Abstract
MRI-targeted biopsy is a promising technique that offers an improved detection of clinically significant prostate cancer over standard non-targeted biopsy. It is established that prostate MRI is of use in both the primary and repeat biopsy setting for the detection of significant prostate cancer. There are three approaches to targeting biopsies to areas of interest seen on prostate MRI. They each rely on the acquisition and reporting of a diagnostic quality multi-parametric MRI scan used to identify areas of interest, and the subsequent use of those diagnostic quality images in combination with real-time images of the prostate during the biopsy procedure. The three techniques are: visual registration of the MRI images with a real-time ultrasound image; software-assisted fusion of the MRI images and the real-time ultrasound images, and in-bore biopsy, which requires registration of a diagnostic quality MRI scan with a real time interventional MRI image. In this paper we compare the three techniques and evaluate those studies where there is a direct comparison of more than one MRI-targeting technique. PubMed was searched from inception to November 2016 using the search terms (cognitive registration OR visual registration OR fusion biopsy OR in-bore biopsy OR targeted biopsy) AND (prostate cancer OR prostate adenocarcinoma OR prostate carcinoma OR prostatic carcinoma OR prostatic adenocarcinoma) AND (MRI OR NMR OR magnetic resonance imaging OR mpMRI OR multiparametric MRI). The initial search included 731 abstracts. Eleven full text papers directly compared two or more techniques of MRI-targeting, and were selected for inclusion. The detection of clinically significant prostate cancer varied from 0% to 93.3% for visual registration, 23.2% to 100% for software-assisted registration and 29% to 80% for in-bore biopsy. Detection rates for clinically significant cancer are dependent on the prevalence of cancer within the population biopsied, which in turn is determined by the selection criteria [biopsy naïve, previous negative biopsy, prostate specific antigen (PSA) selection criteria, presence of a lesion on MRI]. Cancer detection rates varied more between study populations than between biopsy approaches. Currently there is no consensus on which type of MRI-targeted biopsy performs better in a given setting. Although there have been studies supporting each of the three techniques, substantial differences in methodology and reporting the findings make it difficult to reliably compare their outcomes.
Keywords: Image-guided biopsy, prostatic neoplasms, magnetic resonance imaging, prostate
Introduction
The current standard technique for prostate cancer detection in men with a high prostate specific antigen (PSA) or an abnormal finding on digital rectal examination (DRE) is a 10–12 core trans-rectal ultrasound-guided biopsy (TRUS-GB).
The use of a standardized 6-core biopsy over an approach targeting lesions visible on ultrasound was first described by Hodge and colleagues in 1989 (1). Cancer was found in 83 men (62%), and random and directed biopsies were done in 57 men. Of those 57 men the biopsy results were in agreement in 49 men (86%), whilst in 5 men (9%) random systematic biopsies found cancer and in 3 men (5%) directed biopsies found cancer missed on systematic biopsy. Although the original paper suggesting combining the use of systematic biopsies with targeted to biopsies to any hypoechoic areas not sampled by systematic biopsy, it became common practice to carry out systematic biopsy alone.
Over time, the number of cores included in systematic biopsies increased, so that many take 10–12 cores (2). This systematic technique has led to an increase in the detection of low-volume and low-risk disease.
Needle biopsy of the prostate is an invasive procedure, which can be associated with complications (including bleeding, pain, and infection) and it is therefore important to maximize the diagnostic information gained.
Moreover, standard TRUS guided biopsy is highly operator-dependent and is systematically poor at sampling tumors of the anterior part of the prostate, as well as the midline and extreme base. In addition, random error can result in tumors in the peripheral zone being missed or undersampled (3). This combination of random and systematic error explains the low values of sensitivity for cancer detection of systematic biopsy (3), which can also lead to a risk of pathological upgrading later on, seen in up to 40% of men at radical prostatectomy (4).
The European Association of Urology advise performing a multiparametric magnetic resonance imaging (mpMRI) study when initial standard biopsy results are negative but the suspicion of prostate cancer persists (2). This has been echoed by a joint consortium of the American Urology Association and the Society of Abdominal Radiology, which states that where high quality mpMRI is available, it should be strongly considered for men in whom a repeat biopsy is considered (5). In addition, the National Comprehensive Cancer Network (NCCN) guidelines advise the use of mpMRI prior to repeat biopsy, with mpMRI-targeted cores to be considered in addition to standard cores (6).
