Abstract
Objective
The aim of this study was to evaluate the distribution, concentration and toxicity of cinnamaldehyde in electronic cigarette (e-cigarette) refill fluids and aerosols.
Methods
The distribution and concentration of cinnamaldehyde were determined in 39 e-cigarette refill fluids plus 6 duplicates using gas chromatography and mass spectrometry (GC/MS). A cinnamaldehyde toxicity profile was established for embryonic and adult cells using a live cell imaging assay, immunocytochemistry, the comet assay and a recovery assay.
Results
Twenty of the 39 refill fluids contained cinnamaldehyde at concentrations that are cytotoxic to human embryonic and lung cells in the MTT assay. Cinnamon Ceylon aerosol produced in a cartomizer-style e-cigarette was cytotoxic. Cinnamon Ceylon aerosols and refill fluid aerosols (80% propylene glycol or cinnamaldehyde/propylene glycol) made using a tank/boxmod e-cigarette were more cytotoxic at 5 V than 3 V. Using GC/MS, aerosols produced at 5 V contained 10 additional peaks not present in aerosol generated at 3 V. One of these, 2,3-butandione (diacetyl), was confirmed with an authentic standard. Cinnamaldehyde depolymerised microtubules in human pulmonary fibroblasts. At concentrations that produced no effect in the MTT assay, cinnamaldehyde decreased growth, attachment and spreading; altered cell morphology and motility; increased DNA strand breaks; and increased cell death. At the MTT IC50 concentration, lung cells were unable to recover from cinnamaldehyde after 2 hours of treatment, whereas embryonic cells recovered after 8 hours.
Conclusions
Cinnamaldehyde-containing refill fluids and aerosols are cytotoxic, genotoxic and low concentrations adversely affect cell processes and survival. These data indicate that cinnamaldehyde in e-cigarette refill fluids/aerosols may impair homeostasis in the respiratory system.
INTRODUCTION
E-cigarettes have undergone relatively little evaluation with respect to their effects on health. E-cigarette aerosols are generated by heating fluids that usually contain propylene glycol and/or glycerine, nicotine and flavourings.12 In 2014, over 8000 refill fluid products were commercially available,3 and the number is undoubtedly higher today. Although studies have dealt with flavouring chemicals in e-cigarette products,4–8 there is little information on how these chemicals affect health during short-term and long-term exposures. Many of the chemicals used for e-cigarette flavouring are generally regarded as safe (GRAS) by the Flavour and Extracts Manufactures Association (FEMA) (all acronyms appear in online supplementary table S1). However, FEMA has cautioned that their GRAS designation is based on ingestion and that the effects of inhaled e-cigarette flavouring chemicals are generally unknown.9
Some e-cigarette products contain flavour chemicals that are toxicants. Diacetyl, which imparts a buttery flavour, is present in a high percentage of refill fluids.8,10 Diacetyl is associated with bronchiolitis obliterans, an irreversible thickening of lung tissue, making gas exchange difficult and potentially leading to death.9,11–13 Benzaldehyde, which imparts a fruity taste, was present in 75% of 145 e-cigarette refill fluids, with the highest concentrations in cherry flavours.6 Benzaldehyde is cytotoxic and genotoxic to cultured human lymphocytes at concentrations ranging from 10 to 50 µg/mL.14 The concentration of flavouring chemicals in 13 out of 30 refill fluids ranged from 1% to 5% (10–50 mg/mL), and a significant number of these were aldehydes, which are associated with respiratory irritation.7
E-cigarette aerosols were generally not cytotoxic to mouse 3T3 cells in the MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay, except for one coffee-flavoured product.15 Similar results were reported for other aerosolised refill fluids when tested with mouse cardiomyocytes, which were adversely affected by only three tobacco and one cinnamon products.16 In our screen of 36 e-cigarette refill fluids, about a third of the refill fluids were highly cytotoxic with embryonic cells were generally being more sensitive than adult lung fibroblasts.17 Of the 36 products screened, Cinnamon Ceylon was the most cytotoxic to 3 cell types. In a follow-up screen of cinnamon-flavoured refill fluids, all products were cytotoxic to human embryonic stem cells (hESC) and adult human pulmonary fibroblasts (hPF).18 Cinnamaldehyde, which was identified as the dominant flavour chemical in Cinnamon Ceylon, was highly cytotoxicity to hESC and hPF in the MTT assay.18
The purpose of this study was to examine the distribution and concentration of cinnamaldehyde in the refill fluid library that was used in our original cytotoxicity screen,17 to evaluate the cytotoxicity and genotoxicity of aerosol made from a cinnamon-flavoured refill fluid and to determine the range action of cinnamaldehyde on adult lung and embryonic cells.
MATERIALS AND METHODS
Refill fluids and authentic standards
Refill fluids were purchased at various times from internet vendors, including Freedom Smoke USA (Tucson, Arizona, USA), Global Smoke (Los Angeles, California, USA), Johnson Creek (Johnson Creek, Wisconsin, USA), Red Oak (a subsidiary of Johnson Creek), Tasty Puff (Albuquerque, New Mexico, USA), e-cigexpress (Orlando, Florida, USA), Vaporbomb.com (Barberton, Ohio, USA), Vapormaxx (Richmond, Virginia, USA) and DIY Flavour Shack (Las Vegas, Nevada, USA).17,18 Refill fluids were stored at 4°C in the dark. Only the sample from Tasty Puff (Sinful Cinnamon) and its duplicate were sold as a do-it-yourself (DIY) product. All others, including the product from DIY Flavour Shack, were sold as refill fluids.
