Abstract
Resilience to stress-related emotional disorders is governed in part by early-life experiences. Here we demonstrate experience-dependent re-programming of stress-sensitive hypothalamic neurons, which takes place through modification of neuronal gene expression via epigenetic mechanisms. Specifically, we found that augmented maternal care reduced glutamatergic synapses onto stress-sensitive hypothalamic neurons and repressed expression of the stress-responsive gene, Crh. In hypothalamus in vitro, reduced glutamatergic neurotransmission recapitulated the repressive effects of augmented maternal care on Crh, and this required recruitment of the transcriptional repressor repressor element-1 silencing transcription factor/neuron restrictive silencing factor (NRSF). Increased NRSF binding to chromatin was accompanied by sequential repressive epigenetic changes which outlasted NRSF binding. chromatin immunoprecipitation-seq analyses of NRSF targets identified gene networks that, in addition to Crh, likely contributed to the augmented care-induced phenotype, including diminished depression-like and anxiety-like behaviors. Together, we believe these findings provide the first causal link between enriched neonatal experience, synaptic refinement and induction of epigenetic processes within specific neurons. They uncover a novel mechanistic pathway from neonatal environment to emotional resilience.
Introduction
Human emotional profiles are generated by both genetics and environment.1, 2 Experience, particularly during sensitive periods early in life, leaves indelible marks on an individual’s coping abilities and on resilience or vulnerability to stress-related emotional disorders.2, 3 There is evidence that neonatal experiences influence the function of neurons in the brain involved in these crucial behaviors by modifying neuronal gene expression via epigenetic processes.2, 3, 4, 5 However, it is not known how neonatal experiences signal to specific brain cell populations and how these signals influence the orchestrated programs of gene expression that mediate phenotypic resilience or vulnerability.
High-quality maternal care in rodents, whether occurring naturally or induced experimentally, results in enhanced memory functions and attenuated neuroendocrine response to stress.4, 6, 7 Molecular correlates of these behaviors include gene-expression changes in neurons involved in regulating the response to stress, such as increased messenger RNA (mRNA) and protein levels of the glucocorticoid receptor (GR) in hippocampus, and decreased mRNA and protein levels of the neuropeptide corticotropin releasing hormone (CRH) in stress-sensitive hypothalamic cell populations.5, 7, 8, 9 Because the repression of CRH expression in hypothalamic neurons is detectable early, immediately after the neonatal enrichment period, we previously investigated if reduction in CRH levels and release had causal relationship to long-lasting resilience to stress. We found that the degree of activation of the CRH receptor CRHR1 by endogenous CRH release (which is governed, in part, by CRH expression levels) contributes to stress-vulnerability and depressive-like behavior in adulthood.8 These findings suggest an important role for transcriptional changes in CRH expression in hypothalamic neurons in mediating the effects of maternal care on adult phenotype.8, 9, 10 Therefore, we examined experience-related changes in these neurons, and found a transient reduction in the numbers of glutamatergic synapses to the CRH neurons in the paraventricular nucleus (PVN) of the hypothalamus.5 This was apparent from decrease in the frequency of miniature excitatory postsynaptic potentials, together with reduction in vGlut2 levels and ultrastructural evidence for reduced numbers of asymmetric synapses in enriched care animals, provided convincing evidence for reduced excitatory neurotransmission onto CRH cells.5 Notably, there was no significant change in inhibitory innervation.5 Yet, whereas reduction of excitatory input and enduring repression of the CRH in stress-sensitive hypothalamic neurons were associated, their causal relationship has remained unclear. In addition, the mechanisms by which neonatal experience repressed CRH expression enduringly, and the identity of genes that, in concert with Crh, are influenced by neonatal experience and may contribute to the resulting emotional phenotype, have remained unknown.
Here, using a controlled in vitro approach, we found that reduction of glutamatergic receptor activation suffices to recapitulate the repressive effects of augmented maternal care on CRH. This phenomenon requires enhanced nuclear levels and recruitment of the transcriptional repressor neuron restrictive silencing factor (NRSF), also known as repressor element-1 silencing transcription factor (REST), to the Crh gene.11, 12 NRSF chromatin binding was accompanied by methyl CpG binding protein 2 (MeCP2) occupancy, and was followed by sequential accumulation of repressive epigenetic marks in hypothalamus in vitro as well as in immature and adult rats experiencing augmented maternal care. Systematic analyses (chromatin immunoprecipitation (ChIP)-seq) of NRSF targets identified the gene networks that may contribute to the phenotypic changes initiated by enriched maternal care. To the best of our knowledge, the current studies are the first to causally connect neonatal environmental experience with synaptic ‘rewiring’ promoting epigenetic processes within select neuronal populations. They provide a novel mechanistic pathway from early-life experience to phenotypic outcomes that govern human health and disease.
Materials and methods
A complete description of each procedure is found in the Supplementary Information.
Animals
Rats of both sexes were handled according to NIH guidelines for care and use of laboratory animals and in accordance with protocol approval from the University of California-Irvine Institutional Animal Care and Use Committee. Subjects were progeny of timed-pregnant Sprague-Dawley rats. Rats were housed under a 12 h light–dark cycle in humidity and temperature controlled rooms, with ad libitum access to food and drinking water. Parturition was checked daily, and the day of birth was considered postnatal day (P) 0. To generate hypothalamic explant cultures, pups were killed on P7.
Augmentation of maternal care via brief daily separation (handling) and observation of maternal care
Augmented maternal care was accomplished by daily handling of pups for 15 min, which promotes barrages of maternal licking and grooming of pups on return of dam and pups to the cage.5, 8, 9 For these experiments, pups were mixed among litters on P1, and 10 pups with were assigned at random to each dam. Care was taken to ensure that each dam had equal numbers of male and female pups. The pups (n=13 dams per group) experienced one of the following early-life rearing conditions: (1) a brief, 15 min separation from the dam (handling), which took place daily from P2 to P9; or (2) undisturbed controls (non-handled) that remained in cages that were not touched between P2 and P9.5, 8, 9 Maternal caring activities during the 30 min following re-unification of dam and pups were observed and scored by two independent investigators. Animals were either killed on P10 between 08:00–10:00 hours, or maintained under standard animal facility rearing conditions, and grown to adulthood.
