Abstract
The extent to which pharmacogenomic-guided medication use has been adopted in various health systems is unclear. To assess the uptake of pharmacogenomic-guided medication use, we determined its frequency across our health system, which does not have a structured testing program. Using a multi-site clinical data repository, we identified adult patients’ first prescribed medications between January 2011 and December 2013 and investigated the frequency of germline and somatic pharmacogenomic testing, by PharmGKB level of FDA label information. There were 268,262 medication orders for drugs with germline pharmacogenomic testing information in their drug labels. Pharmacogenomic testing was detected for 1.5% (129/8,718) of medication orders with recommended or required testing. Of the 3,817 medication orders associated with somatic pharmacogenomic testing information in their drug labels, 20% (372/1,819) of required tests were detected. The low rates of detectable pharmacogenomic testing suggest that structured testing programs are required to achieve the success of precision medicine.
Keywords: FDA drug labels, Electronic health record, Germline, Pharmacogenomics, Somatic
INTRODUCTION
The promise of precision medicine to optimize medication use has been recognized for decades because of the significant impact of large genetic variability on drug efficacy and adverse events. To support the use of pharmacogenomic information in prescribing practice, US Food and Drug Administration (FDA) drug labels include information about how prescribing practices may be impacted by genetic and protein biomarkers, which we collectively refer to as pharmacogenomics (PGx). As of November 2016, 54 distinct PGx tests are described in the labels of 165 drugs for which pharmacogenomic data were available1. Use of such information has the potential to significantly impact prescribing practices, with up to one-quarter of patients in an ambulatory setting receiving a medication with a PGx recommendation in the drug label2. In another study, greater than 65% of 52,000 patients in a medical home setting received at least one medication with a PGx association, and the authors determined that a pre-emptive PGx program could prevent almost 400 adverse events3. Despite significant numbers of patients potentially impacted by PGx-guided medication use, only 13% of physicians reported ordering or recommending PGx testing in the six months prior to being surveyed and only 15% reported receiving any instruction in PGx testing during training4. Forty percent of these surveyed physicians reported obtaining PGx testing information from drug labels, either exclusively or in addition to other resources5, reflecting the importance of drug labelling in clinical decision making. Another study broadly surveying the general utility of drug labels (outside of PGx testing situations) found that physicians did use label information in prescribing situations6.
The relative lack of education4 and familiarity with PGx testing among physicians has prompted some institutions to develop systemic solutions to perform prospective testing and coordinate appropriate medication utilization through clinical decision support7. Investigators at Vanderbilt University Medical Center estimated that with an effective preemptive PGx program in place, almost 400 serious adverse events and hundreds of additional less serious events could be prevented3, which has helped to serve as an impetus to establish a prospective PGx program for eligible groups of patients8. Greater than 10,000 patients have been genotyped in the Vanderbilt program, and greater than 90% of patients had at least one actionable genotype, further underscoring the impact of PGx on medication use9. While other institutions have also implemented programs that integrate clinical decision support and prospective genotyping7,10–14, this is not currently standard practice across medical centers in the US.
While a handful of institutions have developed programs to prospectively genotype patients and have studied their impact, the adoption of PGx testing across other settings has only previously been studied through self-report. We therefore sought to objectively ascertain rates of PGx test utilization in an academic medical center setting by analyzing laboratory and medication order data from our institutional clinical data repository (CDR). The objective of our work was to determine how frequently PGx biomarker testing was performed according to the FDA label in the absence of a structured program to prospectively determine patient genotypes. We stratified our analyses by type of PGx test (germline or somatic), and applied the level of actionability assigned to each medication-test pair by the Pharmacogenomics Knowledgebase (PharmGKB), which classifies the recommendation for testing from the FDA labels as informative, actionable, recommended, or required. We characterized each medication-test order by PharmGKB level and therapeutic class, and examined the temporal relationship between medication use and PGx testing. Finally, we determined the attributes of specific medication orders and PGx tests that were associated with an increased frequency of PGx testing to better understand if any factors could predict the adoption of PGx testing.
RESULTS
Study Population
The patient cohort consists of 132,340 unique patients cared for at all four sites across the study period, collectively totaling 3,211,797 hospital/clinic visits. The median age of the cohort at first medication order was approximately 50 years and the distribution of patient sex was 54% female and 46% male (among patients for whom sex was recorded). There were a total of 272,079 index medication orders, where index is defined as the absence of a medication order in the previous twelve months. (Table 1) The average number of index medications per patient was 2 (range: 1 to 14). Of the 128 medications marketed during the study timeframe, 122 were represented in the dataset; one was excluded due to the difficulty in querying co-orders (rifampin/isoniazid/pyrazinamide with a PharmGKB level of actionability of Informative only), four were not prescribed (chorpropaminde, naladixic acid, pegloticase, thioguanine), and the sixth does not carry a PharmGKB level of actionability (PEG-3350). In general, the latter five are infrequently prescribed.
Table 1.
