Abstract
Objectives
To understand the effect of tight glycemic control (TGC) on cardiac surgery-associated acute kidney injury (CS-AKI).
Design
Secondary analysis of data from the Safe Pediatric Euglycemia after Cardiac Surgery (SPECS) trial of TGC vs. standard care
Setting
Pediatric cardiac intensive care units (ICU) at University of Michigan (UM) C.S. Mott Children’s Hospital and Boston Children’s Hospital (BCH).
Patients
Children 0–36 months of age undergoing congenital cardiac surgery.
Interventions
None.
Measurements and Main Results
CS-AKI was assigned using the Acute Kidney Injury Network criteria with the modification that a >0.1 mg/dL increase in serum creatinine was required to assign CS-AKI. We explored associations between CS-AKI and TGC and clinical outcomes. Of 799 patients studied, CS-AKI occurred in 289 (36%) patients, most of whom had Stage II or III disease (72%). CS-AKI rates were similar between treatment groups (36% vs. 36%, p=0.99). Multivariable modeling showed that patients with CS-AKI were younger (p=0.002), underwent more complex surgery (p=0.005) and had longer cardiopulmonary bypass times (p=0.002). CS-AKI was associated with longer mechanical ventilation and ICU and hospital stays and increased mortality. Patients at UM had higher rates of CS-AKI compared to BCH patients (66% vs. 15%, p<0.001) but UM patients with CS-AKI had shorter time to extubation and ICU and hospital stays compared to BCH.
Conclusions
TGC did not reduce the CS-AKI rate in this trial cohort. We observed significant differences in CS-AKI rates between the two study sites, and there was a differential effect of CS-AKI on clinical outcomes by site. These findings warrant further investigation to identify causal variation in perioperative practices that affect CS-AKI epidemiology.
Keywords: acute kidney injury, cardiac intensive care, cardiopulmonary bypass, congenital heart disease, tight glycemic control, pediatric critical care, cardiac surgery
Introduction
Cardiac surgery-associated acute kidney injury (CS-AKI) is associated with adverse outcomes including prolonged mechanical ventilation and longer intensive care unit and hospital stays and mortality (1–4). Previous work identifies prematurity, cardiopulmonary bypass, surgical complexity, perioperative morbidities, delayed sternal closure and markers of inadequate systemic oxygen delivery as risk factors for postoperative pediatric CS-AKI (1–8).
Few preventative or therapeutic measures for CS-AKI exist and standard care is supportive, requiring fluid restriction, diuresis and avoidance of nephrotoxins. Given the association between CS-AKI and outcome after pediatric cardiac surgery, efforts aimed at modifying CS-AKI risk may yield important reductions in postoperative mortality, cardiac ICU and hospital length of stay (LOS). Though some factors may be modifiable, efforts to identify practices to reduce CS-AKI rates and improve patient outcomes are ongoing.
Tight glycemic control (TGC) showed promise as a therapy to prevent CS-AKI. Retrospective data suggest an association between intraoperative hyperglycemia and CS-AKI (9) for adult surgical patients. Two prior clinical trials demonstrated a reduction in renal replacement therapy and new AKI in critically ill adults randomized to TGC in surgical and medical ICUs (10, 11). Multiple studies have explored associations between hyperglycemia, renal dysfunction and clinical outcome with conflicting results (10–15). The Safe Pediatric Euglycemia after Cardiac Surgery (SPECS) study demonstrated no benefit of TGC on health care-associated infection (HAI) rates in the overall study cohort, the relationship between TGC and CS-AKI in this population has not been explored (12).
To characterize the relationship between TGC and CS-AKI, we performed a planned secondary analysis of the SPECS trial, in which children were randomized to TGC versus standard care in the postoperative period after cardiac surgery. We hypothesized that patients in the TGC group would have lower CS-AKI risk. Also, we sought to explore center variation in CS-AKI epidemiology, including differences in CS-AKI and associated clinical outcomes.
Materials and Methods
Details of the SPECS trial were published previously (12, 16). In brief, 980 children less than 36 months of age undergoing cardiac surgery requiring cardiopulmonary bypass at University of Michigan (UM) C.S. Mott Children’s Hospital and Boston Children’s Hospital (BCH) were enrolled from September 2006 to May 2012. Children were randomized to receive TGC (80–110 mg/dL) or standard care postoperatively. Bedside clinicians were not blinded to treatment group assignments. The trial’s primary outcome was HAI incidence, including bloodstream infections, urinary tract infections, pneumonia, and surgical-site infections. TGC did not reduce HAI incidence compared to standard care (12). Patients in the TGC group had lower time-weighted glucose averages (median [interquartile range (IQR)] 112 [104–120] vs. 121 [109–136] mg/dL; p<0.001) and more hypoglycemia (any [<60 mg/dL]: 19% vs. 9%; p<0.001; severe [<40 mg/dL]: 3 vs. 1; p=0.03). The original study was IRB approved at both institutions and parents or legal guardians gave written informed consent.
The current study is a planned secondary analysis of existing SPECS data. Additional laboratory data not part of the study database were extracted from the hospitals’ medical record, including preoperative (baseline) and daily highest postoperative creatinine levels and use of modified ultrafiltration (MUF). All patients with the requisite serum creatinine data to assign postoperative CS-AKI were included. Preoperative AKI and remote AKI history were not assessed in the parent study, so AKI status prior to randomization is unknown. IRB approval was obtained for collection of additional study variables and retrospective review of data from the original study.
