Abstract
We conducted a cluster-randomized trial to estimate effects of directly observed combination antiretroviral therapy (DOT-cART) on retention with viral suppression among HIV-positive adults in Peru.
We randomly allocated facilities to receive the 12-month intervention plus the standard of care, including adherence support provided through accompaniment. In the intervention arm, health workers supervised doses, twice daily, and accompanied patients to appointments.
Among 356 patients, intention-to-treat analyses showed no statistically significant benefit of DOT, relative to no-DOT, at 12 or 24-months (adjusted probability of primary outcome: 0.81 vs. 0.73 and 0.76 vs. 0.68, respectively). A statistically significant benefit of DOT was found in per-protocol and as-treated analyses at 12-months (0.83 for DOT vs. 0.73 for no DOT, p-value: 0.02 per-protocol, 0.01 as-treated), but not 24-months.
Rates of retention with viral suppression were high in both arms. Among adults receiving robust adherence support, the added effect of time-limited DOT, if any, is small-to-moderate.
Keywords: HIV, antiretroviral, treatment support, directly observed treatment
Resumen
Realizamos un estudio clínico aleatorizado por grupos para estimar los efectos que tiene la terapia antirretroviral combinada directamente observada (DOT-cART) sobre la retención con supresión viral entre adultos VIH-positivos en el Perú.
Cada establecimiento fue aleatorizado para recibir la intervención de 12 meses, adicional a la atención estándar, incluyendo acompañamiento como soporte para la adherencia. En el brazo de intervención, los trabajadores de salud supervisaron la toma de dosis, dos veces diariamente, acompañando a los pacientes a sus citas médicas.
Entre 356 pacientes, el análisis por intención-de-tratar no mostro un beneficio estadísticamente significativo del DOT respecto al no-DOT tanto a los 12 como a los 24 meses (probabilidad ajustada del indicador primario: 0.81 vs. 0.73 y 0.76 vs 0.68, respectivamente). Un beneficio estadísticamente significativo del DOT se encontró en el análisis por-protocolo y en el análisis ‘tratado en el estudio’ a los 12 meses (0.83 para DOT vs. 0.73 para no-DOT; valor-p: 0.02 por-protocolo, 0.01 tratado en el estudio), pero no a los 24 meses.
Las tasas de retención con supresión viral fueron altas en los dos brazos del estudio. Entre adultos que recibieron un robusto soporte para asegurar la adherencia, el efecto adicional de DOT-cART de tiempo-limitado, si lo hay, es menor a moderado.
INTRODUCTION
Directly observed therapy (DOT), in which a health worker observes the ingestion of medications in order to guarantee adherence, has been implemented for combination antiretroviral therapy (cART) delivery for HIV with varying success.(1-6) Two meta-analyses revealed no statistically significant effect of DOT-cART on HIV outcomes in randomized trials; however, there was heterogeneity across studies, including differences in the target population, duration and site of the intervention, study outcome, and preparation for the post-DOT period.(3, 4) When non-randomized studies with control groups were included, DOT-cART participants were more likely to experience undetectable viral loads, greater increases in CD4 cell count, and optimal adherence.(3) Both meta-analyses suggested DOT-cART may be more effective in populations at higher risk of non-adherence.(3, 4) The few studies exploring whether DOT-cART benefits are sustained beyond the intervention suggest a waning effect.(3, 4)
DOT implementation strategy (e.g., community-based versus clinic-based; DOT delivered by nurse versus layperson) may be a critical determinant of success. In community-based DOT, home visits by a community health worker (CHW) provide an opening for intensive emotional support and follow-up for hard-to-reach patients, which supplements DOT.(7) A key foundation of community-based support is the notion of accompaniment, defined by Paul Farmer as long-term support to vulnerable patients by CHWs.(8) Accompaniment, argues Farmer, is particularly important for individuals living in poverty,(8) who face obstacles to accessing and adhering to treatment.(9-11) Financial constraints contribute to food insecurity, lack of money for transportation, extended work hours, and an inability to negotiate time off for medical appointments—all of which may challenge routine clinic visits and daily adherence.
