Abstract
Prostate cancer (PCa) is one of the few neoplasms that are not well served by 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET). As a result, a number of PET tracers have been developed to target particular biological features of PCa. Such agents can be used for diagnosis, staging, identification of biochemical recurrence (BCR) and evaluation of metastatic disease. Here, we focus on primary disease and local staging. To date, magnetic resonance imaging (MRI) has proven superior to PET in the imaging of primary PCa. However, some PET agents have shown remarkable promise in staging high-risk PCa (defined as any combination of a clinical T3, a PSA score >20 ng/mL, or a Gleason score of 8–10), as well as biochemical relapse after definitive therapy and metastatic PCa. PET agents can be divided into those that interrogate tumor metabolism (18F-FDG, 11C-Choline, 18F-Choline, 11C-Acetate, 18F-FACBC), hormone receptors (18F-FDHT), and other targets such as prostate specific membrane antigen (PSMA) (68Ga-PSMA, 18F-DCFBC, 18F-DCFPyl) or gastric releasing peptide (18F-GRP or 18F-Bombesin). In this review, we compare the available PCa targeted PET tracers utilized in staging of high risk tumors.
Keywords: Prostate cancer (PCa), positron emission tomography (PET), staging, prostate specific membrane antigen (PSMA)
Introduction
Prostate cancer (PCa) is the most common non-cutaneous malignancy among American men with 180,890 new cases and 26,120 deaths estimated for 2016 (1). In the ongoing effort to understand, control, and to cure this disease, better imaging tools for detecting PCa in its various states are constantly evolving to assist clinical decisions. PCa demonstrates a wide range of biologic activity ranging from indolent to highly aggressive, although the vast majority of PCa are clinically insignificant, meaning that they do not pose a threat to the patient’s longevity. In addition to having markedly different aggressiveness among tumors, there is also a great deal of heterogeneity within tumors. Thus, it is not sufficient to simply localize the tumor but it is also important to find regions within the tumor that are the most aggressive. To date, neither MRI nor PET has been sufficient for this task. For patients with low risk tumors (e.g., Gleason 3+3 low volume), there is increasing emphasis on using active surveillance instead of definitive treatment. For patients with organ-confined PCa that is considered intermediate or high risk, primary curative approaches, including radical prostatectomy (RP) or radiation therapy (RT), are advised provided that there is no evidence of metastatic disease. On the other hand, when the disease is metastatic, systemic therapies are more suitable (2).
The initial routine screening evaluation for PCa includes prostate specific antigen (PSA) screening followed by transrectal ultrasonography (TRUS)-guided systematic biopsy of the gland (10–12 cores) (3). Due to the high false positive rate of PSA and the non-guided nature of TRUS biopsy many low risk cancers are “overdiagnosed” even while higher risk tumors are underdiagnosed. Multi-parametric MRI (mpMRI), was initially introduced to improve detection of these underdiagnosed tumors. mpMRI is indicated after a negative TRUS-guided biopsy but with persistent clinical suspicion of PCa and the results can be used to guide prostate biopsy. However, the mpMRI simply identifies the location of the lesion without specifically identifying particular biologic “hot spots” within the tumor. Moreover, staging with regard to nodes or bony metastases are limited. Despite the high success rates of primary definitive therapy options including radiation therapy (RT) and RP, PCa mortality has not decreased significantly and about 15–25% of cases still experience biochemical failure, or biochemical recurrence (BCR), following primary definitive therapy (4-9). Thus, there is increasing interest in PET imaging in PCa (10), as it provides more functional information than other imaging modalities and can detect metastases. In PET, radiolabeled biomolecules pertinent to cellular processes are used to detect metabolic activity or cell surface molecules that are usually associated with cancer. PET imaging is usually combined with CT or MRI which improves anatomic localization of any abnormal tracer uptake. Furthermore, CT or MRI helps differentiate physiologic activity (e.g., activity in the ureters) from pathologic uptake (11). Clinical experience with PET in PCa is increasing. PET might also be useful for identifying patients suitable for active surveillance (AS), for accurate pre-radical prostatectomy/pre-radiation therapy staging. However, the increased costs of PET compared with other imaging methods means that the choice of PET in an imaging algorithm must be judiciously considered.
The most well-known PET agent is 18F-fluorodeoxyglucose (18F-FDG) which was introduced as a routine clinical imaging method in the early 2000s. It has been the mainstay of clinical molecular imaging in cancer. However, PCas are not particularly avid for 18F-FDG. Recently, several newer tracers have been introduced. They are able to target a variety of metabolites (e.g., glucose, fatty acids, and amino acids), antigens (e.g., prostate-specific membrane antigen and prostate-specific stem cell antigen), angiogenesis, hypoxia, and gene-based pathways (10). Still, very few of these agents are available clinically and even fewer are reimbursed (12). The most common PET radiotracers in the imaging evaluation of PCa are summarized in Table 1.
Table 1. Potential PET tracers for prostate cancer.
| PET tracer | Mechanism | Cyclotron requirements |
|---|---|---|
| 18F-FDG | Glucose metabolism | Regional |
| 11C/18F-acetate | Lipid metabolism | On site/regional |
| 11C/18F-choline | Lipid metabolism | On site/regional |
| 11C-methionine | Amino acid transport | On site |
| 18F-FACBC | Amino acid transport | Regional |
| 18F-DCFBC; 18F-DCFPyL; 64Cu/89Zr-J591 | PSMA inhibitors/antibodies | Regional |
| 68Ga-PSMA | PSMA inhibitors/antibodies | Generator (cyclotron independent) |
| 18F-FDHT | Androgen receptor | Regional |
| 18F-NaF | Calcium analog | Regional |
| 68Ga-Bombesin | Gastrin releasing peptide | Generator (cyclotron independent) |
Here, we review the current state-of-art in PET imaging in localized PCa staging and risk assessment, pointing out the most important achievements and highlighting the remaining complexities.