Demographics
| Academic Medical Center |
Public Hospital, Level I Trauma Center |
Primary Care Clinic Network |
Cancer Center |
Total | ||
|---|---|---|---|---|---|---|
| Number of patients* |
2011 | 41,261 | 34,517 | 33,660 | 11,123 | 120,561 |
| 2012 | 45,681 | 38,600 | 39,734 | 12,006 | 136,021 | |
| 2013 | 46,459 | 38,868 | 41,166 | 13,270 | 139,763 | |
| Number of visits |
2011 | 342,355 | 302,007 | 229,745 | 146,634 | 1,020,741 |
| 2012 | 362,704 | 298,338 | 248,382 | 170,327 | 1,079,751 | |
| 2013 | 364,970 | 296,489 | 268,829 | 181,017 | 1,111,305 | |
| Total | 1,070,029 | 896,834 | 746,956 | 497,978 | 3,211,797 | |
| Number of index medication orders |
2011 | 34,921 | 31,418 | 27,092 | 1,058 | 94,489 |
| 2012 | 33,918 | 28,248 | 26,584 | 1,086 | 89,836 | |
| 2013 | 32,374 | 27,835 | 26,292 | 1,253 | 87,754 | |
| Total | 101,213 | 87,501 | 79,968 | 3,397 | 272,079 | |
| Mean age (Interquartile range) |
55 (43, 67) |
53 (42, 65) |
45 (28, 59) |
57 (49, 66) |
51 (38, 65) |
|
| % male/female | 45%/55% | 56%44% | 47%/53% | 36%/64% | 46%/54% | |
Patients are counted based on the first time they are seen at a given facility in a given year. A patient can therefore be counted in more than one year. 132,340 unique patients were seen across all four facilities during the study period.
Medication Usage and Pharmacogenomic Test Utilization
Germline Biomarker Results
Of the PGx testing performed on germline samples (testing for inherited disease), 95 associated medications appear in our dataset and are coupled with 38 distinct PGx tests, such that we evaluated 100 medication-test pairs (Table 2). There were 268,262 index medication orders (Table 3). Although 12% of medications are classified at the PharmGKB required level, 4% at the recommended level, 58% at the actionable level, and 26% at the informative level, the percentage of index medication orders at each of these levels in our dataset were 1%, 2%, 61%, and 36%, respectively (Tables 2, 3). In total, 1,217 PGx tests associated with index medications were captured in our dataset. Less than 1% of index medications were associated with a PGx test (Table 3).
Table 2.
Frequency of medications, PGx tests, and of PGx-medication test pairs, by PharmGKB level
| Number (%) of medications |
Number (%) of PGx tests |
Number (%) of PGx- medication test pairs |
||
|---|---|---|---|---|
| Germline | ||||
| Informative | 26 (27%) | 9 (24%) | 26 (26%) | |
| Actionable | 55 (58%) | 15 (39%) | 58 (58%) | |
| Recommended | 4 (4%) | 3 (8%) | 4 (4%) | |
| Required | 10 (11%) | 11 (29%) | 12 (12%) | |
| Total | 95 (100%) | 38 | 100 | |
| Somatic | ||||
| Informative | 5 (19%) | 4 (20%) | 5 (16%) | |
| Actionable | 3 (11%) | 4 (20%) | 4 (13%) | |
| Recommended | 0 | 0 (0%) | 0 (0%) | |
| Required | 19 (70%) | 12 (60%) | 23 (72%) | |
| Total | 27 (100%) | 20 | 32 | |
PGx = Pharmacogenomics; Numbers may sum to slightly more than 100% due to rounding.
Table 3.
Count and frequency of medication orders associated with PGx test orders, by PharmGKB level
| Medication order associated with PGx test order (% of all orders in level) |
||||
|---|---|---|---|---|
| Yes | No | Total number (%) of medication orders |
||
| Germline | ||||
| Informative | 5 (<1%) | 95,110 (>99%) | 95,115 (36%) | |
| Actionable | 1,083 (<1%) | 163,346 (>99%) | 164,429 (61%) | |
| Recommended | 95 (2%) | 5,110 (98%) | 5,205 (2%) | |
| Required | 34 (1%) | 3,479 (99%) | 3,513 (1%) | |
| Total | 1,217 (<1%) | 267,045 (>99%) | 268,262 (100%) | |
| Somatic | ||||
| Informative | 503 (43%) | 677 (57%) | 1,180 (31%) | |
| Actionable | 34 (4%) | 784 (96%) | 818 (21%) | |
| Recommended | 0 | 0 | 0 | |
| Required | 372 (20%) | 1,447 (80%) | 1,819 (48%) | |
| Total | 909 (24%) | 2,908 (76%) | 3,817 (100%) | |
PGx = Pharmacogenomics
Gastrointestinal (29%), psychiatry (22%), and cardiology (17%) medications were the three most frequently prescribed therapeutic classes. For the majority of index medications, testing was classified as either informative or actionable, with the exception of neurology, for which 43% of tests were required. (Supplementary Table 1) Within a therapeutic class, PGx tests were drawn for the greatest percentage of medication orders in the infectious disease category (4%), followed by oncology (2%). (Supplementary Table 2)
For medications with an associated germline test, six of the top ten medication orders for which a PGx test was ordered were infectious disease medications, three were oncology medications, and one was a rheumatology medication (Table 4). Among these top 10, the percentage of medication orders for which a PGx test was drawn ranged from 58% to 8%, with glucose 6-phosphate dehydrogenase (G6PD) being ordered for 58% of dapsone orders and for 55% of primaquine orders. Both medications carry an actionable level of evidence and both are in the infectious disease therapeutic category.
Table 4.
