Abstract
Robot assisted thyroid surgery has the advantage of a superior field vision and technical advancements of robotic technology that have permitted novel remote access thyroid surgical approaches. Gasless trans-axillary robot-assisted thyroidectomy has been proved to be among the most current feasible approaches. This approach offers an excellent cosmetic outcome, with comparable outcomes to conventional surgical approaches. This review aims to provide details of this specific remote access technique for thyroid resection with most recent evidences in the literature.
Keywords: Robotic-assisted, gasless trans-axillary, remote access, thyroidectomy, da Vinci
Introduction
Conventional open thyroidectomy has been the standard of care for thyroid surgery for decades. However, the visible neck scar is undesirable for many patients especially young patients with history of healing with keloid. With technical advancement, thyroid procedures transitioned from conventional to video-assisted thyroidectomy and, lately, to robot-assisted approaches for better cosmetic outcome. Before the era of robot, endoscopic remote access thyroidectomy was associated with several technical drawbacks in its implementation (1,2). In early 2000s, after lunching the da Vinci robotic system (Intuitive Surgical, Inc., Sunnyvale, CA, USA), Surgical robots have been increasingly used because of their greater image, fine articulation and stable operative view by hand-tremor filtration technology (3,4).
The gasless surgical robotic system has emerged as a new remote access surgery in which this new technique provides an adequate stereo-optic three-dimensional view of the operative field, and decreases complications associated with gas insufflation previously used in other endoscopic approaches, such as hypercapnia, respiratory acidosis, and air embolism (5).
Equipment
It is vital to have all the required equipment prior to the implementation of the technique in clinical practice. Equipment used in this procedure are listed in Table 1.
Table 1. The procedure equipment.
| Flab creation set |
| #15-scalpel blade |
| Adson tissue forceps with teeth |
| Long DeBakey forceps |
| Army-Navy/Richardson retractors |
| Chung retractor or Modified thyroidectomy retractor (Marina Medical, Sunrise, FL, USA) |
| Fiber-optic mammary retractors |
| Bovie electrocautery with short and long tip extension |
| Vessel sealing device |
| Endoscopic suction/irrigation device |
| Surgical sleds and foams |
| Nerve monitoring leads and surface electrode primed endotracheal tube |
| Extended nerve stimulator tip |
| Robotic part set |
| The da Vinci S, Si, or Xi system (Intuitive Surgical Inc., Sunnyvale, CA, USA) |
| 30° dual-channel endoscope (Intuitive Surgical, Sunnyvale, CA, USA) |
| Prograsper (Intuitive Surgical, Sunnyvale, CA, USA) |
| Maryland dissector (Intuitive Surgical, Sunnyvale, CA, USA) |
| Harmonic curved shears (Ethicon, Cincinnati, OH, USA) |
The technique
Positioning
The patient is laid on the operating room (OR) table in a supine positioning. Following intubation, hyperextension of the neck is achieved by use of shoulder roll underneath patients upper back. First, the contralateral arm is padded and tucked to the side of the patient. The arm ipsilateral to the axillary incision is raised, so that the shoulder is internally rotated and the elbow is flexed and forearm placed over the forehead with proper padding (Figure 1). We routinely perform nerve monitoring for median, radial and ulnar nerves to avoid brachial plexus injury by using somatosensory evoked potential (SSEP).
Figure 1.
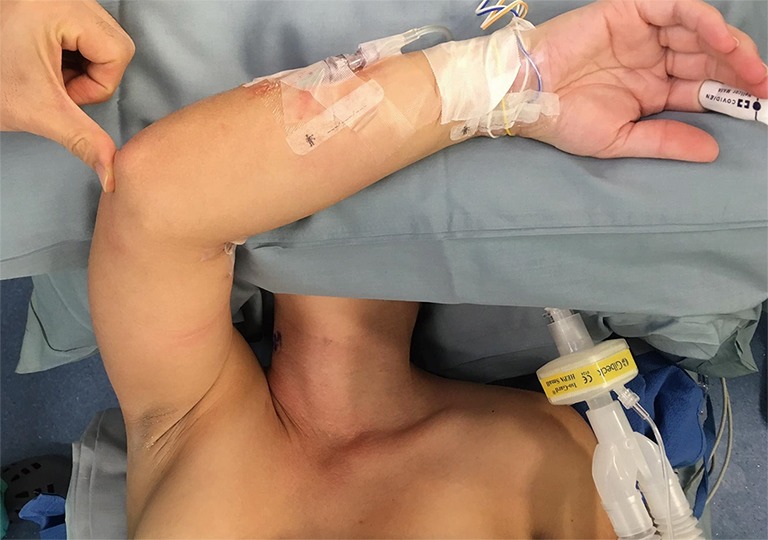
Forearm placement and proper padding (SSEP electrodes attached). SSEP, somatosensory evoked potential.