Recent advances in mpMRI [e.g., the combination of multiple sequences, such as T2-weighted, diffusion-weighted imaging (DWI) and dynamic contrast-enhancement] have improved the sensitivity of mpMRI for the detection of clinically significant prostate cancer. However, the diagnostic capability of prostate mpMRI is inherently dependent on a number of factors including the technical acquisition of the mpMRI images, the expertise of the radiologist reporting the images, the threshold used to define a lesion on mpMRI, and the definition of histologically significant prostate cancer (7).
The European Society of Urogenital Radiology (ESUR) has published recommendations on how to conduct and report an mpMRI study of the prostate (8). The ESUR was joined by the American College of Radiologists (ACR) when the Prostate Imaging-Reporting and Data System (PI-RADS) classification version 2.0 was recently updated (9). PI-RADS describes the standards for image acquisition and reporting, including the precise parameters required to predict the likelihood of significant disease using a 1–5 scale.
A systematic review in 2012 by Moore et al. (10) has shown that MRI-guided biopsies in the biopsy naïve prostate detect clinically significant prostate cancer in an equivalent number of men to standard biopsy. This is achieved using fewer biopsies in fewer men, and is associated with a reduction in the diagnosis of clinically insignificant cancer.
Wegelin et al. recently led a systematic review (11) to evaluate whether MRI-guided biopsies have increased detection rates of clinically significant prostate cancer compared to TRUS-GB, analyzing which MRI-guided technique has the highest detection rate. The Authors concluded that on a per patient basis MRI-guided biopsies had higher detection rates of clinically significant prostate cancer compared to TRUS-GB, as MRI-guided biopsies missed 10% of significant cancers whilst TRUS-GB missed 21%. The shift in emphasis between the two reviews shows the change in the literature over time, and indicates that the diagnostic efficiency of prostate MRI is improving over time.
In a study of 1,140 men (12) with a raised PSA it was shown that the proportion of men with cancer was higher among those randomized to mpMRI and MRI-targeted biopsy (417/570, 73%) compared to those randomized to TRUS-guided biopsy (215/570, 38%).
In another report of 1,003 men, Muthigi et al. (13) have recently reported that software-assisted MRI-targeted biopsy rarely missed clinically significant prostate cancer, as only 62 of 1,003 cases (6.2%) were upgraded to clinically significant disease by systematic biopsy.
MRI-targeted biopsy has been shown to significantly improve risk stratification by reducing sampling error (14) and evidence is accumulating in recommending mpMRI as a means of directing either initial or repeat biopsies of the prostate, following a previous negative TRUS-GB (15,16).
Some studies of MRI-targeted prostate biopsies have significantly higher rates of detection for clinically significant cancer, being associated with a higher percentage of positive cores and longer maximum cancer core length compared to systematic biopsies (10,17). Use of targeted cores alone can also reduce the detection of incidental, clinically insignificant tumors, although there is no widespread agreement that this is the optimal strategy (18).
Types of MRI-targeted biopsy
According to the Standards of Reporting for MRI-targeted Biopsy Studies (START) guidelines (19), an MRI-targeted biopsy is defined as a technique where an mpMRI scan is used to determine the location of a suspicious target within the prostate prior to biopsy.
There are three practical approaches to MRI-targeted biopsy: (I) visual registration; (II) software-assisted (fusion) registration and (III) direct in-bore biopsy. Currently, there is no consensus on whether any one type of MRI-targeted biopsy is superior in cancer detection or other areas, although visual registration is likely to be the least expensive of the three approaches.
In fact, all of these methods have shown the potential to address the drawbacks of TRUS-GB (i.e., false-negative biopsies and erroneous risk stratification due to under- or over-sampling) (20).
Visual registration
In visual registration (also referred to as “cognitive” registration in the literature) a suspicious lesion is identified on mpMRI by a radiologist prior to biopsy, and then targeted using TRUS guidance by the biopsy operator, who may be a radiologist, urologist, or other trained operator (e.g., nurse practitioner). The information on the location of the area of interest may be shown on a diagram (Figure 1) which can be hand drawn, or created using customized software, or snapshots of the areas of interest can be saved within the mpMRI file. Some reports will describe the location in prose (e.g., between 5 and 7 o’clock), although it is generally agreed that a visual report is the most helpful. Direct review of the mpMR images immediately prior to the biopsy is highly recommended.
Figure 1.