Top 10 medications ordered, by PGx test, PharmGKB level, and therapeutic class
| Biomarker (HUGO Symbol) |
Corresponding Medication |
PharmGKB level | Therapeutic class |
Total number of medication orders |
Number (%) of medication orders for which PGx test was ordered |
|---|---|---|---|---|---|
| Germline | |||||
| G6PD | DAPSONE | Actionable | ID | 760 | 438 (58%) |
| G6PD | PRIMAQUINE | Actionable | ID | 29 | 16 (55%) |
| G6PD | RASBURICASE | Required | Oncology | 82 | 24 (29%) |
| UGT1A1 | NILOTINIB | Required | Oncology | 15 | 3 (20%) |
| IFNL3 | BOCEPREVIR | Informative | ID | 16 | 2 (13%) |
| CCR5 | MARAVIROC | Required | ID | 29 | 3 (10%) |
| TPMT | AZATHIOPRINE | Recommended | Rheuma- tology |
781 | 79 (10%) |
| IFNL3 | TELAPREVIR | Actionable | ID | 22 | 2 (9%) |
| HLA-B | ABACAVIR | Required | ID | 145 | 13 (9%) |
| G6PD | METHYLENE | Actionable | Oncology | 94 | 7 (8%) |
| Somatic | |||||
| TNFRSF8 | BRENTUXIMAB | Informative | Oncology | 21 | 15 (71%) |
| MS4A1 | TOSITUMOMAB | Informative | Oncology | 11 | 7 (64%) |
| ERBB2 | TRASTUZUMAB | Required | Oncology | 262 | 165 (63%) |
| PML/RARA | ARSENIC | Required | Oncology | 12 | 6 (50%) |
| ERBB2 | PERTUZUMAB | Required | Oncology | 43 | 21 (49%) |
| MS4A1 | RITUXIMAB | Informative | Oncology | 958 | 450 (47%) |
| MS4A1 | OFATUMUMAB | Actionable | Oncology | 25 | 11 (44%) |
| KRAS | PANITUMUMAB | Required | Oncology | 24 | 9 (38%) |
| BRAF | VEMURAFENIB | Required | Oncology | 15 | 5 (33%) |
| ERBB2 | LAPATINIB | Required | Oncology | 10 | 3 (30%) |
HUGO = Human Genome Organization; ID = Infectious Disease; PGx = pharmacogenomic
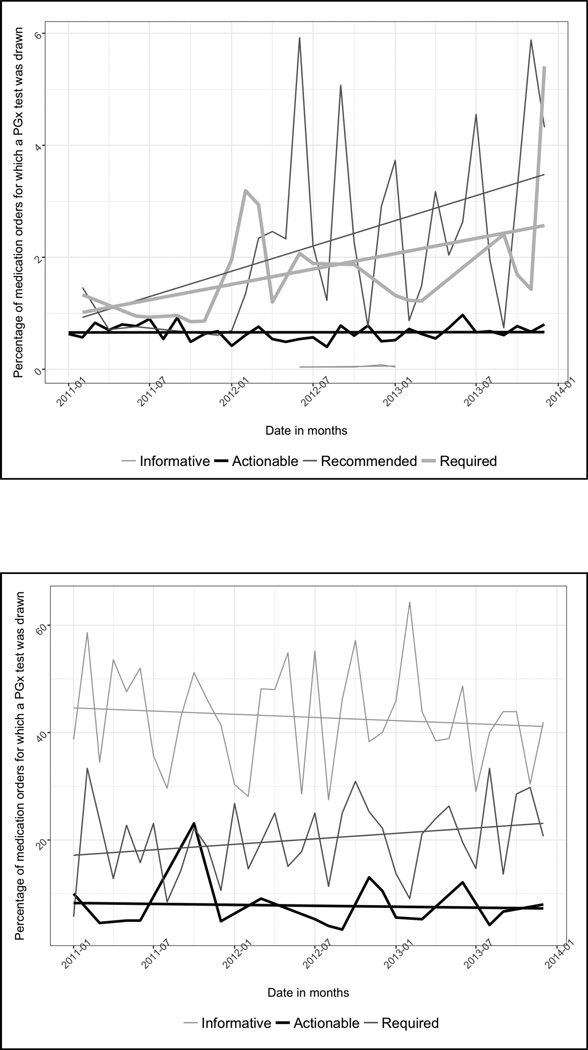
A review of the percentage of medication orders for which a PGx test was drawn, by month, shows an increase from the start to end of the study period for tests in the recommended and required levels, from 1.46% to 4.32%, and from 1.33% to 5.41%, respectively. There was no increase for tests with informative and actionable levels of evidence (Figure 1a).