Prior studies in Peru and Rwanda have reported improved retention and viral load suppression with community-based accompaniment including DOT, as compared to a clinic-based standard of care for cART delivery.(12, 13) These observational studies were unable to examine the independent impact of DOT because DOT was included in the community-based adherence support received by all intervention participants. We conducted a cluster-randomized control trial to study whether there was an added benefit of DOT on HIV treatment outcomes among impoverished adults receiving community-based adherence support. The primary outcome was retention with viral load suppression and secondary outcomes included change in CD4 cell count from baseline, perceived social support, depression, time to attrition, time to HIV-related complication, and time to virologic failure. We followed participants for a period of 24 months in order to estimate the immediate and longer-term effects of community-based DOT-cART on outcomes among HIV-positive adults.
METHODS
Study population
We undertook this study in ten of 43 districts in Lima, Peru, which were selected on the basis of their higher burden of HIV. Health facilities in the catchment area were eligible to participate if they were not a tertiary referral center. We recruited HIV-positive adults ≥18 years, who were living in poverty (score < 45 based on Progress Out of Poverty Index(14)), starting a new cART regimen (a first or salvage regimen), residing and receiving HIV care within the study catchment area and open to receiving CHW visits for DOT-cART. Patients who were imprisoned or could not give informed consent due to neuropsychiatric impairment were excluded. Eligible participants were selected consecutively from March 2010 through July 2012, and followed until July 2014.
Study design
The study area contained two tertiary referral hospitals and 90 health centers and posts. Health facilities were assigned to the intervention or control arm using a computer-based randomization sequence. Intervention assignment was not revealed to the participant until after s/he had been enrolled and baseline data collection was completed. Treatment facilities were not informed of their study arm. Because the study was not blinded, it is possible that health facility personnel learned of the treatment assignment from patients or DOT workers; however, randomization occurred after facilities were recruited, thus study assignment did not influence each facility’s decision to participate in the trial. Separate teams were responsible for the recruitment, enrollment and data collection for each arm. A baseline assessment was performed, and primary and secondary outcomes were assessed after 12 and 24 months to estimate the immediate and sustained intervention effect.
Standard of care plus accompaniment
All participants received the standard of HIV care, according to national guidelines,(15) which included free clinic-based cART and CD4 cell counts and viral loads every six months. National guidelines specified that prospective cART patients identify a treatment supporter who can offer emotional and adherence support. Additionally, all study participants received community-based accompaniment in the form of monthly home visits by trained health promoters employed by Socios En Salud, a local non-governmental organization. During home visits, health promoters reviewed treatment adherence, reminded patients of upcoming medical encounters, and screened patients and family members for health problems. Health promoters coordinated with healthcare providers to manage acute issues and assisted in hospital navigation.
To our knowledge, there were no changes to national HIV treatment and care guidelines during the study period that would have influenced interpretation of our findings. Although changes to national guidelines for cART eligibility were updated in 2012, this occurred following completion of study enrollment and therefore would not have influenced cART initiation among study participants.
DOT-cART intervention
Individuals receiving cART at facilities assigned to the intervention arm received 12 months of community-based DOT-cART, delivered by a team of 61 DOT workers selected from the Ministry of Health CHW program, which identifies and trains lay health promoters from local communities to participate in vaccination campaigns, community-based DOT for TB treatment, and health promotion efforts. The DOT-cART workers received four days of training about HIV care, adherence, the role of DOT, and factors that could affect patients’ adherence and well-being such as domestic violence and substance use. They participated in refresher workshops twice a year. Further details regarding the training are found in Electronic Supplementary Materials.
Patients selected a DOT worker from those in close proximity to their residence, but were not given additional information about the DOT workers aside from his/her name. DOT workers’ primary responsibility was the supervision of all cART doses, twice daily, in the participant’s home or mutually agreed upon location. Additional responsibilities included recording doses and notifying providers of missed or self-administered doses, and accompanying patients to medical appointments to relay information, advocate, and help the patient understand and complete provider recommendations. During encounters, DOT workers triaged for side effects and HIV complications, and identified and notified providers of any psychosocial and medical problems. DOT workers were supervised by both the health promoters and a nurse. In addition, a random selection of approximately 7% of patient participants and their health promoters and DOT workers were observed and interviewed throughout the study period by study staff. After eight months of daily DOT-cART, patients began to transition to self-administration, during which time the frequency of DOT visits tapered to every other day, weekly, and every two weeks. DOT workers prepared treatment supporters to support the patient during the taper and transition to self-administration.