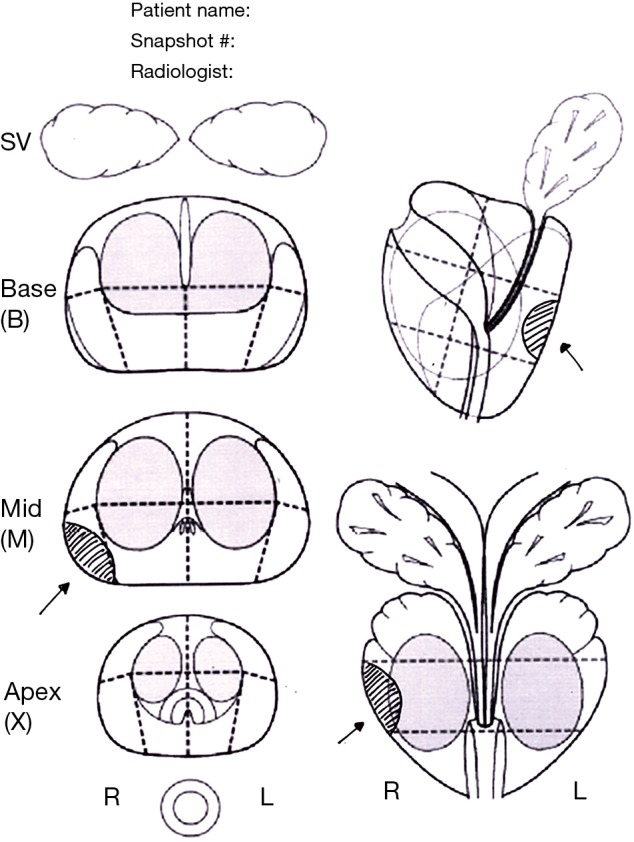
Hand drawn diagram made by the reporting radiologist that can be saved within the MRI file together with snapshots of the suspicious areas to be targeted. SV, seminal vesicles.
This approach requires a trained operator who is able to cognitively transfer the visual information from one format to another. MRI-targeted biopsies conducted by visual registration have been shown to increase the detection rates and accuracy for significant prostate cancer when compared with systematic biopsies (17,21).
Software-assisted fusion registration
Suspicious lesions are identified and contoured on the mpMR images—together with the whole prostate—prior to biopsy. The contours are then uploaded into the fusion software platform and converted into a 2D or 3D model, which is matched or registered (fusion) with the real-time US image during the procedure. This allows the operator to identify and target lesions deemed suspicious on mpMRI. This approach is also called MRI/TRUS fusion biopsy (22).
Three commonly used software-based platforms are Artemis (Eigen, Grass Valley, California, USA), Urostation (Koelis, LaTronche, France), and UroNav (Philips Electronics, Amsterdam, the Netherlands) (23,24).
Registration can be either rigid, where there is a direct overlay of the mpMR images over the ultrasound image, or elastic. Elastic registration takes into account the difference in the shape of the gland between the MRI scan (in supine position with or without an endorectal coil) and the ultrasound scan (done in lithotomy or left lateral position), where the ultrasound probe can deform the prostate. In addition, some systems aim to compensate for swelling and motion during the procedure.
MRI/TRUS fusion biopsies have demonstrated detection rates superior to systematic biopsies, particularly for higher-grade disease (25,26).
In-bore biopsy
This technique is carried out within the mpMRI scanner, and diagnostic quality images used to identify areas of interest are registered with the interventional images (often acquired at 1–1.5T). The target lesion is biopsied using guidance from serial scans during the procedure (27).
Different anatomical approaches can be used: transrectal (the most common), transperineal and transgluteal. One of the main advantages of this technique is the ease of registration of two sets of mpMR images acquired in a similar position. It is common practice in this approach that only a few targeted cores are taken, reducing the detection of insignificant tumors. However, this approach requires significant additional training for the radiologist and time in the MRI scanner, as well as training of the biopsy operator.
A systematic review by Overduin et al. (28) has shown that the cancer detection rates of in-bore biopsies range from 8% to 59% (median 42%) and that the majority of tumors detected are clinically significant (81–93%)
Potential methodological differences between studies
During MRI-targeted prostate biopsies the operator is privy both to mpMRI and ultrasound findings and has a real-time image of the lesion during the biopsy process. This has shown significant advantages over systematic TRUS-GB (29). However, there is considerable heterogeneity in the study designs reported in the literature. This is due to a number of differences that range from the definition of clinically significant disease to the mpMRI protocols used.