Figure 1.
a. Time trends in the percentage of index medication orders for which a PGx test was drawn, stratified by PharmGKB level of actionability: Germline tests
b. Time trends in the percentage of index medication orders for which a PGx test was drawn, stratified by PharmGKB level of actionability: Somatic tests
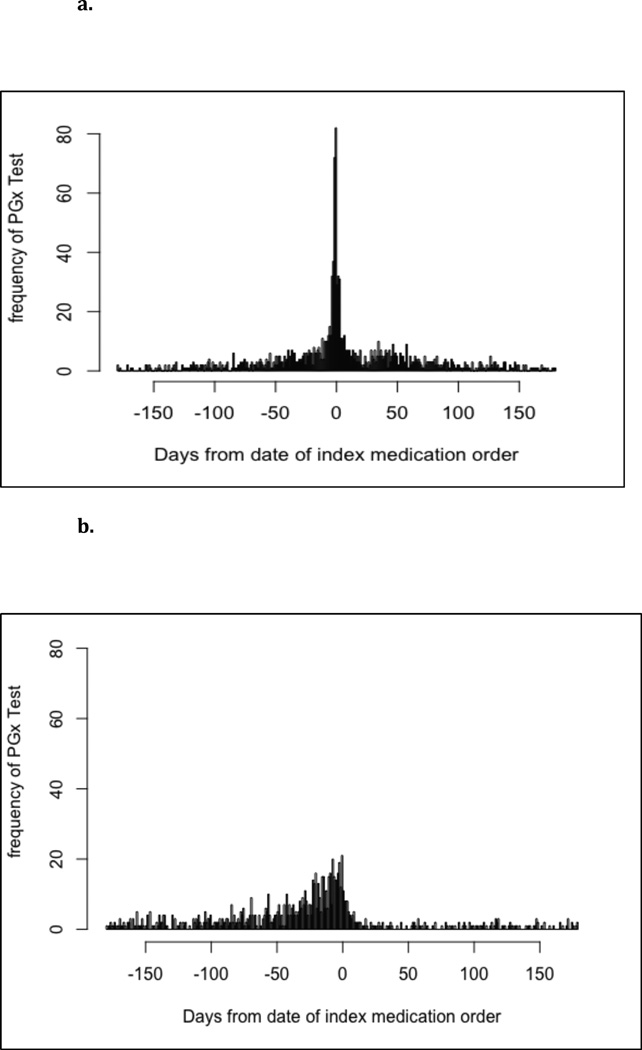
Review of the temporal association of testing with the index medication order date reveals that a majority of testing (72.6%) was completed within 60 days (before or after) of the index medication order(Figure 2a).
Figure 2.
a. Distribution of the number of days between a PGx test order and the index medication order: Germline tests
b. Distribution of the number of days between a PGx test order and the frequency of index medication order: Somatic tests
Somatic Biomarker Results
Twenty-seven medications with linked somatic biomarker testing (tissue testing associated with cancers) appear in our dataset, along with 20 distinct tests, which combine into 32 medication-test pairs (Table 2). There were 3,817 index orders for these medications (Table 3). Seventy-two percent of the medications are classified at the required level, 13% at the actionable level, and 16% at the informative level. No medication was classified at the recommended level. The corresponding distributions for index medication orders in our dataset were 48%, 21%, and 31%, respectively (Tables 2 & 3). In total, 909 index medications associated with PGx tests were captured, representing 24% of index medications with an associated somatic biomarker (Table 3).
All somatic tests were for oncology medications. Almost half (48%) of oncology index medications were for medications for which testing was required by PharmGKB (Supplementary Table 1).
For the top ten medications for which a PGx test was ordered, the percentage of medication orders for which a PGx test was ordered ranged from 71% to 30%, with TNFRSF8 (CD30) ordered for 71% of brentuximab orders and MS4A1 (CD20) ordered for 64% of tositumomuab orders. Both biomarkers are considered informative by PharmGKB interpretation of FDA label (Table 4). A complete listing of the frequency of test orders for both germline and somatic tests, by PharmGKB level, medication, and therapeutic category appears in Supplementary Table 3.
A review of the percentage of medication orders for which a PGx test was drawn, by month, reveals a slight increase from the start to end of the study period for tests in the required level, from 5.71 % to 20.69 %. There was a slight decrease for tests with informative and actionable levels of evidence. (Figure 1b)
Review of the temporal association of testing with the index medication order date reveals that over half of testing (54.8%) was completed within 60 days before the index medication order (Figure 2b). Testing conducted outside of this period reflected different patterns of testing, with some evidence of drugs matching with PGx tests that were drawn for other reasons. Twenty percent of patients with a drug-test pair outside of the 60 day window also appeared within the 60 day window, suggesting that prescribing decisions may continue to be influenced by past tests. Many somatic biomarkers tested by immunohistochemistry appeared outside of the 60 day window, which is not unexpected because some cancer therapies may be initiated months or years after the initial diagnosis. In addition, patients frequently have multiple biopsies during the course of their treatment and our association of medication with test would capture all of those biopsies (some of which are likely to fall outside the window). This suggests a pattern of care wherein the patient receives testing at one facility, then seeks care in another system before eventually returning to the system at which they received their test, perhaps as their condition advanced.
Regression Analysis
Results of the regression analysis suggest that the odds of a test being ordered when the test is somatic versus germline is approximately 74 times greater (Odds ratio (OR) 73.49; 95% confidence interval (CI) 60.79, 88.85). Results also suggest a significant association between PharmGKB level and the ordering of a PGx test. When compared to a test with an informative level of evidence, the odds of a test being ordered when the level of evidence is actionable is approximately 2.8 times (OR 2.78; 95% CI 2.43, 3.18), for recommended is 11.18 times (OR 11.18; 95% CI 8.86, 14.10), and for required is 1.7 times (OR 1.68; 95% CI (1.31, 2.17). The odds of a test being drawn was approximately 1.2 times (OR 1.19; 95% CI 1.11, 1.28) for each year beyond 2011 (2012, 2013). The odds of a test being drawn when the duration of index medication use was ≥ 7 days was 2.4 times (OR 2.43; 95% CI 2.12, 2.77). Each yearly increase in age did not significantly increase the odds of a test being ordered. (Table 5)
Table 5.