Hypothalamic organotypic/explant procedures
Hypothalamic explants were cultured according to a modified stationary hypothalamic slice culture protocol as previously described.13 In brief, rat pups were decapitated on P7–P8, and hypothalamic blocks were dissected and cut into 350 μm coronal sections. Explants were maintained at 37 °C in 5% CO2 enriched air in an incubator. On day in vitro (DIV) 6, cultures were transferred to serum-free media and treated with either sterile, nuclease-free tissue culture grade water (vehicle), or 50 μm MK-801 (Sigma, Sigma-Aldrich, St Louis, MO, USA) and 50 μm CNQX (Sigma, Sigma-Aldrich). Media containing either vehicle or glutamate receptor antagonist(s) were refreshed every 12 h for 52 h. Cultures were harvested and frozen.
For oligodeoxynucleotide (ODN) applications, the previously described phosphothioated ODNs consisting of either a control randomly ordered sequence (scrambled; SCR), or a sequence coding for the NRSF binding site (NRSE), were used.14, 15 Hypothalamic explants were maintained as described above and exposed to one of the following conditions on DIV 6: [Veh+SCR], [Veh+NRSE], [CNQX/MK-801+SCR] or [CNQX/MK-801+NRSE] for 12 h. Dose-response analysis determined 10 nm as the optimal ODN dose. To examine if the ODNs entered neurons and neuronal nuclei, dissociated neuronal cultures were generated as previously described,16 incubated with BODIPY-tagged ODNs, and visualized using fluorescence microscopy.
RNA extraction, reverse transcription and qRT-PCR
RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and complementary DNA prepared using a cDNA synthesis kit (Roche, Basel, Switzerland), both according to the manufacturer’s instructions. Primer sequences used for quantitative real-time PCR (qRT-PCR) are provided in Supplementary Table 1.
Semiquantitiative in situ hybridization
In situ hybridization histochemistry was conducted on hypothalamic explants (n=5–6 per group) using an established protocol for deoxyoligonucleotide (ODNs) probes.5, 8, 9, 17 Analysis was carried out without knowledge of treatment group as previously described.5, 8, 9, 17
Immunocytochemistry and analysis
Detailed methodology of the immunocytochemistry (ICC) and of the analyses are in the Supplementary Material. In brief, double-labeling ICC of CRH and vGlut2 was performed and analyzed as previously described (without knowledge of early-life treatment).5 For CRH ICC in vitro, hypothalamic slice cultures (n=4–5 per group) were subjected to standard avidin–biotin complex methods.5 Each culture flattened to ~70–100 μ in thickness, and its analysis was performed using stereological principles as described previously and in the Supplementary Material.
Western blotting
For detection of transcription factors or nuclear proteins, samples were enriched for nuclear and cytoplasmic fractions using the NE-PER kit (Thermo Fisher, Waltam, MA, USA) according to the manufacturer’s instructions. Standard SDS/PAGE technique was used with the following primary antibodies: Rabbit anti-NRSF antibody (1:1000, Santa Cruz; sc-25398, Dallas, TX, USA), Rabbit anti-Actin (1:10 000, Sigma-Aldrich; Ab8227).
Chromatin immunoprecipitation
Hypothalamic explants treated with either vehicle or CNQX/MK-801 were cross-linked, homogenized, and the nuclei were harvested by centrifugation. Nuclei were sonicated and precleared with Protein-A/G (Santa Cruz). They were then immunoprecipitated with 10 μg of either control non-immune serum (IgG) (Cell Signaling; 2729S, Danvers, MA, USA), anti-NRSF (Santa Cruz; sc-25398x), anti-Histone3 Lysine 9 dimethyl antibody (Abcam; Ab1220, Cambridge, MA, USA), anti-Histone3 Lysine 27 tri-methyl antibody (Abcam; Ab6002), or anti-Histone3 Lysine 9 tri-methyl antibody (Abcam; Ab8898) overnight at 4 °C. Precleared protein A/G beads (Santa Cruz; sc-2003) were added to the lysate for 2 h. The beads were washed to remove non-specifically bound protein, then subjected to SDS elution. Eluates were reverse cross-linked, and the bound DNA was purified using the QiaQuick MinElute PCR purification kit (Qiagen, Valencia, CA, USA). Quantitative PCR (qPCR) amplification was done using SYBR Green chemistry (Roche, Indianapolis, IN, USA). Primer sequences used for ChIP analyses are provided in Supplementary Table 2.
Restraint stress and corticosterone assay
Young adult male rats from the control and enriched care groups were subjected to a 30 min restraint stress (without knowledge of early-life treatment), and corticosterone assay was performed as previously described using commercial RIA kits (INCSTAR, Stillwater, MN, USA, and ICN, Irvine, CA, USA).8, 17
Elevated plus-maze test
‘Anxiety-like’ behaviors, manifested as reduced time and entries into the open arm of the elevated plus-maze, were tested on young adult male rats without knowledge of early-life treatment as previously described.18, 19, 20
Porsolt forced swim test
‘Depression-like’ behaviors were tested on young adult male rats without knowledge of early-life experience.20 The forced swim test consisted of two sessions separated by 24 h. The habituation session (Day 1), lasted 15 min. Rats were placed in a glass cylinder (20 cm in diameter and 60 cm high) containing water (23–25 °C) filled to a depth of 45 cm. The test session occurred 24 h later, and rats were placed in the cylinder for 5 min. Behavior was monitored using a video camera, and the duration of immobility was scored.20
ChIP sequencing
The ChIP protocol described above was modified for ChIP sequencing (ChIP-seq) experiments. After hypothalamic explants were treated with vehicle or CNQX/MK-801 as described above, explants from 10 animals were pooled from each treatment, and ChIP was performed. DNA concentration, quality, and sonication profile was verified by Bioanalyzer (Agilent, Santa Clara, CA, USA) and Qubit. Libraries were prepared using NextFlex Rapid DNA seq kit (Bio Scientific, Austin, CA, USA) according to the manufacturer’s protocol. Sequencing was performed through the University of California-Irvine Genomics High Throughput Facility (UCI GHTF) using Illumina HiSeq 2500. The libraries were multiplexed to 4 per lane, and SR50 run was performed. Sequencing data was analyzed as described.21 The ChIP-seq data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE87709.
Statistical considerations
Rats were assigned to groups randomly, and group sizes were determined a priori based on expected effect size and variance. All analyses were performed without knowledge of treatment group. Grubbs or confidence-limit outlier tests were used to determine outliers. Beyond ChIP-seq analysis, we employed the Prism Graphpad software package (San Diego, CA, USA). One-way or two-way analysis of variance (ANOVAs) or Student’s t-test were used to assess statistical significance as appropriate. For ChIP analysis of NRSF activity, two-way repeated measure ANOVA was used to account for batch effects in the assay. Following a significant F value for an interaction in a two-way ANOVA, Bonferroni post hoc test was used to distinguish among groups. Following a significant F value in a one-way ANOVA, a Dunnett’s post hoc test was conducted to compare experimental groups to control. For behavioral tests, in the Porsolt swim test, one-way ANOVA with post hoc Bonferroni tests were used, in elevated plus maze the two groups were compared using a t test. Significance for all analyses was set at P<0.05, and data are expressed as mean and s.e.m. unless noted otherwise.
