Abstract
Objectives
Preclinical studies have suggested that non-antineoplastic medication use may impact pancreatic cancer biology. We examined the association of several medication classes on pancreatic cancer survival in a large medical claims database.
Methods
Histologically confirmed pancreatic adenocarcinoma diagnosed between 2006 and 2009 were analyzed from the Surveillance, Epidemiology and End Results (SEER)-Medicare database with available Part D data. Drug use was defined as having two prescriptions filled within 12 months of pancreatic cancer diagnosis. The following medication classes/combinations were analyzed: beta-blocker, statin, insulin, metformin, thiazolidinedione (TZD), warfarin, heparin, beta-blocker/statin, metformin/statin and beta-blocker/metformin. Multivariable Cox proportional hazard models adjusting for age, gender, race, stage at diagnosis, site of cancer, and Charlson comorbidity index were constructed to test the association between medication classes and overall survival.
Results
13,702 patients were included in the study; median age 76 years, 42.5% males, 77.1% white. The most common anatomical site and stage at diagnosis were head of the pancreas (49.9%) and stage 4 (49.6%) respectively. 94% of patients died in the follow-up period (median overall survival 5.3 months). Multivariable Cox regression analysis showed that use of beta blockers, heparin, insulin and warfarin were significantly associated with improved survival (p<0.05 for each one), whereas metformin, TZD, statin and combination therapies were not.
Conclusions
In this study, use of beta blockers, heparin, insulin and warfarin were associated with improved survival in patients with pancreatic cancer. Additional studies are needed to validate these findings in the clinical setting.
Keywords: pancreatic cancer, diabetes, medications, pharmacoepidemiology
INTRODUCTION
In 2015 there were an estimated 48,960 new cases of pancreatic cancer and 40,560 deaths from pancreatic cancer in the United States making it the fourth highest cause of cancer-related deaths [1]. Most patients present with advanced incurable disease and the effectiveness of current palliative systemic therapy is limited [2, 3]. With a median age of diagnosis of 71 years, a high proportion of pancreatic cancer patients are elderly, and have high rates of chronic non-neoplastic comorbid conditions requiring regular medication use [4]. There is mounting preclinical evidence that medications conventionally used for chronic non-cancer indications such as diabetes, hypertension, hypercholesterolemia and thromboembolic disease may have an effect on pancreatic cancer biology, and can potentially impact cancer outcomes [5–7].
Validating whether these non-cancer medications have effects on pancreatic cancer in prospective clinical trials is difficult due to resource and time constraints. Availability of large clinical datasets allows the impact of medications to be evaluated on large numbers of cancer patients, but institutional cancer registries often do not capture details on individual patient medication use[8]. As a result, prior studies assessing the relationship of medication use and cancer outcome have been limited to small database analysis. Since 2006, Medicare beneficiaries had the option to obtain outpatient prescription drug benefits under the Part D program, making Medicare claims files a unique data set to enable such analyses. The aim of our study was to utilize data available from Part D beneficiaries to assess the impact of outpatient medications on survival in a large sample of elderly pancreatic cancer patients.
METHODS
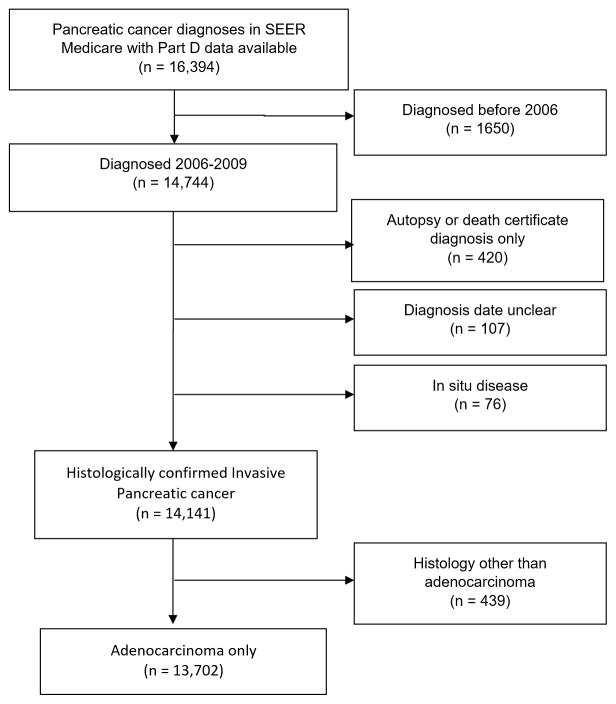
We used data from the Surveillance, Epidemiology and End Results (SEER)-Medicare linked database.[9] The National Cancer Institute’s SEER program contracts with population-based cancer registries to provide data on all incident cancers cases and their tumor characteristics. Individuals aged 66 or above, with pancreatic adenocarcinoma and available Part D data diagnosed between 2006 and 2009 were included (Figure 1). Patients with primary site of cancer as pancreas were identified; and patients with adenocarcinoma were selected for using ICD-O-3 histology codes. Cases with non-adenocarcinoma histology, carcinoma in situ, and diagnosis by autopsy/ death certificate only data were excluded. Cases with multiple prior cancers were included regardless of the sequence of pancreatic cancer in relation to other cancers and the number of prior/future cancers. This study was conducted in accordance with a SEER-Medicare data use agreement, and was approved by the University of Texas Southwestern Medical Center Institutional Review Board.