Adjusted association between PharmGKB level and PGx test ordering
| PharmGKB level | Odds Ratio |
Standard Error |
95% Confidence Interval* |
||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Somatic (referent germline) |
73.49 | 7.12 | 60.79 | 88.85 | |
|
PharmGKB level (referent informative) |
|||||
| Actionable | 2.78 | 0.19 | 2.43 | 3.18 | |
| Recommended | 11.18 | 1.33 | 8.86 | 14.10 | |
| Required | 1.68 | 0.22 | 1.31 | 2.17 | |
| Year index medication prescribed (referent 2011) |
1.19 | 0.04 | 1.11 | 1.28 | |
| Duration of index medication (≥ 7 days) (referent < 7 days) |
2.43 | 0.17 | 2.12 | 2.77 | |
| Age at date of index medication | 1.00 | <0.01 | 0.99 | 1.00 | |
All p<0.001, except age
DISCUSSION
In this retrospective cohort study, we analyzed a large volume of medication and laboratory data from a multi-site CDR over three years to establish rates of PGx testing for all drugs containing an FDA label with PGx biomarker information. Because the presence of biomarker information in the drug label alone does not provide context for the clinical relevance of testing, we relied on PharmGKB levels of actionability to better classify how strongly the FDA drug label endorses PGx testing for each drug-biomarker pair.
For germline tests, greater than 95% of the index medication orders we captured were for medications with weaker guidance for PGx testing (informative or actionable), and tests were associated with fewer than 1% of index medication orders. This may not be surprising given the fact that less than 5% of index medication orders were for medications for which testing was classified by PharmGKB as recommended or required. Overall, the rate of biomarker testing has increased slowly over time, but is still below 3%. These findings are consistent with the fact that only 16% of medications in our dataset contain a label requiring or recommending testing.
In contrast, for somatic tests weak evidence was provided for just over 50% of index medication orders, while PGx tests were associated with 24% of index medication orders, overall. The rate of increase in required testing has risen to just over 20%, while the rate of informative testing has declined to 40% of index medication orders. As expected, the highest rates of biomarker testing occurred with somatic tests, and among only oncology medications, since 72% of these medications in our dataset contain a label requiring testing.
Our regression analysis supports a higher likelihood of PGx testing if the test was somatic versus germline, demonstrating the significant impact of oncology medications on the landscape of pharmacogenomic-guided medication use. We also found a positive association between PharmGKB level and test ordering, highlighting the importance of information found in medication labeling.
Among medications associated with germline biomarker testing, infectious disease therapies had six of the ten highest testing frequencies. (Table 4) Four of these top ten medications are associated with G6PD testing, which is a well-established assay to detect patients at risk for hemolytic reactions to multiple drugs. Rates of germline genetic testing for UGT1A1 in patients with nilotinib and for TPMT in patients on azathioprine were only 20% and 10%, respectively.
In contrast to the medications associated with germline testing, of which only the top two drugs (dapsone and primaquine) had testing rates above 30%, all of the top 10 medications with the highest rates of somatic biomarker testing had rates above 30% (Table 4). Interestingly, three of these top ten medications with the highest somatic biomarker testing rates were classified as informative, which is the lowest PharmGKB level. However, Vivot et al. reviewed the FDA drug labels and disagreed with the informative classification of three drugs (rituximab, tositumomab, and lenalidomide), reclassifying them as required15, and two of these three medications appear in the top ten in our data set. The authors also reclassified three medications with recommended or required classifications (mercaptopurine, carglumic acid, and velaglucerase) to no guidance, and all three have low rates of testing in our data set (Supplementary Table 3), suggesting that these reclassifications may better reflect current practice. Among the medications that require testing but had low PGx testing rates, two high volume medications – carbamazepine and valproic acid – drove the overall rate of testing down to 1%. Careful reading of the labels for carbamazepine and valproic acid indicates that testing is required for populations with a high pretest probability of a specific HLA-type or possible metabolic syndrome, respectively. Without these medications, the rate of testing for required medications would be significantly higher at 24%, but still well below a rate near 100% that might be expected for medications in which PGx testing is required.
Our study is the first of which we are aware that integrates medication ordering information with laboratory test order information over a large number of drugs and multiple sites within a large academic medical system. As most institutions have not established broad programs for prospective genotyping, the rates of PGx biomarker utilization we observe likely reflect utilization rates at other academic medical institutions. While our CDR does not strictly standardize medication orders or laboratory tests into widely used vocabularies, another strength of our study was the use of a diverse team with content knowledge across pharmacy, laboratory medicine, and information systems to ensure relevant data capture and analysis. Finally, although there is a diverse set of provider settings covered in our study, laboratory services are centralized in our medical system, which allowed us to reliably capture testing ordered in our patient population within our study locations.