Data availability statement
The ChIP-seq data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE87709.
Results
Reduction in glutamatergic synaptic neurotransmission onto stress-sensitive hypothalamic neurons represses CRH expression
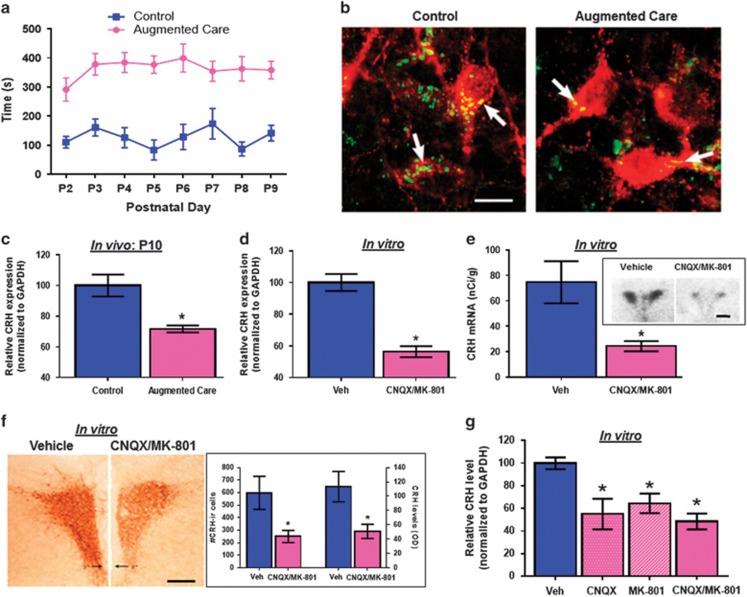
We implemented the brief daily-handling paradigm,5, 6, 7, 8, 9, 17, 22 resulting in a robust, 300–350% augmentation of maternal care on reunification of pups and dams (Figure 1a). The augmented maternal care, in turn, led to a reduction in the number of glutamatergic synapses contacting CRH neurons in the PVN, in line with our prior observations (Figure 1b).5 To examine if the decline in glutamatergic input to CRH neurons (Figure 1b) directly repressed CRH mRNA and protein levels as observed in neonatal rats experiencing augmented maternal care (Figure 1c),5 we employed organotypic hypothalamic slice cultures and reduced glutamatergic neurotransmission by incubating the cultures with ionotropic glutamate receptor blockers. Chronic exposure to AMPA receptor (CNQX) and NMDA receptor (MK-801) blockers resulted in significant reduction of CRH mRNA levels, as assessed by qRT-PCR (Figure 1d) and in situ hybridization histochemistry (Figure 1e). The mRNA changes translated into reduced peptide expression (Figure 1f), and were selective to CRH, because no changes were found for a second hypothalamic stress peptide, arginine-vasopressin (Supplementary Figures 1a–c). We further assessed the role of individual types of ionotropic glutamate receptor-mediated neurotransmission by incubating the hypothalamic explants with either NMDA receptor or AMPA receptor blockers individually. Interestingly, blockade of either NMDA receptors or AMPA receptors was sufficient to decrease CRH expression (Figure 1g; one-way ANOVA F[3,24]=5.98; P=0.0037; all treatments different from control, Dunnett’s post hoc). Together, these findings indicated that a reduction in ionotropic glutamatergic receptor activation over a few days was sufficient to decrease the expression of a stress-sensitive molecule, CRH, in hypothalamic neurons. Because this early modulation of CRH-expressing stress-sensitive neurons seems to contribute to a life-long phenotype of improved memory and resilience to stress, as shown using pharmacological approaches,8 we proceeded to study the mechanisms responsible for this reduction and the identity of the gene networks that, together with CRH, change with early-life experience and may contribute to the resulting phenotype.
Figure 1.
Augmented maternal care reduces excitatory synapses on the paraventricular nucleus-corticotropin releasing hormone (PVN-CRH) neurons, and the blockade of ionotropic glutamatergic input is sufficient to decrease CRH levels in vitro. (a) Sensory stimulation (licking and grooming) of pups by the dam is enhanced daily following a brief separation of the pups from their mother (n=13 dams per group). (b) Merged confocal microscope images of sections at the level of the PVN double labeled for CRH (red) and vGlut2 (green) in control and augmented early-life experience P10 rats (n=3 per group, scale bar 10 μm). (c) quantitative real-time PCR (qRT-PCR) analysis of CRH expression in the PVN of control and augmented care rats at P10 (n=9 per group). (d) qRT-PCR analysis (n=26 per group) and (e) in situ hybridization for CRH expression in hypothalamic explants incubated with vehicle (n=5) or CNQX/MK-801 (n=6; scale bar 450 μm). (f) ICC measuring CRH peptide levels in control (n=4) and CNQX/MK-801 (n=5) exposed explants (scale bar 300 μm). (g) qRT-PCR analysis for CRH expression in hypothalamic explants incubated with either CNQX or MK-801 (n=7 per group).
