Abstract
With the advancement and adaptation of technology, there has been a tremendous evolution in the surgical approaches for thyroidectomy. Robotic thyroidectomy has become increasingly popular worldwide attracting both surgeons and patients searching for new and innovative techniques for thyroidectomy with a superior cosmetic result when compared to the conventional open procedures. In this review, we describe the following surgical approaches for robotic thyroidectomy: transaxillary, retroauricular (facelift) and transoral. The advantages and disadvantages as well as limitations of each approach are examined, and future directions of robotic thyroidectomy are discussed.
Keywords: Thyroidectomy, robotic, minimally invasive, remote-access
Introduction
With the advancement and adaptation of technology, there has been a tremendous evolution in the surgical approaches for thyroidectomy over the past two decades. The first endoscopic thyroidectomy was first performed in 1997, introducing the era of minimally invasive and remote-access approaches for thyroidectomy (1-4). The Da Vinci robotic system (Intuitive Surgical, Sunnyvale, California) was first utilized for transaxillary thyroidectomy by Chung in 2007.
Chung and his colleagues have since performed over 5,000 robotic transaxillary thyroidectomy cases demonstrating the safety and feasibility of the robotic procedure for thyroidectomy. Using a robotic system helped to overcome some of the limitations of the endoscopic procedures such as reduced range of motion, and impaired eye-hand coordination (while relying on an unstable 2-dimentional view) (3,5-7). Because of this, robotic thyroidectomy has become increasingly popular around the world (8-10) attracting both surgeons and patients searching for new and innovative procedures and allowing for the removal of thyroid glands with a superior cosmetic result (4,6,8) when compared to the conventional open thyroidectomy procedures. Many studies have described the safety of the remote-access robotic thyroidectomy procedures and have demonstrated comparable oncologic outcomes between the robotic and open conventional thyroidectomy (11-14).
Since the initial transaxillary approach, new approaches to robotic thyroidectomy have been developed, including retroauricular (aka “facelift”), axillary-breast, and the latest approach to be described in the literature: transoral (4,12,15). There have also been descriptions of combinations and modifications of these approaches (4). Recently, the American Thyroid Association published a statement indicating that remote-access thyroidectomy may be performed safely in high-volume centers (10). They have acknowledged the role of robotic thyroidectomy in selected patients and emphasized the importance of strict selection criteria (10).
This review describes surgical approaches for robotic thyroidectomy: transaxillary, retroauricular and transoral. A literature review was performed, and the relevant papers were selected and summarized (Tables 1-3). The ad vantages and limitations of each approach and future directions of robotic thyroidectomy are discussed.
Table 1. Summary of selected studies of transaxillary robotic thyroidectomy.
| Author [year] (ref.) | Study design (cases) | Pathology [cases] | Extent of surgery | Operative time (mean total ± SD) | Complication profile [cases] | Hospital stay (median) |
|---|---|---|---|---|---|---|
| Kuppersmith [2011] (16) | Case series | PTC [3], FN [1], benign [27] | HT [20], TT [11] | Range: 132–328 | Transient radial nerve neuropraxia [1], transient vocal cord paresis [1] | 1 |
| Ciabatti [2012] (17) | Case series | PTC [29] | TT [29] | 178.51±24.18 | Transient hoarseness [2] | 3 |
| Foley [2012] (18) | Case series | PTC [4], HCTC [1], benign [6] | HT [5], TT [6] | 232±65.7 | Transient hypocalcemia [1], chest paresthesia [2], shoulder discomfort [1] | |
| Kandil [2012] (19) | Case series | N/A | HT [69], TT/near total [22], completion [9] | HT: 108.1±60.5; TT: 118.1±51.3 | Conversion to open [2], tracheal injury [1], transient hoarseness [7], transient hypocalcemia [10], seroma [4], infection [1], transient radial nerve neuropraxia [1] | 1 |
| Landry [2012] (20) | Case controls | PTC [4], benign [17], uncertain malignant potential [4] | HT [25] | 121 | Hematoma [3], Infection [2], seroma [3], temporary hoarseness [5], brachial plexus injury [2] | 1 |
| Terris [2012] (21) | Case series | PTC [1], benign [4] | HT [5] | 196±38.1 | Chest wall numbness [5] | 1 |
| Axente [2013] (3) | Case series | PTC [4], benign [46] | Lobectomy [41]; total [9] | 159±38.2 | Conversion to open [1], Transient brachial plexus neuropraxia [1], transient vocal cord paresis [1], transient hypocalcemia [1], chest paresthesia/ hypoesthesia [5], hematoma [1]. Postoperative wound complications [4]: seroma [3]; wound infection [1] | 4 |
| Giannopoulos [2013] (22) | Case series | Benign [44] | HT [27]; TT [5]; subtotal [12] | 129.8±39.5 | Transient hypocalcemia [1], transient hoarseness [1], permanent RLN injury [1] | 3 |
| Aliyev [2013] (23) | Case control | PTC [10], FTC [1], benign [5] | Total [16] | 183±11 | Asymptomatic hypocalcemia at 2 weeks post op [2], hematoma [1] | 1 |
| Arora [2016] (24) | Case control | PTC [3], benign [13] | Lobectomy [16] | 228 | Transient RLN paresis [1], temporary shoulder dysfunction [1], seroma [1] | NA |
| Ban [2014] (5) | Case series | PTC [2,979], FTC [12], HCTC [1], MTC [8] | Less than total [1,910], total [1,090] | 124.78±32.50 | Transient hypocalcemia [408], permanent hypocalcemia [12], transient RLN injury [37], permanent RLN injury [8], seroma [52], hematoma [11], chyle leak [11], trachea injury [6], Horner’s syndrome [1], major vessel injury [2], brachioplexus neuropraxia [4], skin flap perforation [3] | 3 |
PTC, papillary thyroid cancer; FN, follicular neoplasm; HCTC, Hurthle cell thyroid cancer; FTC, follicular thyroid cancer; MTC, medullary thyroid cancer; HT, hemithyroidectomy; TT, total thyroidectomy; RLN, recurrent laryngeal nerve.