18F-fluorodeoxyglucose (18F-FDG)
FDG is a glucose analog with replacement of the oxygen in the C-2 position with 18-fluorine. Its uptake is elevated in most primary and metastatic cancers due to malignancy-related increased glucose uptake due to Warburg physiology or aerobic glycolysis that is inherently less efficient than oxidative phosphorylation (13). 18F has a half-life of 110 minutes making it ideal for clinical use and 18F also has favorable imaging characteristics. However, its excretion into the urinary system poses a problem for pelvic malignancies. Although, 18F-FDG shows an excellent performance in many malignant lesions, in PCa, this tracer is limited due to generally low glucose utilization in PCa cells (14-17). Moreover, in 18F-FDG PET/CT studies, the tracer showed overlapping uptake in normal, benign and malignant tissues resulting in poor specificity (18-20). A recent systematic review and meta-analysis of 3,586 men with PCa compared the diagnostic accuracy among four PET/CT radiotracers (18F-FDG, 11C-choline, 18F-choline and 11C-acetate) suggesting diagnostic superiority of 18F-choline, ranked as the most favorable with the highest value of AUC (AUC =0.94; 95% CI: 0.92–0.96), whereas 18F-FDG was the least favorable (AUC =0.73; 95% CI: 0.69–0.77) for PCa detection (2). On the other hand, a recent review suggests that, 18F-FDG might be helpful for restaging purposes in patients with advanced PCa metastatic disease. However, evidence for its value in the initial staging of PCa remains scant and it is not recommended (21,22). Despite its wide availability and use in cancer imaging, 18F-FDG PET/CT has low specificity for PCa, and consequently, its use is reserved for late stage metastatic disease.
11C/18F-acetate
Acetate is a vital part of fatty acid metabolism. It is converted by Acetyl-CoA synthetase to Acetyl-CoA, which is further converted by fatty acid synthetase (FAS) into fatty acids, which are then incorporated into the cellular membrane. The increased cell turnover in malignant cells results in relatively more uptake in cancers compared to normal tissue. Increased acetate uptake due to increased FAS activity in cells has been shown to correlate with aggressiveness of PCa (23). Acetate can be labeled with either 11C or 18F although the most viable tracer by far is 11C-Acetate. 11C-acetate is excreted in the pancreas, liver and bowel, with relatively minor kidney uptake and urinary system excretion (24). In PCa imaging of primary tumors, the absence of 11C-acetate in the urinary tract is advantageous, especially if local recurrent disease is suspected.
Overall, 11C-acetate has shown some promise in advanced PCa detection, but low specificity in evaluating localized disease. In a study by Mena et al. (25), 39 patients with localized PCa underwent 11C-Acetate PET/CT prior to radical prostatectomy to characterize the difference in the tracer uptake between PCa lesions, BPH and normal prostate tissue. The average SUVmax values were correlated to mpMRI findings, whole mount histopathology, fatty acid synthase expression and clinical markers. Although there was a higher tracer uptake in tumor foci compared with unaffected prostate tissue, the difference in 11C-Acetate uptake between cancer lesions and BPH nodules was not significant, with considerable overlap in uptake. Furthermore, on a sector-based comparison with histopathology for all lesions >0.5 cm, 11C-Acetate PET/CT showed much lower sensitivity and specificity compared to mpMRI, respectively 61.1% and 80.0% vs. 82.3% and 95.1%, suggesting low utility of 11C-Acetate PET/CT as an independent modality for detecting and staging localized PCa. Additionally, no significant correlation was found between 11C-Acetate uptake and clinical markers such as PSA levels (r=−0.128) or fatty acid synthase expression in tumor. Similarly, Oyama et al. (26) studied the potential of 11C-acetate to image primary and metastatic PCa. In their study, 22 patients with PCa underwent 11C-acetate PET imaging and the primary PCa lesions were identified in all patients, with high sensitivity for detection of metastatic PCa lymph nodes (100%) and bone metastases (86%). However, there was no analysis of sensitivity for metastatic disease as a function of PSA which is a critical index when comparing PET agents. In general, primary PCa and metastatic sites were detected with higher sensitivity with 11C-acetate than 18F-FDG. Furthermore, no 11C-acetate accumulation in the urine was present (26). Haseebuddin et al. (27), reported on 107 biopsy-proven PCa patients with intermediate/high risk tumors who underwent staging 11C-acetate PET/CT before radical prostatectomy (RP). They found a sensitivity of only 68% and specificity of 78% for detection of pelvic lymphadenopathy. Moreover, patients with positive PET scans had a 3.3-fold higher risk for therapy failure after RP (27).
Recent studies suggest that lipogenesis tracers may be useful in the detection of tumor recurrence in patients with suspicion of BCR, who had been treated previously with RP or RT. However, only a few studies have investigated its role in metastatic PCa.
18F-labeled acetate has also been reported as a potential PCa imaging agent due to its desirable physical properties, although experience with this tracer remains limited. The Swedish Uppsala University group (28) studied the biodistribution of 18F-acetate and 11C-acetate in cynomolgus monkeys and one domestic pig. In this study, 18F-acetate had protracted blood retention, rapid clearance from liver, excretion in bile and urine, and defluorination (i.e., high bone uptake). Thus, 18F-acetate is not a functional equivalent of 11C-acetate and therefore, is not likely a viable clinical imaging agent.