The transcriptional repressor NRSF/REST mediates experience-dependent repression of CRH expression
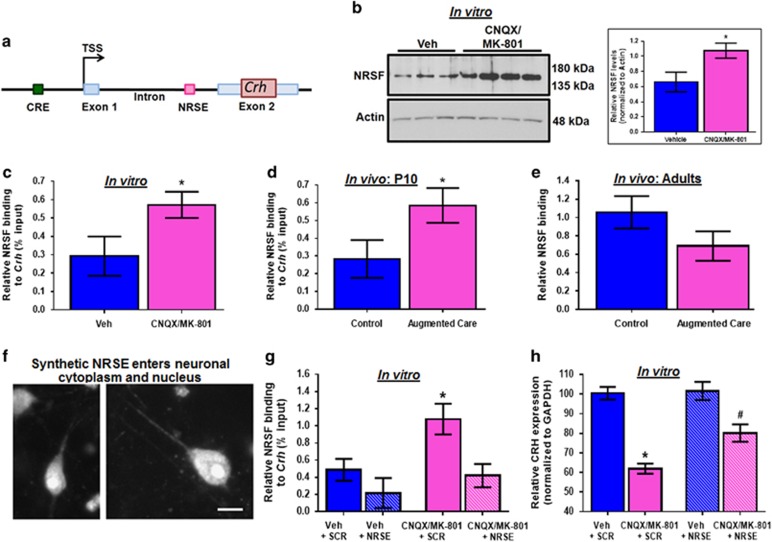
The Crh gene has a limited number of regulatory sequences, including a consensus sequence for the transcriptional repressor NRSF (Figure 2a).11, 12 NRSF interacts with co-repressor complexes to epigenetically silence target gene expression,23, 24, 25, 26, 27, 28 and we have found increased NRSF levels in hypothalami of infant and adolescent rats that experienced augmented maternal care.5 To examine if NRSF plays a role in CRH repression, we first quantified NRSF levels in hypothalamic explants exposed to ionotropic glutamate receptor blockers. NRSF levels were increased in the nuclear (0.91±0.05 vs 0.34±0.09; P=0.0016), but not in the cytoplasmic (0.96±0.02 vs 0.88±0.06; P=0.2) cell fractions of the experimental group (Figure 2b). Next, we assessed if NRSF directly interacted with the Crh gene, using ChIP. NRSF binding to the Crh gene was increased after blocking glutamatergic neurotransmission in vitro (Figure 2c), and in hypothalami of immature rats experiencing enhanced maternal care (Figure 2d). Notably, augmented NRSF binding to Crh was no longer present in 3-month-old adult rats that had experienced increased maternal care (Figure 2e). NRSF binding was not detected at the Avp gene, which does not contain an NRSE (Supplementary Figures 1d and e).
Figure 2.
Increased neuron restrictive silencing factor (NRSF) binding is required to repress corticotropin releasing hormone (CRH) expression following ionotropic glutamate receptor blockade in vitro and after augmented care. (a) A schematic of Crh gene, highlighting the NRSE. (b) Representative western blot showing NRSF levels in the nuclear fraction prepared from hypothalamic explants exposed to vehicle (n=9) or CNQX/MK-801 (n=10; several lanes for each group are shown). Chromatin immunoprecipitation (ChIP) followed by quantitative PCR (qPCR) was performed to measure NRSF binding at the Crh gene in (c) hypothalamic explants incubated with vehicle or CNQX/MK-801 (n=8 per group, presented as %input−1), (d) the PVN of P10 (n=10 per group, presented as %input−2), and (e) adult (n=10 per group) animals that received normal or enriched care early in life. (f) Dissociated neurons incubated with BODIPY-tagged oligodeoxynucleotides (ODNs), and visualized using fluorescence microscopy (Scale bar 10 μm). (g) ChIP followed by qPCR for NRSF binding to the Crh gene in hypothalamic explants incubated with [vehicle+ODNs] or [CNQX/MK-801+ODNs] (n=5 per group, presented as %input-2). (h) qRT-PCR analysis (n=10 per group) revealed a significant reduction in CRH expression in explants incubated with [CNQX/MK-801+Scr] relative to controls (indicated by * one-way ANOVA with post hoc Dunnet’s multiple comparisons test). Incubation with the NRSE ODN caused a significant increase in CRH mRNA, so that the [CNQX/MK-801+ODN was no longer significantly different from the controls (indicated by # Dunnet’s multiple comparison test).
To examine if increased NRSF occupancy at the Crh gene was required for CRH repression, we interfered with NRSF chromatin binding. This was accomplished using ODNs coding for the NRSF binding sequence (NRSE), that were chemically modified for stability.14, 15 These NRSE-ODNs as well as random-sequence control ODNs entered neurons and neuronal nuclei (Figure 2f), and acted as decoys, binding cellular NRSF and preventing its interaction with the chromatin.14, 15 Dose-response analysis testing the effects of 2, 10, and 100 nm was performed to determine the optimal ODN dose (Supplementary Figure 2a). We then queried if application of these ODNs to the hypothalamic explants would prevent the observed CNQX/MK-801 induced CRH suppression. The NRSE ODNs influenced NRSF binding to the Crh gene (Figure 2g. Because the experiment was performed in four ‘batches’ we used repeated measure two-way ANOVA. We found significant glutamate-receptor-block X NRSE interaction, F[1,8]=6.64 P<0.05. Specifically, blocking ionotropic glutamate receptors significantly increased NRSF binding to Crh (Figure 2g; [Veh+SCR] vs [CNQX/MK-801+SCR]: Bonferroni post hoc test, P<0.05). The addition of the NRSE ODNs to controls [Veh+NRSE group] had little effect, likely as a result of low ambient levels and binding of the repressor under control conditions. However, the NRSE-ODNs reduced NRSF binding to the Crh gene in the presence of glutamate receptor blockers (Figure 2g; [CNQX/MK-801+NRSE] vs [CNQX/MK-801+SCR]: Bonferroni post hoc test, P<0.05). Importantly, the NRSE-ODNs largely rescued CRH expression from its repression after reduced glutamate receptor function (Figure 2h; two-way ANOVA; significant CNQX/MK-801 X ODN interaction, F[1,35]=5.04, P<0.05): Specifically, the 40% reduction of CRH expression induced by the glutamate receptor blockers ([Veh+SCR] vs [CNQX/MK-801+SCR]: Bonferroni post hoc test, P<0.05) was significantly attenuated, (to a 20% reduction) with the addition of NRSE ODNs ([CNQX/MK-801+NRSE] vs [CNQX/MK-801+SCR]: Bonferroni post hoc test, P<0.05). Both the gene repression and the rescue were not observed for a separate, stress-related hypothalamic peptide, arginine-vasopressin (Supplementary Figure 2b). These results indicated that the NRSF binding to Crh was required for the repression of this gene by reduced glutamate-receptor function.
Transient NRSF binding to chromatin initiates enduring epigenetic modifications
The binding of NRSF to the Crh gene, required for its repression upon CNQX/MK-801 treatment in vitro (Figure 2c), also took place in immature rats immediately following augmented maternal care (Figure 2d). However, the increased occupancy of NRSF at the Crh gene did not persist in adult animals (Figure 2e). Therefore, we reasoned that the initial NRSF binding might promote enduring chromatin changes that last into adulthood,3, 23, 25, 26, 28 maintaining the continued silencing of Crh. To test this possibility, we assessed the Crh gene for evidence of putative gene-repressing epigenetic processes employed by NRSF.