Table 2. Summary of selected studies of retroauricular (facelift) robotic thyroidectomy.
| Author [year] (ref.) | Study design [cases] | Pathology [cases] | Extent of surgery | Operative time (mean total ± SD) | Complication profile [cases] | Hospital stay (median) |
|---|---|---|---|---|---|---|
| Terris [2011] (25) | Case series | PTC [2], FTC [1], benign [15] | HT [13], TT [1], completion [3] | Range: 97–193 | Seromas [2], transient VC dysfunction [1], temporary periauricular hypesthesia [17] | 0 |
| Kandil [2015] (26) | Case series | PTC [1], FTC [2], benign [9] | HT [12] | 145.4±10.08 | Transient hypocalcemia [3], transient hoarseness [1], seroma [2], hypoesthesia [3] | 1 |
| Byeon [2016] (27) | Case series | PTC [75], FTC [2], benign [10] | TT + MRND [16], total [15], HT [56] | HT: 88.1±51.2, TT: 173.8±75.2 | Transient VC palsy [5], permanent VC palsy [3], Temporary hypoparathyroidism [12], temporary MM nerve injury [5], earlobe numbness [6], seroma [21], hematoma [2], chyle leak [1], skin flap ischemia [2] | TT or less: 6; TT + MRND: 10 |
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; HT, hemithyroidectomy; TT, total thyroidectomy; MRND, modified radical neck dissection.
Table 3. Summary of selected studies of transoral robotic thyroidectomy.
| Author [year] (ref.) | Study design (cases) | Pathology [cases] | Extent of surgery | Operative time (min) | Complication profile [cases] | Hospital stay (median) |
|---|---|---|---|---|---|---|
| Lee [2015] (28) | Case series | PTC [1], benign [3] | HT [4] | Range: 190–390 | Lower lip and chin paresthesia [4] | 5 |
PTC, papillary thyroid cancer; HT, hemithyroidectomy.
Comprehensive review of robotic thyroidectomy
Surgical techniques
The most commonly used approaches are transaxillary, retroauricular and the combination of both approaches. The newest approach is the transoral approach. The selection of approach is largely dependent on the training, skill, and preference of the surgeon and/or patient. Both the transaxillary and retroauricular approaches are performed in a gasless fashion, while the transoral technique requires CO2 gas insufflation for the procedure. Many recommend that the intraoperative nerve monitoring (IONM) is used for robotic thyroidectomy cases. It is however important to note that the IONM tube can be moved or shifted with the change in the neck position, especially for the transaxillary and the retroauricular approaches. Therefore, the placement and the position of the IONM tube must be verified especially in the absence of the signals when the nerve is being stimulated.
The surgical techniques of the three aforementioned approaches using the Da Vinci robots are described below. Regardless of the selected approach, there are three consistent steps to robotic thyroidectomy: (I) working space formation; (II) docking; and (III) console stages.
Transaxillary approach
Working space formation
After general anesthesia induction, the patient is in the supine position with slight extension of the neck (6,29,30). This neck extension can be achieved by using either a shoulder roll or by using a thyroid pillow which also offers additional supports to the upper back and scapula (29). The ipsilateral arm is then extended and rotated cephalad fully exposing the axilla (Figure 1). It is important to assess the extent of the patient’s arm extension/abduction without applying additional force to prevent unnecessary over-extension of the arm which can lead to inadvertent brachial plexus injury. A 5–6 cm curved-vertical line is drawn just posterior to the anterior axillary fold (Figure 1) (14). Once the incision is drawn, the arm can be repositioned in the adduction position to verify if that the incision is well hidden in the axillary crease for the maximal cosmetic outcome (Figure 2A,B). The arm is then re-extended and secured on an arm board. It is important to make sure that no extra tension is placed on the extended arm to avoid brachial plexus injury. This arm is padded and secured using tape.
Figure 1.
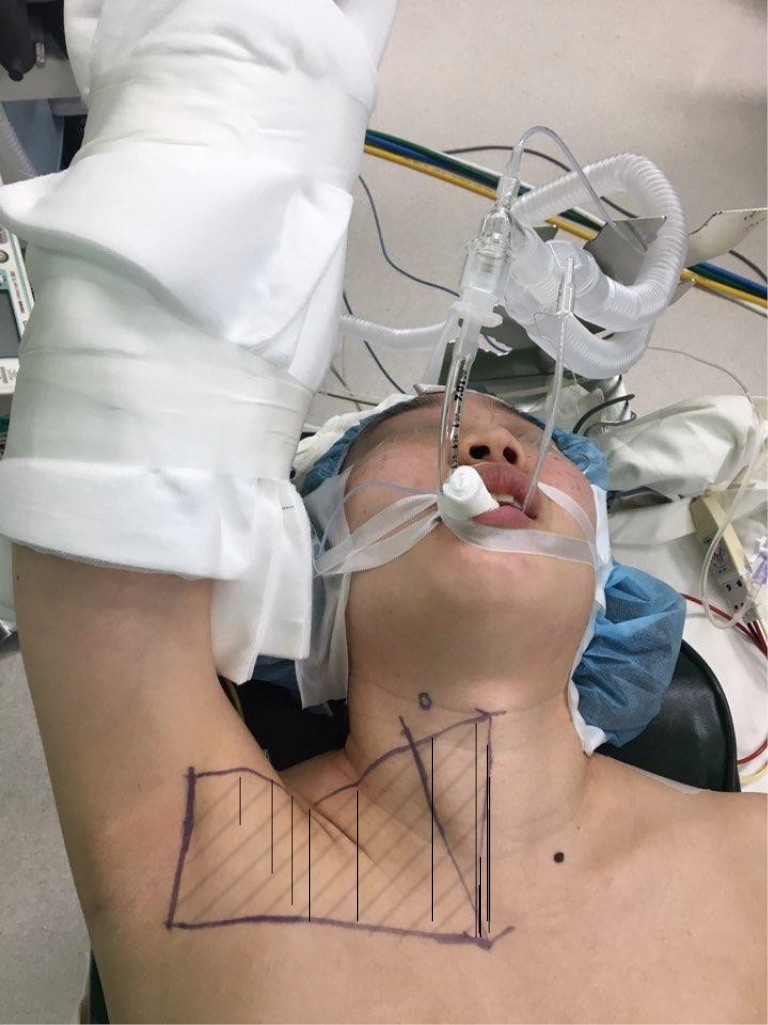
Transaxillary robotic thyroidectomy: patient positioning and anatomical landmarks. The ipsilateral arm is extended to expose the axilla. The incision is marked along the posterior edge of the anterior axillary fold; (A) thyroid cartilage, (B) superior border of thyroid lobe, (C) suprasternal notch. The shaded area represents the surface area where the subcutaneous/subplatysmal flap needs to be elevated for working space formation.