CS-AKI was assigned using the Acute Kidney Injury Network (AKIN) criteria, which was the most frequently used AKI classification system at the time of study design (17): Stage I (mild CS-AKI) was defined as an increase in serum creatinine from baseline by ≥0.3 mg/dL or 150–199%, Stage II (moderate CS-AKI) as an increase from baseline by 200–299% and Stage III (severe CS-AKI) as an increase from baseline by ≥300%, a serum creatinine of ≥4.0 mg/dL or need for renal replacement therapy. Due to the relatively low serum creatinine levels in our population, we modified the AKIN criteria to require an increase in serum creatinine from baseline of >0.1 mg/dL to assign CS-AKI of any stage. This was performed in order to prevent small variations in serum creatinine from overestimating the true incidence of CS-AKI (e.g., a rise in serum creatinine from 0.2 mg/dL to 0.3 mg/dL, which, using the AKIN criteria, would be considered Stage I AKI) (17). Accurate urine output data were unavailable and not used to assign CS-AKI. CS-AKI duration was defined as time to normalization of serum creatinine to baseline. Surgical complexity was assessed using the Risk Adjustment in Congenital Heart Surgery-1 (RACHS-1) (18) score as this was the most common case-mix adjustment method used when the trial protocol was developed.
Statistical Methods
Treatment group comparisons of CS-AKI incidence, CS-AKI stage, first day of CS-AKI and CS-AKI duration were made using Fisher’s exact tests or Wilcoxon rank sum tests. We found no statistically significant differences in LOS or mortality outcomes between Stage I patients and Stage II or III patients (Supplemental Table 1), so patients with any CS-AKI were combined into one group and compared to patients with no CS-AKI in most analyses. Univariable logistic regression was used to compare patient characteristics between children with and without CS-AKI. Stepwise multivariable logistic regression with adjustment for treatment group and site was performed to predict CS-AKI incidence. Additional predictors were defined a priori and included age at surgery, weight-for-age z-score, gender, prematurity, RACHS-1 category ≥3 or not assignable, single ventricle physiology, cardiopulmonary bypass (CPB) time ≥120 minutes, cross-clamp time ≥60 minutes, use of deep hypothermic circulatory arrest (DHCA), highest intraoperative glucose ≥250 mg/dL, use of intraoperative steroids and delayed sternal closure (postoperative characteristics were not considered). Modified ultrafiltration was considered, but was found to be highly collinear with site, so could not be evaluated as an independent predictor.
A p-value of <0.05 was required for a covariate to remain in the model. Kaplan-Meier curves and proportional hazards regression with adjustment for site were used to evaluate the impact of CS-AKI incidence and duration (0, 1, 2 or ≥3 days) on the outcomes of duration of mechanical ventilation and cardiac ICU and hospital LOS, and logistic regression with adjustment for site was used for in-hospital mortality. CS-AKI duration was categorized due to the rapid onset and resolution in the population, with shorter CS-AKI duration hypothesized to be associated with less adverse outcomes. Outcomes longer than 30 days were censored at day 30. Multivariable proportional hazards regression was used to further evaluate the effect of CS-AKI on the length of stay outcomes after controlling for other factors known to be associated with worse outcomes. Site comparisons of CS-AKI incidence, CS-AKI stage, first day of CS-AKI, CS-AKI duration and patient characteristics were made using Fisher’s exact tests or Wilcoxon rank sum tests. Analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC).
Results
Patient and Clinical Characteristics
Among the 980 patients enrolled in the SPECS study, 799 patients (82%) had the requisite serial creatinine measurements to determine CS-AKI status and were included in this study. Excluded patients were older (8.1 vs. 4.2 months, p<0.001) and had less complex surgeries (RACHS-1 category ≥3 or not assignable 32% vs. 57% and single ventricle 10% vs. 22%, p<0.001 for both). Patients were similar with respect to sex, prematurity, chromosomal or non-cardiac anomalies and cross-clamp time. The missing data rate was similar between treatment groups (TGC 19% vs. standard care 18%, p=0.51). The TGC group accounted for 49% of the cohort (n=395/799); UM patients numbered 327 (41%).
CS-AKI Epidemiology
CS-AKI occurred in 289 patients (36%). Stage I occurred in 28% with CS-AKI (n=80/289), Stage II in 47% (n=137/289) and Stage III in 25% (n=72/289). The median CS-AKI duration was 2 days, with most (83%) developing CS-AKI on postoperative day 1.
Any CS-AKI (36% vs. 36%, p=0.99) and Stage II/III CS-AKI rates (28% vs. 25%, p=0.38) were similar between treatment groups. CS-AKI lasted a median duration of 2 days (IQR 1–3) for both treatment groups (p=0.35), but patients in the TGC group developed CS-AKI on postoperative day 1 more frequently (89% vs. 78%, p=0.02). Adjustment for site did not change treatment group comparisons, nor were there any significant treatment group by site interactions. CS-AKI rates were similar between treatment groups at each site (TGC vs. standard care; UM: 66% vs. 66%, p=0.91; BCH: 15% vs. 16%, p=0.70).