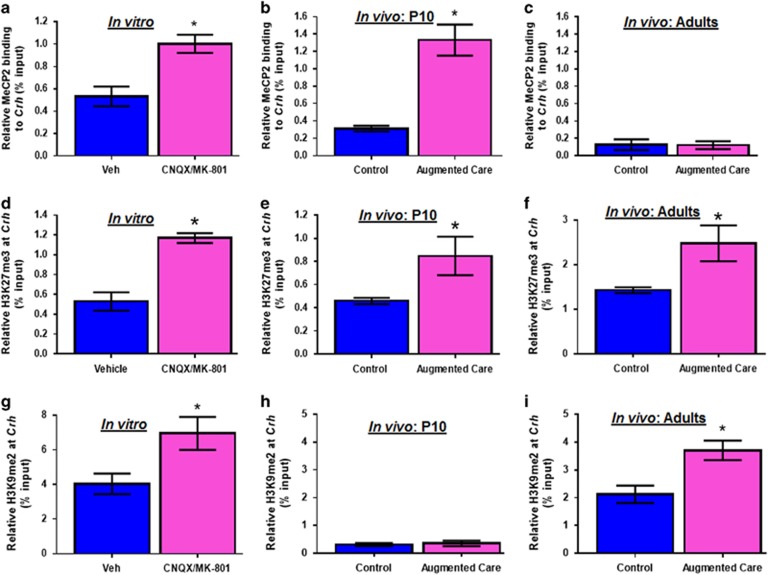
NRSF recruits MeCP2,26 a protein that binds methylated DNA29 and contributes to repression of gene expression.27, 28, 29 Indeed, MeCP2 binding to Crh was enhanced in hypothalamic explants exposed to glutamate receptor blockers (Figure 3a). MeCP2 binding to Crh was also augmented in hypothalami of immature augmented maternal care rats (Figure 3b). However, similar to NRSF, enrichment of MeCP2 binding was no longer present in adult rats experiencing augmented maternal care early in life (Figure 3c). Looking for mechanisms of persistent epigenetic repression, we focused on several histone methylation marks at specific lysine residues, which influence activation or repression of genes.3, 28, 30 We identified early and enduring increase of histone-3 lysine 27 tri-methylation (H3K27me3) at the Crh gene in vitro after incubation with glutamate receptor blockers, as well as after augmented care in both immature and adult rats (Figures 3d–f). Additionally, increased histone-3 lysine 9 dimethylation (H3K9Me2), a second mark of transcriptional silencing,30 was evident at the Crh gene in glutamate receptor blockers exposed hypothalamic explants (Figure 3g), and in hypothalami of adult rats experiencing augmented maternal care (Figure 3i). This repressive mark seemed to arise later in vivo, as it was not apparent immediately following the neonatal enrichment period (Figure 3h). Finally, incubation of PVN explants with glutamate receptor blockers increased histone-3 lysine 9 trimethylation (H3K9me3) at the Crh gene in vitro (data not shown). However, there was no evidence of changes in H3K9 trimethylation at the Crh gene in either P10 or adult animals following enriched care (data not shown). No changes in MeCP2 binding, H3K27 trimethylation, or H3K9 dimethyation were detected at the Avp gene (Supplementary Figures 3a–g).
Figure 3.
NRSF promotes lasting epigenetic changes on the Crh gene that persist into adulthood. Chromatin immunoprecipitation (ChIP) followed by qPCR was used to measure MeCP2 binding at the Crh gene in (a) hypothalamic explants exposed to vehicle or CNQX/MK-801 (n=8 per group, presented as %input–1), (b) PVN of P10 (n=6 per group, presented as %input–2), and (c) adult (n=5 per group, presented as %input-2) animals that received normal or augmented care early in life. ChIP followed by qPCR was used to measure H3K27 trimethylation at the Crh gene in (d) hypothalamic explants incubated with vehicle or CNQX/MK-801 (n=7 per group), (e) PVN of P10 (n=6 per group), and (f) adult animals that experienced control (n=5) and augmented care (n=6). ChIP followed by qPCR was used to measure H3K9 dimethylation at the Crh gene in (g) hypothalamic explants incubated with CNQX/MK-801 (n=8 per group), (h) PVN of P10 (n=5 per group), and (i) adult (n=6 per group) animals that received control or enriched care early in life. NRSF, neuron restrictive silencing factor; PVN, paraventricular nucleus.
Synaptic and epigenetic changes in CRH-expressing neurons are associated with enduring resilience
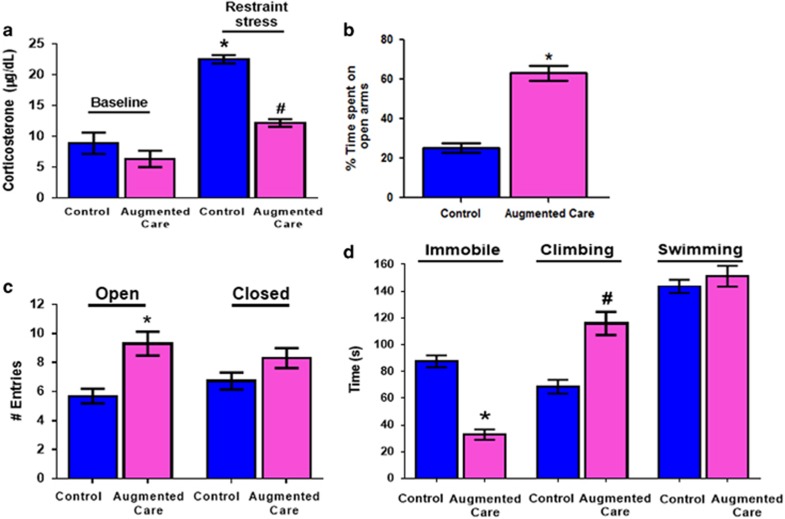
The above lasting epigenetic changes following augmented maternal care were accompanied with enduring repression of Crh5 and a stress-resilient adult phenotype.6, 7, 8, 9, 11 Thus, adult rats experiencing augmented maternal care had reduced plasma corticosterone levels in response to an acute restraint stress (Figure 4a), with no change in basal hormone levels, in line with reports by others.6, 7 They also exhibited behaviors attributable to reduced anxiety-like and resilience to depressive-like behaviors. Thus, augmented-care adult rats spent more time in (Figure 4b), and made more entries into (Figure 4c), the open arms of the elevated plus-maze compared with controls, indicating reduced anxiety-like behaviors.20, 31 They also swam/climbed longer and floated less in the forced swim test (one-way ANOVA F=58.98. P<0.0001; post hoc P<0.01 Figure 4d), considered indications of resilience to depressive-like behaviors in rodents.18, 19, 20, 31 Notably, these behaviors were not associated with hyperactivity: total distance traveled by controls and augmented group did not differ (6122±420 cm vs 6687±365 cm respectively; P=0.33).