11C/18F-choline
Radiolabeled choline tracers are perhaps the most widely available PCa PET agents worldwide and they have been broadly, if not deeply, studied in recent years. Choline tracers bear a strong resemblance to acetate tracers in their performance (29). Choline is a precursor for the biosynthesis of phospholipids, which are major components of the cellular membrane. Choline binds to choline transporters which internalizes it. It is believed, that the biologic basis for the accumulation of radiolabeled choline in tumors is, in part, due to overexpression of choline kinase which is necessary for cellular membrane synthesis (10,30). Though both 11C- and 18F-choline tracers are similar in principle they vary greatly from each other in physical half-life and physiologic excretion patterns. 11C-labeled choline has a short half-life (20 minutes), and is primarily excreted via the hepatobiliary system with only little urinary excretion, which is advantageous for the evaluation of the prostate gland (31-33). 18F-fluorocholine is excreted by the urinary tract leading to higher accumulation of the tracer in the bladder, which is less favorable for PCa imaging. However, 18F-fluorocholine has a longer half-life (110 minutes) which makes it more practical (31).
A wide range of overall sensitivity (73–91%) and specificity (43–86%) have been reported for 11C-/18F-labelled choline derivatives (34). In primary disease, specificity is reduced by high uptake of these agents in BPH and other benign conditions. However, 11C-/18F-cholines are useful in patients with suspected recurrence after first line or salvage therapies (34-36), and for that reason the FDA approved the use of 11C-choline for BCR evaluation in September 2012. The studies that led to this approval showed that in patients with BCR, 11C-choline PET/CT detected more sites of recurrence than 18F-FDG PET/CT and many of these were validated by biopsy or surgery as metastases (37). On the other hand, Castellucci et al. (38) performed 11C-choline PET in 605 patients evaluated for early BCR after RP with a resulting detection rate of only 28%, likely reflecting a much earlier cohort of recurrence. In addition to limited sensitivity there are issues with specificity. Suardi et al. (39) reported a 20% false positive rate for lymph nodes with 18F-choline PET. However, most studies showed that the cholines are useful in the detection of skeletal bone metastases (31,40). Overall, the role of radiolabeled choline tracers is to detect recurrences in BCR, while use in the detection of primary PCa and staging has marked limitations. Nevertheless, the cholines, while better than no study at all, are not sufficient sensitive or specific to warrant routine use. No commercial source for 18F-choline is available in the U.S. and 11C-choline is only available at selected sites.
Anti-1-amino-3-(18F)-fluorocyclobuate-1-carboxylic (18F-FACBC)
18F-FACBC (also known as fluciclovine or Axumin) is a synthetic isoleucine analog that is taken up by amino acid transporters leading to intracellular accumulation. Renal excretion is delayed relative to imaging creating a favorable imaging “window” wherein there is relatively little bladder activity at the time of imaging (41). The normal biodistribution of 18F-FACBC is mainly in the pancreas and liver, with lesser activity in the bone marrow (42). Similar to acetate and choline, 18F-FACBC-PET shows high sensitivity for primary PCa but lacks specificity with regard to BPH and inflammation (42-48). In a prospective study by Turkbey et al. (49) 21 men with localized PCa, BPH, and normal prostate tissue were scanned with 18F-FACBC-PET and MR imaging with histopathology available in all patients which was analyzed on a per-lesion and per-sector basis. 18F-FACBC PET/CT detected localized PCa with a sensitivity and specificity of 67% and 66%, localizing dominant prostate tumors with a sensitivity of 90%. However, MRI was indispensable as the combined use of 18F-FACBC-PET/CT and mp-MRI yielded a positive predictive value of 82% for tumor localization in the sector-based analysis. In a retrospective analysis of Kairemo et al. (42), 18F-FACBC-PET/CT images of 26 patients were analyzed and compared to PSA concentrations and PSA doubling times (PDT). There was no statistically significant difference in PSA level between the patients with positive and negative findings; on the other hand, patients with positive FACBC PET findings showed significantly shorter PDT comparing to patients with negative PET indicating that a positive FACBC potentially indicates a more aggressive tumor. For more advanced disease 18F-FACBC performs similarly to 11C-acetate and 11C/18F-choline, however for BCR, 18F-FACBC seems to be more sensitive than 11C-choline based on the sensitivity vs PSA in the BCR setting (48). Consequently, 18F-FACBC has been recently approved by the FDA for the detection of recurrent PCa (50). However, a meta-analysis by Ren et al. (51) of 251 patients showed a relatively high false positive rate for 18F-FACBC in detecting recurrent PCa, with a pooled sensitivity of 87% but a specificity of only 66%. Currently, the evidence in the literature suggests that 18F-FACBC PET may play a limited role in staging of primary PCa, however, with its regulatory approval more data will likely emerge especially in comparison to PSMA-based agents, discussed next.
Prostate-specific membrane antigen (PSMA)
PSMA is a type II membrane glycoprotein that is overexpressed on prostate tumor cells and thus provides a rational target not only for diagnosis and monitoring but also for targeted therapy. PSMA expression appears to correlate with disease aggressiveness (52). A variety of ligands targeting PSMA have been developed but they all target the enzymatic portion of PSMA and therefore mimic the substrate that normally binds to PSMA. 68Ga-PSMA has been mostly studied in Germany whereas 18F-DCFPyL has been studied mostly in the United States. 68Ga-labeled PSMA HBED-CC (68Ga-PSMA) was developed in Germany and has been widely studied there. It consists of a targeting moiety and a chelate to which is added 68Ga. 68Ga is obtained from generators that are on site. At first glance this seems like an advantage but it requires that each dose of the conjugate be made separately. Moreover, the approximately 70 minutes half-life of 68Ga means that the agent must be used quickly after radiolabeling and likely cannot be distributed from a central geographic source. Recent studies demonstrate excellent performance of 68Ga-PSMA PET/CT compared to conventional imaging including PET with other tracers (e.g., 18F-Choline, 11C-Choline) with regard to local staging (53-58). 68Ga-PSMA PET/CT has been reported to clearly improve detection of lymph node metastases compared to morphological imaging for staging primary PCa (55). 68Ga-PSMA PET/CT had a high specificity of 98% and a moderate sensitivity of 56% for LN-detection (59). There is considerable tracer uptake seen in other solid tumors such as thyroid, colon, kidney, glioblastoma (55,57,60,61) and in normal anatomical structures (e.g., coeliac, cervical ganglia, salivary glands, kidney). The alternative to the Gallium-based PSMA tracers is 18F-DCFPyL which has the advantages of being cyclotron produced with a longer half-life and more favorable energy levels, improving resolution. Larger batches of the 18F-PSMA agents can be made once during the day and distributed around a metropolitan area which has practical importance. However, unlike the 68Ga compounds, the 18F-PSMA agents are directly fluorinated and there is no chelate. In a prospective study, Rowe et al. (62) studied the uptake of a precursor to 18F-DCFPyL, 18F-DCFBC, and found focal 18F-DCFBC uptake in high-volume lesions with Gleason score ≥7 while focal uptake was rarely seen in small-volume and Gleason score 6 disease (ρ-coefficient =0.65). Additionally, they observed significantly higher 18F-DCFBC uptake in high-grade PCa lesions compared to BPH. However, large-scale prospective studies are needed to prove the diagnostic efficacy and accuracy of this tracer, before implementing in clinical practice.