Authentic standards were purchased to produce ‘lab-made’ refill fluids. Trans-Cinnamaldehyde was purchased from TCI (Tokyo, Japan), and propylene glycol was from Acros Organics (New Jersey, USA). With exception of the aerosol MTT assays, all toxicity assessment assays were performed at the no observed adverse effect levels (NOAEL) and the inhibitory concentrations at 50% (IC50), which we reported previously for cinnamaldehyde.18 The NOAEL values for the hESC and hPF were 7.6×10−6 and 3×10−6 M, respectively, while the IC50 values were 4×10−5 and 3.7×10−5 M.
Identification and quantification of organic chemicals using GC/MS
After dilution with acetonitrile (Fisher Scientific, Fair Lawn, New Jersey, USA), refill fluids were analysed by GC/MS. Using internal standard-based calibration procedures similar to those described elsewhere,19 analyses were performed with an Agilent (Santa Clara, California, USA) 7693 autosampler, Agilent 7890A GC, and Agilent 5975C MS. A DB-VRX phase GC capillary column was used (60 m×250 mm×1.4 mm film). For each replicate sample, 50 mL of each fluid was dissolved in 1 mL of acetonitrile, and 1 mL was then injected into the GC with a 10:1 split. The GC temperature programme for all analyses was as follows: 45°C hold for 5 min; 12°C/min to 189°C; hold at 189°C for 2 min; then 5°C/min to 245°C and hold for 10 min at 245°C. The MS was operated at electron ionization mode. The ion source temperature was 250°C. The scan range was from 34 to 400 amu; for quantitation of each analyte, use of scan mode facilitated verification so that no co-eluting peaks were affecting the results. Each target analyte was quantitated using authentic standard material, and an internal standard (1,2,3-trichlorobenzene) normalised non-linear multipoint calibration curve based on peak area. The quantitation ion for cinnamaldehyde was 131. The electron multiplier voltage was 1350 V. Cinnamon Ceylon aerosols with 3 and 5 V were collected in 0.4 mL water and added into 0.8 mL of acetonitrile before GC/MS analysis; with an injection of only 1 mL, and the injector split, the water caused no problems in the analyses. Concentrations of the chemicals detected in the 5 Vaerosol were estimated by accounting for dilutions into water and acetonitrile and using the amount of fluid consumed by weighing the tank before and after aerosol production.
E-cigarette aerosols
E-cigarette aerosols were produced with fresh unused cartomizers or tanks using a smoking machine.2,20,21 The Vea cartomizer device and unfilled cartomizers (Johnson Creek, Hartland, Wisconsin) operated at 2.9 V, 2.1 Ω and 4 W. Cartomizers were loaded with 1 mL of refill fluid as recommended by the vendor and used in a manner that avoided dry puffing. An Innokin iTaste MVP 3.0 battery with variable voltage and wattage and Innokin iClear 16D bottom dual coil clearomizers (tanks) were operated at 3 V, 2.1 Ω and 4.2 W or at 5 V, 2.1 Ω and 11.9 W. For each sample, 2 mL of fluid was pipetted into new clean tanks. Puff duration was 4.3 s, the average for e-cigarette users,22 and flow rate was adjusted to produce consistent robust puffs (eg, cartomizer 30 mL puffs and tank 56 mL puffs). Aerosols were collected in a round-bottom flask containing culture medium and submerged in an ice bath or dry ice bath. Aerosol solutions were made up to six total puff equivalents (TPE), where TPE are the number of puffs fully dissolved in 1 mL of culture medium. For the ice bath method, 12 puffs were collected in 2 mL of medium, while in the dry ice bath method, 24 puffs were collected into 4 mL of medium.
Culturing hPF, A549 and hESC
hPF were chosen as a differentiated adult lung cell that is often more sensitive to e-cigarette products than lung epithelium (unpublished data). hPF (ScienCell, Carlsbad, California, USA) were cultured on poly-L-lysine-coated flasks and dishes using the manufacturer’s protocol in complete fibroblast medium containing 2% fetal bovine serum, 1% fibroblast growth serum and 1% penicillin/streptomycin.18 In experiments, hPF were dispersed into single cells and plated at a density of 4000 cells/0.32 cm2 using a BioMate 3S Spectrophotometer (Thermo Fisher Scientific, Chino, California, USA)-based standard curve.
A549 CCL-185 cells (ATCC, Manassas, Virginia, USA), a line of lung epithelial cells often used in toxicological testing, were cultured using the distributors’ protocol in ATCC F-12K Medium and 10% fetal bovine serum on tissue culture flasks. In experiments, cells at 80% confluency were rinsed in 0.25% trypsin and plated as single cells at a density of 50 000 cells/ 0.32 cm2 using a BioMate 3S Spectrophotometer-based standard curve.
hESC were used as a model for early postimplantation human embryos. hESC (H9) (WiCell, Madison, Wisconsin, USA) were cultured on Matrigel in mTeSR1 medium in six-well plates.23,24 For experiments, wells at 60–80% confluency were washed with Dulbecco’s phosphate-buffered saline, and cells were enzymatically detached using Accutase (eBioscience, San Diego, California, USA). Large cell clumps were mechanically dispersed with sterile glass beads to form small colonies of 2–10 cells. For MTT experiments, cell concentration was adjusted using a BioMate 3S Spectrophotometer to produce 40 000 cells/ 0.32 cm2.23,25
Cytotoxicity in the MTT assay
Dose–response experiments using the MTT assay were performed using aerosols made from Cinnamon Ceylon refill fluid and a laboratory-made refill fluid containing cinnamaldehyde plus propylene glycol. Cinnamon Ceylon was used as a representative cinnamon flavour with high toxicity. Aerosols were tested at 0.06, 0.2, 0.6, 2 and 6 TPE. Cells were seeded in 96-well plates containing control wells, vapour effect control wells (wells adjacent to the highest concentration to ensure vapours did not affect neighbouring wells) and treatment wells.25 MTT reagent was added after 48 hours of exposure, and 2 hours later MTT solution was added to the medium. Formazan crystals were solubilised in dimethyl sulfoxide, and absorbance was read at 570 nm. For each variable tested, three independent experiments were performed.