Figure 1.
Flow Diagram for Inclusion of Patients Diagnosed with Primary Pancreatic Adenocarcinoma in SEER Medicare
Outcome
The primary end point was overall survival. Overall survival was defined as the time (in months) from date of pancreatic cancer diagnosis till death from all causes as defined in SEER files. Due to the high mortality rate of pancreatic cancer this was expected to closely reflect pancreatic cancer related survival. Patients alive at the cutoff date (February 28, 2012) were censored.
Covariates
Data including date of diagnosis, date of death or last follow-up, demographics (age, gender, ethnicity), tumor characteristics (site, stage, histology), comorbidities and pancreatic cancer surgery were extracted from SEER files. Outpatient medication use was assessed from prescription drug event (PDE) records. PDE files are derived from prescription drug claims and include prescription fill dates, costs and day’s supply on covered drugs. Drug use was dichotomized as a yes/ no variable and defined as having two prescriptions of that drug filled within 12 months of pancreatic cancer diagnosis, till death. Based on available preclinical and prior clinical reports of effect on pancreatic cancer biology, the following medications classes were analyzed: beta-blocker, statin, insulin, metformin, thiazolidinediones (TZDs), warfarin and heparin. As most elderly patients are on multiple drug combinations, the most commonly occurring combinations were also explored. The Charlson comorbidity score was obtained from Medicare files [10].
Statistical Analyses
Descriptive statistics were used to report baseline characteristics of our cohort. Cox proportional hazards models were constructed to examine the unadjusted and multivariable adjusted associations between different classes of drugs and OS, and are reported as hazard ratios (HR) and 95% confidence intervals (CI). Pancreatic cancer surgery was only included in the univariate Cox regression, not in multivariable Cox regression since 69.3% of pancreatic cancer surgery information was missing. After the unadjusted model, models were adjusted for age, gender, race, stage, site, Charlson comorbidity index and each medication class. All analyses were performed using SAS version 9.2 (SAS Corporation, Cary, North Carolina).
RESULTS
There were 13,702 patients that had available Part D data and met inclusion criteria. A study flowchart is presented in Figure 1. Median age of the cohort was 76 years, 42.5% were males and 77.1% were white. Head of the pancreas tumors accounted for 49.9% cases, and 61.9% had advanced stage III/IV disease. At the time of study cutoff, 93.5% of patients had died. Median overall survival of the cohort was 5.3 months (95% CI 5.1–5.4). Baseline patient characteristics are summarized in Table 1.
Table 1.
Clinical and demographic characteristics of sample, with univariate analysis describing the hazard ratio for overall survival
| Baseline Characteristic | Value | HR (95% CI) | P value |
|---|---|---|---|
| Age at diagnosis, years | 76.1 (9.2) | 1.03 (1.03–1.03) | <.0001 |
| Race | |||
| White | 10,545 (77.1%) | Reference | 0.0436 |
| Others | 3137(22.9%) | 1.04 (1.00–1.08) | |
| Sex | |||
| Male | 5820 (42.5) | Reference | 0.3718 |
| Female | 7882(57.5%) | 1.02 (0.98–1.05) | |
| Year of diagnosis | |||
| Diagnosis 2006 | 1459 (10.7) | Reference | <.0001 |
| Diagnosis 2007 | 3827 (27.9) | 1.95 (1.83–2.08) | |
| Diagnosis 2008 | 4180 (30.5) | 1.93 (1.81–2.05) | |
| Diagnosis 2009 | 4236 (30.9) | 1.89 (1.77–2.01) | |
| Stage at diagnosis | |||
| Stage I/II | 4261 (38.1) | Reference | <.0001 |
| Stage III/IV | 6914 (61.9) | 2.49 (2.39–2.59) | |
| Primary site | |||
| Head of pancreas | 6842 (49.9) | Reference | <.0001 |
| Other | 6860 (50.1) | 1.34 (1.30–1.39) | |
| Pancreatic cancer surgery | |||
| Pancreatic cancer surgery | 400 (69.3%) | 0.74 (0.62–0.88) | 0.001 |
| No pancreatic cancer surgery | 177(30.7%) | Reference |
HR (95% CI) - hazard ratio (95% confidence interval)
Data are number (%) of patients or median (standard deviation) unless otherwise indicated. Percentages are excluding ‘missing data’.