Figure 4.
Synaptic and epigenetic changes in corticotropin releasing hormone (CRH)-expressing neurons are associated with enduring stress resilience. (a) Corticosterone levels after acute restraint stress in control (n=5) and augmented care animals (n=7). Restraint stress causes a significant increase in corticosterone levels in control animals (indicated by *). Augmented care animals have significantly lower levels of corticosterone after restraint stress as compared with stressed controls (indicated by #). (b) Percentage of time spent on the open arms, and (c) total number of entries in the open and closed arms of elevated plus maze by control and augmented care animals (n=19–27 per group). (d) Time spent climbing, immobile or swimming by control (n=19) and augmented care animals in the forced swim test (n=27). Early-life augmented-care animals spent significantly more time climbing (indicated by #), and were less immobile (indicated by *), relative to the control animals (one-way ANOVA with post hoc Bonferoni’s tests).
Early-life experience promotes expression changes of numerous genes via NRSF-dependent mechanisms
Repressed CRH expression classically leads to reduced peptide release during stress and thus attenuated secretion of adrenal stress hormones.6, 7, 9, 17 Therefore, the molecular mechanisms promoting reduced CRH levels after maternal care likely contribute to the phenotype. However, the enduring resilient phenotype generated by augmented maternal care via reduced excitatory innervation to hypothalamic neurons and modulation of epigenetic mechanisms within these cells is unlikely to involve the Crh gene alone. We reasoned that common mechanisms might regulate Crh and the other genes involved, and sought to identify the complement of genes influenced by NRSF by performing NRSF ChIP-seq.
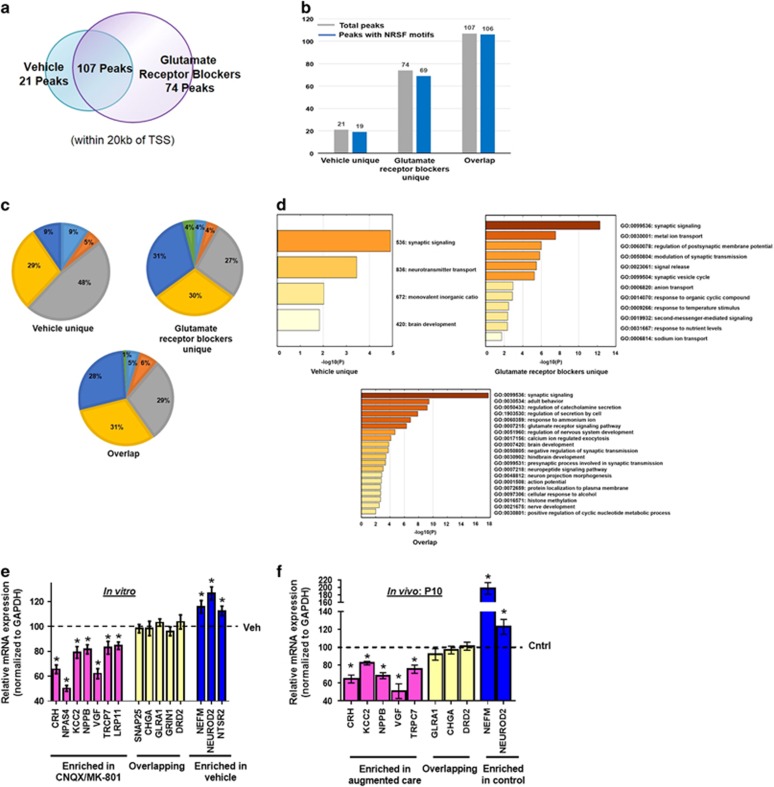
ChIP-seq analyses (two independent replicates) revealed 277 and 407 NRSF-bound peaks in control hypothalami and those exposed to blockade of glutamatergic neurotransmission, respectively, consistent with augmented levels and function of NRSF in the latter group (Supplementary Tables 3; Supplementary Figures 3, 4). One hundred and ninety-five peaks were unique to the glutamatergic receptor blocker group, 65 peaks were found only in control hypothalami and 212 peaks were common to both conditions (Supplementary Table 4). NRSF binding sites were distributed throughout the genome.27 When we focused on sites that were within 20 kb of gene transcription start sites, we identified 74 peaks that were unique to the glutamate-receptor blocked group, 21 that were unique to vehicle controls and 107 peaks that were common to both conditions (Figure 5a; Supplementary Table 4 and Figure 5). The large majority of these peaks contained NRSF binding motifs (Figure 5b; Supplementary Table 4). In addition, the majority (52–73%) of the NRSF-bound peaks were located within genes, inhabiting promoters (defined by Homer software as −1000 bp to +100 bp surrounding transcription start sites), introns, exons, untranslated regions and transcription termination sites (Figure 5c). Interestingly, location within the promoter was much higher (31%) in the glutamate-receptor blocked group compared with the controls (9%). In contrast, intergenic location was much higher in the vehicle controls (48%) compared with peaks unique to glutamate receptor blocked group (27%) or overlapping between groups (29%). Intronic location was similar across groups (29, 30 and 31% respectively). Thus, combined promoter and intronic location of NRSF-binding peaks comprised 61% of glutamate-blocker unique peaks but only 38% of vehicle-unique peaks (Figure 5c). Together, these findings suggested that, in conjunction with repression of the Crh gene, reduction of glutamatergic neurotransmission not only increased the number of sites bound by NRSF, but also drove NRSF binding preferentially to sites throughout the genome that are more effective in influencing gene expression.
Figure 5.
In addition to Crh, NRSF ChIP-seq uncovers a repertoire of genes and pathways that contribute to resilience later in life. Two independent replicates of ChIP-seq for NRSF-bound genes was performed in pooled hypothalamic explants exposed to vehicle or CNQX/MK-801 (n=10 per group per replicate). (a) Venn diagram illustrating that 74 peaks were unique to glutamate receptor blocked slices, 21 were unique to controls and 107 peaks were common to both conditions (within 20 kb of transcription start sites). (b) The number of total peaks (in gray) compared with the number of peaks having NRSF motifs (in blue) shows an enrichment of NRSE sites under each condition. (c) Genome-wide locations of NRSF bound peaks under each condition. (d) Significant gene ontology terms of protein-coding genes uniquely occupied by NRSF under each condition. The augmented binding of the transcriptional repressor NRSF led to preferential repression of the affected genes in (e) hypothalamic explants exposed CNQX/MK-801 (n=12) relative to vehicle incubated slices (n=8), and (f) PVN of P10 rats experiencing augmented maternal care (n=11) as compared with controls (n=9). Shown are fold changes in expression (assessed by qRT-PCR) of several genes in each group, in comparison to controls. NRSF, neuron restrictive silencing factor.