Recovery experiments
The hPF and hESC were plated in wells containing medium or medium with cinnamaldehyde (MTT IC50 concentration). Periodically a well containing medium with cinnamaldehyde was washed with PBS, and fresh medium without cinnamaldehyde was added. This procedure was repeated hourly for 3 hours for hPF and every 2 hours for 8 hours for hESC. Cells were imaged after 24 and 48 hours to observe recovery. For each cell type, three independent experiments were performed.
Effect on cytoskeleton
The hPF and hESC were plated in chamber slides for 40 hours and then treated for 2 hours at control, MTT NOAEL and MTT IC50 concentrations. After treatment, cells were fixed with 4% paraformaldehyde and blocked in goat serum for 30 min at room temperature. Cells were labelled using phalloidin-Alexa 488 and a β-tubulin TRITC-conjugated antibody. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). Phase and fluorescent images were taken on a Nikon Eclipse inverted microscope. Four independent labelling experiments were performed.
Live cell imaging assay
When hESC cultures reach 80% confluency, colonies were passaged using ReLeSR (StemCell Technologies, Vancouver, Canada). In total, 600 colonies containing 10–20 cells were allowed to attach overnight. Colonies were treated with 7.6×10−6 M of cinnamaldehyde. Time-lapse phase contrast images of control and treated cells were taken every hour for 70 hours in a Nikon BioStation CT using 3×3 tiling. Videos were generated and analysed using StemCellQC video bioinformatics software.26 Totally, 10–15 colonies/group were analysed in three independent experiments.
Alkaline comet assay
Comet assays were performed to determine if cinnamaldehyde induced strand breaks in DNA. A549 cells and hPF were cultured 48 hours then treated for 3 hours using 3×10−6 M cinnamaldehyde (MTT NOAEL concentration for hPF).18 One group of hESC were treated with the MTT NOAEL concentration for 3 hours, while a second treated group was allowed to recover for 24 hours after treatment. Cells were harvested, suspended in agarose, lysed, subjected to alkaline electrophoresis (Trevigen) and stained with SYBR green. Fluorescent images were taken using an inverted microscope, and the percentage of cells with comet tails, comet tail length and olive moment (tail length×-fraction of DNA in tail) were determined with CometScore (Sumerduck, Virginia, USA). Single cells from 12 images were used to determine the percentage of cells with comet tails, and 100 cells/group were evaluated to determine comet tail length and olive moment. Three independent experiments were performed with each cell type.
Data analysis
For dose–response experiments, IC50 values were computed with Prism software (GraphPad, San Diego, California, USA) using the log inhibitor vs normalised response-variable slope. An analysis of variance (ANOVA) on three independent experiments of the dose–response MTT and hESC comet assay data were performed using Graph Pad Prism. When significance was found, treated groups were compared to the lowest concentration using Dunnett’s post hoc test, and means were considered significantly different for p<0.05. For the comet assay using hPF, hESC and A549 cells, unpaired one-tailed t-tests were used to compare control to NOAEL test groups. Means were considered significantly different when p<0.05.
RESULTS
Identification and quantification of cinnamaldehyde in refill fluids
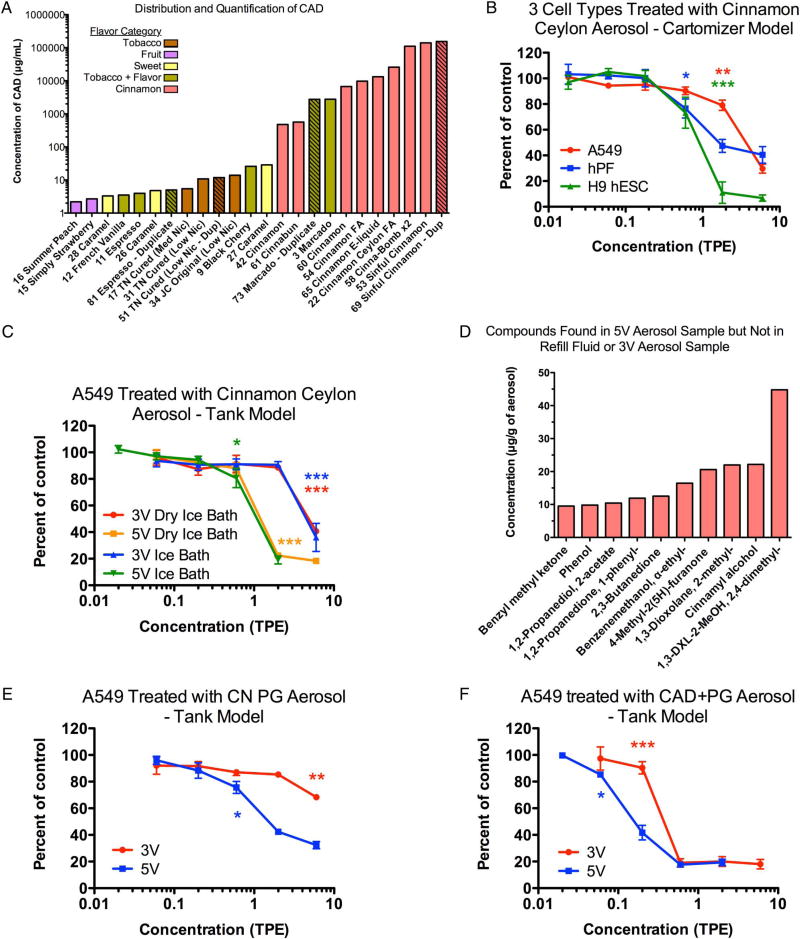
Twenty of 39 refill fluids contained cinnamaldehyde ranging in concentration from 2.2 to 140 000 µg/mL (1.7×10−5 to 1.1 M) (figure 1A; also see online supplementary table S2). All products containing cinnamaldehyde had concentrations that were higher than the lowest observed adverse effect level (LOAEL), and 14 products had concentrations greater than the IC50 value in the MTT assay.18 The general reactivity of cinnamaldehyde complicates its quantitative determination, so the values reported here for cinnamaldehyde are less certain than for more stable compounds. The products containing cinnamaldehyde fell within five flavouring categories: tobacco, fruit, sweet, cinnamon and flavoured tobacco. The flavoured-tobacco category had additional flavours such as fruit or coffee. The concentrations of cinnamaldehyde in four duplicate refill fluid products purchased at different times were similar (figure 1).