P-value based on univariate Cox regression analysis
Medications that were most commonly used included beta-blockers (38.0%) followed by statins, insulin and metformin (Table 2). The most common combination medication use was beta blocker/statin (18.9%).
Table 2.
Cox proportional hazard models of medication use and overall survival in patients with pancreatic cancer
| Medication class | Number (%) of patients | Model 1 * HR (95% CI) |
Model 2 ** HR (95% CI) |
|---|---|---|---|
| Individual medication class | |||
| Beta-blocker | 5209 (38.0%) | 0.92 (0.88, 0.95) | 0.90 (0.85,0.95) |
| Statin | 4720 (35.5%) | 0.96 (0.93, 1.00) | 0.98 (0.92, 1.04) |
| Insulin | 2319 (16.9%) | 0.79 (0.75, 0.82) | 0.87 (0.82, 0.92) |
| Metformin | 2277 (16.6%) | 0.94 (0.89, 0.98) | 1.02 (0.93, 1.11) |
| Thiazolidinedione | 1037 (7.6%) | 1.03 (0.97, 1.10) | 1.08 (1.00, 1.17) |
| Warfarin | 1857 (13.6) | 0.88 (0.83, 0.92) | 0.90 (0.85, 0.95) |
| Heparin | 746 (5.4%) | 0.75 (0.70, 0.81) | 0.75 (0.69, 0.82) |
| Combination drug class | |||
| Beta-blocker/statin | 2590 (18.9) | 0.93 (0.89, 0.98) | 1.05 (0.97, 1.15) |
| Metformin/statin | 1255 (9.2%) | 0.95 (0.89, 1.01) | 1.00 (0.90, 1.11) |
| Beta-blocker/metformin | 1094 (8.0%) | 0.90 (0.84, 0.96) | 0.99 (0.89, 1.10) |
HR (95% CI) - hazard ratio (95% confidence interval)
Model 1- univariate Cox regression analysis
Model 2- multivariable Cox regression analysis adjusting for age, gender, race, stage, site, and Charlson comorbidity index
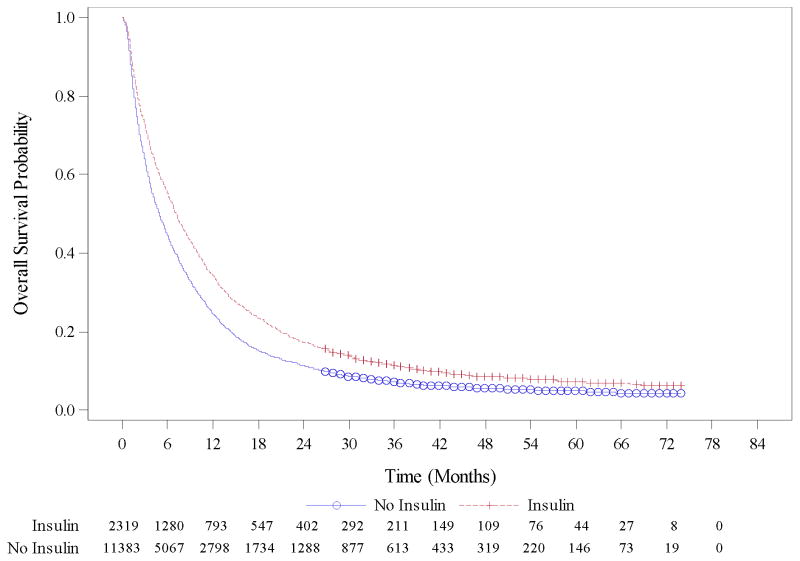
On univariate analysis age, race, stage, primary site of the tumor, surgery and Charlson Comorbidity Index (CCI) score were associated with survival (Table 1) whereas statin and TZD use were not (Table 2). Among the three combination medication groups tested, beta-blocker/statin and beta-blocker/metformin were associated with improved survival while metformin/statin was not in univariate Cox regression analysis. On multivariable Cox regression analysis, beta-blocker (HR 0.90, 95% CI 0.85–0.95), heparin (HR 0.75, 95% CI 0.69–0.82), insulin (HR 0.87, 95% CI 0.82–0.92) and warfarin use (HR 0.90, 95% CI 0.85–0.95) were associated with improved survival (Table 2). Kaplan-Meier curve for overall survival with insulin use versus no insulin use is presented in Figure 2. Metformin use, which had a weak association with survival in univariate analysis, was not associated with survival in multivariate analysis. None of the combination therapies were associated with pancreatic cancer survival in multivariable models.