Figure 2.
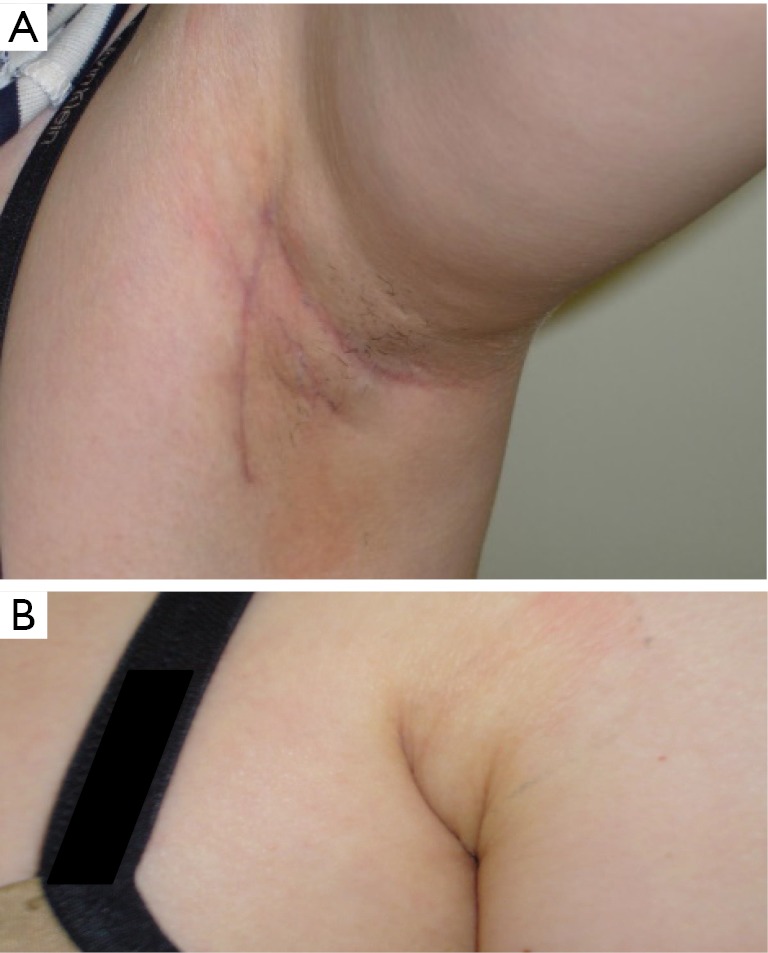
Transaxillary robotic thyroidectomy: (A) 3 months postoperative results; (B) the axillary scar is well hidden and not visible when the arm is adducted.
Once the ideal position is achieved, the patient’s arm, neck and chest is prepped and draped exposing the axilla, neck and upper chest. The incision is made using a 15 blade, and the subcutaneous flap is raised using electrocautery. It is very important to preserve the fascia overlying the pectoralis major muscle to prevent postoperative adhesions which may lead to increased discomfort and pain over the chest area. Once the pectoralis major is exposed, a careful dissection over the clavicle is performed until the sternocleidomastoid (SCM) muscle is exposed. It is important to preserve the posterior triangle soft tissue structures to avoid unnecessary injury to the external jugular vein and increased risk of postoperative hematoma. Once the SCM is exposed, the dissection proceeds by opening the avascular plane between the clavicular and sternal heads of the SCM. It is crucial for the surgeons to enter this avascular space correctly to avoid possible injury to the major vessels that are laying directly below the route of dissection. Strap muscles are then encountered. The dissection is then carefully proceeded directly underneath the strap muscles exposing the thyroid gland. The working space is considered to be safe and adequate if a sufficient space is created exposing the superior pole of thyroid and central neck. Once the dissection is completed, Chung retractor or an equivalent retractor is placed holding the subcutaneous flap, anterior SCM and strap muscles upwards to keep the working space exposed. In the case of total thyroidectomy, the contralateral lobe should be completely exposed and the position of the retractor should be appropriately modified to allow safe resection of the contralateral lobe. The art of robotic total thyroidectomy requires significantly more skills and knowledge due to the complexity of the procedure, and the details of surgical steps are beyond the scope of this review. The authors recommend that transaxillary robotic total thyroidectomy be performed by robotic surgeons with advanced robotic endocrine training background.
Docking stage
Once adequate working space is achieved, the robot is advanced towards the patient from the contralateral side in preparation for the docking of the arms. For the transaxillary procedure, all four robotic arms are used. For the right sided approach, the arm closest to the head of the patient carries the Maryland dissector, followed by the second arm holding the 30 degree endoscope (Figure 3). On the arm next to the endoscope arm, the ProGrasp forceps is inserted. The arm closest to the patient’s feet carries the Harmonic scalpel (Figure 3). The order is reversed when the procedure is approached via the left side. This docking method ensures that the surgeon operates using the Harmonic scalpel using his right hand, similarly to the open procedures. The Maryland and ProGrasp forceps provide constant countertraction for safe and accurate dissection, following the same surgical principles used in conventional open surgeries. In the authors’ opinion, there is no significant docking method difference between the Si and Xi models. It is important to keep sufficient distance between each robotic arm to avoid fighting between the instruments.
Figure 3.