Table 1 shows univariable associations. Patients were similar with respect to prematurity, previous cardiac surgery, chromosomal and non-cardiac anomalies. CS-AKI patients underwent more complex surgery (p<0.001) with shorter cross-clamp times (p=0.02) and more frequent use of hypothermic circulatory arrest (p=0.004). Few patients (n=11) required renal replacement therapy (Table 1). CS-AKI patients had more frequent intraoperative hyperglycemia (blood glucose ≥250 mg/dL), MUF and delayed sternal closure and were less likely to have had intraoperative steroids (p<0.001 for each). There was no difference in time-weighted blood glucose average or hypoglycemia rates between patients with CS-AKI and those without.
TABLE 1.
Characteristics of patients with and without cardiac surgery-associated acute kidney injury
| Characteristic | No CS-AKI (n = 510) | CS-AKI (n = 289) | Mild CS-AKI (n = 80) | Moderate/Severe CS-AKI (n = 209) | P value* |
|---|---|---|---|---|---|
| Preoperative characteristics | |||||
| Age at surgery, months, median (IQR) | 4.6 (2.0–8.8) | 3.5 (0.3–6.4) | 2.4 (0.2–5.9) | 3.7 (0.7–6.5) | .004 |
| Preoperative weight, kg | 5.4 (3.8–7.3) | 4.6 (3.4–6.4) | 3.9 (3.3–5.7) | 4.8 (3.5–6.7) | .003 |
| Weight-for-age z-score | −1.5 (−2.6 to −0.5) | −1.3 (−2.4 to −0.3) | −1.2 (−2.5 to −0.0) | −1.3 (−2.4 to −0.4) | .31 |
| Female gender, n (%) | 233 (46) | 136 (47) | 41 (51) | 95 (45) | .71 |
| Premature birth | 91 (18) | 50 (17) | 12 (15) | 38 (18) | .85 |
| Chromosomal anomaly | 96 (19) | 62 (21) | 20 (25) | 42 (20) | .37 |
| Non-cardiac structural abnormality | 66 (13) | 39 (13) | 11 (14) | 28 (13) | .82 |
| Baseline serum creatinine, mg/dL | 0.3 (0.2–0.4) | 0.3 (0.2–0.3) | 0.4 (0.3–0.4) | 0.2 (0.2–0.3) | <.001 |
| RACHS-1 category | |||||
| 1–2 | 246 (48) | 98 (34) | 21 (26) | 77 (37) | |
| 3 | 165 (32) | 90 (31) | 29 (36) | 61 (29) | |
| 4 | 50 (10) | 65 (22) | 17 (21) | 48 (23) | |
| 5–6 | 30 (6) | 31 (11) | 13 (16) | 18 (9) | |
| Not assignable | 19 (4) | 5 (2) | 0 | 5 (2) | |
| RACHS-1 category ≥3 or not assignable | 264 (52) | 191 (66) | 59 (74) | 132 (63) | <.001 |
| Single ventricle physiology | 100 (20) | 75 (26) | 22 (28) | 53 (25) | .04 |
| Previous cardiac surgery | 128 (25) | 75 (26) | 18 (23) | 57 (27) | .79 |
| Operative characteristics | |||||
| Site | <.001 | ||||
| University of Michigan | 111 (22) | 216 (75) | 50 (63) | 166 (79) | |
| Boston Children’s Hospital | 399 (78) | 73 (25) | 30 (38) | 43 (21) | |
| Cardiopulmonary bypass time ≥120 minutes | 202 (40) | 122 (42) | 38 (48) | 84 (40) | .47 |
| Cross-clamp time ≥60 minutes | 265 (52) | 126 (44) | 41 (51) | 85 (41) | .02 |
| Deep hypothermic circulatory arrest | 81 (16) | 70 (24) | 22 (28) | 48 (23) | .004 |
| Lowest intraoperative temperature <24°C | 109 (21) | 91 (31) | 26 (33) | 65 (31) | .002 |
| Highest intraoperative glucose ≥250 mg/dL | 55 (11) | 93 (32) | 25 (31) | 68 (33) | <.001 |
| Intraoperative steroids | 303 (59) | 107 (37) | 40 (50) | 67 (32) | <.001 |
| Modified ultrafiltration | 134 (26) | 203 (70) | 48 (60) | 155 (74) | <.001 |
| Delayed sternal closure | 58 (11) | 60 (21) | 15 (19) | 45 (22) | <.001 |
| Postoperative characteristics | |||||
| Tight glycemic control group | 252 (49) | 143 (49) | 34 (43) | 109 (52) | .99 |
| Unanticipated reoperation | 6 (1) | 27 (9) | 4 (5) | 23 (11) | <.001 |
| Need for CPR | 7 (1) | 12 (4) | 2 (3) | 10 (5) | .02 |
| Need for ECMO | 8 (2) | 5 (2) | 1 (1) | 4 (2) | .86 |
| pRBC transfusion on postoperative day 1 | 190 (37) | 129 (45) | 29 (36) | 100 (48) | .04 |
| Time-weighted blood glucose average (mg/dL) | 115 (106–128) | 116 (107–127) | 116 (105–126) | 117 (108–127) | .74 |
| Hypoglycemia (<60 mg/dL) | 66 (13) | 50 (17) | 16 (20) | 34 (16) | .09 |
| Severe hypoglycemia (<40 mg/dL) | 7 (1) | 9 (3) | 4 (5) | 5 (2) | .10 |
| Renal replacement therapy | 0 | 11 (4) | 0 | 11 (5) | <.001 |
Values are median (interquartile range) or n (%). CS-AKI, cardiac surgery-associated acute kidney injury; IQR, interquartile range; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; pRBC, packed red blood cells.