Figure 1.
Cinnamaldehyde distribution, quantification and cytotoxicity of Cinnamon Ceylon and cinnamaldehyde aerosols. (A) Distribution and quantification of cinnamaldehyde (CAD) containing refill fluids from a library of 45 samples. Numbers were assigned to each sample when purchased. Duplicate products are crosshatched. Dup, duplicate; FA, flavour art; TN, tennessee; Nic, nicotine. (B) A549 cells, hPF and hESC treated with Cinnamon Ceylon aerosol made using a cartomizer-style e-cigarette. (C) A549 cells treated with Cinnamon Ceylon aerosol from a tank/boxmod e-cigarette at 3 and 5 V using two methods of aerosol collection. (D) Compounds identified in the 5 V aerosol sample of Cinnamon Ceylon that were not in the 3 V aerosol and the refill fluid. The chemical denoted as ‘1,3-DXL-2-MeOH, 2,4-dimethyl-’ is 1,3-dioxolane-2-methanol, 2,4-dimethyl-. (E) A549 cells treated with 80% PG/20% distilled water aerosols made at 3 and 5 V in a tank-style e-cigarette. (F) A549 cells treated with 75% PG/25% cinnamaldehyde aerosols made at 3 and 5 V using a tank-style e-cigarette. Asterisks indicate the LOAEL concentrations that are significantly different from the lowest concentration tested in the concentration range. *p<0.05, **p<0.01, ***p<0.001. Each dose-response curve is the average of three experiments±SEM.
Cytotoxicity of Cinnamon Ceylon and cinnamaldehyde aerosols
Cinnamon Ceylon aerosols produced in the Vea cartomizer-style e-cigarette were cytotoxic in the MTT assay across three cell types (figure 1B) with hESC being more sensitive (IC50=0.862 TPE) than hPF (IC50=2.55 TPE) followed by A549 cells (IC50=3.66 TPE). Aerosols produced in a tank-style variable voltage e-cigarette and collected using either an ice bath or dry ice bath then exposed to A549 cells had similar cytotoxicity. The IC50s ranged from 4.8 to 5.0 TPE for both protocols at 3 V and from 1.1 to 1.3 TPE for both protocols at 5 V (figure 1C). This demonstrates that Cinnamon Ceylon was more cytotoxic at 5 V than at 3 V (figure 1C). The aerosols made with the cartomizer and the tank-style e-cigarettes set at 3 V had similar cytotoxicity (figure 1B, C).
Using GC/MS, 10 chemicals were detected in the 5 V aerosol of Cinnamon Ceylon that were not present in the corresponding aerosol made at 3 V or in the parent refill fluid (figure 1D). Of these, 2,4-dimethyl-1,3-dioxolane-2-methanol was in the highest concentration at 44.8 µg/g of aerosol, and 2,3-butanedione (diacetyl) was detected at a concentration of 12.5 µg/g, as confirmed with an authentic standard.
Laboratory-made refill fluids were tested with A549 cells using 3 and 5 V aerosols of an 80% propylene glycol/20% distilled water refill fluid (IC50=3 V not determinable and 5 V=1.92 TPE) and a 75% propylene glycol/25% cinnamaldehyde refill fluid (IC50 for 3 V=0.389 TPE and 5 V=0.186 TPE) (figure 1E, F). In the propylene glycol control (figure 1E) and in the cinnamaldehyde-containing refill fluid (figure 1F), the 5 V aerosols were more cytotoxic than the corresponding 3 V aerosols. Both aerosols containing cinnamaldehyde (3 and 5 V) were more cytotoxic than the 3 and 5 V propylene glycol aerosols (figure 1E, F).
The following studies were performed at either the MTT IC50 or MTT NOAEL concentrations determined from prior dose–response curves.18 The NOAELs for the hESC and hPF were 7.6×10−6 and 3×10−6 M, respectively, and the IC50s were 4×10−5 and 3.7×10−5 M.