Landmarks
Two lines are drawn to mark the inferior and superior limits of the axillary incision; the first one is made horizontally from the suprasternal notch toward the axilla. The second line is made obliquely from thyroid cartilage toward the axilla. Approximately, a 2-inch longitudinal mark is made between the two lines; along the lateral border of pectoralis major muscle (Figure 2).
Figure 2.
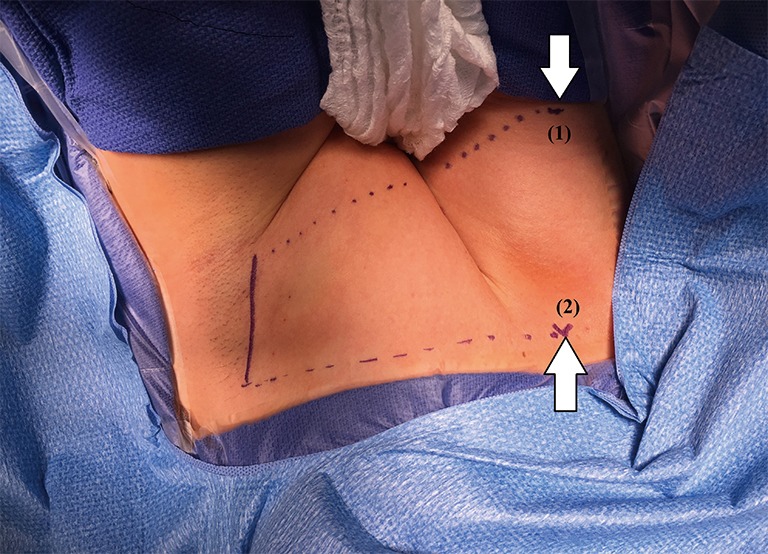
Landmarks: (1) thyroid cartilage, (2) suprasternal notch.
Flap creation
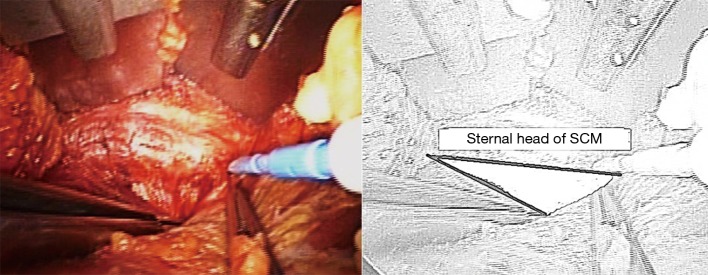
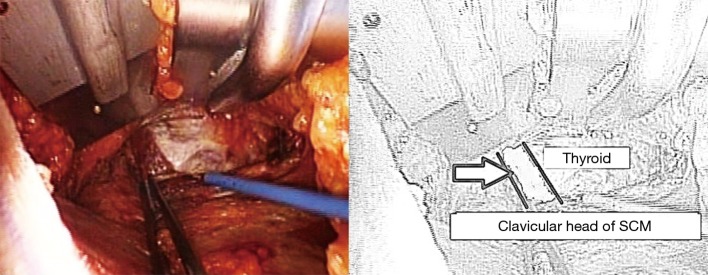
The dissection starts through the subcutaneous tissue to expose the lateral border of the pectoralis major muscle. The flap is created superficial to the pectoralis fascia. As dissection advances, electrocautery with long tip extension and lighted mammary retractors are used to facilitate flap creation until the clavicle is identified and followed medially till the sternal notch. The platysma is then identified in the neck and subplatysmal dissection continues, which eventually leads to the identification of the sternocleidomastoid (SCM). The avascular plane between the sternal and clavicular heads of the SCM is then identified and a plane between the two heads of SCM is developed (Figure 3). While the sternal head of SCM is retracted anteriorly, omohyoid muscle identification is crucial since it’s a good landmark for the superior pole of the thyroid gland (Figure 4). Cephalad retraction or division of the omohyoid muscle, leads to exposure of the upper thyroid pole. Careful avoidance of internal jaguar vein injury is essential during this part of the dissection. The ipsilateral thyroid lobe and lateral border of strap muscles are exposed, and the strap muscles are dissected off the thyroid using electrocautery or vessel sealing device. The dissection is continued until the contralateral thyroid lobe is visualized.