Outcomes
Primary and secondary outcomes were determined at 12 and 24 months following cART initiation, reflecting the times at which the intervention was discontinued in DOT-cART patients and one year of post-intervention follow-up, respectively. The primary outcome was retention with HIV viral load suppression (<400 copies/mL). Individuals who died, became lost to follow-up, or defaulted from treatment were not considered retained. Death was defined as death from any cause. Loss to follow-up and default were established after an individual missed 60 consecutive days of treatment. Virologic failure was defined as two consecutive detectable viral loads (≥400 copies/mL) after achieving virologic suppression or cART change due to virologic failure. Individuals for whom we were unable to obtain primary outcome data (i.e., due to a missing viral load result or withdrawal from the study) but who remained on treatment according to health facilities records were not considered lost in primary endpoint analyses. Rather, their primary outcome data was considered missing, and we conducted sensitivity analyses to examine the impact of these missing data. Participants who were lost to follow-up or discontinued cART were visited monthly, if possible, to assess clinical status and encourage cART re-initiation.
Secondary outcomes included change in CD4 cell count from baseline, perceived social support, depression, time to attrition, time to HIV-related complication, and time to virologic failure. Social support and depression were assessed via face-to-face interviews using an adapted version of the Duke UNC Social Support Questionnaire,(16) and the Hopkins symptom checklist, respectively.(17) The Duke UNC questionnaire consists of 14 items with a 5-point Likert scale (1= much less than I would like, to 5 = as much as I would like) that was originally developed among patients attending a family medicine clinic in the United States. Time to attrition was the number of days from cART initiation to death, loss to follow-up, or default. Time to HIV-related complication was the number of days from cART initiation to new opportunistic infection, HIV-related hospitalization, or HIV-related death. Time to virologic failure was the number of days from cART initiation to the first of two consecutive detectable viral loads after achieving virologic suppression or the date of cART change due to virologic failure, whichever occurred first.
Data collection
Outcome and covariate data were collected using standardized forms, via medical chart review and face-to-face interviews with participants. Alcohol and drug use disorders were measured at baseline and monthly using the 20-item alcohol and drug modules of the Mini International Neuropsychiatric Interview, which has previously been validated in Spanish.(18) These modules consist of yes or no questions that can be summed to determine whether the participant meets DSM-IV criteria (The Fourth Edition of the Diagnostic and Statistical Manual of Mental Disorders) for alcohol and/or substance abuse or dependence. HIV-related stigma was measured using an abbreviated version of the Berger HIV Stigma Scale.(19) The original English-language version of this stigma scale contains 40 items that used a four-point likert scale and covers the following domains: personalized sigma, disclosure concerns, negative self - image, and concern with public attitudes toward people with HIV. It was originally developed and validated in an adult HIV-infected population in the United States. Members of our study team subsequently translated the scale to Spanish, demonstrated its internal reliability and construct validity in an urban Spanish-speaking population in Peru, and created an abridged 21-item version of the scale.(19)
Sample size calculations
Sample size calculations were based on the primary outcome, using estimates of intra-cluster correlation (0.05) from other precedents.(20) Based on this and an average cluster size of 7.4 (standard deviation, 10.7) in a pilot study,(12) we estimated an inflation factor of 2.09 for cluster randomization. A detectable risk difference of 0.15 with 80% power and a two-sided alpha-test of 0.05 required a sample of 340 HIV patients. Estimating fewer than 5% missing outcomes, we increased this estimate to 356.
Statistical analyses
Primary analyses were intention-to-treat. To estimate the effect of DOT-cART on the primary outcome as well as binary secondary outcomes (depression versus no depression), we constructed separate marginal models for 12- and 24-month outcomes using generalized estimating equations with a binary response, logit link, and robust standard errors. We used an exchangeable correlation matrix, which presumes individuals are uncorrelated across clusters, while individuals within the same cluster share a correlation coefficient. For continuous secondary outcomes (change in CD4 cell count, social support) we used generalized estimating equations with a normal distribution and identity link. To quantify differences in time to attrition, HIV-related complication, and virologic failure, we used marginal Cox models and maximum partial likelihood estimates under an independent working assumption and robust sandwich covariance matrix estimate to account for the intra-cluster dependence. We conducted multivariable analyses in which we adjusted for baseline variables that appeared imbalanced across the study arms. Because neither the primary outcome nor its inverse were rare events, the odds ratio did not approximate the risk ratio, and we therefore reported predicted probabilities and 95% confidence intervals.