Recoverability of hESC and hPF after short-term exposures to cinnamaldehyde
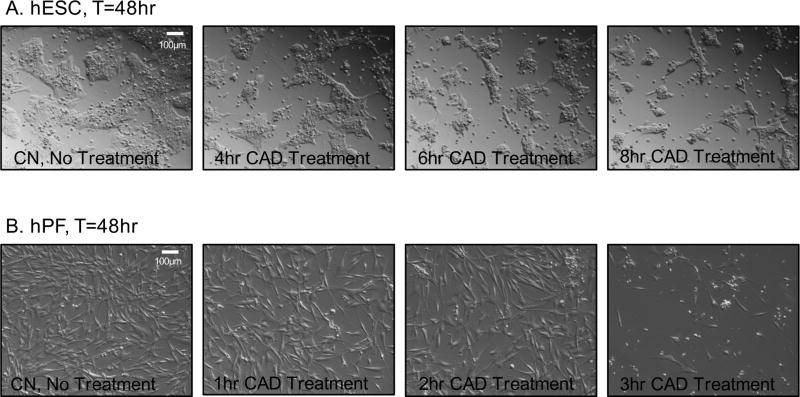
The ability of hESC and hPF to recover from short-term MTT IC50 treatments of cinnamaldehyde was studied using live cell imaging (figure 2A, B). hESC were less sensitive to short-term cinnamaldehyde exposures than hPF. hESC colonies were able to survive and remain viable after 8 hours of cinnamaldehyde exposure followed by 40 hours of recovery with cinnamaldehyde-free medium (figure 2A). In contrast, hPF were unable to recover after 3 hours of cinnamaldehyde treatment followed by 45 hours of recovery in cinnamaldehyde-free medium (figure 2B). The hPF treated for 2 hours recovered, while most hPF treated with cinnamaldehyde for 3 hours became round or died.
Figure 2.
Effect of MTT IC50 concentration of cinnamaldehyde on survival of hESC and hPF after short-term exposure. (A) hESC recovered from 8 hours of exposure to cinnamaldehyde (CAD) which was removed and replaced with fresh medium every 2 hours for 8 hours. Images were taken at 10× after 48 hours to allow cell recovery following cinnamaldehyde exposure. (B) hPF recovered from 2 hours of exposure to cinnamaldehyde which was removed and replaced with fresh medium every 2 hours for 3 hours. Images were taken at 10× after 48 hours to allow cell recovery following cinnamaldehyde exposure.
Effect of cinnamaldehyde on the cytoskeleton
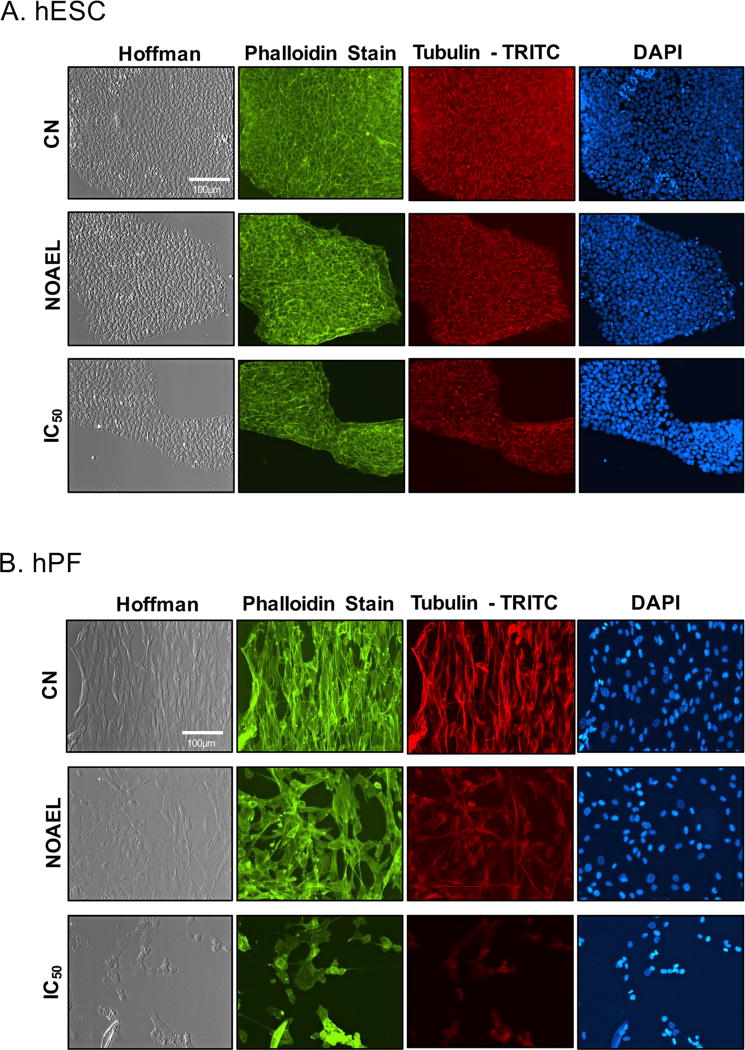
Since cinnamaldehyde treatment caused rounding of hPF, the effect of cinnamaldehyde on actin microfilaments and microtubules was examined at the MTT NOAEL and IC50 concentrations determined previously.18 In treated hESC, F-actin was intact with hot spots due to contraction or depolymerisation of microfilaments, while microtubules appeared unchanged (figure 3A). hPF treated with cinnamaldehyde rounded up, and microtubules, which appeared more sensitive than the microfilaments, depolymerised as cinnamaldehyde concentration increased (figure 3B). In hESC and hPF, nuclei were smaller and brighter in cinnamaldehyde treatments (figure 3A, B).
Figure 3.
Cinnamaldehyde altered morphology and depolymerised microtubules in hPF. (A) hESC treated with cinnamaldehyde at the MTT IC50 and MTT NOAEL concentrations and stained for actin (phalloidin), tubulin (β-tubulin conjugate) and DNA (DAPI). (B) hPF treated with cinnamaldehyde at MTT IC50 and NOAEL concentrations and stained for actin (phalloidin), tubulin (β-tubulin conjugate) and DNA (DAPI).