Figure 3.
The plane between the sternal and clavicular heads of the SCM (shaded space in the illustration). SCM, sternocleidomastoid.
Figure 4.
Identification of omohyoid muscle (arrowed in the illustration). SCM, sternocleidomastoid.
Retractor placement
A special self-retaining bladed thyroid retractor; Chung retractor or modified thyroidectomy retractor (Marina Medical, Sunrise, USA) is used. It is mounted at the contralateral side of the bed and deployed below the sternal head of the SCM and strap muscles to maintain working space (Figure 5). This retractor should be connected to a suction tube to remove the smoke from the sealing devices used during the procedure (Figure 6).
Figure 5.
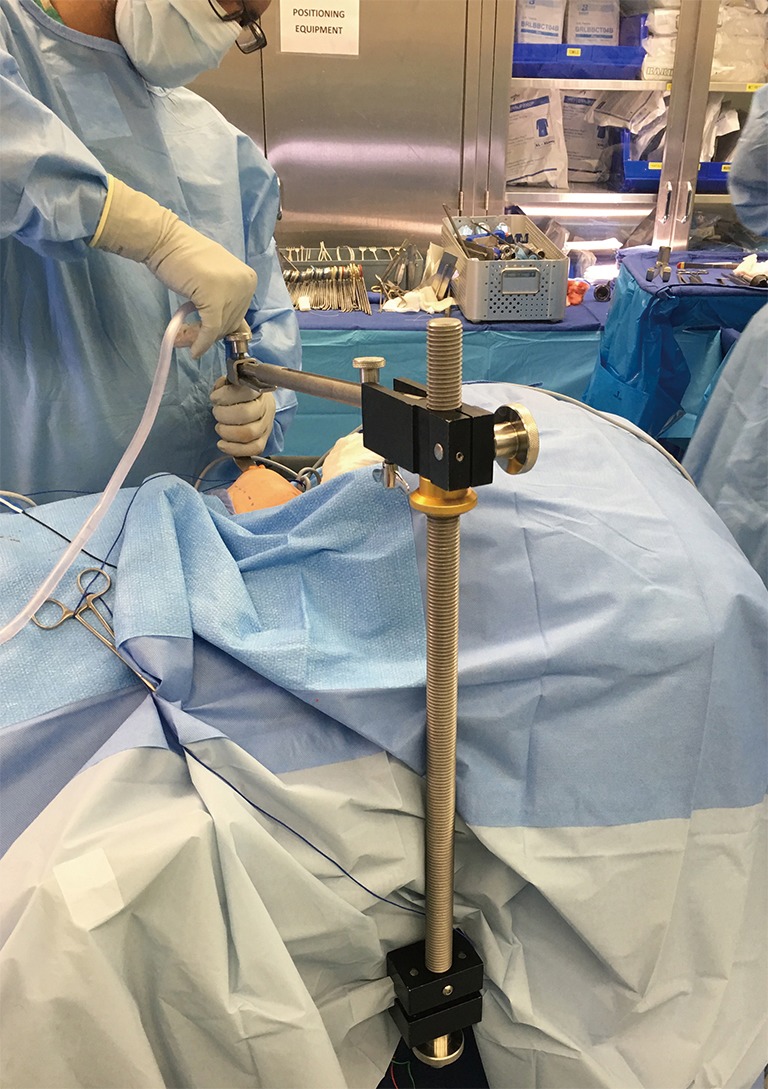
Chung or modified thyroidectomy retractor is mounted at the contralateral side of the bed.