The definition of clinically significant prostate cancer is often based on the Epstein criteria (PSA density >0.15; more than a third of biopsy cores positive; more than 50% of involvement of any core) (30) or the d’Amico classification (Gleason score >6; PSA >10 ng/mL; > stage T1) (31). These were defined in the setting of a systematic biopsy. Of note, these classifications were derived from a previous analysis by Stamey et al. (32) where the tumor volume threshold of 0.5 cm3 for insignificant prostate cancer was obtained from 139 consecutively sampled radical cystoprostatectomy specimens. The Authors found that 55/139 men (40%) had incidental prostate cancer and—basing on epidemiological data—suggested that tumors measuring <0.5 cm3 were unlikely to reach a clinically significant size within a man’s life.
Due to the introduction of MRI-targeted biopsies, the definition of clinically significant prostate cancer has been changing and a number of new definitions have been proposed, though none have been widely recognized so far (18,33,34).
The true prevalence of prostate cancer in any given population is another confounding factor when assessing which technique is the best to assess the prevalence of clinically significant prostate cancer. For example, a screening population will have a lower prevalence of disease if compared to an unscreened population, or a selected group of men with a prior positive biopsy. Some studies on MRI-targeted biopsies are focused only on biopsy-naïve men with a raised PSA (primary biopsy) whilst others only on those men with a previous biopsy, regardless of the presence of prostate cancer (secondary biopsy).
The accuracy of mpMRI scans is another potential methodological issue, as studies not complying to the ESUR guidelines (8) could result in a lower detection rate of cancer at imaging.
The accuracy of biopsy sampling is also important; histologic information from MRI-targeted biopsies tends to show longer cancer core length and higher Gleason sum than TRUS-GB, due to the tendency to target the center of a tumor which tends to have the highest grade disease (35). Vargas et al. (36) reported that 20% of a group of 388 low-risk men had their cancers upgraded through the use of mpMRI and confirmatory biopsy.
In addition, because multiple cores are directed at a highly suspicious area, it is common to get a higher absolute number and proportion of positive cores than when the intention is to sample the gland in a systematic manner. This could result in different risk stratification of men, if a risk stratification system based on standard biopsy is used.
Evidence acquisition
In this paper, we look at each of the three techniques of MRI-targeted biopsy and report those studies which have compared outcomes of using more than one technique. We searched MEDLINE/PubMed for manuscripts published up to November 2016. The search terms used were (cognitive registration OR visual registration OR fusion biopsy OR in-bore biopsy OR targeted biopsy) AND (prostate cancer OR prostate adenocarcinoma OR prostate carcinoma OR prostatic carcinoma OR prostatic adenocarcinoma) AND (MRI OR NMR OR magnetic resonance imaging OR mpMRI OR multiparametric MRI).
If it was not clear from the abstract whether the paper might contain relevant data, the full paper was assessed. Overall, 731 publications were found. The literature search and study pre-selection is graphically displayed in Figure 2. As the majority of studies assessed one MRI-targeting technique against standard biopsies this is also discussed.
Figure 2.
Flow diagram showing the outcome of the initial searches resulting in the full studies included in the review. MRI, magnetic resonance imaging.
Evidence synthesis
Among the 189 reports which were assessed in full, 57 (30%) used visual registration, 108 (57%) used software assisted registration and 13 (7%) used in-bore MRI-targeted biopsies, with 11 (6%) reporting a comparison of different techniques.
Visual registration compared to standard biopsy
In a study by Park et al. (21) of biopsy-naïve men randomized to standard TRUS (n=41) or mpMRI-targeted biopsies (n=44), prostate cancer was detected in 30% (13 of 44) of the mpMRI-targeted group compared with 10% (4 of 41) of the standard TRUS biopsy group. The Authors concluded that in men with rising PSA and no previous biopsy, the use of mpMRI before TRUS-GB contributed to the detection of prostate cancer.
Haffner et al. (17) compared MRI-targeted biopsy with systematic biopsy in 555 men, and reported that the detection accuracy of significant prostate cancer by targeted biopsies is significantly higher than that by extended systematic biopsies. Moreover, 13% of clinically insignificant cancers would have been avoided with the use of targeted biopsy alone.
Watanabe et al. (37) have reported interesting results on the use of DW-MRI from mpMRI to guide MRI-targeted biopsies. The population comprised 1,448 men suspected of having prostate cancer based on PSA level, and was split into two groups. Group A (890 patients with low apparent diffusion coefficient—ADC—lesions on mpMRI) underwent both targeted and systematic biopsies, whereas group B (558 patients with no ADC lesion on mpMRI) underwent systematic biopsies. Overall prostate cancer detection was 70.1% for group A compared with 13.1% for group B (P<0.001). Interestingly, the use of ADC maps resulted in the significantly greater cancer detection rates observed in group A.