Figure 2.
Kaplan Meier survival curves demonstrating median overall survival (OS) in months in pancreatic cancer patients receiving insulin (red) versus not receiving insulin (blue).
DISCUSSION
Our results of a large claims based database of elderly pancreatic cancer patients demonstrate an association between outpatient medication use and outcome of pancreatic cancer. This is the first population-based analysis testing the association between multiple medication use and pancreatic cancer survival. We identified a statistically favorable effect of use of beta-blockers, heparin, warfarin and insulin while oral anti-diabetes medications including TZD and metformin, and statins were not associated with survival. There has been mounting evidence that medications prescribed and intended for management of chronic non-cancer conditions have potential biological effect on pancreatic cancer. Understanding these relationships can allow practitioners to manage medical comorbidities using agents that can best complement existing cancer therapies.
The immense interest in diabetes medications on pancreatic cancer outcome results from the high prevalence of a type 2 diabetes mellitus phenotype in these patients (up to 70–80%). [11, 12] This is believed to be secondary to a marked decline in pancreatic β-cell function along with profound peripheral insulin resistance. Data regarding the association of diabetes mellitus and pancreatic cancer survival is conflicting[13, 14] and studies reporting its association with insulin use are limited[15]. Our group has previously demonstrated improved survival of pancreatic cancer patients with diabetes mellitus in a large study based on the Veterans Affairs Central Cancer Registry.[13] However, a recent meta-analysis demonstrated worse outcomes in pancreatic cancer patients with diabetes mellitus.[14] A source of further confusion is that pancreatic cancer is associated with both insulin resistance and hyperinsulinemia (largely type 2 diabetes mellitus) and ultimately endocrine pancreatic destruction associated with insulin deficiency (type 1 diabetes mellitus). Interpreting exogenous insulin administration is thus fraught with difficulty. A recent study has also demonstrated improved survival in pancreatic cancer patients with diabetes mellitus and non-inferior outcomes in pancreatic cancer patients receiving insulin therapy.[16] We observed a protective association of insulin use on pancreatic cancer survival. Metformin has shown potential antitumor activity in cancer through modulation of the AMP-activated protein kinase (AMPK) pathway, and inhibition of complex 1 of the mitochondrial respiratory chain and mammalian target of rapamycin (mTOR) signaling [17–19]. Despite encouraging preclinical rationale, a study on pancreatic cancer patient derived xenografts (PDX) did not demonstrate an antitumor effect or metformin[20]. Retrospective studies suggested metformin improved pancreatic cancer survival [6, 21, 22] but these findings were not validated in a prospective randomized phase 2 study which did not reveal an impact of metformin on clinical outcomes in pancreatic cancer[23]. TZDs are high affinity ligands for the nuclear receptor, peroxisome proliferator-activated receptor gamma (PPARγ). PPARγ is commonly up-regulated in pancreatic cancer and preclinical studies have shown PPARγ agonists can potentiate the effect of chemotherapy on pancreatic cancer cell lines and xenograft model [24–26]. Our analysis did not support a survival benefit of oral diabetes medications (metformin and TZDs).