Figure 6.
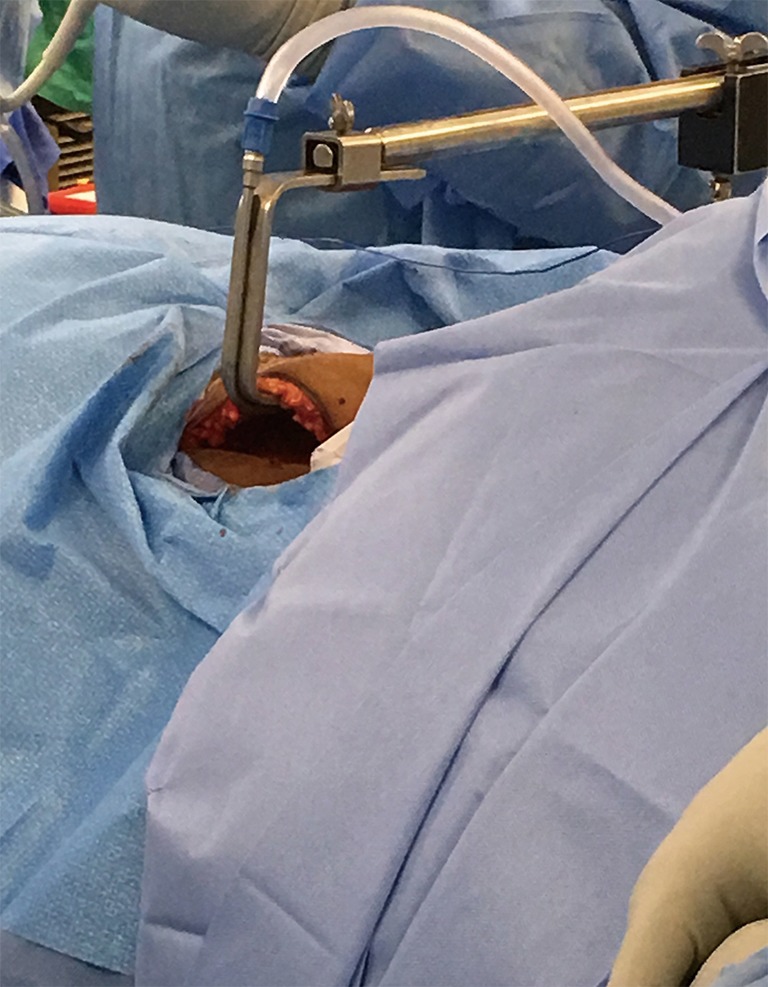
The retractor is connected to a suction tube.
Robot docking
The robot is docked from the contralateral side of the lesion. The instruments are deployed, with the dual-channel camera being placed and docked first; at the center of the lower edge of the incision (Figure 7). After docking the camera arm, instrument arms are positioned in place; to allow maximum range of motion of the arms and avoid collision. The sealing device, ideally the harmonic scalpel in our practice, is placed on the lateral edge of the incision; at the surgeon’s dominant side. The Maryland dissector on the opposite lateral edge of the incision, and the Prograsper on the upper central edge of the incision to the same side of the lesion in relation to the retractor blade (e.g., to the right of the retractor blade in case of right approach).
Figure 7.