P values for the comparison of the no CS-AKI and CS-AKI groups were determined by logistic regression, except for renal replacement therapy which used Fisher’s exact test.
Using multivariable analysis, younger age, RACHS-1 ≥3 or not assignable, CPB time ≥120 minutes and site predicted CS-AKI (Table 2). Site was the most important contributor to the model with an odds ratio of 15.7 (p<0.001). Treatment group was not a significant predictor of CS-AKI, nor was there a significant treatment group by site interaction.
TABLE 2.
Multivariable model to predict cardiac surgery-associated acute kidney injury
| Variable* | Odds ratio (95% CI)† | P value |
|---|---|---|
| Tight glycemic control group | 0.92 (0.65–1.31) | .65 |
| University of Michigan | 15.8 (10.5–23.6) | <.001 |
| Age at surgery (per month) | 0.96 (0.94–0.99) | .002 |
| RACHS-1 category ≥3 or not assignable | 1.8 (1.2–2.6) | .005 |
| Cardiopulmonary bypass time ≥120 minutes | 2.0 (1.3–3.1) | .002 |
CI, confidence interval;
After adjusting for treatment group and site, potential additional predictors of acute kidney injury included age at surgery, weight-for-age z-score, female gender, premature birth, RACHS-1 category ≥3 or not assignable, single ventricle physiology, cardiopulmonary bypass time ≥120 minutes, cross-clamp time ≥60 minutes, deep hypothermic circulatory arrest, highest intraoperative glucose ≥250 mg/dL, and delayed sternal closure. Postoperative characteristics were not included in the model to predict cardiac surgery-associated acute kidney injury.
An odds ratio > 1 indicates a greater risk of acute kidney injury.
Clinical Outcomes Related to CS-AKI
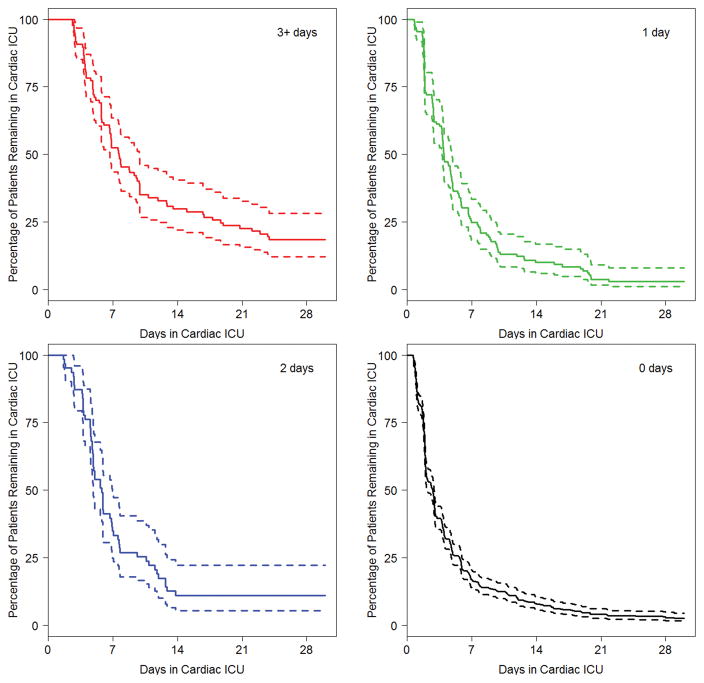
In site-adjusted analysis, CS-AKI patients experienced higher mortality (7% vs. <1%, p<0.001) and had longer mechanical ventilation and cardiac ICU and hospital LOS (Table 3). After adjustment for other factors associated with worse outcomes, including age, cardiac support time, surgical complexity, delayed sternal closure, need for ECMO and unanticipated reoperation, CS-AKI was associated with longer mechanical ventilation (median 4 vs. 2 days), cardiac ICU LOS (5.6 vs. 2.8 days) and hospital LOS (12 vs. 7 days; p<0.001 for each). Further, duration of CS-AKI predicted prolonged mechanical ventilation, cardiac ICU LOS (Figures 1 and 2) and hospital LOS. We found no association between TGC and these secondary outcomes in the multivariable analysis.
TABLE 3.
Association of cardiac surgery-associated acute kidney injury and outcomes
| Outcome | No CS-AKI (n = 510) | CS-AKI (n = 289) | Site-adjusted models | Multivariable models* | ||
|---|---|---|---|---|---|---|
| Hazard ratio† (95% CI) | P value‡ | Hazard ratio† (95% CI) | P value‡ | |||
| Duration of mechanical ventilation, days | 2 (1–4) | 4 (3–7) | 0.47 (0.40–0.56) | <.001 | 0.56 (0.46–0.67) | <.001 |
| Length of cardiac ICU stay, days | 3 (2–6) | 6 (4–10) | 0.49 (0.42–0.58) | <.001 | 0.58 (0.48–0.70) | <.001 |
| Length of hospital stay, days | 7 (5–12) | 12 (8–23) | 0.51 (0.43–0.62) | <.001 | 0.65 (0.53–0.78) | <.001 |
Values are median (interquartile range). CS-AKI, cardiac surgery-associated acute kidney injury; CI, confidence interval; ICU, intensive care unit.