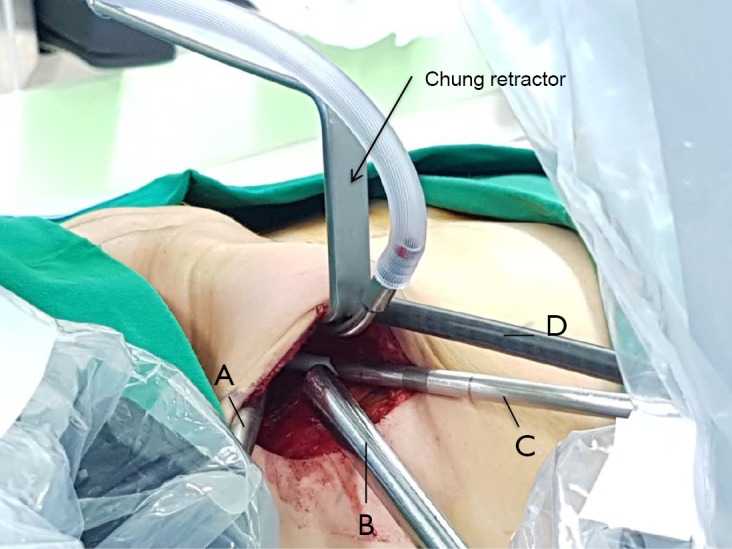
Transaxillary robotic thyroidectomy: docking of the robot for right sided approach. Chung retractor is inserted in the middle of the incision and is secured to expose the working space (arrow). The Maryland dissector (A) and the Harmonic scalpel (C) are placed at the cephalad and caudal extremities of the incision respectively. The 30-degree endoscope (B) is inserted in the middle of the working space inferiorly, sitting right above the pectoralis muscle. The ProGrasp forceps (D) is inserted beneath the Chung retractor: this instrument is inserted off the midline slightly towards the feet of the patient.
The docking stage is heavily dependent on the skills and experience of the surgeon and his operative team. Even a small difference in the docking position can affect the efficiency of the console stage, and this statement holds true regardless of the robotic thyroidectomy approach. The authors recommend that same nursing and surgical assistant team is used if possible for every robotic thyroidectomy cases to improve the safety and efficiency of the procedure.
Console stage
Once the ideal docking of the robotic arms is achieved, the surgeon may proceed to the console stage. The superior pole is first dissected by addressing the superior vessels. This first step is crucial in order to identify the superior parathyroid gland and safely preserve it. Once the superior pole is released, then the attention is turned to the inferior pole. If the central neck dissection (CND) is indicated, then this is performed en bloc prior to the inferior pole dissection. The recurrent laryngeal nerve (RLN) is identified inferiorly and is dissected superiorly towards the ligament of Berry while carefully dissecting the thyroid gland off of the adjacent structures. The isthmus is then divided which completes the hemithyroidectomy. The entire CND content and thyroid gland are removed en bloc. Once the specimen is removed, careful examination of the surgical field is performed for verification of hemostasis
Retroauricular approach
Working space formation
The patient is intubated under general anesthesia and positioned supine with the head turned gently to the contralateral side from the approach, exposing the posterior auricular sulcus with the posterior portion of the neck facing the surgeon. The incision is marked along the posterior auricular sulcus extending over the mastoid and inferiorly parallel to the occipital hairline. The authors recommend that the incision be marked at least 5 mm posterior to the occipital hairline for optimal cosmesis. The patient is prepped and draped exposing the incision line, neck and the ipsilateral half of the face. Once the incision is made, the subcutaneous retroauricular flap is raised anteriorly exposing the parotid tail and the SCM. Care must be given to preserve the greater auricular nerve and the marginal mandibular nerve. The subplatysmal flap is then dissected towards the midline of the neck exposing the inferior border of the mandible and strap muscles down to the sternal notch inferiorly. Broad dissection underneath the strap muscles is performed to expose the thyroid gland. A modified Chung retractor, in our practice, is inserted underneath the strap muscles (Figure 4).
Figure 4.
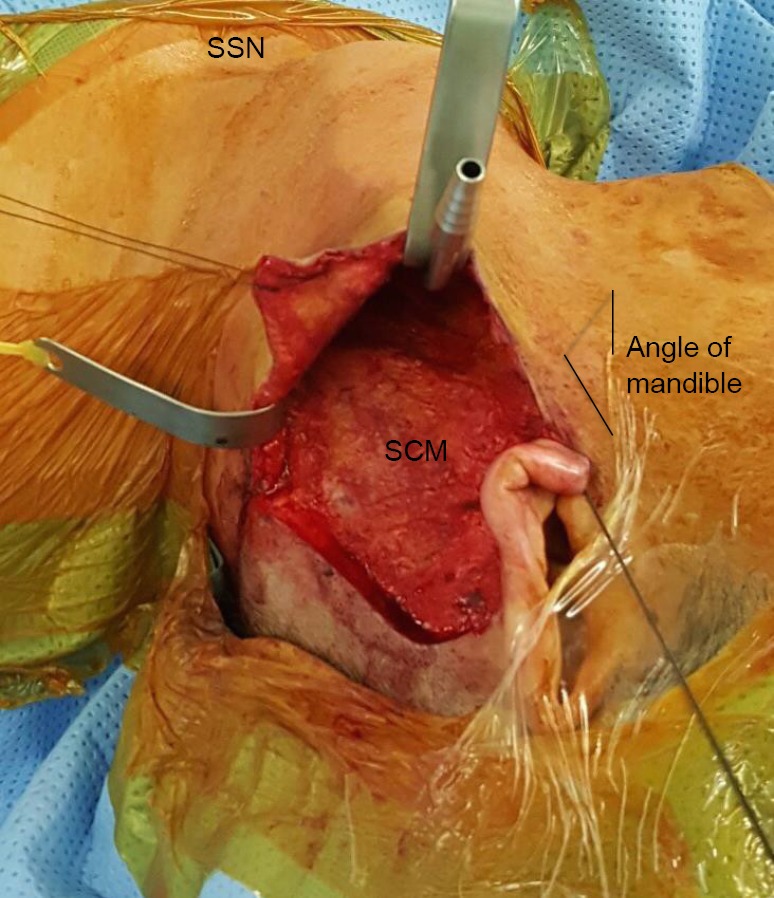
Retroauricular robotic thyroidectomy: working space formation. A modified Chung retractor is inserted and secured to expose the working space. Additional retraction is achieved using vicryl sutures to prevent the retroauricular skin flap and the ear lobe from obscuring the working space. SCM, sternocleidomastoid muscle; SSN, suprasternal notch.
Docking stage
Once an adequate working space is established, the robotic system is docked. If the working space allows, it is always preferable to use all three robotic arms (30 degree endoscope with three instrument arms) in order to facilitate the surgery (Figure 5). If the working space is limited, the surgery can still be done using only two instrument arms without the ProGrasp. The authors have found that operating with two instrument arms instead of three is technically more challenging. The docking method is similar to the transaxillary approach where the Maryland dissector and the Harmonic scalpel are controlled by the surgeon’s left and right hands respectively (Figure 5).