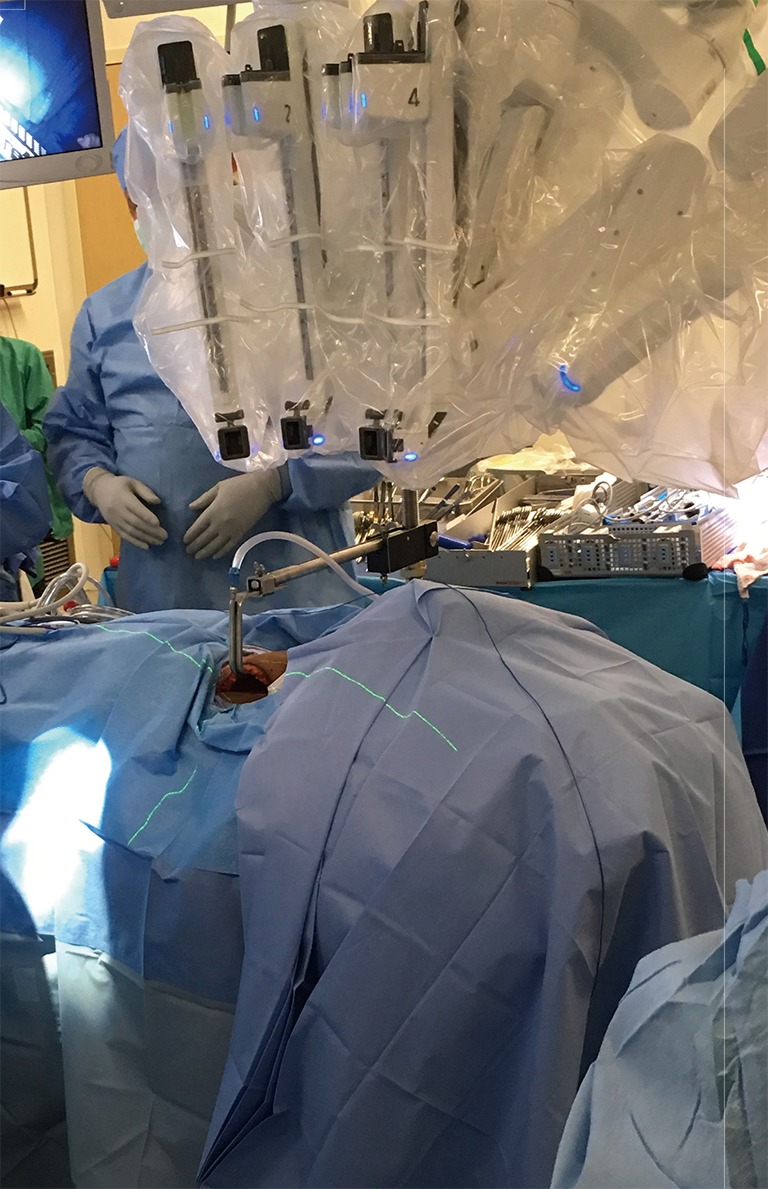
Robot docking.
Console time
The assistant surgeon rule is vital, due to the need of introduction of the laparoscopic suction/irrigation device through the axillary incision, which is used to retract the clavicular head of the SCM or trachea downward during the dissection in addition to placement of the nerve monitor probe and troubleshoots robotic arm positioning. The carotid sheath is then exposed, and the assistant surgeon then introduces a nerve monitor probe into the field for initial stimulation of the vagus nerve. In some cases where bilateral or central lymph node dissection is planned, we elect to choose the placement of an electrode on the vagus nerve for continuous nerve monitoring.
The Prograsper is used to medially and inferiorly retract the upper pole of thyroid. The Harmonic and Maryland dissector are then used to dissect and divide the superior thyroid vessels close to the thyroid; in order to avoid injury of the external branch of the superior laryngeal nerve. The upper pole is freed from the cricothyroid muscle with further dissection. Identification and preservation of the superior parathyroid gland is carried out. Then the Prograsper is repositioned to retract the thyroid medially, and the middle thyroid vein is dissected and divided using Harmonic. The recurrent laryngeal nerve (RLN) is then identified at the tracheoesophageal groove after meticulous dissection and is carefully followed until its insertion into the cricothyroid muscle (Figure 8). Surgeon should always be aware of the non-RLN variation in dissection of the right tracheoesophageal groove, but this should be predicted with short latency from the vagal nerve stimulation. The nerve’s functional integrity is confirmed with use of the nerve monitor probe. The inferior pedicle is then dissected and divided using the Maryland and Harmonic, and the inferior parathyroid gland is bluntly separated away from the thyroid tissue. Following that the thyroid is dissected carefully medial to the RLN. Then it is shaved from the trachea until reaching the contra lateral side. The thyroid lobe and isthmus are divided from the remaining thyroid lobe and the specimen is extracted through the axillary incision. If central lymph node dissection is planned, the central lymph nodes are dissected enblock circumferentially from the nerve and removed enblock with the thymus and the thyroid.