Gene ontology analyses of the three groups (glutamate-receptor-blocked, vehicle and overlapping) was conducted (Figure 5d). It revealed an enrichment of genes that code for molecules contributing to neuronal function and plasticity among the NRSF-enriched genes unique to the hypothalami exposed to reduced glutamatergic neurotransmission. Specifically enriched in the analysis of this group were genes involved in synaptic signaling, ion channel function and intercellular communication via synaptic processes, indicating neuronal plasticity (see ‘Discussion’ section).
Expression levels of several genes from each of the three groups was assessed in vehicle and CNQX/MK-801 incubated explants in vitro (Figure 5e), and in P10 rats experiencing augmented maternal care (Figure 5f), as validation of the ChIP-seq results. Expression of genes in which NRSF occupancy was enriched on CNQX/MK-801 incubation was indeed downregulated relative to controls (Figure 5e, pink). In contrast, for genes in which NRSF binding was higher in the vehicle treated vs glutamatergic transmission-blocked explants, gene expression of NRSF targets was less repressed after CNQX/MK-801 treatment (Figure 5e, blue). mRNA levels of genes in the overlapping group did not change upon incubation with glutamate receptor antagonists (Figure 5e, yellow). Similar results were obtained in vivo, in the PVN of P10 rats belonging to either control or augmented care groups: increased binding of NRSF generally led to the repression of gene expression (Figure 5f). Together, these data support a role of NRSF as an initiator of an epigenetic process leading to large-scale, experience-dependent neuronal plasticity that contributes to behavioral phenotypes.
Discussion
Here we find that neonatal experience promotes enduring resilience via sequential intercellular and intracellular mechanisms. Refinement of maturing brain circuits by modulation of synaptic innervation in the hypothalamus initiates intracellular epigenetic processes that govern the expression of a panoply of genes, including the stress-mediator Crh. The transcriptional repressor NRSF contributes critically to the initiation of the epigenetic cascades that modify the chromatin, and these evolve and persist into adulthood. Thus, transient intercellular and intracellular mechanisms orchestrate lasting changes in gene expression in specific neuronal populations that then contribute to phenotypic resilience or vulnerability later in life.
Early-life experience-dependent changes in the number and strength of synaptic input onto a neuron have been previously described in visual,32, 33 tactile,34 and olfactory35 sensory systems.36 In analogy, we find that enhanced maternal care early in life causes a reduction in excitatory synapses that enduringly changes the sensitivity of stress-responsive hypothalamic neurons to future stress.5 The role of hypothalamic CRH cells in orchestrating behavioral responses to stress is now firmly established,37, 38 and our data suggests that reduction of excitatory synapses and consequent repression of the expression of the stress peptide CRH (and numerous other genes) contributes to governing the ‘set-point’ of the response of CRH-expressing neurons to stressful signals.37 The altered stress-sensitivity is apparent in behavioral measures, as well as by blunted corticosterone (CORT) release in response to stresses later in life (resilience), without a change in basal plasma CORT levels.7, 17 The maintenance of stable low levels of basal CORT in the face of reduced or augmented CRH expression in hypothalamus is likely a result of complex regulatory systems at both hypothalamic39, 40, 41 and pituitary levels.41, 42 Additional homeostatic mechanisms that dissociate plasma CORT from hypothalamic CRH levels have been found also in transgenic animals43 and include the CREB/CREM family of molecular mediators.41, 44
We have previously found that it is the barrages of maternal care on reunification of dam and pups, rather than the brief separation during the ‘handling’ procedure that is responsible for the enduring reduction of CRH expression in the augmented maternal care group.9 Interestingly, increased excitatory synaptic input and augmented CRH expression were described in hypothalamic neurons after abnormal neonatal maternal care, supporting the bidirectional role of glutamatergic synapse function in influencing neuronal gene expression.45 Notably, the authors of the above study reported on CRH levels only during the third week of life whereas we found unaltered18 or lower46 levels of hypothalamic CRH in adult rats and mice subjected to the same protocol. Notably, the origin of excitatory neurotransmission onto CRH cells of the PVN nucleus is not fully elucidated. Both extra-and intra-hypothalamic cells are likely involved in vivo.47, 48 The persistence of these synapses in hypothalamic explants cultured for over a week provides strong support for the functional role of intra-hypothalamic excitatory neurons innervating the CRH-expressing cells.
The transcription factor identified here as a mediator of changes in gene expression induced by diminished excitation to CRH-expressing neurons, NRSF, is an ideal candidate to fine-tune expression of neuronal genes.23, 25, 26, 28 First, its canonical role is to silence neuronal genes in non-neuronal tissues, so that many crucial neuronal genes are endowed with NRSEs and are responsive to NRSF binding.12, 24, 25, 26, 27 Second, variation in the binding probabilities of different neuronal genes to NRSF in the brain enables selective repression of subsets of NRSE-containing genes depending on NRSF levels in mature neurons.15 Thus, moderate, activity- or insult-induced increase of NRSF levels in mature neurons14, 15, 49 preferentially influences partially bound genes with moderate ‘affinity’ to this repressor, enabling selective regulation of subsets of NRSE-containing genes, with important implications to neuronal and behavioral phenotypes.15, 49, 50
How might reduced excitatory input onto CRH-expressing neurons augment NRSF expression and function? There is a large yet inconclusive literature about the transcriptional regulation of NRSF expression.25, 27, 51 Bioinformatics analysis has suggested that excitatory neurotransmission might regulate NRSF expression via non-canonical intracellular signals. Among these, microRNAs, including microRNA-124, are appealing candidates51 that will be a target of future studies.