Warfarin imparts its anticoagulant activity through vitamin K antagonism by inhibiting the formation of the biologically active, gamma carboxylated coagulation factors[27]. Growth arrest-specific gene 6 (gas6) is a member of the plasma vitamin K dependent protein family, similar to the plasma anticoagulant protein S, and functions as a ligand to the Axl pathway. Activation of the Axl receptor tyrosine kinase pathway has been associated with tumor progression and metastasis[28, 29]. Inhibitors to Axl are an emerging class of small molecules currently in early phase clinical development in oncology[30]. Preclinical studies have demonstrated that inhibition of gas6 reduced Axl signaling leads to subsequently reduced pancreatic cancer tumor cell migration, invasiveness and proliferation, while increasing apoptosis and sensitivity to chemotherapy[31]. This effect has been seen in retrospective analysis which demonstrated low dose warfarin improved pancreatic cancer survival, regardless of the type of chemotherapy received and was attributed to the potential of warfarin in prevention of thromboembolic events[32]. A retrospective study of low molecular weight heparin (LMWH) has also shown a survival benefit in patients with metastatic pancreatic cancer receiving LMWH as opposed to those who did not[33]. A Cochrane review on the effect of parenteral blood thinners on cancer outcome assessed 15 trials spanning 7622 patients with multiple tumor types including non-small cell lung cancer, small cell lung cancer and pancreatic cancer[5]. This analysis demonstrated a small improvement of parenteral anticoagulants (including unfractionated heparin and LMWH) on mortality across tumor types. A similar analysis of seven studies of oral anticoagulant (6 on warfarin) composed of a total of 1539 patients with multiple cancers did not show an effect of therapy on cancer outcome[34]. The effect of warfarin on cancer survival is of particular interest due to the high rate of venous thromboembolic (VTE) events in pancreatic cancer, and the traditional preference towards LMWH for management of cancer-associated VTE. Given these study findings, the optimal anticoagulant therapy in pancreatic cancer patients warrants further study.
Beta-adrenergic signaling increases pancreatic cancer progression and invasion while pharmacologic blockade of beta-adrenergic signaling stopped tumor invasion in preclinical orthotopic model of pancreatic cancer[35, 36]. A favorable effect was not observed in a population based retrospective cohort of multiple cancers, but notably only a small proportion of patients in this study had pancreatic cancer[7]. We did observe a survival benefit with beta-blocker use
Preclinical evidence suggests that statins have anti-neoplastic properties by inhibiting cell proliferation, and inducing apoptosis[37]. Statin use has been associated with improved survival in several other cancers[38]. However, like our study, a previous large population based study also did not find an overall protective benefit of statin use in patients with pancreatic cancer[39], although certain subgroups (statin-naïve prior to diagnosis, early stage disease, those undergoing surgery) did have improved survival in that study. Biological tumor differences may explain these results; it is known that tumor subtypes respond differently to therapy[40]. Metformin and statin use combined, was also not associated with improved survival in our study. Due to lack of detailed information on medication use (pre vs post-diagnosis), dose, duration and potential for lag-time effect where medication use takes time to establish its protective efficacy (especially in post-diagnosis use in patients with pancreatic cancer who had median survival of 5.3 months), these results should be interpreted with caution.
Strengths of the study include a large nationally representative sample and detailed information on prescriptions. Our study population comprised exclusively of elderly pancreatic cancer patients; although the Medicare database only includes patient >65 years of age, the median age of pancreatic cancer diagnosis in our study and the US population were similar, and our cohort is expected to represent a large proportion of real world patients. Limitations of this study reflect those inherent to the use of a claims database, where there is lack of information on whether patients actually took the prescribed medications and its retrospective nature. Part D enrollees historically may be less healthy than non-Part D enrollees[41]. As Medicare beneficiaries were not mandated to acquire their outpatient medications through Part D, a proportion of SEER-Medicare cases who were not Part D enrollees were excluded from this analysis. Also for patients who were enrolled in the Part D program we were not able to account for over the counter medications (aspirin, acetaminophen, vitamins) or medications which may have been purchased out of the Part D program. Patients labeled as ‘medication-non-users’ may be taking other medications of similar/ different mechanism of action, which may affect the analysis of user vs non-user on pancreatic cancer survival. Medication non-users may also lack the indication for that medication lending itself to unmeasured confounding. Information of pancreatic cancer surgery was missing in a majority of patients and it was not included in the multivariable analysis. The advancement of pancreatic cancer directed therapy since 2009 (the last year included in our study) including refinements in surgical techniques and approval of abraxane in 2013 may affect the validity of our findings. A dose-response relationship would add strength to the associations found in the study.