Figure 8.
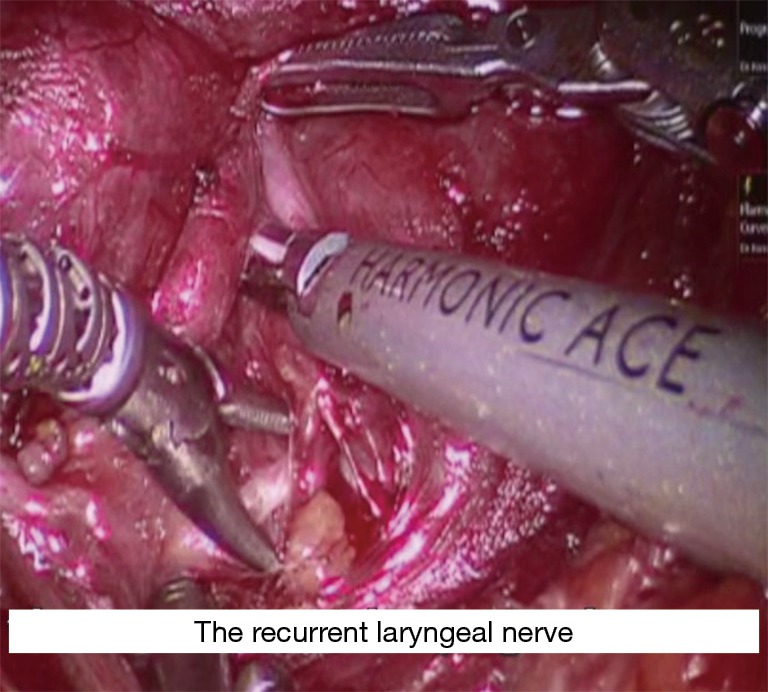
Identification of RLN. RLN, recurrent laryngeal nerve.
If total thyroidectomy is planned, after removing the ipsilateral lobe subcapsular dissection of the contralateral lobe from the trachea is performed until reaching the contralateral tracheoesophageal groove. Then the RLN is identified and stimulated using the nerve monitor probe. The operating table can be tilted 10–15° to improve the exposure of the tracheoesophageal groove in certain conditions; like prominent trachea or male patients. However, we rarely tilt the table at this stage of our practice. Following that the superior and inferior thyroid pedicles are dissected, RLN is traced to cricothyroid membrane and the remaining thyroid is dissected and extracted through the axillary incision. Final stimulation of the RLN and vagus nerves is performed. At the end, hemostasis is secured, a drain is placed and the incision is closed in two layers; interrupted subcutaneous and continuous subcuticular closure.
Procedure safety
Several studies have addressed the safety of robot-assisted thyroid surgery and found that Robotic approach is as safe and oncologically sufficient as the conventional open approach (6-9). The rates of locoregional recurrence of thyroid cancer on long-term follow-up at 5 years were similar between robotic thyroidectomy and conventional cervical thyroidectomy (1.2% vs. 1.2%) (10).
Benefits and drawbacks
Obviously, the perceptible advantage of robot-assisted transaxillary approach over conventional cervical thyroidectomy is cosmetic by avoiding a visible neck scar. This cosmetic characteristic makes this approach more attractive to young female patients and those with a propensity toward healing with keloid.
The robot-assisted transaxillary approach has several technical advantages when compared to the open and endoscopic approaches. First, the robotic system provides three-dimensional vision of the surgical field with up to 10 times magnification and wristed instrumentation, which empowers identification of the RLN and parathyroid glands and enables a wider range of motion; second, it eliminates the natural surgeon tremor via a distinct software with motion scaling and tremor filtration; and, third, design innovations of Fluorescence Imaging System of the new da Vinci family provides surgeons with real-time visualization and analysis of tissue perfusion. In addition, the improved surgical ergonomics provided by the robot resulted in reduced musculoskeletal discomfort to the surgeon compared to open and endoscopic surgery.