Software-assisted fusion registration compared to systematic biopsy
Pinto et al. (22) have compared standard 12-core TRUS biopsy with MRI/TRUS fusion biopsy with an electromagnetic tracking device in 101 men. Prostate cancer was identified in 55 out of 101 men and MRI/TRUS fusion detected more cancer per core than systematic 12-core TRUS biopsy for patients with low, moderate and high suspicion of cancer on mpMRI. The targeted approach required a mean of 2.6 cores compared with 12 for the standard series, potentially reducing the detection of insignificant disease.
Another research group (38) performed MRI/TRUS fusion biopsies, followed by conventional standard transrectal or transperineal biopsies, in 85 men with a previous negative biopsy. Prostate cancer was detected in 52 out of 85 men (61%). The evaluation of biopsy cores showed that the detection rate per core was significantly higher in MRI/TRUS fusion biopsy (62/192 positive biopsy cores; 32%) compared to standard biopsy (75/833 positive cores; 9%) (P< 0.01).
Hadaschik et al. (39) investigated the use of transperineal MRI/US fusion biopsies in addition to standard transperineal cores in a population of 106 men; prostate cancer was detected in 63 out of 106 men (59%), with cancer detection per core rates of 25% (101/410 positive cores) versus 9% (179/2,051 positive cores) for the MRI-targeted and standard cores, respectively.
The use of MRI/TRUS fusion biopsy has been shown to be of particular help in sampling midline lesions (40), and to rule out the presence of prostate cancer on a second MRI/TRUS fusion biopsy in mpMRI visible lesions initially found to be pathologically benign on first MRI/TRUS fusion biopsy (41).
Cool et al. (42) have recently evaluated the clinical benefit of MRI/TRUS fusion biopsy over systematic biopsy between first-time (n=50) and repeat prostate biopsy (n=50) men with prior atypical small acinar proliferation (ASAP). They concluded that, whilst the detection rate of clinically significant disease was higher in the first time biopsy group (17/50) compared to the biopsy after ASAP group (7/50), the addition of MRI/TRUS fusion biopsy had greater clinical utility in the repeat biopsy group, as fusion detected more cancers missed by standard biopsy. This is in keeping with other series, where at first biopsy many tumors will be detected on both standard and targeted biopsy, but when men are re-biopsied after an initial biopsy negative for cancer the tumors are detected on targeted biopsy rather than repeat biopsy. This suggests that targeting corrects the systematic error of missing midline, anterior, apical and extreme basal tumors, more often than the random error of missing a peripheral zone tumor.
In-bore registration
In a systematic review, Overduin et al. (28) have reported that the majority of tumors detected by this technique are clinically significant (81–93%). They concluded that in-bore MRI-guided biopsy is an accurate and safe diagnostic tool to detect clinically significant prostate cancer but, due to the limited general availability, this procedure should be reserved for specific patients.
There is growing evidence that missed cancer rates of in-bore MRI guided biopsies are low, ranging from 6% to 10% (42,43).
In a study by Hambrock et al. (14), specimens obtained by in-bore biopsies were found to be highly representative of true tumor grade, exactly matching prostatectomy histopathology in 88% of cases.
A recent study by Schimmöller et al. (43) has retrospectively evaluated the utility of two targeted biopsy cores per mpMRI-lesion within in-bore guided biopsy in 290 men. They demonstrated that taking only one biopsy core per lesion using the in-bore approach does not significantly affect the final Gleason classification when compared to 2 cores. In almost 90% of the men included in the study there was no upgrading of the Gleason score by the second targeted biopsy core of a particular intraprostatic lesion. In only 2% there was an upgrade to a clinical significant prostate cancer (Gleason score ≥4+3=7).
Felker et al. (44) have reported interesting results on a cohort of 461 men (381 with no previous prostate cancer and 80 under active surveillance), who underwent in-bore magnetic resonance biopsy. With a mean of 1.7 sampled targets per gland, the Authors concluded that in-bore targeted biopsy appears most useful in men with high or very high suspicion lesions, with detection rates for clinically significant disease of 43% and 84%, respectively.