Multivariable models adjust for treatment group, site, age at surgery, weight-for-age z-score, female gender, premature birth, chromosomal anomaly, non-cardiac structural abnormality, RACHS-1 category ≥3 or not assignable, cardiopulmonary bypass time ≥120 minutes, cross-clamp time ≥60 minutes, delayed sternal closure, unanticipated reoperation, cardiopulmonary resuscitation, extracorporeal membrane oxygenation, packed red blood cell transfusion on postoperative day 1 and hypoglycemia (blood glucose <60 mg/dL).
A hazard ratio < 1 indicates that acute kidney injury is associated with a longer duration of mechanical ventilation, cardiac ICU stay or hospital stay.
P values for the comparison between groups were determined by proportional hazards regression.
Figure 1. Cardiac intensive care unit length of stay by duration of cardiac surgery-associated acute kidney injury.
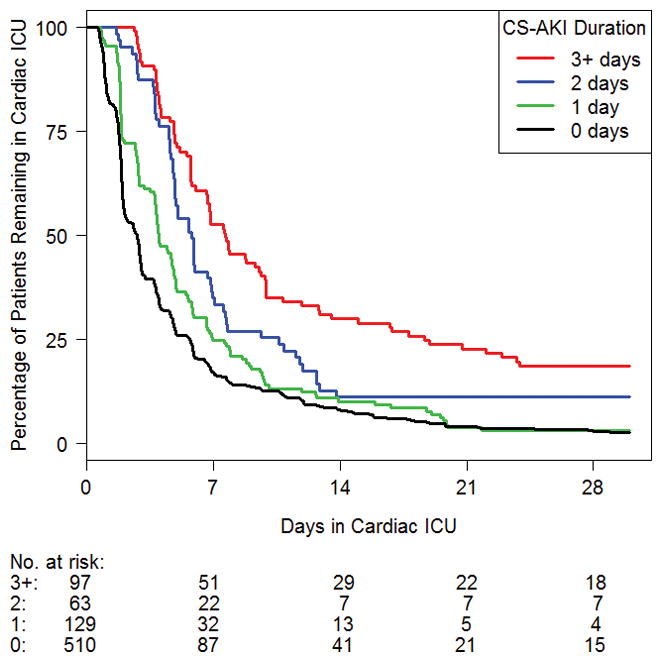
CS-AKI, cardiac surgery-associated acute kidney injury; ICU, intensive care unit.
Figure 2. Confidence intervals for Figure 1 by duration of cardiac surgery-associated acute kidney injury.
CS-AKI, cardiac surgery-associated acute kidney injury; ICU, intensive care unit.
Site Differences
Site strongly predicted CS-AKI (Table 4). Both incidence (66% vs. 15%) and severity of CS-AKI (51% vs. 9%; p<0.001 for both) were greater at UM. Median CS-AKI duration was 2 days (IQR 1–3) at both sites. In general, CS-AKI occurred by postoperative day 2 at both sites, though UM patients developed CS-AKI on postoperative day 1 more frequently. CS-AKI occurred on postoperative day 1 in 241 patients; 93% of UM patients and 83% of BCH patients reached maximum CS-AKI stage on this day.
TABLE 4.
Cardiac surgery-associated acute kidney injury by site
| AKI Status | Overall (n = 799) | University of Michigan (n = 327) | Boston Children’s Hospital (n = 472) | P value* |
|---|---|---|---|---|
| CS-AKI, n (%) | 289 (36) | 216 (66) | 73 (15) | <.001 |
| Maximum CS-AKI stage | ||||
| Stage I (mild) | 80 (28) | 50 (23) | 30 (41) | .01 |
| Stage II (moderate) | 137 (47) | 107 (50) | 30 (41) | |
| Stage III (severe) | 72 (25) | 59 (27) | 13 (18) | |
| First day of CS-AKI | <.001 | |||
| Postoperative day 1 | 241 (83) | 200 (93) | 41 (56) | |
| Postoperative day 2 or later | 48 (17) | 16 (7) | 32 (44) | |
| Duration of CS-AKI, days, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | .61 |
Values are n (%) or median (interquartile range). CS-AKI, cardiac surgery-associated acute kidney injury; IQR, interquartile range.
P values were determined by Fisher’s exact tests or Wilcoxon rank sum tests.
UM patients were more likely to have single ventricle physiology (28% vs. 17%, p<0.001), but preoperative characteristics were otherwise similar (Supplemental Table 2). Patients at UM had shorter cardiac support times (CPB time ≥120 minutes, 27% vs. 50%; cross-clamp time ≥60 minutes, 27% vs. 64%), more intraoperative hyperglycemia (33% vs. 8%), lower intraoperative steroids use (19% vs. 74%) and more frequent MUF (91% vs. 8%) (p<0.001 for each).