The true clinical value of PSMA PET/CT lies in its much higher sensitivity for recurrent disease. A recent meta-analysis involving several 68Ga-PSMA-11 PET articles covering 1309 BCR patients reported a summary sensitivity and specificity of 80% and 97%, respectively on a per-lesion analysis (63). Even patients with very low levels of PSA were identified on 68Ga-PSMA-11 PET. In a retrospective study Eiber et al. (56) studied 248 men with BCR using 68Ga-PSMA and detected a malignant lesion in 90%. Higher PSA levels and higher PSA velocity were correlated with higher tumor detection rates, but no significant association was seen with PSA doubling time. Interestingly, 68Ga-PSMA-11 PET/CT was more frequently positive in patients receiving ADT at the time of the scan than in patients without such treatment (57). The relationship between PSMA expression and ADT is not yet completely understood. In preclinical studies, antiandrogens initially upregulated PSMA expression (64), but prolonged ADT seemed to downregulate PSMA expression over time (65). This controversy will require additional study in the near future.
There is a general consensus that PSMA is superior to the other agents used for PCa in terms of sensitivity and particularly specificity although there is a paucity of head-to-head comparisons. Afshar-Oromieh et al. (53), compared 68Ga-PSMA and 18F-choline in patients with BCR. Overall, 68Ga-PSMA outperformed 18F-choline in terms of detection of metastatic lesions. Despite its theoretical advantages there are few studies comparing 18F-DCFPyL and 68Ga-PSMA (66). PSMA-based tracers show outstanding sensitivity and specificity for PCa and among all the novel radiotracers, this class of agent is most likely to become universally available in the clinic in the next few years (Figure 1).
Figure 1.
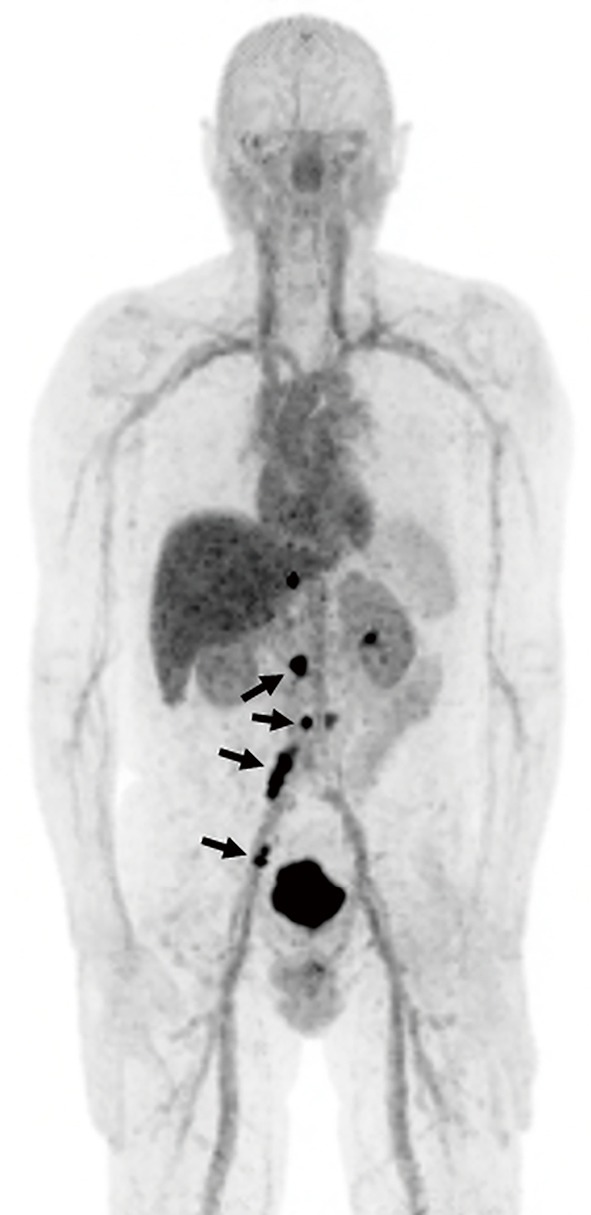
A 66-year-old man with metastatic prostate cancer. 18F DCFBC PET scan demonstrates metastatic para-aortic and iliac lymph nodes with overexpression of PSMA (arrows).