To assess potential bias due to missing outcome data, we examined whether the rates of missing outcome data varied by study arm. We also conducted sensitivity analyses where participants who were missing primary outcome data were considered (1) not retained with viral load suppression and; (2) retained with viral load suppression. Because there were some missing covariate data and complete case analyses may be biased if data are not missing completely at random, we performed sensitivity multivariable analyses on data sets multiply imputed (N=5) using covariate and outcome data. Imputation was conducted using the Markov Chain Monte Carlo methods, and effect estimates were pooled across data sets.
Fourteen participants never initiated DOT although they received cART at a DOT health facility. Reasons included concern that others would discover their diagnosis, perceived inconvenience of DOT, and employment scheduling conflicts. Nine individuals incompletely followed their allocated treatment because they changed residences during the 12-month intervention period and began receiving care at a health facility assigned to the other study arm. Four of these individuals moved from a DOT to a no-DOT health facility, and five moved from a no-DOT to a DOT health facility. To account for infidelity to allocated study arm, we conducted a per-protocol analysis in which we excluded patients who did not follow their intervention assignment; and an as-treated analysis in which patients were assigned the exposure they received for the majority of the 12-month intervention period. From the latter, we excluded two people in the DOT arm who never initiated DOT due to hospitalization and early death. We considered this to be a conservative approach because these patients would have been counted as deaths in the control arm. Because perceived HIV-related stigma might contribute to DOT refusal, we adjusted for HIV stigma score in multivariable per-protocol and as-treated analyses.
SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used for statistical analyses. Study procedures were approved by Partners Healthcare Human Research Committee and the Ethics Committee of the Peru National Institute of Health. This trial is registered at ClinicalTrials.gov, NCT01070017.
RESULTS
Health facility allocation and inclusion
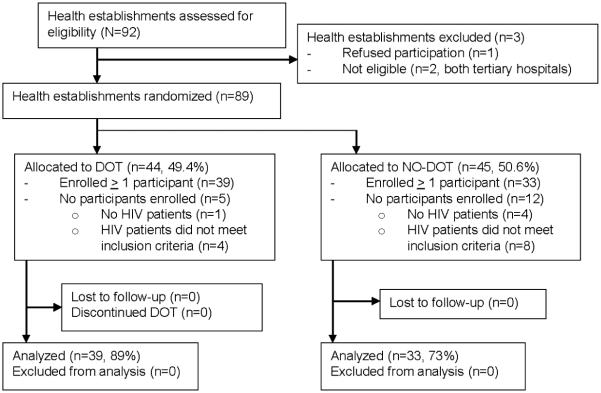
Of 92 health facilities assessed for eligibility, two tertiary hospitals were excluded and one health facility elected not to participate. Of the 89 health facilities randomized, 72 enrolled one or more HIV-patients and were subsequently included for analysis (39 DOT sites and 33 no-DOT sites; Figure 1). The median cluster size was four participants, with a range from one to 18. Thirteen clusters included only one participant.
Figure 1.
Cluster randomization of health establishments to DOT-cART intervention or control in Lima, Peru
Baseline characteristics and treatment allocation
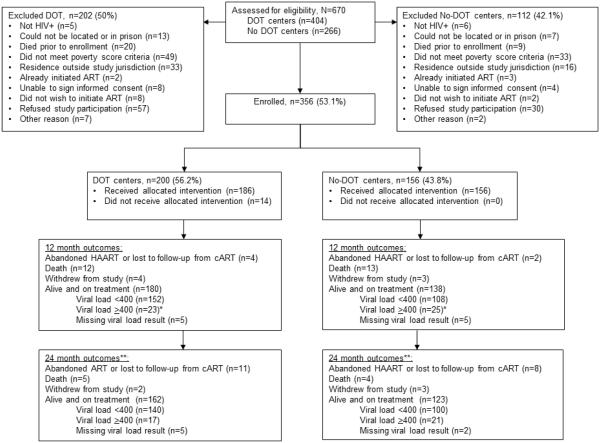
Of 670 individuals screened for inclusion, over half (n=356) enrolled (Figure 2). Of those enrolled, 200 (56.2%) belonged to DOT facilities and 156 (43.8%) belonged to no-DOT facilities. Baseline characteristics of participants are shown in Table 1. Although the two groups were generally similar, patients in the DOT arm were more often originally from Lima, had a higher median CD4 count, more depression, fewer substance use disorders, less unemployment and less severe food insecurity. Levels of perceived HIV-related stigma were comparable in the two groups.