We found that CS-AKI affected clinical outcome at each site differently (Table 5). UM patients with CS-AKI had shorter mechanical ventilation (median 4 vs. 6 days, p=0.004), cardiac ICU LOS (4.9 vs. 7.7 days, p<0.001) and hospital LOS (11.5 days vs. 15 days, p=0.02) compared to BCH patients with CS-AKI. Mortality in CS-AKI patients was similar between sites (6% vs. 11%, p=0.12).
TABLE 5.
Outcomes in patients with and without cardiac surgery-associated acute kidney injury stratified by site
| Outcome | University of Michigan | Boston Children’s Hospital | ||||
|---|---|---|---|---|---|---|
| No CS-AKI (n = 111) | CS-AKI (n = 216) | P value* | No CS-AKI (n = 399) | CS-AKI (n = 73) | P value* | |
| Duration of mechanical ventilation, days | 2 (1–4) | 4 (3–7) | <.001 | 2 (1–4) | 6 (3–14) | <.001 |
| Length of cardiac ICU stay, days | 3 (1.8–5.7) | 5 (3–8) | <.001 | 3 (2–6) | 8 (5–20) | <.001 |
| Length of hospital stay, days | 8 (5–14) | 12 (8–19) | <.001 | 7 (5–11) | 15 (9–30) | <.001 |
Values are median (interquartile range). CS-AKI, cardiac surgery-associated acute kidney injury; ICU, intensive care unit.
P values for the comparison between groups were determined by proportional hazards regression.
Discussion
In this planned secondary analysis of 799 patients from the SPECS trial (12, 16), we found that CS-AKI occurred in 36% patients with most (72%) having Stage II or III disease. To our knowledge, this is the first study examining the relationship between TGC and pediatric CS-AKI. While previous studies in adults suggested a protective effect of TGC (9–11) we found no association between TGC and CS-AKI. Consistent with previous work, we observed worse clinical outcomes in CS-AKI patients. However, we did observe striking differences in CS-AKI rates and differential impact on clinical outcomes across the two study sites.
Though investigators have reported disparate CS-AKI rates following congenital heart surgery (1–5), there are no prior data on site differences within relatively homogenous populations using a standard data collection method. Our study allows some investigation into potentially modifiable practice variations. While we identified some site-specific practice variations (e.g., use of MUF, intraoperative steroids) that may contribute to CS-AKI, we found that site and MUF were collinear with highly discrepant MUF rates (UM 91% vs. BCH 8%) precluding evaluating MUF in the multivariable analysis. The role of ultrafiltration on CS-AKI has not been fully explored to date. Paugh found that patients receiving intraoperative continuous ultrafiltration had higher AKI rates, with increasing fluid removal associated with more AKI in patients with pre-existing renal dysfunction (19). Kuntz showed no relationship between intraoperative ultrafiltration and intraoperative urinary flow rate, but the study did not define AKI using classification systems (20). Given that infants have late renal maturation, volume shifts associated with MUF may confer additional CS-AKI risk.
In part, MUF utilization may explain the discrepant CS-AKI rates, CS-AKI timing and the transient CS-AKI we observed. We postulate that early transient CS-AKI may indicate that MUF predisposes vulnerable patients to CS-AKI. Another plausible explanation is that the early postoperative rise in serum creatinine reflects MUF-related volume depletion and does not reflect true injury. At UM, MUF is commonly used following cardiopulmonary bypass, which predictably resulted in rising serum creatinine within the first 24 postoperative hours. Potential additional factors contributing to the difference in CS-AKI incidence include intraoperative and postoperative intravascular volume status, CPB strategy, postoperative fluid administration, residual heart disease, blood loss and total blood product administration (21–23). For example, excessive fluid overload (which was unmeasured in our study) may result in prolonged mechanical ventilation and LOS, but which may be modified by MUF, reducing the impact of CS-AKI on those outcomes. We postulate that some of these unmeasured variables are contributory to the CS-AKI site difference we found. Future work aimed at utilizing novel biomarkers and uncovering more granular intraoperative and postoperative practices will undoubtedly help us understand why CS-AKI affects clinical outcomes differently at different cardiovascular programs.
Despite finding higher CS-AKI rates at UM, UM CS-AKI patients had shorter mechanical ventilation and cardiac ICU and hospital LOS compared to BCH CS-AKI patients. While CS-AKI itself may be an important morbidity, the site-specific impact on other clinical outcomes appears variable. Moreover, this discrepancy between higher CS-AKI rates and shorter mechanical ventilation and LOS at UM suggests that not all CS-AKI is created equal. Multiple studies have described discrepant CS-AKI incidence and outcome associations (1–4, 7). Our data lend credence to the hypothesis that MUF-related early increases in serum creatinine may represent volume shifts rather than glomerular or interstitial injury. While these patients met qualifications for AKI using creatinine-based criteria, this may not represent true kidney injury. To further investigate this hypothesis it will be important to corroborate these findings by pairing serum creatinine-based AKI definitions with AKI biomarkers in future studies. Patients receiving MUF with a small serum creatinine rise 24 hours after surgery may have biomarker negative AKI (creatinine rise without damage), potentially explaining the divergent outcomes at both sites. Pediatric CS-AKI rates vary widely from 15% to 52%, much of which is limited to mild disease, with investigators previously demonstrating no association between mild CS-AKI and outcome (12, 13). Our outcome data, however, are more consistent with adult data, which found that critically ill post-surgical patients with mild AKI are at risk for poor outcomes (6). The reasons underlying our discrepant findings are unclear, but may be due to differing patient demographics, as well as unmeasured institutional practice (1–4). Taylor reported on a large, heterogeneously aged cohort with significantly lower rates of CS-AKI and without an effect of mild CS-AKI on postoperative outcome (3). Though previous work described significant differences in outcome with increasing CS-AKI severity, we did not observe this phenomenon, which suggests that center-specific variables may modulate its effect on outcome.