Evaluating the impact of concurrent medications on cancer outcome is an area of intense clinical interest due to the high rate of concurrent medication use in pancreatic cancer patients. Prospective trials to evaluate these associations may help answer mechanism of action questions but larger randomized controlled studies are not feasible due to limitations in resources and multiple types of medications and the possible combinations. The availability of large databases such as SEER-Medicare allows researchers to analyze real world effects of medications in a manner not possible in the past. Analysis such as this help in adding to the body of evidence and can be readily applied in clinic to help guide clinical practice.
Acknowledgments
Support: UL1TR001105 (MSB)
Dr. Mortensen were supported in part by a grant from the Agency for Healthcare Research and Quality (R24 HS022418) and the University of Texas Southwestern Center for Patient-Centered Outcomes Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Veterans Affairs. This material is the result of work supported with resources and the use of facilities at the VA North Texas Health Care System. The funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database
Footnotes
Prior Presentations: ‘Impact of concurrent medications use on outcome of pancreatic cancer – SEER Medicare analysis. Gastrointestinal Cancers Symposium, San Francisco January 2016
Disclaimers or conflicts of interest: None
Conflict of Interest:
The authors declare that they have no conflict of interest
Author contributions:
First Author: Beg
Corresponding Author: Beg
Conception, Design, Analysis, and Interpretation of Data: Beg, Gupta, Gao, Ahn
Drafting, Revision, and Final Approval: Beg, Gupta, Sher, Ali, Khan, Gao, Stewart, Anh, Berry, Mortensen
Article guarantor:
Beg and Gupta had full access to all of the data in the study and take responsibility for the integrity of the study.
Research involving Human Participants and/or Animals:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
Informed consent:
Not applicable
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M U. Groupe Tumeurs Digestives of, P. Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Facts and Figures. [accessed 02/17/2016];Special Section: Pancreatic cancer. 2013 < http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-038828.pdf>.
- 5.Akl EA, Kahale LA, Ballout RA, Barba M, Yosuico VE, van Doormaal FF, Middeldorp S, Bryant A, Schunemann H. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev. 2014;12:CD006652. doi: 10.1002/14651858.CD006652.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Yoon SH, Lee HS, Chung MJ, Park JY, Park SW, Song SY, Chung JB, Bang S. Can metformin change the prognosis of pancreatic cancer? Retrospective study for pancreatic cancer patients with pre-existing diabetes mellitus type 2. Dig Liver Dis. 2015 doi: 10.1016/j.dld.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72(1):157–61. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facility Oncology Data Standards (FORDS) manual. available at < https://www.facs.org/quality-programs/cancer/ncdb/registrymanuals/cocmanuals>.
- 9.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knopf KB. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Bartosch-Harlid A, Andersson R. Diabetes mellitus in pancreatic cancer and the need for diagnosis of asymptomatic disease. Pancreatology. 2010;10(4):423–8. doi: 10.1159/000264676. [DOI] [PubMed] [Google Scholar]
- 12.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):981–7. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beg MS, Dwivedi AK, Ahmad SA, Ali S, Olowokure O. Impact of diabetes mellitus on the outcome of pancreatic cancer. PLoS One. 2014;9(5):e98511. doi: 10.1371/journal.pone.0098511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen H, Zhan M, Wang W, Yang D, Wang J. Impact of diabetes mellitus on the survival of pancreatic cancer: a meta-analysis. Onco Targets Ther. 2016;9:1679–88. doi: 10.2147/OTT.S95744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng CH. Diabetes, insulin use, smoking, and pancreatic cancer mortality in Taiwan. Acta Diabetol. 2013;50(6):879–86. doi: 10.1007/s00592-013-0471-0. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y, Kim TY, Oh DY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res Treat. 2016;48(1):171–9. doi: 10.4143/crt.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Transl Oncol. 2013;6(6):649–59. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipner MB, Marayati R, Deng Y, Wang X, Raftery L, O'Neil BH, Yeh JJ. Metformin Treatment Does Not Inhibit Growth of Pancreatic Cancer Patient-Derived Xenografts. PLoS One. 2016;11(1):e0147113. doi: 10.1371/journal.pone.0147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18(10):2905–12. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak MM, Anderson EM, von Eyben R, Pai JS, Poultsides GA, Visser BC, Norton JA, Koong AC, Chang DT. Statin and Metformin Use Prolongs Survival in Patients With Resectable Pancreatic Cancer. Pancreas. 2016;45(1):64–70. doi: 10.1097/MPA.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 23.Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora P, Sordi V, Lampasona V, Ceraulo D, Di Terlizzi G, Doglioni C, Falconi M, Piemonti L. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22(5):1076–85. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 24.Kristiansen G, Jacob J, Buckendahl AC, Grutzmann R, Alldinger I, Sipos B, Kloppel G, Bahra M, Langrehr JM, Neuhaus P, Dietel M, Pilarsky C. Peroxisome proliferator-activated receptor gamma is highly expressed in pancreatic cancer and is associated with shorter overall survival times. Clin Cancer Res. 2006;12(21):6444–51. doi: 10.1158/1078-0432.CCR-06-0834. [DOI] [PubMed] [Google Scholar]
- 25.Shimazaki N, Togashi N, Hanai M, Isoyama T, Wada K, Fujita T, Fujiwara K, Kurakata S. Anti-tumour activity of CS-7017, a selective peroxisome proliferator-activated receptor gamma agonist of thiazolidinedione class, in human tumour xenografts and a syngeneic tumour implant model. Eur J Cancer. 2008;44(12):1734–43. doi: 10.1016/j.ejca.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Koga H, Selvendiran K, Sivakumar R, Yoshida T, Torimura T, Ueno T, Sata M. PPARgamma potentiates anticancer effects of gemcitabine on human pancreatic cancer cells. Int J Oncol. 2012;40(3):679–85. doi: 10.3892/ijo.2011.1237. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 Suppl):8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 28.Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer. 2014;134(5):1024–33. doi: 10.1002/ijc.28246. [DOI] [PubMed] [Google Scholar]
- 29.Pinato DJ, Mauri FA, Lloyd T, Vaira V, Casadio C, Boldorini RL, Sharma R. The expression of Axl receptor tyrosine kinase influences the tumour phenotype and clinical outcome of patients with malignant pleural mesothelioma. Br J Cancer. 2013;108(3):621–8. doi: 10.1038/bjc.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Liu X, Koul S, Lee CY, Zhang Z, Halmos B. AXL kinase as a novel target for cancer therapy. Oncotarget. 2014;5(20):9546–63. doi: 10.18632/oncotarget.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirane A, Ludwig KF, Sorrelle N, Haaland G, Sandal T, Ranaweera R, Toombs JE, Wang M, Dineen SP, Micklem D, Dellinger MT, Lorens JB, Brekken RA. Warfarin Blocks Gas6-Mediated Axl Activation Required for Pancreatic Cancer Epithelial Plasticity and Metastasis. Cancer Res. 2015;75(18):3699–705. doi: 10.1158/0008-5472.CAN-14-2887-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakchbandi W, Muller H, Singer MV, Lohr M, Nakchbandi IA. Effects of low-dose warfarin and regional chemotherapy on survival in patients with pancreatic carcinoma. Scand J Gastroenterol. 2006;41(9):1095–104. doi: 10.1080/00365520600575720. [DOI] [PubMed] [Google Scholar]
- 33.von Delius S, Ayvaz M, Wagenpfeil S, Eckel F, Schmid RM, Lersch C. Effect of low-molecular-weight heparin on survival in patients with advanced pancreatic adenocarcinoma. Thromb Haemost. 2007;98(2):434–9. [PubMed] [Google Scholar]
- 34.Akl EA, Kahale L, Terrenato I, Neumann I, Yosuico VE, Barba M, Sperati F, Schunemann H. Oral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2014;7:CD006466. doi: 10.1002/14651858.CD006466.pub4. [DOI] [PubMed] [Google Scholar]
- 35.Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, Hollande F, Sloan EK. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40–7. doi: 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang D, Ma QY, Hu HT, Zhang M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther. 2010;10(1):19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–32. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, Singh S, Singh P. Statin use and mortality in patients with colorectal cancer: A systematic review and meta-analysis of observational studies. J Clin Oncol. 2015;33(suppl 3) abstr 686. [Google Scholar]
- 39.Jeon CY, Pandol SJ, Wu B, Cook-Wiens G, Gottlieb RA, Merz CN, Goodman MT. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One. 2015;10(4):e0121783. doi: 10.1371/journal.pone.0121783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riley GF, Levy JM, Montgomery MA. Adverse selection in the Medicare prescription drug program. Health Aff (Millwood) 2009;28(6):1826–37. doi: 10.1377/hlthaff.28.6.1826. [DOI] [PubMed] [Google Scholar]