Fluorine-18-labeled sodium fluoride (18F-NaF)
18F-NaF is an old tracer first approved for clinical use by the FDA in 1972 (67). This agent is used exclusively for imaging bone metastases and is not specific for PCa. It is mostly used in staging of high risk cancers. 18F-NaF-PET/CT provides rapid, bone-specific uptake and excellent visualization of the axial skeleton compared to 99mTc-MDP bone scan. But, 18F-NaF is not a comprehensive PET agent and is only capable of detecting bone metastases. In a recent prospective study, Apolo et al. (68) evaluated the ability of 18F-NaF-PET/CT to detect and monitor bone metastases over time in 60 patients with advanced PCa, who received primary definitive therapy. 18F-NaF-PET/CT detected more bone metastases than 99mTc-based bone scans, in particular in patients with high metastatic risk without any known bone metastases on standard imaging (PSA of ≥10 ng/mL or a PSA doubling time of <6 months). The baseline number of malignant lesions and changes in SUV on follow-up 18F-NaF-PET/CT scans significantly correlated with clinical impression and overall survival. For bone metastases 18F-NaF compares favorably with other imaging agents. Azad et al. (69) concluded that 18F-NaF, 11C-choline and 18F-choline PET/CT have equal sensitivities in finding PCa bone metastases although the specificity is higher for the choline-based agents. However, while sensitive, 18F-NaF is criticized for its non-specificity and has not received Medicare reimbursement approval in the United States. In summary, 18F-NaF-PET/CT is most useful in high-risk patients with a negative or equivocal 99mTc-MDP bone scan because of its ability to detect occult bony metastatic disease (Figure 2).
Figure 2.

A 60-year-old man with metastatic prostate cancer. 18F NaF PET demonstrates focal uptake corresponding to bone metastasis but also areas of benign degenerative disease.
Dihydrotestosterone analogs (18F-FDHT)
The androgen receptor (AR) is crucial for the growth of PCa. Typically, PCa cells require testosterone and its derivative dihydrotestosterone (DHT) for growth and androgen deprivation therapy (ADT) interferes with tumor growth by blocking AR until castration resistant clones develop (70). ADT is thus, a first line therapy for patients with advanced PCa eventually resulting in castration resistant prostate cancer (CRPC) (71). AR overexpression is present in the majority of CRPC patients indicating there are alternate pathways to activating the AR axis. AR-targeted imaging with PET can predict AR expression levels, and consequently show the potential to image and assess the cancer, as well as to detect the therapeutic effect of AR-targeted drugs in specific patients. 18F-16β-fluoro-5α-dihydrotestosterone (18F-FDHT) is chemically similar to DHT. In a clinical trial conducted by Larson et al. (72) which included patients with advanced aggressive PCa, 18F-FDHT showed lower sensitivity for PCa detection compared to 18F-FDG (86% vs. 97%, respectively). However, there were lesions seen by both scans, lesions seen by on only one and lesions seen by neither. This is a tantalizing result that is still unclear in its meaning. However, for in vivo estimation of the AR expression in patients on ADT, 18F-FDHT may be the better PET tracer for the assessment of treatment response (72). To date, there is limited clinical data regarding the role of 18F-FDHT. However, this radiotracer showed utility in the assessment of AR blockade with 2nd line anti-androgens (31,73).
Bombesin/gastrin-releasing peptide
Gastrin-releasing peptide (GRP) is a 10-amino acid peptide involved in multiple physiological and pathophysiological processes. GRP binds to 7-transmembrane G-protein coupled receptor which promote tumor growth (74). High GRP receptor (GRPR) overexpression is present in many different cancers, such as breast, lung, urinary tract and prostate. Not all PCas express GRPR equally. de Visser et al. reported low GRPR expression levels in poorly differentiated PCa (75). However, peptides and their receptors present advantages for PET imaging such as exceptional stability and rapid clearance from the blood, rapid tissue penetration, as well as low immunogenicity, which are well suited for both diagnostic and therapeutic purposes (76).
Two classes of GRP/bombesin analogues labeled with a variety of different PET radioisotopes have been developed, GRPR radioagonists and radioantagonists. Prior studies have shown high binding affinity of agonists to GRPRs, and their subsequent cell internalization was believed to enhance the uptake, because the radioactivity was trapped in the intracellular compartment. However, other studies proved that antagonists are superior to agonists, because of their considerably higher binding affinity to the GRPR (77,78). One of the most investigated radioantagonists for PCa imaging is statine-based JMV594 (DPhe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2), first designed by Llinares et al. (79) and then modified. Moreover, labeling antagonists with 64Cu, 68Ga and 18F led to optimization of pharmacokinetic properties. Overall, studies have shown high efficiency of antagonists in the detection of primary PCa, while for bone metastases and BCR, the detection rates were much lower (74,80-82). However, more clinical data is needed on the use and efficacy of GRP targeted imaging in combination with PET in patients with PCa compared to PSMA based agents.
Summary/conclusions
Early diagnosis and accurate staging of clinically significant PCas are the most pivotal factors determining outcome. Because 18F-FDG performs poorly in PCa numerous PET agents have been developed to identify primary tumors, stage them, detect recurrence after treatment and monitor metastases. The clinical use of these agents in PCa is being investigated, however, it is very difficult to compare different agents as patient populations and scanner variability limit comparisons and it is difficult to combine 2 or more PET agents in one study due to the expense and radiation exposure. Despite the considerable limitations, PET seems to be useful for diagnosis and staging of known or suspected primary PCa with high Gleason scores, in the detection of BCR in locally recurrent or metastatic disease in patients with rising PSA levels, in monitoring response to therapies and in prognostication. The role of PET imaging in patients with PCa is likely to expand particularly in improving initial staging and in more accurate localization of sites of recurrence both of which may enable more focal therapies for recurrence. However, most radiolabeled agents are still in the early stage of clinical evaluation and therefore it is difficult to comment on which agent is the most useful for imaging of primary disease and risk stratification. Moreover, the expense of such agents will slow their development in an era of medical cost containment.