Studies comparing multiple MRI-targeted biopsy techniques
Eleven studies were identified which compared two different MRI-targeting techniques. One study was a simulation study, and two studies selected men for one technique or another in a non-randomised fashion, and are described below. The eight remaining studies are summarized in Table 1 and Table S1. Only two studies (47,52) investigated a totally biopsy-naïve cohort of men (n=451). All mpMRI scans were performed on either a 1.5T or 3T scanner and had T2-weighted scans, DWI and DCE. In the study by Lee et al. (45) 12% of men did not have a DCE study. Standard TRUS biopsy was used as an additional comparator in 6 studies.
Table 1. Comparison between the different MRI-targeted biopsies and the standard comparator in the full studies included in the review.
| Type of MRI-targeted biopsy | Study (ref.) | Comparator standard (biopsy) | Number of patients | Detection of clinically significant disease (MRI-targeted biopsy technique) | Detection of clinically significant disease (comparator) |
|---|---|---|---|---|---|
| Visual registration biopsy vs. transperineal or standard TRUS biopsy | Lee et al. (45) | 286 | 78/286 (27%) | ||
| Oberlin et al. (46) | Transrectal ultrasound | 150 (visual registration) | 25/150 (17%) | ||
| Pepe et al. (47) | Transperineal | 60 | 56/60 (93%) | 59/60 (98%) | |
| Valerio et al. (48) | Transperineal | 50 | 32/50 (64%) | 38/50 (76%) | |
| Wysock et al. (49) | Transrectal ultrasound | 67 (only biopsy-naïve men) | 18/67 (27%) | 22/67 (33%) | |
| Oderda et al. (50) | Transrectal ultrasound | 25 | 8/25 (32%) | ||
| Software-assisted biopsy vs. transperineal or standard TRUS biopsy | Lee et al. (45) | 286 | 74/286 (26%) | ||
| Oberlin et al. (46) | Transrectal ultrasound | 81 (software-assisted) and 100 (standard TRUS) | 24/81 (30%) | 32/100 (32%) | |
| Pepe et al. (47) | Transperineal | 60 | 40/60 (67%) | 59/60 (98%) | |
| Valerio et al. (48) | Transperineal | 50 | 34/50 (68%) | 38/50 (76%) | |
| Wysock et al. (49) | Transrectal ultrasound | 67 (only biopsy-naïve men) | 19/67 (28%) | 22/67 (33%) | |
| Oderda et al. (50) | Transrectal ultrasound | 25 | 12/25 (48%) | ||
| In-bore biopsy (no comparator) | Arsov et al. (51) | 106 | 31/106 (29%) |
TRUS, transrectal ultrasound.
Two studies compared biopsy techniques across different men biopsied at different time periods in the same institution, rather than comparing techniques in the same men, or randomizing men to one approach or another. With a sequential design, it is possible that the selection criteria for men having an MRI or biopsy may differ between groups, or that there may be a learning curve effect both in terms of MRI and biopsy, over time.
Delongchamps and colleagues (52) compared the accuracy of targeted biopsies in 391 patients using visual registration (n=127), rigid (n=131) and elastic (n=133) fusion, over three consecutive time periods. The positive mpMRI rate increased over the three time periods, with a rate of 54/127 (42%) in the first cohort who had visual registration, 78/131 (59%) in the second cohort who had rigid registration and 82/133 patients (62%) in the third cohort who had elastic registration. Clinically significant cancer on mpMRI-targeted biopsies was found in 18/127 (14%), 33/131 (19%) and 27/133 (20%) of each cohort respectively. They reported that visual registration did not improve cancer detection over systematic biopsies (P=0.66) and that there was a significantly increased cancer detection rate with rigid (P=0.006) and elastic (P=0.001) fusion biopsies when compared with random biopsies, with the added value of requiring fewer cores and an associated decreased detection of clinically insignificant prostate cancer.
The higher detection rate in the later cohorts of rigid and elastic fusion techniques could be ascribed to the more accurate delineation of mpMRI suspicious lesions, although the lower positive MRI rate in the first group may be a factor. The Authors concluded that mpMRI-targeted biopsy alone provided a significantly higher cancer detection rate than random biopsies and that mpMRI-targeted biopsies increased the detection rate of high Gleason score cancer, while decreasing the detection rate of small and well differentiated tumors.
Mouraviev and colleagues (53) compared mpMRI targeted prostate biopsy in 32 men using visual registration (n=9), software-assisted registration (n=13) and in-bore biopsy (n=10). This is the only report comparing all three techniques, but the population seems to differ between the groups, which limits the applicability of the findings. The cancer detection rate was 33% (3/10 men) for visual registration and 46% (6/13 men) for software-assisted registration (P=0.005), and 80% (8/10 men) for in-bore biopsy (P=0.005 when compared to software-assisted registration). However, the Authors reported that the majority of men undergoing in-bore biopsy (7/10) had a previously diagnosed prostate cancer on TRUS-guided 12-core biopsy. In addition, the detection rates for visually targeted and fusion biopsies are low compared to other series.