Because our study relies on multiple data sources in a CDR that include data from multiple institutions, there are some limitations of our work. First, since some of our sites are referral centers, patients transition in and out of our health system for care, so not all relevant medication, laboratory, and patient information is available. Patients visiting our cancer center often visit with laboratory and pathology studies performed at other institutions; these are never translated into a structured format in the EHR, which will be a recurring problem for studies of this type in the absence of interoperability. Although our study may have underestimated the number of tests drawn, the centers from which patients are referred are generally lower complexity clinics and therefore less likely to draw biomarkers. Another limitation is the nature of the medication information stored in our CDR: medication administration orders for our cancer care center were captured but outpatient prescriptions were not, which prevents us from capturing a subset of eligible medications. However, a large percentage of those patients are admitted to our tertiary care center for chemotherapy, and medications for those inpatients were captured in our dataset. Third, we reduced the complexity of the data to associate only index medications with a PGx test, which may not accurately reflect the reason a test was performed. For example, a test performed out of concern for an adverse event after the subsequent administration of a drug is not associated with that particular medication order and instead would appear as testing well after the index medication order. Indeed, establishing an association between a variant-medication order and a specific adverse event is a non-trivial research challenge, and is an area for future investigation.
With the continued emphasis on developing precision medicine to improve patient care, the development of infrastructure to support the use of genomics within clinical workflows is paramount. While our work illustrates that overall use of PGx testing is increasing, we found that a majority of medications with a PharmGKB level of required testing did not have the corresponding biomarker test performed in either a structured or semi-structured (by searching relevant strings in pathology reports) format in our EHR. Our study does not ascertain whether such testing data is present in an unstructured format, which is possible given the fact that multiple referral centers were included in the study. However, even if the percentage of required testing among these medications was much higher, in our current state less than one-fifth of the test orders queried in this study were available in a structured format with a defined laboratory test code, and searching of free text strings was required to detect many testing orders. In order to adopt enabling tools such as genomic clinical decision support, the presence of structured results, which are more complex to represent than orders, will be required. Furthermore, to make this information useful for all patients, genomic information will need to move between institutions, emphasizing the importance of standards such as Logical Observation Identifier Names and Codes (LOINC)16. Our work demonstrates that although PGx testing has expanded rapidly, particularly in the realm of oncology, there are significant hurdles to integrating EHRs within PGx testing workflows to promote testing for all patients who may benefit.
In conclusion, we performed a retrospective cohort analysis to determine patterns of PGx-guided medication use using a CDR that covered approximately 200,000 patients in diverse settings. We found that overall, 1% of eligible index medication orders had associated test orders recorded in a structured format. Among medications with associated germline PGx testing, less than 1% of index medication orders had associated test orders, although this number was largely driven down by two high volume drugs for which testing is required only in specific subpopulations. Among oncology medications with associated somatic biomarker testing, only 24% of index medications were associated with a test order. However, the percentage of required testing performed in both the germline and somatic groups has increased over the 3-year study period. Oncology medications have impacted these trends to a greater degree than any other therapeutic class, as somatic biomarker testing was 73 times more likely compared to medications with germline biomarkers. Our work illustrates that in the absence of a structured PGx testing program, biomarker testing adoption appears relatively low, but more importantly, there are significant technical hurdles to fully supporting precision medicine.
METHODS
Setting
UW Medicine owns or operates four hospitals and multiple outpatient care clinics, with approximately 65,000 admissions and more than 1.6 million outpatient and emergency room visits each year. Selected electronic health record (EHR) data from a subset of UW Medicine's institutions are available in the institutional CDR including Harborview Medical Center (413-bed publicly owned hospital with Level I trauma center), University of Washington Medical Center (450-bed tertiary care center), Seattle Cancer Care Alliance (over 70,000 yearly patient visits), and UW Neighborhood Clinics, and a network of ten primary care clinics. In addition to providing inpatient care, both Harborview Medical Center and University of Washington Medical Center house outpatient specialty care clinics.
Study Design
This is a retrospective cohort study of adult patients with at least one outpatient prescription or inpatient medication order (herein after both referred to as orders) on record for a drug that contains any PGx biomarker testing information in the FDA label, from January 2011 to December 2013. Inclusion criteria included age > 18 years old, at least one medication record for drug(s) of interest. As this study did not meet the federal regulatory definition of human subjects research, review by the University of Washington Institutional Review Board was not required.
Data Collection
Data were collected from the UW Medicine CDR in a multi-step process to create an analytic data set without patient identifiers. In preparation, we first, we created a reference table to support queries of the CDR that included two primary elements: 1) generic drug names for each of the 128 medications containing an FDA drug label with information about biomarker testing that were available during the study timeframe, and 2) the associated biomarker recommended, with a translation to local laboratory and pathology test order names. One drug combination carrying an “Informative Only” PharmGKB level of actionability – rifampin, isoniazid, and pyrazinamide - was excluded due to the challenge in consistently querying for orders that overlap one another for this combination. Biomarkers were captured via one of three methods: 1) a defined test code in the laboratory information system, 2) matching a string for a biomarker name in send-out tests that do not have a defined test code, or 3) matching a string for a biomarker name in pathology reports since structured immunohistochemistry (IHC) data were not available (eg. for the MS4A1 gene the report must contain “CD20” to be captured). Each of these three methods either alone or in combination was used regardless of whether biomarkers were germline or somatic, based on preliminary analysis of the data repository to determine which method(s) accurately detected each individual biomarker. Generic drug names were then mapped to medication names in inpatient and outpatient EHRs by first querying the medication catalog of each system for the list of names, followed by manual search, addition, and curation of any generic drug names that did not return a catalog drug. Finally, since the FDA does not explicitly use a standard nomenclature to classify the strength of recommendation for PGx testing according to information on drug labels, we relied on classifications provided by the Pharmacogenomics Knowledgebase (PharmGKB)17. PharmGKB classifies four levels of actionability for PGx testing: testing required, testing recommended, testing provides actionable information (without stating or implying a requirement or recommendation), or testing is only informative. For each medication-PGx test pair, we included PharmGKB level. Separately, we categorized medications by therapeutic class, using the taxonomy provided in the Healthcare Cost and Utilization Project (HCUP) databases17. The Supplementary Figure illustrates the linked files that comprise the analytic dataset.