Figure 5.
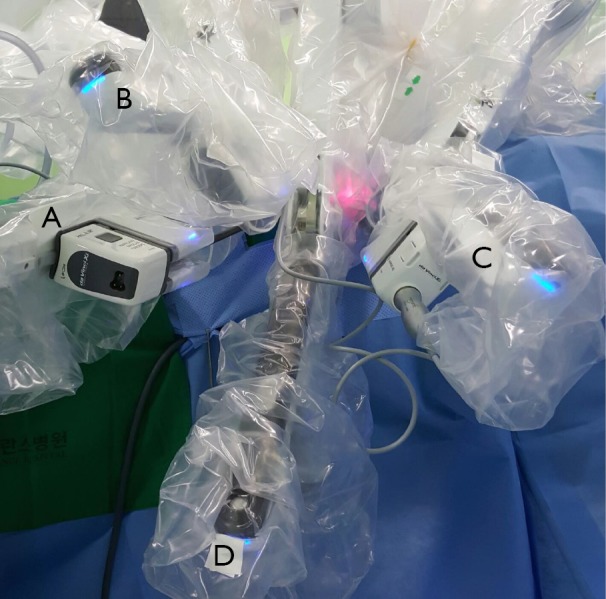
Retroauricular robotic thyroidectomy: docking of the robot for the left sided approach. Similar to the transaxillary approach, the Maryland dissector (A) and the Harmonic scalpel (C) are inserted at each extremity of the incision. In the center of the working space, the 30-degree endoscope (D) is inserted. Above the endoscope, the ProGrasp forceps (B) is inserted slightly off the midline towards the feet of the patient.
Console stage
The steps of the hemithyroidectomy via the retroauricular approach begin with the identification and dissection of the superior pole. The superior pole is gently retracted superiorly, and the superior pole vessels are carefully ligated one vessel at a time. During these steps, the surgeon must identify superior parathyroid gland and preserve it. Careful and gentle dissection is recommended to decrease the risk of thermal injury to the parathyroid gland.
Once the superior pole is mobilized and the cricothyroid muscle is exposed, the isthmusectomy is then performed. This will facilitate the identification and dissection of the RLN. The nerve is identified in the tracheoesophageal groove near the cricothyroid joint where the nerve enters the larynx. The IONM probe can be used to confirm the RLN. Once the nerve is correctly identified and confirmed via IONM, the rest of the thyroid gland is dissected off of its surrounding soft tissue while keeping the RLN intact along its course. With the dissection of the inferior pole, the procedure is completed.
Transoral approach
Working space formation
Once the patient is placed under general anesthesia, the neck is placed in slight extension. Three incisions are made in the gingival-buccal sulcus: one in the midline, approximately 2 cm above the frenulum labii inferioris, and two laterally near the angle of mouth (Figure 6). The central incision is addressed first. A submental subplatysmal pocket is formed to create a tunnel towards the edge of the mandible. Blunt dissection is performed to elevate the platysma off the strap muscles all the way down towards the suprasternal notch. This blunt dissection is facilitated via injections of saline mixed with epinephrine into the subplatysmal layer. Once an adequate flap is created, the endoscope (30 degrees, down facing) cannula is inserted. CO2 insufflation (8–10 L/min) is introduced and maintained via the central port. A similar blunt dissection is also performed from the two lateral incision sites allowing insertion of the instrument cannulae into the subplatysmal working space. A few vicryl stitches can be used to help retract the subplatysmal flap superiorly in order to create a larger working space (Figure 7).
Figure 6.
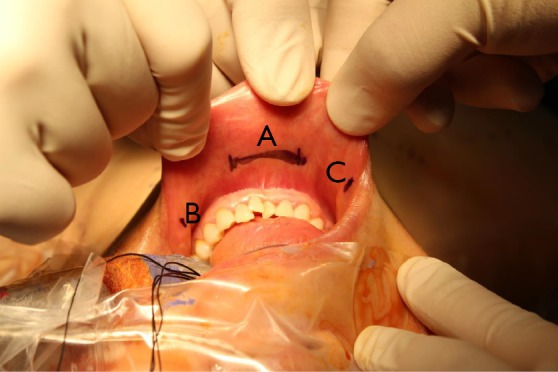
Transoral robotic thyroidectomy: working space formation. Three incisions are made in the gingival-buccal sulcus: one in the midline (A), approximately 2 cm above the frenulum labii inferioris, and two smaller incisions (B,C) laterally near the angle of mouth.
Figure 7.
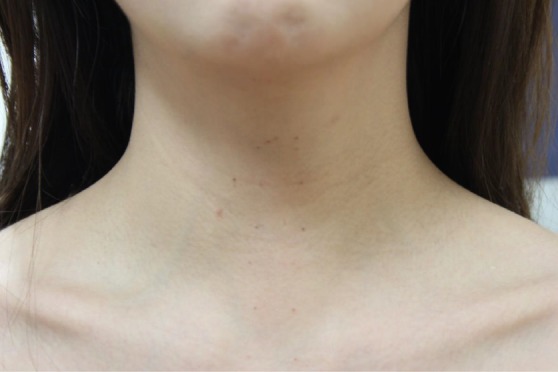
Transoral robotic thyroidectomy: 1 week postoperatively. There is evidence of suture marks from the vicryl sutures that were applied intraoperatively to retract the subplatysmal flap.
Docking stage
Once the working space formation is complete, the robotic system is deployed. The cannulae are inserted into the robotic arms, starting with the central cannula to secure the position of the endoscope. A Maryland dissector and the Harmonic scalpel are inserted into the left and right ports respectively.
Console stage
Dissection in the midline raphe is performed to separate the strap muscles. The strap muscles are dissected off the thyroid gland, exposing the lobe(s) of interest. The pyramidal lobe is dissected off the thyroid cartilage and isthmusectomy is performed. Once the thyroid lobe is freed off the trachea medially, the superior pole is addressed. Careful dissection of the superior lobe is performed ligating one vessel at a time. The superior parathyroid gland is identified and preserved. The thyroid lobe is retracted inferiorly to facilitate the identification of the RLN at its entry point into the larynx. Once the RLN is identified and carefully preserved, the Berry’s ligament is addressed. The dissection is then carried out inferiorly preserving the inferior parathyroid gland. Once the inferior lobe is free off of its surrounding soft tissue, hemithyroidectomy is complete.