Robot-assisted transaxillary approach was found to yield better patient outcomes, including reduced pain and increased cosmetic satisfaction (11-15), postoperative voice change (16), and swallowing discomfort (11,17).
In addition to that, particularly with elimination of anterior chest wall incision, the single incision robot-assisted approach was found to be technically safe and feasible compared with the two incisions technique (15).
On the contrary, the new approach has few drawbacks; the most evident drawback is the longer operative time in comparison with the conventional open approach. However, it is clearly shown that the time spent performing this approach depends on the surgeon experience and how frequent this procedure done (6,7). There is clear evidence for gradual decrease of operation times, reaching a plateau after 20 robotic less-than-total thyroidectomies for surgeons with little or no experience in endoscopic surgery (18). Robot-Assisted Transaxillary Approach introduces potential new complications such as chest paresthesia due to the extensive skin flap dissection (19). Tracheal and esophageal injuries have been reported but these are complications that are also reported with conventional thyroid surgery (6). In addition, due to the ipsilateral arm position, there is a risk of brachial plexus neuropathy. This risk can be reduced by avoiding overextension of the shoulder and placing the arm in a flexed overhead position, thereby reducing the chance of stretching the nerves. Intra-operative monitoring of the ulnar, radial, and median nerves may further reduce the possibility of brachial plexus injury, by identification of any impending damage to these nerves and enabling the patient to be repositioned (7,20).
Contraindications
On initial implementation of this approach, it has been suggested that patients should be screened for contraindications such as rotator cuff issues, shoulder/neck mobility problems, cervical spine disease, previous neck, chest wall or axillary surgery, and possible complicating conditions like obesity, certain thyroid pathologies such as locally advanced cancers, Graves’ disease, Hashimoto’s thyroiditis, or substernal goiter (21). However currently and with gaining the experience over time, most of those that used to be contraindications are not contraindications any more (22). Here we stratify the contradictions to absolute and relative ones (Table 2). Most of our patients are young female patients with breast implants who are concerned about cosmetic outcomes and we never encountered any problems with performing this procedure on patients with breast implants. Additionally, we routinely perform this procedure on patients with Hashimoto’s thyroiditis and Graves’ disease.
Table 2. Contraindications.
| Absolute contraindications | Relative contraindications |
|---|---|
| Previous neck surgery | Limitation of shoulder or neck mobility |
| Substernal goiter | Thyroid nodule >4 cm |
| Advanced thyroid cancer (invasion of adjacent structures, or metastasis to retropharyngeal or substernal lymph nodes) | Morbid obesity |
| Medullary or anaplastic thyroid cancer | Graves’ disease with substernal extension |
Conclusions
Gasless trans-axillary robot-assisted thyroid surgery is a safe and feasible option in a wide range of patients. Proficiency can be obtained during a short period, leading to low conversion and complication rates. After all, we recommend a team approach of more than one experienced surgeon participating during the learning phase and a dedicated OR team for incorporating this technique.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lang BH. Minimally invasive thyroid and parathyroid operations: surgical techniques and pearls. Adv Surg 2010;44:185-98. 10.1016/j.yasu.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 2.Linos D. Minimally invasive thyroidectomy: a comprehensive appraisal of existing techniques. Surgery 2011;150:17-24. 10.1016/j.surg.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 3.Tanna N, Joshi AS, Glade RS, et al. Da Vinci robot-assisted endocrine surgery: novel applications in otolaryngology. Otolaryngol Head Neck Surg 2006;135:633-5. 10.1016/j.otohns.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Hinson AM, Kandil E, O'Brien S, et al. Trends in Robotic Thyroid Surgery in the United States from 2009 Through 2013. Thyroid 2015;25:919-26. 10.1089/thy.2015.0066 [DOI] [PubMed] [Google Scholar]