Live cell imaging assay
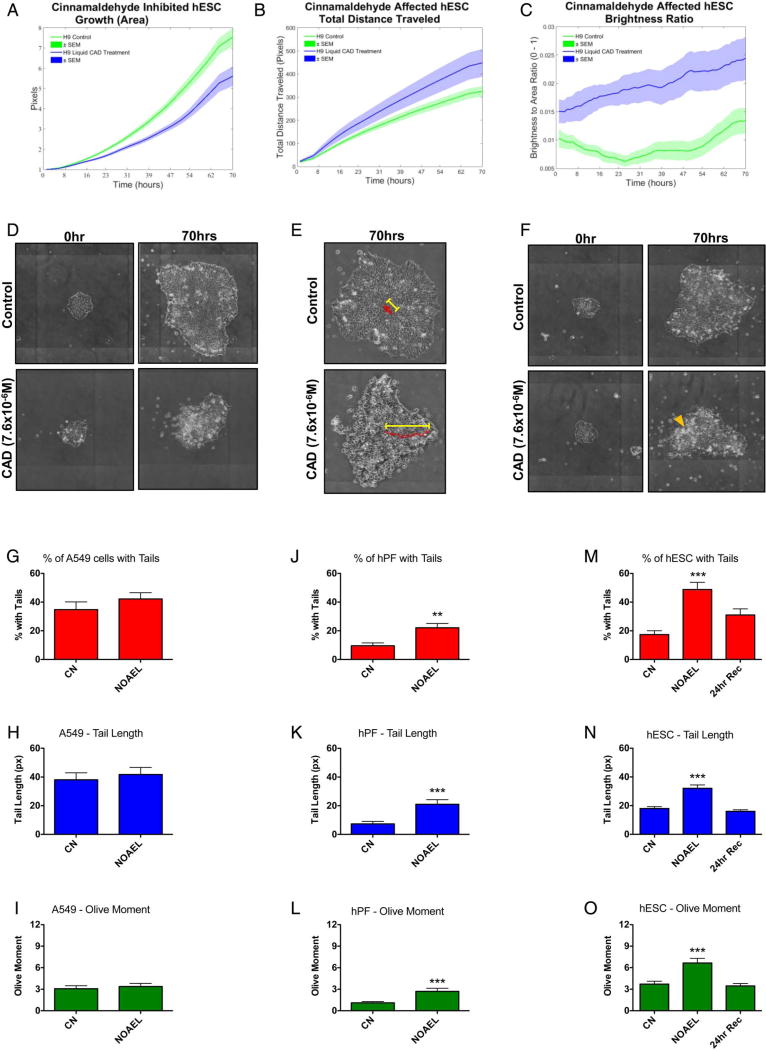
hESC were exposed to the MTT NOAEL concentration of cinnamaldehyde and imaged every hour for 70 hours. StemCellQC analysis of time-lapse videos showed that cinnamaldehyde treatment inhibited growth but increased total distance travelled by colonies and cell death (figure 4A – F). Brightness to total area ratio was higher in the cinnamaldehyde-treated group than the control at the beginning of the experiment because colonies were treated with cinnamaldehyde for 2 hours before imaging began.
Figure 4.
The effect of the MTT NOAEL concentration of cinnamaldehyde on growth, motility, apoptosis and DNA damage. StemCellQC software analysis of time-lapse videos of control and cinnamaldehyde (CAD)-treated colonies showing effects on (A) growth (area), (B) motility (total distance travelled) and (C) apoptosis (brightness ratio). Phase contrast images showing the effect of cinnamaldehyde on growth (D), motility (E) and apoptosis (F). Yellow arrowhead (F) indicates cell debris/dead cells. The alkaline comet assay showing the percentage of cells with comet tails, the comet tail length and the olive moment across three cell types. For A549 cells (G–I) and hPF (J–L), the MTT NOAEL concentrations for hPF dose– response curve were used. For hESC (M–O), the MTT NOAEL concentration was used, and additional recovery group was added in which 3 hours after treatment with cinnamaldehyde, medium was replaced with cinnamaldehyde-free medium, and cells were allowed to recover (24-hour Rev). CN, control. NOAEL, no observed adverse effect level. **p<0.01, ***p<0.001. Averages of three experiments+SEM plotted in live cell assays and column graphs.
Genotoxicity of cinnamaldehyde
To test the genotoxicity of cinnamaldehyde at non-cytotoxic concentrations, the comet assay was performed with three cell types at the MTT NOAEL concentration of cinnamaldehyde (figure 4G – O). The percentages of cells with comet tails, comet tail length and olive moment were all significantly increased by cinnamaldehyde treatment and were significantly different from the control for the hPF and hESC. When hESC were allowed to recover for 24 hours after treatment, each parameter approached control values and the significance was lost (figure 4M – O).
DISCUSSION
Cinnamaldehyde is the major chemical in cinnamon-flavoured e-cigarette products, and our current study corroborates the finding that cinnamaldehyde is one of the most cytotoxic flavour chemicals in e-cigarette refill fluids. Cinnamaldehyde, which was present in 51% of the products sampled, was more widely distributed in e-cigarette refill fluids than expected. In addition to cinnamon-flavoured refill fluids, cinnamaldehyde was present in 3 of 7 tobacco flavours, 3 of 10 sweet flavours, 4 of 6 flavoured tobacco products and 2 of 4 fruit flavours. The concentration of cinnamaldehyde in one tobacco product (Marcado) was higher than in two of the cinnamon-flavoured products. Its use in caramel and some fruit flavours was unexpected. Its concentration was very high in most of the cinnamon-flavoured products. However, even the lowest concentration of cinnamaldehyde in Summer Peach (1.7×10−5 M or 2.2 µg/mL) would be toxic in the live cell imaging and comet assays and would show a significant decrease in cell viability in the MTT assay.