Eligible patients were selected by querying outpatient EHR prescriptions and inpatient medication orders between January 2011 and December 2013 for all medications of interest. For each medication order, the medication name, date and time of order, start, date and time of order end were collected. In addition, the following information for the patient encounter associated with the medication order was collected: visit type (inpatient, office visit, refill), location, arrival and departure time, and age at time of medication start date. For each patient with an eligible medication order, any laboratory test orders for a PGx biomarker recommended by the FDA for each of the eligible medications between January 2010 and June 2014 were collected. As a final data extraction step, patient identifiers and visit numbers were replaced with randomly generated numbers that were consistent between medication order, visit, and biomarker orders to preserve the relationships among these three data elements.
The medication dataset was analyzed to find the first and last medication order for every unique patient-medication combination. An index medication was defined as the first instance of a medication order for a given patient between January 1, 2011 through December 31, 2013, with no order for the same medication within the previous 12 months. The index medication may therefore have been either the patient's first ever order of the drug, the first order within the study period (for a drug that was first ordered prior to January 2010), or the first order since 12 months had elapsed since the previous order. Information from the laboratory and pathology test order dataset was linked, then used to determine whether the patient had a PGx test relevant to a given medication and, if so, what test was performed and the number of days it was ordered before or after the first order of the medication. Validation steps included ensuring temporal associations between medication ordering and biomarker testing were within expected timeframes, ensuring consistency in the number of visits and frequency of medication orders over time, and confirming that counts for all send-out tests were the same in both the laboratory system and the dataset.
Statistical Analysis
An analytic dataset was then created. Each test was categorized as being germline or somatic based on whether the biomarker testing is typically performed on a blood sample or a tissue sample, respectively. The unit of analysis was each medication-biomarker pair. Each pair was associated with a patient ID number. The timing of each index medication order was compared to the timing of relevant PGx testing, if any occurred. The look-back and look-forward periods were six months each, from the date of index medication order. If more than one PGx test relevant to a given medication was conducted, the test that occurred closest to the date of the index medication (before or after) was used for further analysis. The level of actionability for each particular medication-test combination, as graded by PharmGKB18, was indicated. Additional relevant information included age, gender, number of visits, first and last dates of medication order and therapeutic class.
Descriptive analyses were conducted using means and standard deviations for continuous variables, counts and percentages for categorical variables. We modeled the association between PharmGKB level and the probability of drawing of a PGx biomarker using generalized estimating equations, clustering on patient, and using an exchangeable correlation structure. We used the Huber-White (sandwich) estimator to adjust standard errors19. Analyses were conducted in R20 and Stata21. An alpha level of 0.05 was considered significant, throughout.
Supplementary Material
Creation of the Analytic Dataset
HCUP = Healthcare Cost and Utilization Project; ID = Identifier; PGx = Pharmacogenomic
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Use of pharmacogenomic biomarkers has not been well characterized outside of formal testing programs at a few academic medical centers.
What question did this study address?
Using data from a multi-site academic health system, we determined the frequency of pharmacogenomic testing for medications that include biomarker information in the FDA drug label.
What this study adds to our knowledge
Rates of pharmacogenomic testing were approximately 1% for required germline and 24% for required somatic biomarkers. Even after capturing unstructured data by searching pathology/laboratory reports, testing rates remained low.
How this might change clinical pharmacology or translational science
Our study reveals that without a formal pharmacogenomic testing program, rates of adoption are low. While clinical decision support is assumed to have a major role in increasing uptake, our work highlights the importance of a formal testing program to achieve the promise of precision medicine.
Acknowledgments
We thank Sally Lee, PhD, and Tony Black, MA, for their assistance in formulating queries and querying the clinical data repository. We thank Christian Bock for his participation in formulating queries in our institution’s de-identified clinical data repository early in the project, and Aasthaa Bansal, PhD for her suggestions on the statistical analysis. We thank Brian Shirts, MD, PhD, and Sean Mooney, PhD, as well as other members of the Precision Medicine Informatics Group at the University of Washington for their continued feedback on our work. Finally, we thank the two reviewers for their helpful comments that greatly strengthened the manuscript.
This specific project received no grant from any funding agency but the work was made possible by the resources provided by the Institute of Translational Health Sciences at the University of Washington, which is supported by grants UL1TR000423, KL2TR000421, and TL1TR000422 from the NIH National Center for Advancing Translational Sciences through the Clinical and Translational Science Awards Program (CTSA). Partial support was also provided by grant number T32HS013853 from the Agency for Healthcare Research and Quality. In addition, partial support for computing resources utilized in this research came from a Eunice Kennedy Shriver National Institution of Child Health and Human Development research infrastructure grant, R24 HD042828, to the Center for Studies in Demography & Ecology at the University of Washington.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
Patrick C. Mathias – I have nothing to disclose.