In summary, 18F-FDG, the most common PET radiotracer is generally limited in the diagnosis and staging of clinically organ-confined PCa, however, it may be able to indicate the aggressiveness of disease. Similarly, radiolabeled acetate and choline tracers as well as 18F-FACBC, are equally useful in imaging locally recurrent or metastatic disease in men with biochemical relapse, but their ability to detect primary PCa showed marked limitations. PSMA is a more sensitive and specific radiotracer with a high sensitivity for early recurrent disease. Dihydrotestosterone analogs have a more limited role in detecting AR and effects of androgen blockage. Finally, GRP targeted PET imaging has shown high efficiency in the detection of primary PCa, while the sensitivity for bone metastases and BCR was much lower. On the other hand, in recent bone metastases seeking studies 18F-NaF showed superiority, comparing to standard imaging, in detecting occult bone metastases, in particular in high-risk patients with PCa. Still, more clinical data is needed to prove the efficacy of these PET tracers. Additional studies for existing PET tracers and the development of other novel radiopharmaceuticals are expected in the future as it is clear that the ideal agent has not yet been developed.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Chen Z, Wang T, et al. Influence of Four Radiotracers in PET/CT on Diagnostic Accuracy for Prostate Cancer: A Bivariate Random-Effects Meta-Analysis. Cell Physiol Biochem 2016;39:467-80. 10.1159/000445639 [DOI] [PubMed] [Google Scholar]
- 3.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol 1994;151:1571-4. [DOI] [PubMed] [Google Scholar]
- 4.Shikanov S, Kocherginsky M, Shalhav AL, et al. Cause-specific mortality following radical prostatectomy. Prostate Cancer Prostatic Dis 2012;15:106-10. 10.1038/pcan.2011.55 [DOI] [PubMed] [Google Scholar]
- 5.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol 2000;163:1632-42. 10.1016/S0022-5347(05)67511-8 [DOI] [PubMed] [Google Scholar]
- 6.Laufer M, Pound CR, Carducci MA, et al. Management of patients with rising prostate-specific antigen after radical prostatectomy. Urology 2000;55:309-15. 10.1016/S0090-4295(99)00465-3 [DOI] [PubMed] [Google Scholar]
- 7.Zumsteg ZS, Spratt DE, Romesser PB, et al. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era for prostate patients undergoing external beam radiotherapy. J Urol 2015;194:1624-30. 10.1016/j.juro.2015.06.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol 2015;67:1009-16. 10.1016/j.eururo.2014.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertan FV, Greer MD, Borofsky S, et al. Multiparametric Magnetic Resonance Imaging of Recurrent Prostate Cancer. Top Magn Reson Imaging 2016;25:139-47. 10.1097/RMR.0000000000000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jadvar H. Molecular Imaging of Prostate Cancer: PET Radiotracers. AJR Am J Roentgenol 2012;199:278-91. 10.2214/AJR.12.8816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vali R, Loidl W, Pirich C, et al. Imaging of prostate cancer with PET/CT using 18F-Fluorocholine. Am J Nucl Med Mol Imaging 2015;5:96-108. [PMC free article] [PubMed] [Google Scholar]
- 12.Bonekamp D, Jacobs MA, El-khouli R, et al. Advancements in MR imaging of the prostate: from diagnosis to interventions. Radiographics 2011;31:677-703. 10.1148/rg.313105139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Townsend DW. Dual-modality imaging: combining anatomy and function. J Nucl Med 2008;49:938-55. 10.2967/jnumed.108.051276 [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Murray I, Brock C, et al. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol 2007;51:1511-20; discussion 1520-1. 10.1016/j.eururo.2007.01.061 [DOI] [PubMed] [Google Scholar]
- 16.von Mallek D, Backhaus B, Muller SC, et al. Technical limits of PET/CT with 18FDG in prostate cancer. Aktuelle Urol 2006;37:218-21. 10.1055/s-2006-932129 [DOI] [PubMed] [Google Scholar]
- 17.Jadvar H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat Rev Urol 2009;6:317-23. 10.1038/nrurol.2009.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avril N, Dambha F, Murray I, et al. The clinical advances of fluorine-2-Ddeoxyglucose--positron emission tomography/computed tomography in urological cancers. Int J Urol 2010;17:501-11. 10.1111/j.1442-2042.2010.02509.x [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Koch S. Positron emission tomography/computed tomography potential pitfalls and artifacts. Curr Probl Diagn Radiol 2009;38:156-69. 10.1067/j.cpradiol.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 20.Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol 2008;179:34-45. 10.1016/j.juro.2007.08.176 [DOI] [PubMed] [Google Scholar]
- 21.Jadvar H. Positron emission tomography in imaging evaluation of staging, restaging, treatment response, and prognosis in prostate cancer. Abdom Radiol (NY) 2016;41:889-98. 10.1007/s00261-015-0563-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu CY, Desai B, Ji L, et al. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging 2014;4:580-601. [PMC free article] [PubMed] [Google Scholar]
- 23.Madigan AA, Rycyna KJ, Parwani AV, et al. Novel nuclear localization of fatty acid synthase correlates with prostate cancer aggressiveness. Am J Pathol 2014;184:2156-62. 10.1016/j.ajpath.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seltzer MA, Jahan SA, Sparks R, et al. Radiation dose estimates in humans for (11)C-acetate whole-body PET. J Nucl Med 2004;45:1233-6. [PubMed] [Google Scholar]
- 25.Mena E, Turkbey B, Mani H, et al. 11C-Acetate PET/CT in localized prostate cancer: a study with MRI and histopathologic correlation. J Nucl Med 2012;53:538-45. 10.2967/jnumed.111.096032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyama N, Akino H, Kanamaru H, et al. 11C-acetate PET imaging of prostate cancer. J Nucl Med 2002;43:181-6. [PubMed] [Google Scholar]