Figure 2.
Allocation status and follow-up of 356 HIV-positive adults enrolled in a cluster randomized trial of DOT-cART
* Virologic failure was defined as two consecutive detectable viral loads after achieving virologic suppression or cART change due to virologic failure.
** Among 180 and 138 participants alive and on treatment at 12-months in the DOT and No-DOT centers, respectively.
Table I.
Baseline characteristics of participants, by study arm
| Characteristic | N |
DOT-HAART group (n=200)
N (%), mean ± SD, or median [IQR] |
Control group (n=156)
N (%), mean ± SD, or median [IQR] |
|---|---|---|---|
| Socio-demographic characteristics | |||
| Female | 356 | 71 (36) | 57 (37) |
| Age (years) | 356 | 33 [27, 40.5] | 32.5 [26, 40] |
| Originally from Lima | 356 | 119 (60) | 86 (55) |
| Married or living with partner | 356 | 70 (35) | 51 (33) |
| Number of children | 356 | 1 (0, 2) | 1 (0, 3) |
| Did not complete high school | 353 | 102 (52) | 79 (51) |
| Poverty score | 356 | 36 (30, 41) | 35 (29.5, 41) |
| Food insecurity status | 348 | ||
| Food secure | 37 (19) | 26 (17) | |
| Mildly insecure | 18 (9) | 11 (7) | |
| Moderately insecure | 64 (33) | 42 (27) | |
| Severely insecure | 75 (39) | 75 (49) | |
| No full-time employment* | 356 | 118 (59) | 103 (66) |
| Difficulty accessing health services | 350 | 177 (90) | 138 (90) |
| Clinical characteristics | |||
| Months with diagnosis at cART initiation | 355 | 3.6 [1.8, 12.0] | 2.6 [1.4, 13.9] |
| BMI (kg/m2) | 355 | 22.4 ± 3.8 | 22.2 ± 3.7 |
| CD4 (cells/μL) | 337 | 174 [69, 262] | 128.5 [57, 254] |
| Viral load (copies/ml) | 336 | 145511 [40103, 356193] | 143571 [36121, 437653] |
| Prior HAART | 356 | 12 (6) | 6 (4) |
| Co-morbidities | |||
| Any substance use disorder | 340 | 54 (28) | 52 (35) |
| TB disease | 356 | 38 (19) | 33 (21) |
| Psychosocial variables | |||
| Social support score | 349 | 27.3 ± 6.1 | 27.2 ± 6.2 |
| Perceived HIV-related stigma | 350 | 52.4 ± 9.8 | 54.3 ± 12.0 |
| Depression | 340 | 25 (13) | 11 (7) |
The following responses were classified as not having full-time employment: house-wife, student, seasonal employees or unemployed.
Individuals were defined as having difficulty accessing health services if they reported any of the following on a standardized Likert-type scale: never being admitted to a hospital without difficulty when in need of hospital services; never easily able to get to places where s/he could receive health services; always or sometimes having to solve own health problems without medical services because they were too expensive; always or sometimes having difficulty accessing health services in case of an emergency; or needing health services in the six months prior to interview but unable to get them.
Fidelity to allocated intervention
Nine individuals changed residences during the 12-month intervention period, resulting in a new health facility that was randomized to an arm differing from their initial assignment. Four of these individuals moved from a DOT to a no-DOT health facility, and five moved from a no-DOT to a DOT health facility.
Intention to treat analyses
The primary outcome of retention with viral load suppression tended to be more common among individuals in the DOT arm at both 12 and 24 months (80% versus 73% and 74% versus 68%, respectively). These differences were not significant in univariable or multivariable analyses (Table 2). Secondary outcomes were similar across the intervention and control groups at 12 and 24 months (Electronic Supplementary Materials Tables S1 and S2). Virologic failure was rare, occurring in three participants (1.5%) in the DOT arm and one participant (0.64%) in the no DOT arm, and therefore was not analyzed using regression methods. Only 4.8% and 3.8% of participants were missing primary outcome data at 12 and 24 months, respectively, and study arm did not predict missing primary or secondary outcome data. Some clusters were dropped from the analyses due to missing outcomes. For the primary outcome this occurred only among cluster sizes of one.