Nathaniel Hendrix – I have nothing to disclose.
Wei-Jhih Wang – I have nothing to disclose.
Katelyn Keyloun– I have nothing to disclose.
Maher Khelifi– I have nothing to disclose.
Peter Tarczy-Hornoch– I have nothing to disclose.
Beth Devine – I have nothing to disclose.
AUTHOR CONTRIBUTIONS
E.B.D and P.C.M. wrote the manuscript; E.B.D. and P.C.M. designed the research; E.B.D., P.C.M., N.H., K.R.K., and M.K. performed the research; E.B.D., P.C.M., N.H., and W-J.W. analyzed the data; P.T-H. contributed new reagents/analytical tools.
REFERENCES
- 1.Center for Drug Evaluation and Research Table of Pharmacogenomic Biomarkers in Drug Labeling. 2014 [Google Scholar]
- 2.Frueh F, et al. Pharmacogenomic Biomarker Information in Drug Labels Approved by the United States Food and Drug Administration: Prevalence of Related Drug Use. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2008;28:992–998. doi: 10.1592/phco.28.8.992. [DOI] [PubMed] [Google Scholar]
- 3.Schildcrout, et al. Optimizing Drug Outcomes Through Pharmacogenetics: A Case for Preemptive Genotyping. Clinical Pharmacology & Therapeutics. 2012;92:235–242. doi: 10.1038/clpt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanek, et al. Adoption of Pharmacogenomic Testing by US Physicians: Results of a Nationwide Survey. Clinical Pharmacology & Therapeutics. 2012;91:450–458. doi: 10.1038/clpt.2011.306. [DOI] [PubMed] [Google Scholar]
- 5.Stanek E, Sanders C, Frueh F. Physician Awareness and Utilization of Food and Drug Administration (FDA)-Approved Labeling for Pharmacogenomic Testing Information. Journal of Personalized Medicine. 2013;3:111–123. doi: 10.3390/jpm3020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan H, O’Donoghue A, Aikin K. Primary Care Physicians’ Use of FDA-Approved Prescription Drug Labels. The Journal of the American Board of Family Medicine. 2014;27:694–698. doi: 10.3122/jabfm.2014.05.140039. [DOI] [PubMed] [Google Scholar]
- 7.Dunnenberger H, et al. Preemptive Clinical Pharmacogenetics Implementation: Current Programs in Five United States Medical Centers. Annu Rev Pharmacol. 2015;55:1–18. doi: 10.1146/annurev-pharmtox-010814-124835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulley, et al. Operational Implementation of Prospective Genotyping for Personalized Medicine: The Design of the Vanderbilt PREDICT Project. Clinical Pharmacology & Therapeutics. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driest V, et al. Clinically Actionable Genotypes Among 10,000 Patients With Preemptive Pharmacogenomic Testing. Clinical Pharmacology & Therapeutics. 2014;95:423–431. doi: 10.1038/clpt.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nutescu E, et al. Feasibility of implementing a comprehensive warfarin pharmacogenetics service. Pharmacotherapy. 2013;33:1156–1164. doi: 10.1002/phar.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielinski SJ, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clinic proceedings. 2014;89:25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell GC, et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J Am Med Inform Assoc. 2014;21:e93–e99. doi: 10.1136/amiajnl-2013-001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman J, et al. PG4KDS: A model for the clinical implementation of pre-emptive pharmacogenetics. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2014;166:45–55. doi: 10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell P, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care–initial results of the University of Chicago ‘1,200 Patients Project’. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2014;166:68–75. doi: 10.1002/ajmg.c.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivot A, Boutron I, Ravaud P, Porcher R. Guidance for pharmacogenomic biomarker testing in labels of FDA-approved drugs. Genetics in Medicine. 2014;17:733–738. doi: 10.1038/gim.2014.181. [DOI] [PubMed] [Google Scholar]
- 16.Deckard, McDonald, Vreeman Supporting interoperability of genetic data with LOINC. Journal of the American Medical Informatics Association. 2015 doi: 10.1093/jamia/ocu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healthcare Cost and Utilization Project (HCUP) [Accessed: November 20, 2016];Agency for Healthcare Research and Quality. Avaialable from URL: http://www.ahrq.gov/research/data/hcup/index.html. [PubMed] [Google Scholar]
- 18.Thorn C, Klein T, Altman R. Pharmacogenomics and bioinformatics: PharmGKB. Pharmacogenomics. 2010;11:501–505. doi: 10.2217/pgs.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers W. Regression standard errors in clustered samples. Stata technical bulletin. 1994;3 [Google Scholar]
- 20.The R Project for Statistical Computing. [Accessed: November 20, 2016];2013 Avaialable from URL: https://www.r-project.org/ [Google Scholar]
- 21.Stata Data Analysis and Stastical Software. [Accessed: November 20, 2016];Release 13. Available from: http://www.stata.com/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Creation of the Analytic Dataset
HCUP = Healthcare Cost and Utilization Project; ID = Identifier; PGx = Pharmacogenomic