Previous work in pediatric CS-AKI has not assessed the effect of CS-AKI duration on outcome. We found that patients developed CS-AKI quickly after surgery (<2 days) with rapid resolution, indicating transient injury. The early onset and short CS-AKI duration may indicate that intraoperative and postoperative volume status may be an important and, as yet, unmeasured determinant of CS-AKI. In particular, ultrafiltration results in intravascular volume contraction following CPB and has been associated with CS-AKI in adults (20). Postoperative hypovolemia may result in rapidly resolving CS-AKI, whereas more prolonged CS-AKI may have a different etiology. Patients with transient moderate to severe CS-AKI may not be significantly different from those with mild CS-AKI. Patients with shorter CS-AKI had shorter mechanical ventilation and cardiac ICU and hospital stay compared to those with longer CS-AKI duration. CS-AKI duration may be another useful measure to determine the clinical burden of CS-AKI and measures aimed at reducing CS-AKI duration (e.g., reducing early nephrotoxin exposure or early re-intervention for residual heart disease) may improve outcomes. More durable CS-AKI may suggest prolonged exposure to residual heart disease, which may leave the kidney exposed to high central venous pressures and limited renal blood flow for a prolonged time. Earlier re-intervention to ameliorate deleterious hemodynamics may reduce clinical impact of CS-AKI.
Consistent with previous work, we found that younger age, higher RACHS-1 category, and longer bypass time were associated with CS-AKI. Similarly, multivariable modeling demonstrated that CS-AKI was associated with prolonged mechanical ventilation and cardiac ICU and hospital LOS. Patients with Stage I CS-AKI were similar to patients with Stage II and III with respect to mechanical ventilation and postoperative cardiac ICU and hospital LOS. We discovered important differences in CS-AKI rates and differential impact on clinical outcomes across the two surgical sites.
Our study has several limitations. First, we performed an analysis from an existing prospectively collected database, limiting our ability to control for important covariates (e.g., postoperative blood loss, fluid overload, CPB strategy, deleterious hemodynamics) that were not included in the data collection. Further, previous AKI and chronic kidney disease history were not obtained in the parent trial, and we were unable to include those data in our analysis.. Second, we cannot prove that CS-AKI or CS-AKI duration are causal with regard to poor outcomes. We modified the AKIN criteria to require a serum creatinine rise of >0.1 mg/dL to prevent over-diagnosis of CS-AKI. Such a small change in creatinine may be within laboratory error, so we would have likely overestimated CS-AKI, particularly in infants and small children. Third, given the disparate CS-AKI rates at the two sites, we could not account for site-specific practices regarding postoperative cardiac ICU care that may explain these findings. Fourth, our analysis excluded 181 patients without available serum creatinine data. The excluded patients were older and underwent less complex operations, so we may be overestimating the CS-AKI incidence. Fifth, while available, serum creatinine is a limited CS-AKI biomarker, and may not sufficiently distinguish clinically important from less clinically relevant CS-AKI. Novel AKI biomarkers may help us understand CS-AKI mechanisms more specifically than serum creatinine (5). Basu et al demonstrated that correcting serum creatinine or fluid overload may help distinguish clinically significant CS-AKI in some patients previously classified as having less severe CS-AKI (24). Moreover, data with respect to intraoperative and postoperative fluid overload status were not collected. Excessive fluid overload may require prolonged mechanical ventilation and may necessitate fluid restriction, which may limit the provision of nutrition. Finally, we could not use urine output to define CS-AKI as the data were unavailable, possibly leading to underestimation of moderate to severe CS-AKI. Previous work has found that urine output criteria may increase the detectable rate of moderate to severe CS-AKI-than using serum creatinine criteria alone (2).
Conclusions
We found no evidence to support the use of TGC to prevent CS-AKI. Our analyses revealed novel, provocative findings related to center-specific differences in CS-AKI rates and a variable impact of CS-AKI on important clinical outcomes across sites. Using the Pediatric Cardiac Critical Care Consortium database, efforts are underway to characterize which modifiable candidate variables may affect CS-AKI, including MUF volume, bypass strategies, blood product utilization and fluid overload (JJB, MG) (25). These data will inform prospective multi-institutional research to determine all relevant covariates, to support future therapeutic trials aimed at reducing the CS-AKI burden on this vulnerable population.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung and Blood Institute (R01HL088448), the American Recovery and Reinvestment Act Supplement (R01HL088448-02S1) and the Harvard Catalyst Clinical and Translational Research Center (National Center for Advancing Translational Sciences grant UL1RR05758).
SPECS ClinicalTrials.gov number: NCT00443599
The institutions of Ms. Asaro and Drs. Wypij, Agus and Gaies received funding from National Institutes of Health (NIH), specifically the National Heart, Lung and Blood Institute (NHLBI), for the clinical trial. The remaining authors have disclosed that they did not receive any funding for this secondary analysis.