Table II.
Analyses of primary outcome, retention with viral load suppression a for DOT-cART versus control at 12 and 24 months
| N |
N
clusters |
DOT- cART, n with outcome (%) |
No DOT- cART, n with outcome (%) |
Unadjusted predicted
probability (95 % CI) |
p-
value |
Adjusted predicted
probability (95% CI) |
p-
value |
|||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| DOT-cART | No DOT-cART | DOT-cART | No DOT-cART | |||||||
| 12 months | ||||||||||
| Intention to treat | 339 | 69 | 152 (79.6) | 108 (73.0) | 0.80 (0.75, 0.84) | 0.73 (0.66, 0.79) | 0.10 | 0.81 (0.68, 0.90)b,c | 0.73 (0.57, 0.85)b,c | 0.08 |
| Per protocol | 319 | 68 | 143 (81.7) | 105 (72.9) | 0.82 (0.76, 0.86) | 0.73 (0.66, 0.79) | 0.05 | 0.83 (0.69, 0.92)d,e | 0.73 (0.55, 0.86)d,e | 0.02 |
| As treated | 337 | 69 | 145 (81.9) | 115 (71.9) | 0.82 (0.76, 0.86) | 0.72 (0.65, 0.78) | 0.03 | 0.83 (0.70, 0.91)d,f | 0.73 (0.55, 0.85)d,f | 0.01 |
| 24 months | ||||||||||
| Intention to treat | 337 | 71 | 140 (74.1) | 100 (67.6) | 0.74 (0.67, 0.80) | 0.68 (0.59, 0.75) | 0.20 | 0.76 (0.61, 0.87)b,g | 0.68 (0.52, 0.81)b,g | 0.19 |
| Per protocol | 316 | 70 | 132 (76.3) | 98 (68.5) | 0.77 (0.69, 0.83) | 0.69 (0.60, 0.76) | 0.15 | 0.79 (0.62, 0.89)d,h | 0.70 (0.50, 0.83)d,h | 0.13 |
| As treated | 335 | 71 | 133 (76.0) | 107 (66.9) | 0.76 (0.69, 0.83) | 0.67 (0.59, 0.74) | 0.07 | 0.78 (0.62, 0.88)d,i | 0.68 (0.50, 0.82)d,i | 0.07 |
Viral load suppression was defined as viral load < 400 copies.
Adjusting for baseline CD4 cell count (continuous), food insecurity category, any substance use disorder, no full time employment, depression and place of origin (originally from Lima versus outside of Lima).
N=306, 68 clusters.
Adjusting for baseline CD4 cell count (continuous), food insecurity category, any substance use disorder, no full time employment, depression, place of origin (originally from Lima versus outside of Lima), and baseline stigma score.
N=288, 66 clusters.
N=304, 67 clusters.
N=302, 69 clusters.
N=283, 67 clusters.
N=300, 68 clusters.
Sensitivity analyses
Estimates and p-values from multiply-imputed datasets were consistent with adjusted analyses without imputation. When we treated individuals lacking primary outcome data as not retained with viral load suppression, we found that individuals in the DOT arm were more likely to be retained at 12 months, even after adjustment for baseline differences between the two groups (predicted probability [pp] DOT: 0.79; 95% confidence interval [CI]: 0.66 – 0.87; pp no DOT: 0.69; 95% CI: 0.53 – 0.82; p-value: 0.04); however the difference at 24 months was not statistically significant (pp DOT: 0.72; 95% CI: 0.57 – 0.83; pp no DOT: 0.64; 95% CI: 0.49 – 0.78; p-value: 0.27). In contrast, when we assumed that those lacking primary outcome data were suppressed, the predicted probability was not statistically significant at 12 months (pp DOT: 0.82; 95% CI: 0.69 – 0.90; pp no DOT: 0.75; 95% CI: 0.59 – 0.86; p-value: 0.12) or 24 months (pp DOT: 0.77; 95% CI: 0.63 – 0.87; pp no DOT: 0.70; 95% CI: 0.54 – 0.83; p-value: 0.21).