While aerosols of Cinnamon Ceylon condensed on ice or dry ice gave equivalent dose–response curves in the MTT assay, capturing aerosol using dry ice has the advantage of being faster. Toxicity increased significantly when Cinnamon Ceylon or cinnamaldehyde aerosol was made in a tank at 5 V rather than 3 V. This was likely due in part to formation of toxicants during heating of propylene glycol at the higher voltage (figure 1E) and/or to a higher concentration of cinnamaldehyde in aerosols made at 5 V. Higher voltages increase formaldehyde levels in aerosols27 and may enable cinnamaldehyde to volatilise more readily. GC/MS revealed 10 new peaks in cinnamaldehyde aerosols made at 5 V, including 2,3-butanedione (diacetyl), which impairs lung function when inhaled.28,29 Sleiman et al30 recently also report the formation of diacetyl in e-cigarette aerosol.
In our prior studies, hESC, which model early postimplantation development, were more sensitive to cinnamon-flavoured refill fluids than differentiated adult cells.17,18 Our current study further shows that hESC were more sensitive than hPF and A549 cells when tested in the 48-hour MTT assay with aerosols of Cinnamon Ceylon. In contrast to data from the MTT assay, hESC tolerated short-term exposure to cinnamaldehyde for a longer time (8 hours) than hPF (2 hours). A similar robustness was seen with the hESC cytoskeleton, which survived short-term exposures to cinnamaldehyde better than the cytoskeleton of hPF. These differences in survival of hESC during short vs long exposures to cinnamaldehyde may be explained by the well-developed defence mechanisms of stem cells,31 which could provide robust short-term protection, but fail when the cellular stress is prolonged.32
Pregnant women often perceive e-cigarettes as safer than conventional cigarettes.33 Although more data are needed on the risk of e-cigarettes to embryos and fetuses, our hESC data suggest that e-cigarette products should be used with caution during pregnancy.
The multiplexing live cell imaging assay detected adverse effects of cinnamaldehyde on cellular processes at concentrations that do not produce an effect in the MTT assay. The reduced colony growth in the cinnamaldehyde group may have been due to decreased proliferation, increased cell death (supported by the brightness/area ratio data) or a combination of these factors. The greater motility in the cinnamaldehyde-treated group likely occurs because the larger control colonies required more energy and coordination for directed movement, and/or because the exposed colonies attempt to evade cinnamaldehyde exposure.26 Similar responses in growth, motility and apoptosis occurred in hESCs treated with sidestream cigarette smoke.26
Concentrations of cinnamaldehyde that were not cytotoxic in the MTT assay increased DNA strand breaks in hPF and hESC, implicating cinnamaldehyde in mutagenicity/genotoxicity. Significant effects did not occur in the A549 cells, perhaps because these cells are less sensitive to cinnamaldehyde. DNA damage was reversible in hESC following 24 hours of incubation in cinnamaldehyde-free medium, suggesting efficient repair of DNA once cinnamaldehyde exposure stops. Our data are in agreement with other reports that cinnamaldehyde induces DNA damage in mammalian cells in vitro and in vivo.33–35 In contrast to our data, genotoxicity was not found in cells exposed to e-cigarette aerosols that were not cinnamon flavoured.36
In summary, cinnamaldehyde was present in 51% of 39 refill fluids at concentrations that would be cytotoxic and genotoxic in multiple assays. Refill fluid flavours containing cinnamaldehyde included tobacco, fruit, sweet, cinnamon and flavoured tobacco. Cinnamon Ceylon or cinnamaldehyde aerosols produced at 5 V were more cytotoxic than those produced at 3 V, and aerosols made with Cinnamon Ceylon contained chemicals not seen in the parent refill fluid or the 3 Vaerosol. The hPF and hESC were able to recover normal morphology and grow after short exposures to cinnamaldehyde with hESC being more robust. MTT IC50 concentrations of cinnamaldehyde caused rounding up of hPF accompanied by depolymerisation of microtubules. In the live cell imaging assay, the MTT NOAEL concentration of cinnamaldehyde reduced growth, increased motility and increased death of treated hESC colonies. MTT NOAEL concentrations of cinnamaldehyde also induced DNA damage in hESC and hPF, an effect that was reversible in hESC. These data support the idea that inhaling heated refill fluids containing cinnamaldehyde adversely affects the health of embryonic and respiratory cells. The relatively widespread use of cinnamaldehyde in refill fluids at concentrations that are toxic in vitro suggests a need for regulation and caution in use of refill fluid products. Additional in vitro exposures using an air–liquid interface model as well as animal and human studies could be performed in the future to verify the responses observed in this study.
Supplementary Material
What this paper adds.
-
▸
Cinnamaldehyde is a GRAS chemical flavouring used in the food industry.
-
▸
Little is known about the toxicity of cinnamaldehyde in e-cigarette aerosols.
-
▸
Cinnamaldehyde was present in about 50% of the refill fluids at concentrations that are cytotoxic in multiple assays.
-
▸
Aerosols produced from refill fluids containing cinnamaldehyde were cytotoxic to human embryonic and adult lung cells.
-
▸
Cinnamaldehyde aerosols were more potent when made at a higher voltage.
-
▸
Cinnamaldehyde produced adverse effects on cell survival, growth, the cytoskeleton, motility and DNA.
Acknowledgments
The authors gratefully acknowledge Antonio Loza for helping with StemCellQC processing and Victor Camberos for making aerosol preparations and GC/MS samples.
Funding This work was supported by grants from the NIH (R01DA036493 and R21DA037365) to PT/JFP and a Core Facility grant from the California Institute of Regenerative Medicine (NE-A0005A-1E) to PT. RZB was supported by an NIH NRSA Individual Predoctoral Fellowship (5F31HL116121-03).
Footnotes
▸ Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/tobaccocontrol-2016-053224).
Contributors PT, JFP and RZB formed the conception and design of this study. Data were collected and interpreted by RZB, WL, SCL, YW, JV, JFP and PT. RZB, WL, SCL, JFP and PT involved in data analysis and writing of the manuscript. RZB, WL, YW and JV performed data processing and sample preparation.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All data will be provided by request to the senior author.