We noted increased binding of NRSF to Crh and 73 additional genes, and repression of such genes on reduction of glutamatergic neurotransmission in vitro and in P10 rats experiencing enriched maternal care. In this context, it is notable that the location of NRSF-binding peaks unique to glutamate-receptor blocked hypothalami was primarily (61%) in promoters and introns, whereas only 38% of vehicle-unique peaks localized to these regions. These observations suggest that reduction of glutamatergic neurotransmission both increases the number of sites bound by NRSF and also drives NRSF binding preferentially to sites throughout the genome that are more effective in influencing gene expression. Distinct NRSF-enriched genes were detected uniquely in peaks observed in glutamate receptor blocked group and vehicle control group. Gene ontology analyses revealed that genes enriched in peaks only found in the vehicle control group, such as Cacna1b, Kcnc1 and Neurod2, were involved in synaptic signaling and neurotransmitter transport. In contrast, NRSF-enriched genes unique to the glutamate receptor blocked group were not only involved in synaptic signaling but also encode molecules contributing to ion channel function and regulation of synaptic transmission. Except for significant ion transport-involved genes in the glutamate receptor blocked group, most gene ontology terms in this group were shared between the two groups, suggesting that ion channel function might specifically contribute to the neuronal plasticity in hypothalami exposed to reduced glutamatergic neurotransmission.
Although augmented NRSF levels persisted during adolescence,5 the increased occupancy of NRSF as well as of MeCP2 at the Crh gene did not endure in adult animals. These data suggest that NRSF and its associated cofactors are required to initiate transcriptional silencing of Crh immediately after augmented maternal care.26, 28 However, the maintenance of Crh repression (as well as that of other genes) involves epigenetic changes to the chromatin that are no longer NRSF and MeCP2 dependent. Rather, histone changes, including tri-methylation of H3K27 and dimethylation of H3K9 may promote persistent repression of target genes.
Finally, these studies highlight the need for examining biological processes resulting in enduring plasticity at early time points, proximate to the inciting experiences.39, 52 The reduction in excitatory synapse number,5 the augmented binding of NRSF and the recruitment of MeCP2 as a result of enhanced maternal care were all transient. If examined during adulthood, when the behavioral phenotype and CRH repression are robust, these evanescent mechanistic clues may no longer be in evidence.
Acknowledgments
We thank Melanie Oaks and her staff at the University of California-Irvine Genomics High Throughput Facility for providing excellent sequencing support. This work was supported by MH73136, NS28912 and P50MS096889. Supported by National Institutes of Health Grants MH73136, NS28912 and P50MS096889.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Insel TR. Mental disorders in childhood: shifting the focus from behavioral symptoms to neurodevelopmental trajectories. J Am Med Assoc 2014; 311: 1727–1728. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry 2010; 68: 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron 2008; 60: 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, La Plante P, Weaver S, Parent A, Sharma S, Diorio J et al. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Mol Cell Endocrinol 2001; 185: 205–218. [DOI] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci 2010; 30: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J Neurosci 1993; 13: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 1993; 18: 195–200. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology 2005; 146: 4090–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic–pituitary–adrenal axis early in life requires recurrent recruitment of stress regulating brain regions. J Neurosci 2006; 26: 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother's love to baby's future. Front Behav Neurosci 2009; 3: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J Biol Chem 2001; 276: 13917–13923. [DOI] [PubMed] [Google Scholar]
- Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science 2007; 316: 1497–1502. [DOI] [PubMed] [Google Scholar]
- Bali B, Kovács KJ. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci 2003; 18: 1518–1526. [DOI] [PubMed] [Google Scholar]
- McClelland S, Flynn C, Dubé C, Richichi C, Zha Q, Ghestem A et al. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol 2011; 70: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Brennan GP, Dubé C, Rajpara S, Iyer S, Richichi C et al. The transcription factor NRSF contributes to epileptogenesis by selective repression of a subset of target genes. eLife 2014; 3: e01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noam Y, Zha Q, Phan L, Wu RL, Chetkovich DM, Wadman WJ et al. Trafficking and surface expression of hyperpolarization-activated cyclic nucleotide-gated channels in hippocampal neurons. J Biol Chem 2010; 285: 14724–14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson K, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology 2001; 142: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 2005; 25: 9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neurosci 2008; 154: 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry 2016; 6: e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 2012; 22: 1813–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 1967; 156: 258–260. [DOI] [PubMed] [Google Scholar]
- Schoenherr CJ, Paquette AJ, Anderson DJ. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci USA 1996; 93: 9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunyak VV, Burgess R, Prefontaine GG, Nelson C, Sze SH, Chenoweth J et al. Corepressor-dependent silencing of chromosomal regions encoding neuronalgenes. Science 2002; 298: 1747–1752. [DOI] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol 2005; 15: 500–506. [DOI] [PubMed] [Google Scholar]
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 2005; 121: 645–657. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res 2006; 16: 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol Learn Mem 2011; 96: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 2006; 103: 18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res 2011; 21: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010; 13: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science 1996; 274: 1133–1138. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 2012; 75: 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 2004; 432: 758–761. [DOI] [PubMed] [Google Scholar]
- Woo CC, Hingco EE, Taylor GE, Leon M. Exposure to a broad range of odorants decreases cell mortality in the olfactory bulb. Neuroreport 2006; 17: 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature 2006; 444: 707–712. [DOI] [PubMed] [Google Scholar]
- Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 2016; 7: 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Asia M, Mahoney CE, Joachim M, Shen Y, Gunner G et al. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol Psychiatry 2016; doi:10.1038/mp.2016.136. [DOI] [PMC free article] [PubMed]
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry 2001; 6: 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin N, Shinsako J, Dallman MF. Corticosterone acts on the brain to inhibit adrenalectomy-induced adrenocorticotropin secretion. Endocrinology 1988; 122: 694–701. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol 2012; 33: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M, Leeman E, Shinsako J, Dallman MF. Steroid inhibition of canine ACTH: in vivo evidence for feedback at the corticotrope. Am J Physiol 1988; 255: E241–E246. [DOI] [PubMed] [Google Scholar]
- Dedic N, Touma C, Romanowski CP, Schieven M, Kühne C, Ableitner M et al. Assessing behavioural effects of chronic HPA axis activation using conditional CRH-overexpressing mice. Cell Mol Neurobiol 2012; 32: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci 2005; 25: 4073–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD et al. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci 2013; 33: 19534–19554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008; 149: 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 1997; 77: 3396–4000. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol 2011; 519: 1301–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Ruano A, Chávez AE, Cossio MJ, Castillo PE, Zukin RS. REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci 2012; 15: 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Nakashima K, Clemenson GD Jr, Mejia E, Mathews E, Ure K et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 2007; 27: 5967–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan GP, Dey D, Chen Y, Patterson KP, Magnetta EJ, Hall AM et al. Dual and opposing roles of microRNA-124 in epilepsy are mediated through inflammatory and NRSF-dependent gene networks. Cell Rep 2016; 14: 2402–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Casey BJ. The impact of developmental timing for stress and recovery. Neurobiol Stress 2015; 1: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ChIP-seq data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE87709.