. doi: 10.1016/j.cmet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Szabo ER, Cservenak M, Dobolyi A. Amylin is a novel neuropeptide with potential maternal functions in the rat. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:272–281. doi: 10.1096/fj.11-191841. [DOI] [PubMed] [Google Scholar]
- 81.Dobolyi A. Central amylin expression and its induction in rat dams. Journal of neurochemistry. 2009;111:1490–1500. doi: 10.1111/j.1471-4159.2009.06422.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.