References
- 1.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2011;20:47–52. doi: 10.1136/tc.2010.037259. [DOI] [PubMed] [Google Scholar]
- 2.Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res. 2010;12:905–12. doi: 10.1093/ntr/ntq114. [DOI] [PubMed] [Google Scholar]
- 3.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(Suppl 3):ii3–9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutzler C, Paschke M, Kruschinski S, et al. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88:1295–308. doi: 10.1007/s00204-014-1294-7. [DOI] [PubMed] [Google Scholar]
- 5.Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, et al. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol. 2015;39:262–9. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 6.Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71:376–7. doi: 10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tierney PA, Karpinski CD, Brown JE, et al. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25:e10–15. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JG, Flanigan SS, LeBlanc M, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124:733–9. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallagan J. Safety assessment and regulatory authority to use flavors: focus on e-cigarettes. 2015 https://www.femaflavor.org/safety-assessment-and-regulatory-authority-use-flavors-focus-e-cigarettes.
- 10.Farsalinos KE, Kistler KA, Gillman G, et al. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17:168–74. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanawal R, Kullman G, Kreiss K, et al. Preventing lung disease in workers who use or make flavorings. 2003 http://www.cdc.gov/niosh/docs/2004-110/pdfs/2004-110.pdf.
- 12.Hubbs AF, Cumpston AM, Goldsmith WT, et al. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am J Pathol. 2012;181:829–44. doi: 10.1016/j.ajpath.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanwal R, Kullman G, Piacitelli C, et al. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup Environ Med. 2006;48:149–57. doi: 10.1097/01.jom.0000194152.48728.fb. [DOI] [PubMed] [Google Scholar]
- 14.Ulker Z, Alpsoy L, Mihmanli A. Assessment of cytotoxic and apoptotic effects of benzaldehyde using different assays. Hum Exp Toxicol. 2013;32:858–64. doi: 10.1177/0960327112470271. [DOI] [PubMed] [Google Scholar]
- 15.Romagna G, Allifranchini E, Bocchietto E, et al. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25:354–61. doi: 10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 16.Farsalinos KE, Romagna G, Allifranchini E, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10:5146–62. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahl V, Lin S, Xu N, et al. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–37. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Behar RZ, Davis B, Wang Y, et al. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28:198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Brown JE, Luo W, Isabelle LM, et al. Candy flavorings in tobacco. N Engl J Med. 2014;370:2250–2. doi: 10.1056/NEJMc1403015. [DOI] [PubMed] [Google Scholar]
- 20.Knoll M, Shaoulian R, Magers T, et al. Ciliary beat frequency of hamster oviducts is decreased in vitro by exposure to solutions of mainstream and sidestream cigarette smoke. Biol Reprod. 1995;53:29–37. doi: 10.1095/biolreprod53.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Knoll M, Talbot P. Cigarette smoke inhibits oocyte cumulus complex pick-up by the oviduct in vitro independent of ciliary beat frequency. Reprod Toxicol. 1998;12:57–68. doi: 10.1016/s0890-6238(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 22.Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22:103–6. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 23.Behar RZ, Bahl V, Wang Y, et al. Adaptation of stem cells to 96-well plate assays: use of human embryonic and mouse neural stem cells in the MTT assay. Curr Protoc Stem Cell Biol Published Online First. 2012 Nov 1; doi: 10.1002/9780470151808.sc01c13s23. [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Talbot P. Methods for culturing mouse and human embryonic stem cells. Methods Mol Biol. 2011;690:31–56. doi: 10.1007/978-1-60761-962-8_2. [DOI] [PubMed] [Google Scholar]
- 25.Behar RZ, Bahl V, Wang Y, et al. A method for rapid dose-response screening of environmental chemicals using human embryonic stem cells. J Pharmacol Toxicol Methods. 2012;66:238–45. doi: 10.1016/j.vascn.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Zahedi A, On V, Lin SC, et al. Evaluating cell processes, quality, and biomarkers in pluripotent stem cells using video bioinformatics. PLoS One. 2016;11:e0148642. doi: 10.1371/journal.pone.0148642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen RP, Luo W, Pankow JF, et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372:392–4. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 28.Hubbs AF, Battelli LA, Goldsmith WT, et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharmacol. 2002;185:128–35. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- 29.Hubbs AF, Goldsmith WT, Kashon ML, et al. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol Pathol. 2008;36:330–44. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- 30.Sleiman M, Logue JM, Montesinos VN, et al. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol Published Online First. 2016 Jul 27; doi: 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- 31.Saretzki G, Walter T, Atkinson S, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–64. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 32.Guo L, Li L, Wang W, et al. Mitochondrial reactive oxygen species mediates nicotine-induced hypoxia-inducible factor-1α expression in human non-small cell lung cancer cells. Biochim Biophys Acta. 2012;1822:852–61. doi: 10.1016/j.bbadis.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 33.King AA, Shaughnessy DT, Mure K, et al. Antimutagenicity of cinnamaldehyde and vanillin in human cells: global gene expression and possible role of DNA damage and repair. Mutat Res. 2007;616:60–9. doi: 10.1016/j.mrfmmm.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mereto E, Brambilla-Campart G, Ghia M, et al. Cinnamaldehyde-induced micronuclei in rodent liver. Mutat Res. 1994;322:1–8. doi: 10.1016/0165-1218(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 35.Neudecker T. The genetic toxicology of cinnamaldehyde. Mutat Res. 1992;277:173–85. doi: 10.1016/0165-1110(92)90042-8. [DOI] [PubMed] [Google Scholar]
- 36.Misra M, Leverette RD, Cooper BT, et al. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health. 2014;11:11325–47. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.