Cool and colleagues reported a retrospective, simulation study performed using a validated TRUS prostate biopsy device (54). The simulator used a tracking system to monitor the 3D position of the probe within a phantom; 3D TRUS and mpMRI images were fused together and shown within the simulator, where a biopsy could be taken by targeting the probe. The initial population included 100 men (50 biopsy naive and 50 with a previous negative biopsy or atypical small acinar proliferation). After defining clinically significant prostate cancer as any core with a Gleason score ≥7 or more than 50% tumor involvement in the core, the final population consisted of 20 men with clinically significant on mpMRI targeted cores, with a total of 25 clinically significant tumors. Three different operators performed simulated biopsies on all 25 lesions using three different methodologies (2D TRUS, 3D TRUS and MRI-TRUS fusion), for a total of 225 targeted biopsies (75 for each technique). The Authors concluded that fusion biopsy (75/75; 100%) would be significantly more accurate than 2D (36/75; 48%) and 3D (34/75; 45%) visual registration under TRUS guidance (P<0.001).
Visual registration vs. software assisted registration of MRI and ultrasound images
Four studies including a total of 556 patients were not able to demonstrate significant differences between the two techniques in clinically significant cancer detection, using definitions of clinically significant cancer ranging from Gleason ≥3+4 to maximum cancer core length of 4 mm. (45,48,49,55).
Of note, Puech et al. (55) reported that of 95 men in the study there was a group of 68 men with 79 targets on MRI. Positivity for cancer per target was 47% for visually targeted biopsy and 53% for fusion biopsy. The Authors demonstrated no difference in cancer detection between techniques when they looked at subgroups of posterior, anterior or smallest imaging targets.
Wysock et al. (49) prospectively reported the two targeting techniques in 125 consecutive men, with a total of 172 mpMRI-suspicious lesions that were targeted using software assisted registration. Targets were then blinded, and a second operator took two visually targeted cores and a 12-core biopsy.
Overall cancer detection rate per patient was 45 (36%) and 40 (32%), and Gleason sum ≥7 cancer detection rate was 29 (23.2%) and 24 (19.2%) by fusion and visual registration, respectively (P=0.36, P=0.27). Also this study demonstrated no difference in cancer detection rate between the two techniques, but a trend toward improved cancer detection rate was observed overall and in all subsets, suggesting a need for a larger sample size.
Lee et al. (45) reported the difference in the detection of high-grade (Gleason ≥7) and any grade cancer between the two techniques in 286 men. The difference between fusion and visually registered biopsy was –1.4% (P=0.6) for high-grade cancer (74 vs. 78 men, respectively) and 3.5% for any grade cancer (P=0.2), neither of which were statistically significant. Interestingly, the Authors found that an MRI/TRUS fusion biopsy could detect tumors that are difficult to be visually targeted (i.e., those in the transition zone) and they concluded that combining both techniques may improve prostate cancer detection.
Similar results have been reported by Valerio and colleagues (48) in a cohort of 50 men undergoing visual, fusion and systematic transperineal biopsies. The Authors found that the detection rate of clinically significant prostate cancer was 32/50 (64%), 34/50 (68%) and 38/50 (76%) respectively and concluded that combining the two targeting approaches could minimize missed cancer rates, with a detection similar to systematic biopsies. Transperineal template prostate mapping was performed using the 20-zone modified-Barzell template, regardless of the position of the targets and the biopsy density previously employed with the targeted strategies. Transperineal template prostate biopsies identified 10 men (20%) and 8 men (16%) with clinically significant disease that was missed or undergraded by visually directed targeted biopsy and by software-based targeted biopsy, respectively.
Other studies did report a difference between the different MRI-targeted approaches, with three studies (total 341 men) reporting a difference between techniques, with two finding higher cancer detection with software assisted biopsies, and one for visually registered biopsies.
Oberlin et al. (46) analyzed a cohort of 231 men undergoing fusion (n=81) or visual MRI-targeted biopsy (n=150). The Authors concluded that the overall detection rate of cancer was significantly higher in the fusion cohort (48.1%) compared to the visual registration group (34.6%) (P=0.04).