- 27.Haseebuddin M, Dehdashti F, Siegel BA, et al. 11C-acetate PET/CT before radical prostatectomy: nodal staging and treatment failure prediction. J Nucl Med 2013;54:699-706. 10.2967/jnumed.112.111153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindhe O, Sun A, Ulin J, et al. [(18)F]Fluoroacetate is not a functional analogue of [(11)C]acetate in normal physiology. Eur J Nucl Med Mol Imaging 2009;36:1453-9. 10.1007/s00259-009-1128-7 [DOI] [PubMed] [Google Scholar]
- 29.Buchegger F, Garibotto V, Zilli T, et al. First imaging results of an intraindividual comparison of (11)C-acetate and (18)F-fluorocholine PET/CT in patients with prostate cancer at early biochemical first or second relapse after prostatectomy or radiotherapy. Eur J Nucl Med Mol Imaging 2014;41:68-78. 10.1007/s00259-013-2540-6 [DOI] [PubMed] [Google Scholar]
- 30.Ackerstaff E, Pflug BR, Nelson JB, et al. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res 2001;61:3599-603. [PubMed] [Google Scholar]
- 31.Lindenberg L, Choyke P, Dahut W. Prostate cancer imaging with novel PET tracers. Curr Urol Rep 2016;17:18. 10.1007/s11934-016-0575-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause BJ, Souvatzoglou M, Treiber U. Imaging of prostate cancer with PET/CT and radioactively labeled choline derivates. Urol Oncol 2013;31:427-35. 10.1016/j.urolonc.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 33.Bauman G, Belhocine T, Kovacs M, et al. 18F-fluorocholine for prostate cancer imaging: a systematic review of the literature. Prostate Cancer Prostatic Dis 2012;15:45-55. 10.1038/pcan.2011.35 [DOI] [PubMed] [Google Scholar]
- 34.Schwarzenböck S, Souvatzoglou M, Krause BJ. Choline PET and PET/CT in Primary Diagnosis and Staging of Prostate Cancer. Theranostics 2012;2:318-30. 10.7150/thno.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evangelista L, Guttilla A, Zattoni F, et al. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol 2013;63:1040-8. 10.1016/j.eururo.2012.09.039 [DOI] [PubMed] [Google Scholar]
- 36.Kjölhede H, Ahlgren G, Almquist H, et al. (18)F-choline PET/CT for early detection of metastases in biochemical recurrence following radical prostatectomy. World J Urol 2015;33:1749-52. 10.1007/s00345-015-1547-y [DOI] [PubMed] [Google Scholar]
- 37.von Eyben FE, Kairemo K. Meta-analysis of (11)C-choline and (18)F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun 2014;35:221-30. 10.1097/MNM.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 38.Castellucci P, Ceci F, Graziani T, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-Choline PET/CT scan before salvage radiation therapy? J Nucl Med 2014;55:1424-9. 10.2967/jnumed.114.138313 [DOI] [PubMed] [Google Scholar]
- 39.Suardi N, Gandaglia G, Gallina A, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol 2015;67:299-309. 10.1016/j.eururo.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 40.Wondergem M, van der Zant FM, van der Ploeg T, et al. A literature review of 18F-fluoride PET/CT and 18F-choline or 11C-choline PET/CT for detection of bone metastases in patients with prostate cancer. Nucl Med Commun 2013;34:935-45. 10.1097/MNM.0b013e328364918a [DOI] [PubMed] [Google Scholar]
- 41.Oka S, Hattori R, Kurosaki F, et al. A preliminary study of anti-1-amino-3-18Ffluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med 2007;48:46-55. [PubMed] [Google Scholar]
- 42.Kairemo K, Rasulova N, Partanen K, et al. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. Biomed Res Int 2014;2014:305182. 10.1155/2014/305182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sörensen J, Owenius R, Lax M, et al. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging 2013;40:394-402. 10.1007/s00259-012-2291-9 [DOI] [PubMed] [Google Scholar]
- 44.Schuster DM, Taleghani PA, Nieh PT, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging 2013;3:85-96. [PMC free article] [PubMed] [Google Scholar]
- 45.Amzat R, Taleghani P, Miller DL, et al. Pilot study of the utility of the synthetic PET amino-acid radiotracer anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid for the noninvasive imaging of pulmonary lesions. Mol Imaging Biol 2013;15:633-43. 10.1007/s11307-012-0606-7 [DOI] [PubMed] [Google Scholar]
- 46.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3- 18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology 2011;259:852-61. 10.1148/radiol.11102023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nanni C, Schiavina R, Boschi S, et al. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging 2013;40:S11-S17. 10.1007/s00259-013-2373-3 [DOI] [PubMed] [Google Scholar]
- 48.Nanni C, Schiavina R, Brunocilla E, et al. 18F-FACBC compared with 11C-Choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer 2014;12:106-10. 10.1016/j.clgc.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 49.Turkbey B, Mena E, Shih J, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology 2014;270:849-56. 10.1148/radiol.13130240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okudaira H, Shikano N, Nishii R, et al. Putative transport mechanism and intracellular fate of trans -1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med 2011;52:822-9. 10.2967/jnumed.110.086074 [DOI] [PubMed] [Google Scholar]
- 51.Ren J, Yuan L, Wen G, et al. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol 2016;57:487-93. 10.1177/0284185115581541 [DOI] [PubMed] [Google Scholar]
- 52.Bouchelouche K, Choyke PL, Capala J. Prostate-specific membrane antigen: a target for imaging and therapy with radionuclides. Discov Med 2010;9:55-61. [PMC free article] [PubMed] [Google Scholar]