Advantages and disadvantages
In this review, the authors have presented three approaches of robotic thyroidectomy: transaxillary, retroauricular and transoral. Each approach has its own advantages and disadvantages given the inherent differences in their dissection fields required for the working space formation. Knowing the advantages and disadvantages of each approach helps the surgeon to make the best recommendations and to individualize the approach for each patient based on patient and tumor characteristics. Being aware of the disadvantages and particularly of the possible complications related to each approach allows the surgeon to give informed consent to the patients desiring to undergo robotic surgery.
The transaxillary approach was originally performed via two separate incisions: the first one in the axillary crease and the second one over the anterior chest. It has since been modified to a single axillary incision without the second chest incision. The main advantages of the transaxillary approach are: the ease of console stage, ease of detecting the RLN, ability to perform total thyroidectomy with central and lateral neck dissections for advanced cancer and then also well-established literature demonstrating safety and outcomes.
Furthermore, this is the only approach among the three approaches described that does not violate the space between the platysma and the strap muscles, which may theoretically result in more favorable postoperative swallowing function when compared to retroauricular, transoral and conventional open approaches (6,7,31,32). Some of the main disadvantages of this approach are the possible risks of anterior chest paresthesia and brachial plexus injury (Table 1). There have been reports of high-volume hemorrhage and injury to esophagus (16,25,33), but these complications may be decreased as the surgeon becomes used to the lateral approach to thyroidectomy with experience. Brachial plexus injury is considered to be preventable with proper arm positioning with extra padded support. Anterior chest paresthesia over the clavicular area, however, occurs with unavoidable injury to the sensory nerves of cervical plexus chain during the working space formation (Table 1). These sensory nerves are encountered as the subplatysmal flap is elevated off the clavicle towards the SCM and need to be sacrificed in order to create a safe working space. For the majority of cases, the paresthesia sensation is only temporary, but in rare cases, this may be permanent. This possible complication should therefore be discussed with the patient when obtaining informed consent.
The retroauricular approach was first described by Terris to avoid the complications that are associated with the transaxillary approach (25,33). Terris has emphasized the benefits of the RA approach, speculating that the significantly reduced field of dissection in the RA approach when compared to the TA approach is associated with a faster recovery and decreased postoperative discomfort (25). He also describes the RA technique to be easier than the TA approach when operating on obese patients (25). This statement seems to be supported by the ATA as their latest statement on remote-access surgery states that remote-access surgery should be performed on thin body habitus patients without excessive body fat with exception of the RA approach (10). Other advantages of the RA approach are reduced risk of injury to the great vessels, the esophagus or the anterior chest sensory nerves, since these structures are not encountered during the working space formation. However, there are some disadvantages that are inherent to this approach: injury to greater auricular and marginal mandibular nerves (4,25,27) (Table 2). These complications are usually temporary and resolve completely within a few months following surgery. But these possible complications should be discussed in detail for informed consent prior to surgery.
The transoral approach is the latest technique to be introduced for remote- access thyroidectomy that has been gaining a lot of interest worldwide. This approach to thyroid was first described by Witzel et al. in 2008 (28,34) and has been performed with an endoscope for the majority of cases performed internationally (35-38). The first robotic series was published by Lee et al. (28).
The initial transoral robotic series included four patients who experienced temporary complications related to mental nerve injury (Table 3). This has since been overcome by modifying the sites of incisions to avoid injury to the mental nerves as they exit the mental foramina spreading in branches towards the lip. The main advantages of this approach are the completely invisible intraoral scars, excellent access and exposure to bilateral thyroid lobes for total thyroidectomy, and a reported lower complication profile compared to other remote-access approaches. The transoral technique offers the unique advantage of accessing the thyroid gland from a natural orifice, and its midline approach allows excellent exposure to the entire thyroid gland, making this the safest approach to perform total thyroidectomy when compared to other approaches. The main disadvantages to this technique are the need for postoperative antibiotics, possibly longer length of stay (LOS) and inability to perform lateral neck dissections. There have been no reports of postoperative infections following transoral endoscopic or robotic thyroidectomy; however, postoperative antibiotics are administered for all patients given the possible risk of infection as the transoral thyroidectomy is not considered to be a clean procedure unlike its conventional open counterpart. The postoperative LOS is also considered to be the longest following transoral robotic thyroidectomy when compared to other remote-access approaches. However, this comparison is based on a single study of four patients, and more study on transoral approaches are required to investigate this further. Another possible downside of this approach is the inability to control massive hemorrhage through the intraoral incision in case of inadvertent great vessel injury. If such hemorrhage were to occur, then an anterior neck incision would have to be made to control the bleeding. The biggest weakness of the transoral approach is the inability to perform lateral neck dissections. This challenge may never be overcome given the anatomical constraints, and may require a combination of approaches when performing neck dissections on patients with extensive lateral neck disease. Lateral neck dissections are performed via remote-access approaches increasingly commonly around the world, excepting the United States. According to the ATA statements, the presence of lateral neck disease is currently a contraindication to remote-access thyroid (10). The question regarding the transoral approach that is yet to be answered is its ease and safety of performing the procedure for obese patients, which would be an important consideration for North American surgeons.
Regardless of the chosen approach, the successful outcome of robotic thyroidectomy largely depends on careful patient selection and the skills of the surgeon. The ideal robotic candidate is not obese and has a small tumor that is contained within the thyroid gland without evidence of thyroiditis. They also have a good neck and arm mobility without history of previous neck surgery or irradiation. The strict selection criteria and a thorough preoperative assessment are especially important for surgeons who are inexperienced in robotic thyroidectomy.
A detailed informed consent process is essential when performing cutting edge techniques. Patients should be informed that robotic thyroidectomy is currently considered to be an off-label use of the da Vinci surgical system in the United States (29). Most importantly, the patients should also be informed of the additional possible complications that are associated with robotic procedures and the possible need to convert to open surgery if the robotic approach is not deemed to be possible intraoperatively.