- 5.Kang SW, Jeong JJ, Yun JS, et al. Gasless endoscopic thyroidectomy using trans-axillary approach: surgical outcome of 581 patients. Endocr J 2009;56:361-9. 10.1507/endocrj.K08E-306 [DOI] [PubMed] [Google Scholar]
- 6.Jackson NR, Yao L, Tufano RP, et al. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck 2014;36:137-43. 10.1002/hed.23223 [DOI] [PubMed] [Google Scholar]
- 7.Kandil E, Hammad AY, Walvekar RR, et al. Robotic Thyroidectomy Versus Nonrobotic Approaches: A Meta-Analysis Examining Surgical Outcomes. Surg Innov 2016;23:317-25. 10.1177/1553350615613451 [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Kwon IS, Bae EH, et al. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab 2013;98:2701-8. 10.1210/jc.2013-1583 [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. 10.1007/s00464-010-1113-z [DOI] [PubMed] [Google Scholar]
- 10.Lee SG, Lee J, Kim MJ, et al. Long-term oncologic outcome of robotic versus open total thyroidectomy in PTC: a case-matched retrospective study. Surg Endosc 2016;30:3474-9. 10.1007/s00464-015-4632-9 [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Lee J, Hakim NA, et al. Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck 2015;37:1705-11. 10.1002/hed.23824 [DOI] [PubMed] [Google Scholar]
- 12.Son SK, Kim JH, Bae JS, et al. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:3022-32. 10.1245/s10434-015-4375-9 [DOI] [PubMed] [Google Scholar]
- 13.Song CM, Ji YB, Bang HS, et al. Long-term sensory disturbance and discomfort after robotic thyroidectomy. World J Surg 2014;38:1743-8. 10.1007/s00268-014-2456-8 [DOI] [PubMed] [Google Scholar]
- 14.Kandil EH, Noureldine SI, Yao L, et al. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 2012;214:558-64; discussion 564-6. 10.1016/j.jamcollsurg.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Ryu HR, Kang SW, Lee SH, et al. Feasibility and safety of a new robotic thyroidectomy through a gasless, transaxillary single-incision approach. J Am Coll Surg 2010;211:e13-9. 10.1016/j.jamcollsurg.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 16.Song CM, Yun BR, Ji YB, et al. Long-Term Voice Outcomes After Robotic Thyroidectomy. World J Surg 2016;40:110-6. 10.1007/s00268-015-3264-5 [DOI] [PubMed] [Google Scholar]
- 17.Tae K, Ji YB, Jeong JH, et al. Robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach: our early experiences. Surg Endosc 2011;25:221-8. 10.1007/s00464-010-1163-2 [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Kim HY, Lee CR, et al. A prospective comparison of patient body image after robotic thyroidectomy and conventional open thyroidectomy in patients with papillary thyroid carcinoma. Surgery 2014;156:117-25. 10.1016/j.surg.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 19.Wang YC, Liu K, Xiong JJ, et al. Robotic thyroidectomy versus conventional open thyroidectomy for differentiated thyroid cancer: meta-analysis. J Laryngol Otol 2015;129:558-67. 10.1017/S002221511500122X [DOI] [PubMed] [Google Scholar]
- 20.Davis SF, Abdel Khalek M, Giles J, et al. Detection and prevention of impending brachial plexus injury secondary to arm positioning using ulnar nerve somatosensory evoked potentials during transaxillary approach for thyroid lobectomy. Am J Electroneurodiagnostic Technol 2011;51:274-9. [PubMed] [Google Scholar]
- 21.Perrier ND, Randolph GW, Inabnet WB, et al. Robotic thyroidectomy: a framework for new technology assessment and safe implementation. Thyroid 2010;20:1327-32. 10.1089/thy.2010.1666 [DOI] [PubMed] [Google Scholar]
- 22.Lee YM, Yi O, Sung TY, et al. Surgical outcomes of robotic thyroid surgery using a double incision gasless transaxillary approach: analysis of 400 cases treated by the same surgeon. Head Neck 2014;36:1413-9. [DOI] [PubMed] [Google Scholar]