Per-protocol and as-treated analyses
In per-protocol and as-treated analyses in which individuals who did not fully comply with their initial treatment assignment were excluded or reclassified, respectively, the mean predicted probability of retention with viral load suppression for the DOT arm was 0.02 to 0.03 higher than those observed in the intention-to-treat analysis. At 12 months, the per-protocol and as-treated adjusted predicted probabilities were 0.83 for the DOT arm and 0.73 for the no DOT arm (p-value for per-protocol analysis: 0.02; p-value for as-treated analysis: 0.01; Table 2). While DOT recipients continued to have a higher probability of the primary outcome at 24 months in both the per-protocol and as-treated analyses, this difference was not statistically significant (Table 2). When we repeated these analyses on multiply imputed datasets, we observed estimates and p-values consistent with the adjusted per-protocol and as-treated analyses without imputation with one exception. In the adjusted multiply imputed per-protocol analysis at 12-months, the difference in the probability of retention with viral load suppression in the DOT (0.82) versus no DOT arm (0.73), was only borderline statistically significant (p=0.06).
DISCUSSION
We observed high rates of retention with viral load suppression in both arms of a cluster randomized trial of HIV-positive adults living in poverty and receiving cART and adherence support provided through accompaniment. Intention-to-treat analyses showed no statistically significant benefit of DOT, when added to this standard of care. Per-protocol and as-treated analyses, and sensitivity analyses imputing missing outcomes as unfavorable endpoints, suggested the possibility of a modest statistically significant benefit of DOT at 12-months. Importantly, the differences across study arms in predicted probabilities for the primary outcome were qualitatively similar in intention-to-treat and per-protocol and as-treated analyses, ranging from 0.07 to 0.10. The discordancy in statistical significance at a strict 0.05 threshold is not surprising given the biases inherent to intention-to-treat and per-protocol and as-treated analyses. Intention-to-treat analyses are a conservative approach for analyzing randomized superiority trial data because significant non-adherence to the allocated intervention may attenuate effect estimates, decreasing power.(21) Per-protocol and as-treated analyses may be biased by confounding and patient selection; however, we sought to limit these biases through statistical adjustment. Taken together, we conclude that the added value of DOT-cART on retention with viral load suppression, if any, is small to moderate in the presence of robust adherence support, such as accompaniment. We did not find evidence that DOT-cART influences outcomes of social support, depression, CD4 cell count, HIV-related complications or attrition from care in this context.
This study differs from prior analyses of DOT-cART in several key ways. First, DOT was provided in patients’ homes or another mutually agreed upon location, rather than requiring participants travel to a health facility. Second, by including a robust model of community-based accompaniment in our control group, our study isolated the effect of DOT alone, disentangled from other elements of community-based support. Third, our primary outcome was retention with viral load suppression, representing the ultimate goal for all HIV treatment programs: to keep patients alive and on treatment with an unsuppressed viral load. Finally, we studied post-intervention outcomes one year after DOT discontinuation and tapered DOT in order to prepare participants for the post-DOT period.
Observed differences in the primary outcome were smaller than those used for pre-enrollment sample size calculations. Therefore, the study was likely underpowered to detect statistically significant differences of the observed magnitude. The control arm, which included accompaniment, likely contributed to this smaller-than-expected difference. We designed this robust control group with input from patients, DOT workers and staff with experience providing community-based care and support for patients with HIV and tuberculosis in Lima.(7, 12, 22) Monthly visits by study workers to conduct research interviews may also have improved outcomes in both arms. Neither per-protocol and as-treated analyses analysis accounted for day-to-day fidelity to DOT by participants or DOT workers. Consideration of missed DOT encounters or DOT discontinuation by study participants would likely further strengthen observed associations between DOT and the primary outcome in per-protocol and as-treated analyses. Last, while we expect results are generalizable to HIV-positive adults living in poverty in urban Latin American settings and beyond, they likely only apply to individuals open to receiving visits for DOT, a factor that may vary across settings. In this study only a small percentage (7%) of eligible DOT candidates opted out of DOT after randomization, and concerns of stigma and unintended disclosure were alleviated once the community health worker was able to establish a trusting rapport.
In conclusion, we observed high rates of retention with viral load suppression among HIV-positive adults living in poverty and receiving cART and accompaniment. Although time-limited DOT-cART did not improve retention with viral load suppression in conservative intention-to-treat analyses, per-protocol and as-treated analyses suggested the possibility of a small-to-modest benefit. Future analyses will include subgroup analyses to determine whether some groups (e.g., patients with a substance use disorder, patients with higher levels of perceived HIV-related stigma) benefit more from DOT.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge and thank the patients, treatment supporters, providers and health promotors who participated in this study. We thank Kevin Savage and Carly Rodriguez for assistance with manuscript preparation.