The main criticisms against robotic thyroidectomy are the additional costs, longer operative times and a steep learning curve (8,39). Cabot et al. reported that the costs associated with transaxillary robotic approach are higher than the costs of conventional open thyroidectomy ($13,670 vs. $9,028) and that the this significant difference in cost was primarily due to high equipment depreciation costs and longer operative times for robotic procedures (39). The authors determined that in order for the robotic procedure to reach cost equivalence to the conventional approach it would have to be nearly twice as fast in terms of current operative times (39).
A steep learning curve of 40 to 45 cases has been described for transaxillary robotic thyroidectomy (19,40). This illustrates the difficulty in learning the procedure even for surgeons that are experienced in conventional open thyroid surgery. A decrease in total operative time and complications rates is found after these initial 40–45 cases, a number that may be difficult to achieve in a relatively short period of time for a surgeon in a North American center.
Despite the downsides associated with robotic procedures, there are distinct advantages in the use of the robotic system for thyroidectomy. Aside from superior cosmetic results, the incorporation of robotic system offers 10-time magnification of the surgical view and tremor elimination, which can enhance the safety and precision of the procedure. Identification and preservation of RLNs and parathyroid glands are much easier to achieve with the assistance of robotic system (5). The subgroup of the population who meet selection criteria will benefit from the advantages of robotic system when receiving thyroidectomy, and the procedures should only be performed with well-trained robotic surgeons for the patients to truly experience those benefits while minimizing the risk of possible complications.
As Terris et al. previously stated, “one size no longer fits all” when it comes to approaches in thyroidectomy and parathyroidectomy (41). The number of robotic and remote-access cases continues to increase around the world, and the general public is more interested in the incorporation of robotic system for surgical procedures. As newer generations of patients are increasingly technologically savvy they want to be informed of all available treatment options and want to be an active participant in the decision-making process. The patients who wish to avoid a visible scar when receiving thyroidectomy should be informed of possible alternative remote-access options and should be guided to where they may receive expert care in the field.
With advancements in robotic technology, the list of advantages of robotic surgery will continue to grow. Performing more complex surgeries of head and neck will become a possibility with greater precision and safety along with the development of more sophisticated and advanced robotic tools. On the forefront of this, the introduction of the highly anticipated single port robotic system will likely change the landscape of the robotic thyroid approaches that exist today. It is prudent for academic physicians to be technologically relevant and remain actively engaged to learn the latest technologies available; to adapt to the continuously evolving technology in order to provide the latest and innovative technology for the advancement of quality of care we provide to our patients.
Conclusions
From the transaxillary to transoral approach, robotic thyroidectomy has continued to evolve and is currently being offered in many academic and private practices worldwide. Robotic thyroidectomy has a definite role in a subgroup of patients while following strict selection criteria to improve success and minimize complications. There are many different approaches to robotic thyroidectomy, and the ideal approach should be based on the patient and tumor characteristics and most importantly on the experience of the surgeon. The major factor associated with successful outcomes following robotic thyroidectomy is the experience and skill of the surgeon. Therefore, robotic thyroidectomy should only be performed by experienced robotic surgeons. More studies with clinical outcome data are needed to compare the safety and oncologic outcomes of various robotic thyroidectomy approaches and to further define indications and selection criteria.
Acknowledgements
The authors would like to thank Dr. Whitney Goldner and Dr. Christopher Gillis for their contribution to the editing of the manuscript, and Dr. Chorok Lee in gathering photos of transaxillary procedure.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hüscher CS, Chiodini S, Napolitano C, et al. Endoscopic right thyroid lobectomy. Surg Endosc 1997;11:877. 10.1007/s004649900476 [DOI] [PubMed] [Google Scholar]
- 2.Chung WY. The evolution of robotic thyroidectomy: from inception to neck dissection. J Robot Surg 2011;5:17-23. 10.1007/s11701-010-0232-9 [DOI] [PubMed] [Google Scholar]
- 3.Axente DD, Silaghi H, Silaghi CA, et al. Operative outcomes of robot-assisted transaxillary thyroid surgery for benign thyroid disease: early experience in 50 patients. Langenbecks Arch Surg 2013;398:887-94. 10.1007/s00423-013-1085-2 [DOI] [PubMed] [Google Scholar]
- 4.Sung ES, Ji YB, Song CM, et al. Robotic Thyroidectomy: Comparison of a Postauricular Facelift Approach with a Gasless Unilateral Axillary Approach. Otolaryngol Head Neck Surg 2016;154:997-1004. 10.1177/0194599816636366 [DOI] [PubMed] [Google Scholar]
- 5.Ban EJ, Yoo JY, Kim WW, et al. Surgical complications after robotic thyroidectomy for thyroid carcinoma: a single center experience with 3000 patients. Surg Endosc 2014;28:2555-63. 10.1007/s00464-014-3502-1 [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Chung WY. Robotic surgery for thyroid disease. Eur Thyroid J 2013;2:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Chung WY. Robotic thyroidectomy and neck dissection: past, present and future. Cancer J 2013;19:151-61. 10.1097/PPO.0b013e31828aab61 [DOI] [PubMed] [Google Scholar]
- 8.Rabinovics N, Aidan P. Robotic transaxillary thyroid surgery. Gland Surg 2015;4:397-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son H, Park S, Lee CR, et al. Factors contributing to surgical outcomes of transaxillary robotic thyroidectomy for papillary thyroid carcinoma. Surg Endosc 2014;28:3134-42. 10.1007/s00464-014-3567-x [DOI] [PubMed] [Google Scholar]