- 53.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2014;41:11-20. 10.1007/s00259-013-2525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eiber M, Weirich G, Holzapfel K, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol 2016;70:829-36. 10.1016/j.eururo.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 55.Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic Efficacy of Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging in Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol 2016;195:1436-43. 10.1016/j.juro.2015.12.025 [DOI] [PubMed] [Google Scholar]
- 56.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015;56:668-74. 10.2967/jnumed.115.154153 [DOI] [PubMed] [Google Scholar]
- 57.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015;42:197-209. 10.1007/s00259-014-2949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roethke MC, Kuru TH, Afshar-Oromieh A, et al. Hybrid positron emission tomography-magnetic resonance imaging with gallium 68 prostate-specific membrane antigen tracer: a next step for imaging of recurrent prostate cancer-preliminary results. Eur Urol 2013;64:862-4. 10.1016/j.eururo.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen PJ, Emmett L, Ho B, et al. Prospective Evaluation of 68Gallium-PSMA Positron Emission Tomography/Computerized Tomography for Preoperative Lymph Node Staging in Prostate Cancer. BJU Int 2017;119:209-15. 10.1111/bju.13540 [DOI] [PubMed] [Google Scholar]
- 60.Demirci E, Ocak M, Kabasakal L, et al. (68)Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 2014;41:1461-2. 10.1007/s00259-014-2766-y [DOI] [PubMed] [Google Scholar]
- 61.Verburg FA, Krohn T, Heinzel A, et al. First evidence of PSMA expression in differentiated thyroid cancer using [(68)Ga]PSMA-HBED-CC PET/CT. Eur J Nucl Med Mol Imaging 2015;42:1622-3. 10.1007/s00259-015-3065-y [DOI] [PubMed] [Google Scholar]
- 62.Rowe SP, Gage KL, Faraj SF, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med 2015;56:1003-10. 10.2967/jnumed.115.154336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016;70:926-37. 10.1016/j.eururo.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 64.Wright GL, Mayer Grob B, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996;48:326-34. 10.1016/S0090-4295(96)00184-7 [DOI] [PubMed] [Google Scholar]
- 65.Liu T, Wu LY, Fulton MD, et al. Prolonged androgen deprivation leads to down regulation of androgen receptor and prostate specific membrane antigen in prostate cancer cells. Int J Oncol 2012;41:2087-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szabo Z, Mena E, Rowe SP, et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol Imaging Biol 2015;17:565-74. 10.1007/s11307-015-0850-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grant FD, Fahey FH, Packard AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med 2008;49:68-78. 10.2967/jnumed.106.037200 [DOI] [PubMed] [Google Scholar]
- 68.Apolo AB, Lindenberg L, Shih JH, et al. Prospective study evaluating Na18F-positron emission tomography/computed tomography (NaF-PET/CT) in predicting clinical outcomes and survival in advanced prostate cancer. J. Nucl. Med 2016;57:886-92. 10.2967/jnumed.115.166512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azad GK, Cook GJ. Multi-technique imaging of bone metastases: spotlight on PET-CT. Clin. Radiol 2016;71:620-31. 10.1016/j.crad.2016.01.026 [DOI] [PubMed] [Google Scholar]
- 70.Dehdashti F, Picus J, Michalski JM, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging 2005;32:344-50. 10.1007/s00259-005-1764-5 [DOI] [PubMed] [Google Scholar]
- 71.Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA guideline. J Urol 2013;190:429-38. 10.1016/j.juro.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 72.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alphadihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366-73. [PubMed] [Google Scholar]
- 73.Talbot JN, Gligorov J, Nataf V, et al. Current applications of PET imaging of sex hormone receptors with a fluorinated analogue of estradiol or of testosterone. Q J Nucl Med Mol Imaging 2015;59:4-17. [PubMed] [Google Scholar]
- 74.Koo PJ, Kwak JJ, Pokharel S, et al. Novel Imaging of Prostate Cancer with MRI, MRI/US, and PET. Curr Oncol Rep 2015;17:56. 10.1007/s11912-015-0480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Visser M, van Weerden WM, de Ridder CM, et al. Androgen-dependent expression of the gastrin- releasing peptide receptor in human prostate tumor xenografts. J Nucl Med 2007;48:88-93. [PubMed] [Google Scholar]
- 76.Krenning EP, Bakker WH, Breeman WA, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989;1:242-4. 10.1016/S0140-6736(89)91258-0 [DOI] [PubMed] [Google Scholar]
- 77.Ananias HJ, de Jong IJ, Dierckx RA, et al. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Curr Pharm Des 2008;14:3033-47. 10.2174/138161208786404335 [DOI] [PubMed] [Google Scholar]
- 78.Mansi R, Minamimoto R, Mäcke H, et al. Bombesin-Targeted PET of Prostate Cancer. J Nucl Med 2016;57:67S-72S. 10.2967/jnumed.115.170977 [DOI] [PubMed] [Google Scholar]
- 79.Llinares M, Devin C, Chaloin O, et al. Syntheses and biological activities of potent bombesin receptor antagonists. J Pept Res. 1999;53:275-83. 10.1034/j.1399-3011.1999.00028.x [DOI] [PubMed] [Google Scholar]
- 80.De Vincentis G, Remediani S, Varvarigou AD, et al. Role of 99mTc-bombesin scan in diagnosis and staging of prostate cancer. Cancer Biother Radiopharm 2004;19:81-4. 10.1089/108497804773391711 [DOI] [PubMed] [Google Scholar]
- 81.Scopinaro F, De Vincentis G, Varvarigou AD, et al. 99mTc-bombesin detects prostate cancer and invasion of pelvic lymph nodes. Eur J Nucl Med Mol Imaging 2003;30:1378-82. 10.1007/s00259-003-1261-7 [DOI] [PubMed] [Google Scholar]
- 82.Sah BR, Burger IA, Schibli R, et al. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J Nucl Med 2015;56:372-8. 10.2967/jnumed.114.147116 [DOI] [PubMed] [Google Scholar]