Abbreviations
- AKIN
Acute Kidney Injury Network
- BCH
Boston Children’s Hospital
- CPB
Cardiopulmonary bypass
- CS-AKI
Cardiac surgery-associated acute kidney injury
- ICU
Intensive care unit
- LOS
Length of stay
- MUF
Modified ultrafiltration
- RACHS-1
Risk Adjustment in Congenital Heart Surgery
- SPECS
Safe Pediatric Euglycemia after Cardiac Surgery
- TGC
Tight glycemic control
- UM
University of Michigan
Footnotes
No conflicts of interest disclosed.
Copyright form disclosure: Drs. Asaro, Wypij, and Agus received support for article research from the National Institutes of Health (NIH). Drs. Asaro and Wypij’s institution received funding from NIH/National Heart, Lung, and Blood Institute. Dr. Selewski disclosed that this was a secondary analysis of a large NIH study. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Aydin SI, Seiden HS, Blaufox AD, et al. Acute kidney injury after surgery for congenital heart disease. Annals of Thoracic Surgery. 2012;94:1589–1595. doi: 10.1016/j.athoracsur.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 2.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. Journal of Thoracic and Cardiovascular Surgery. 2012;143:368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Taylor ML, Carmona F, Thiagarajan RR, et al. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. Journal of Thoracic and Cardiovascular Surgery. 2013;146:146–152. doi: 10.1016/j.jtcvs.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Zappitelli M, Bernier PL, Saczkowski RS, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney International. 2009;76:885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 5.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. Journal of the American College of Cardiology. 2014;64:2753–2762. doi: 10.1016/j.jacc.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Critical Care Medicine. 2008;36:1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 7.Toth R, Breuer T, Cserep Z, et al. Acute kidney injury is associated with higher morbidity and resource utilization in pediatric patients undergoing heart surgery. Annals of Thoracic Surgery. 2012;93:1984–1990. doi: 10.1016/j.athoracsur.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Ruiz Gil-Esparza MA, Alcaraz Romero AJ, et al. Prognostic relevance of early AKI according to pRIFLE criteria in children undergoing cardiac surgery. Pediatric Nephrology. 2014;29:1265–1272. doi: 10.1007/s00467-014-2757-z. [DOI] [PubMed] [Google Scholar]
- 9.Lecomte P, Van Vlem B, Coddens J, et al. Tight perioperative glucose control is associated with a reduction in renal impairment and renal failure in non-diabetic cardiac surgical patients. Critical Care. 2008;12:R154. doi: 10.1186/cc7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. New England Journal of Medicine. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 11.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. New England Journal of Medicine. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 12.Agus MS, Steil GM, Wypij D, et al. Tight glycemic control versus standard care after pediatric cardiac surgery. New England Journal of Medicine. 2012;367:1208–1219. doi: 10.1056/NEJMoa1206044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. New England Journal of Medicine. 2009;26:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 14.Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;12:547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 15.Song JW, Shim JK, Yoo KJ, et al. Impact of intraoperative hyperglycaemia on renal dysfunction after off-pump coronary artery bypass. Interactive Cardiovascular and Thoracic Surgery. 2013;17:473–478. doi: 10.1093/icvts/ivt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaies MG, Langer M, Alexander JL, et al. Design and rationale of safe pediatric euglycemia after cardiac surgery: a randomized controlled trial of tight glycemic control after pediatric cardiac surgery. Pediatric Critical Care Medicine. 2013;14:148–156. doi: 10.1097/PCC.0b013e31825b549a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. Journal of Thoracic and Cardiovascular Surgery. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 19.Paugh TA, Dickinson TA, Martin JR, et al. Impact of ultrafiltration on kidney injury after cardiac surgery: the Michigan experience. Annals of Thoracic Surgery. 2015;100:1683–1688. doi: 10.1016/j.athoracsur.2015.04.120. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz RA, Holt DW, Turner S, et al. Effects of Conventional Ultrafiltration on Renal Performance During Adult Cardiopulmonary Bypass Procedures. Journal of Extracorporeal Technology. 2006;38:144–153. [PMC free article] [PubMed] [Google Scholar]
- 21.Bojan M, Vicca S, Boulat C, et al. Aprotinin, transfusions, and kidney injury in neonates and infants undergoing cardiac surgery. British Journal of Anaesthesia. 2012;108:830–837. doi: 10.1093/bja/aes002. [DOI] [PubMed] [Google Scholar]
- 22.Kanji HD, Schulze CJ, Hervas-Malo M, et al. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac-surgery associated acute kidney injury. Journal of Cardiothoracic Surgery. 2010;5:71. doi: 10.1186/1749-8090-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson R, Dip HE, Meeran H, et al. Goal-directed therapy after cardiac surgery and the incidence of acute kidney injury. Journal of Critical Care. 2014;29:997–1000. doi: 10.1016/j.jcrc.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Basu RK, Andrews A, Krawczeski CD, et al. Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediataric Critical Care Medicine. 2013;14(5):e218–e224. doi: 10.1097/PCC.0b013e3182772f61. [DOI] [PubMed] [Google Scholar]
- 25.Gaies MG, Cooper DS, Tabbutt S, et al. Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4) Cardiology in the Young. 2015;25:951–957. doi: 10.1017/S1047951114001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.