- 10.Berber E, Bernet V, Fahey TJ, 3rd, et al. American Thyroid Association Statement on Remote-Access Thyroid Surgery. Thyroid 2016;26:331-7. 10.1089/thy.2015.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang BH, Wong CK, Tsang JS, et al. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol 2014;21:850-61. 10.1245/s10434-013-3406-7 [DOI] [PubMed] [Google Scholar]
- 12.Adam MA, Speicher P, Pura J, et al. Robotic thyroidectomy for cancer in the US: patterns of use and short-term outcomes. Ann Surg Oncol 2014;21:3859-64. 10.1245/s10434-014-3838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Lee CR, Lee SC, et al. Surgical completeness of robotic thyroidectomy: a prospective comparison with conventional open thyroidectomy in papillary thyroid carcinoma patients. Surg Endosc 2014;28:1068-75. 10.1007/s00464-013-3303-y [DOI] [PubMed] [Google Scholar]
- 14.Tae K, Song CM, Ji YB, et al. Comparison of surgical completeness between robotic total thyroidectomy versus open thyroidectomy. Laryngoscope 2014;124:1042-7. 10.1002/lary.24511 [DOI] [PubMed] [Google Scholar]
- 15.Richmon JD, Holsinger FC, Kandil E, et al. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg 2011;5:279-82. 10.1007/s11701-011-0287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope 2011;121:521-6. 10.1002/lary.21347 [DOI] [PubMed] [Google Scholar]
- 17.Ciabatti PG, Burali G, D’Ascanio L. Single-incision robot-assisted transaxillary surgery for early-stage papillary thyroid cancer. Ann Otol Rhinol Laryngol 2012;121:811-5. 10.1177/000348941212101207 [DOI] [PubMed] [Google Scholar]
- 18.Foley CS, Agcaoglu O, Siperstein AE, et al. Robotic transaxillary endocrine surgery: a comparison with conventional open technique. Surg Endosc 2012;26:2259-66. 10.1007/s00464-012-2169-8 [DOI] [PubMed] [Google Scholar]
- 19.Kandil EH, Noureldine SI, Yao L, et al. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 2012;214:558-64. 10.1016/j.jamcollsurg.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Landry CS, Grubbs EG, Warneke CL, et al. Robot-assisted transaxillary thyroid surgery in the United States: is it comparable to open thyroid lobectomy? Ann Surg Oncol 2012;19:1269-74. 10.1245/s10434-011-2075-7 [DOI] [PubMed] [Google Scholar]
- 21.Terris DJ, Singer MC. Qualitative and quantitative differences between 2 robotic thyroidectomy techniques. Otolaryngol Head Neck Surg 2012;147:20-5. 10.1177/0194599812439283 [DOI] [PubMed] [Google Scholar]
- 22.Giannopoulos G, Kang SW, Jeong JJ, et al. Robotic thyroidectomy for benign thyroid diseases: a stepwise strategy to the adoption of robotic thyroidectomy (gasless, transaxillary approach). Surg Laparosc Endosc Percutan Tech 2013;23:312-5. 10.1097/SLE.0b013e31828b8b20 [DOI] [PubMed] [Google Scholar]
- 23.Aliyev S, Taskin HE, Agcaoglu O, et al. Robotic transaxillary total thyroidectomy through a single axillary incision. Surgery 2013;153:705-10. 10.1016/j.surg.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 24.Arora A, Garas G, Sharma S, et al. Comparing transaxillary robotic thyroidectomy with conventional surgery in a UK population: a case control surgey. Int J Surg 2016;27:110-7. 10.1016/j.ijsu.2016.01.071 [DOI] [PubMed] [Google Scholar]
- 25.Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 2011;121:1636-41. 10.1002/lary.21832 [DOI] [PubMed] [Google Scholar]
- 26.Kandil E, Saeed A, Mohamed SE, et al. Modified robotic-assisted thyroidectomy: an initial experience with the retroauricular approach. Laryngoscope 2015;125:767-71. 10.1002/lary.24786 [DOI] [PubMed] [Google Scholar]
- 27.Byeon HK, Kim DH, Chang JW, et al. Comprehensive application of robotic retroauricular thyroidectomy: the evolution of robotic thyroidectomy. Laryngoscope 2016;126:1952-7. 10.1002/lary.25763 [DOI] [PubMed] [Google Scholar]
- 28.Lee HY, You JY, Woo SU, et al. Transoral periosteal thyroidectomy: cadaver to human. Surg Endosc 2015;29:898-904. 10.1007/s00464-014-3749-6 [DOI] [PubMed] [Google Scholar]
- 29.Holsinger FC, Chung WY. Robotic thyroidectomy. Otolaryngol Clin North Am 2014;47:373-8. 10.1016/j.otc.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: The operative outcomes of 338 consecutive patients. Surgery 2009;146:1048-55. 10.1016/j.surg.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. 10.1007/s00464-010-1113-z [DOI] [PubMed] [Google Scholar]
- 32.Son SK, Kim JH, Bae JS, et al. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:3022-32. 10.1245/s10434-015-4375-9 [DOI] [PubMed] [Google Scholar]
- 33.Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech 2011;21:237-42. 10.1097/SLE.0b013e3182266dd6 [DOI] [PubMed] [Google Scholar]
- 34.Witzel K, von Rahden BH, Kaminski C, et al. Transoral access for endoscopic thyroid resection. Surg Endosc 2008;22:1871-5. 10.1007/s00464-007-9734-6 [DOI] [PubMed] [Google Scholar]
- 35.Anuwong A. Transoral endoscopic thyroidectomy vestibular approach: a series of the first 60 human cases. World J Surg 2016;40:491-7. 10.1007/s00268-015-3320-1 [DOI] [PubMed] [Google Scholar]
- 36.Inabnet WB, 3rd, Suh H, Fernandez-Ranvier G. Transoral endoscopic thyroidectomy vestibular approach with intraoperative nerve monitoring. Surg Endosc 2017;31:3030. 10.1007/s00464-016-5322-y [DOI] [PubMed] [Google Scholar]
- 37.Nakajo A, Arima H, Hirata M, et al. Trans-Oral Video-Assisted Neck Surgery (TOVANS). A new transoral technique of endoscopic thyroidectomy with gasless premandible approach. Surg Endosc 2013;27:1105–1110. 10.1007/s00464-012-2588-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm T, Metzig A. Endoscopic minimally invasive thyroidectomy (eMIT): a prospective proof-of-concept study in humans. World J Surg 2011;35:543-51. 10.1007/s00268-010-0846-0 [DOI] [PubMed] [Google Scholar]
- 39.Cabot JC, Lee CR, Brunaud L, et al. Robotic and endoscopic transaxillary thyroidectomies may be cost prohibitive when compared to standard cervical thyroidectomy. Surgery 2012;152:1016-24. 10.1016/j.surg.2012.08.029 [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Yun JH, Nam KH, et al. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol 2011;18:226-32. 10.1245/s10434-010-1220-z [DOI] [PubMed] [Google Scholar]
- 41.Terris DJ. Surgical approaches to the thyroid gland: which is the best for your and your patient? JAMA Otolaryngol Head Neck Surg 2013;139:515-7. 10.1001/jamaoto.2013.289 [DOI] [PubMed] [Google Scholar]