Funding: This study was funded by National Institute of Mental Health (R01MH083550-01A2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors report no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Partners Human Research Committee, and the Research Ethics Committee of the Peru National Institutes of Health.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Author contributions
Design of the work: SS, MMunoz, ET, JA, ES, JLS.
Acquisition of data for the work: AKN, OS, MW.
Data analysis: MMcLaughlin, MF, ZZ.
Interpretation: SS, MMunoz, OS, JSC, LL.
Drafting and revising the work: MMcLaughlin, MF, SS.
Critical for important intellectual content: SS, MMunoz, AKN, OS, JSC, MW, ZZ, LL, ET, JA, ED, JLS.
All authors approved the final version to be published.
REFERENCES
- 1.Sarna A, Luchters S, Geibel S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;48(5):611–9. doi: 10.1097/QAI.0b013e3181806bf1. [DOI] [PubMed] [Google Scholar]
- 2.Gross R, Tierney C, Andrade A, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169(13):1224–32. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart JE, Jeon CY, Ivers LC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr. 2010;54(2):167–79. doi: 10.1097/QAI.0b013e3181d9a330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374(9707):2064–71. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 5.Mathes T, Antoine SL, Pieper D. Adherence-enhancing interventions for active antiretroviral therapy in sub-Saharan Africa: a systematic review and meta-analysis. Sex health. 2014;11(3):230–9. doi: 10.1071/SH14025. [DOI] [PubMed] [Google Scholar]
- 6.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007;46(2):238–44. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin S, Munoz M, Zeladita J, et al. How does directly observed therapy work? The mechanisms and impact of a comprehensive directly observed therapy intervention of highly active antiretroviral therapy in Peru. Health Soc Care Community. 2011;19(3):261–71. doi: 10.1111/j.1365-2524.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer P. Partners In Help: Assisting the Poor Over the Long Term. Foreign Aff. 2011 Available at: https://www.foreignaffairs.com/articles/haiti/2011-07-29/partners-help. Accessed March 21, 2016.
- 9.Gitahi-Kamau NT, Kiarie JN, Mutai KK, Gatumia BW, Gatongi PM, Lakati A. Socio-economic determinants of disease progression among HIV infected adults in Kenya. BMC public health. 2015;15(1):733. doi: 10.1186/s12889-015-2084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joy R, Druyts EF, Brandson EK, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. J Acquir Immune Defic Syndr. 2008;47(4):500–5. doi: 10.1097/QAI.0b013e3181648dfd. [DOI] [PubMed] [Google Scholar]
- 11.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 12.Munoz M, Finnegan K, Zeladita J, et al. Community-based DOT-HAART accompaniment in an urban resource-poor setting. AIDS Behav. 2010;14(3):721–30. doi: 10.1007/s10461-009-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke MF, Kaigamba F, Socci AR, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56(9):1319–26. doi: 10.1093/cid/cis1193. [DOI] [PubMed] [Google Scholar]
- 14.Schreiner M. Progress out of Poverty Index: A Simple Poverty Score for Peru. Grameen Foundation; St. Louis, MO: 2008. [Google Scholar]
- 15.Ministerio de Salud Peru Norma Técnica para el Tratamiento Antiretroviral de Gran Actividad - TARGA en Adultos infectados por el virus de la Inmunodeficiencia Humana. 2005 [Google Scholar]
- 16.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26(7):709–23. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7(0):79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 18.Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12(5):224–31. [Google Scholar]
- 19.Franke MF, Munoz M, Finnegan K, et al. Validation and abbreviation of an HIV stigma scale in an adult spanish-speaking population in urban Peru. AIDS Behav. 2010;14(1):189–99. doi: 10.1007/s10461-008-9474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannheimer SB, Morse E, Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention. Results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S41–7. doi: 10.1097/01.qai.0000245887.58886.ac. [DOI] [PubMed] [Google Scholar]
- 21.Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48–55. doi: 10.1177/1740774511420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin S, Munoz M, Espiritu B, et al. Psychosocial impact of poverty on antiretroviral nonadherence among HIV-TB coinfected patients in Lima, Peru. J Int Assoc Physicians AIDS Care (Chic) 2008;7(2):74–81. doi: 10.1177/1545109708315326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.