Oderda et al. (50) retrospectively evaluated 50 men (16 with a previous negative biopsy and 5 with a previous negative transurethral resection of the prostate) undergoing fusion (n=25) or visually registered (n=25) MRI-targeted biopsy. The cancer detection rate of mpMRI targets was significantly higher in fusion (59%) than visually registered biopsies (27%) (P=0.03).
Pepe et al. (47) reported on the detection rate for clinically significant prostate cancer of transperineal (visually registered) vs. transrectal (rigid fusion) MRI-guided biopsies, comparing these two techniques to transperineal saturation biopsy in 60 men. Transperineal visually targeted biopsy detected a greater percentage of clinically significant prostate cancer of the anterior zone when compared to transrectal fusion biopsy (93.3% vs. 25% respectively, P<0.001).
One might argue that the anatomical approach could have influenced these results, as a transperineal approach permits the operator to sample the anterior zone of the gland more easily.
MRI/TRUS fusion biopsy vs. in-bore
We found only one published study which directly compared an MRI-ultrasound method with an in bore approach. This study, by Arsov et al. (51), compared in-bore and software-assisted fusion biopsies in men with prior negative biopsies. The Authors investigated 210 men (106 undergoing in-bore and 104 fusion plus TRUS biopsies) and observed that detection rates for clinically significant prostate cancer (Gleason ≥3+4) (29% vs. 32%; P=0.7) and the highest percentage tumor involvement per biopsy core (48% vs. 42%; P=0.4) were similar between the arms.
Future studies
It is important to mention that there is also an ongoing large randomized controlled trial (planned recruitment of 675 patients) comparing all three techniques in men with a persistent clinical suspicion of prostate cancer and at least one negative TRUS biopsy (56). The hypothesis of this trial is that MRI/TRUS fusion biopsy demonstrates a similar cancer detection rate of prostate cancer compared to in-bore biopsy, whilst demonstrating an increased cancer detection rate compared to visual TRUS biopsy (56).
Conclusions
Ideally, the optimal biopsy technique should have the highest detection rate of clinically significant prostate cancer, while simultaneously having the lowest detection rate of clinically insignificant disease. Currently there is no consensus on which type of MRI-targeted biopsy performs better in a given setting. Although there have been studies supporting each of the three techniques, substantial differences in methodology and reporting the findings make it difficult to reliably compare their outcomes. The economic implications of using software assisted registration or in-bore registration should be borne in mind when choosing an approach.
Table S1. Details of magnetic resonance imaging and biopsy techniques, and histologic outcomes in the full studies included in the review.
| Study (ref.) | Type of study | Allocation to biopsy methods | Inclusion criteria | Comparator (standard test) | Sampling route | No. of patients for the analysis | Previous biopsy | MRI | MRI-targeted biopsy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Magnet strength | Endorectal coil | Sequences | Navigational system for biopsy | Targeted cores per lesion | Overall prostate cancer detection rate per patient | Overall prostate cancer detection rate per core/per target | Definition of clinically significant disease | Detection rate clinically significant disease per patient | Detection rate clinically significant disease per core/per target | Significant difference between MRI-targeted techniques | |||||||||
| Arsov et al. (51) | Prospective | Randomized | Negative TRUS-biopsy and persistent rising PSA | In bore vs. fusion +TRUS | Transrectal | 210 | Negative TRUS biopsy | 3T | No | T2, DWI, DCE | (I) In bore (106/210; 50%); (II) fusion + TRUS 12 cores (104/210; 50%) |
2 (for each technique) | (I) 39/106 (37%); (II) 35/104 (34%) only fusion |
(I) 123/595 (21%); (II) 210/1,808 (12%) |
Gleason ≥3+4 | (I) 31/106 (29%); (II) 27/104 (26%) only fusion |
NR | No | |
| Lee et al. (45) | Prospective | Sequential | Lesion scoring ≥3 at MRI | NR | Transrectal | 286 | 75/286 biopsy naive; 186/286 positive biopsy; 16/286 negative biopsy | 1.5T/3T | No | T2, DWI, (DCE) | Visual registration ((I) + fusion ((II) in all | 2 (for each technique) | NR | (I) 131/396 (33%); (II) 145/396 (37%) |
Gleason ≥3+4 | (I) 78/286 (27%); (II) 74/286 (26%) |
90/396 (25%); 82/396 (21%) | No, although VR performed better at the base, and FB better in the TZ | |
| Oberlin et al. (46) | Retrospective | Randomized | Rising PSA, abnormal DRE, active surveillance | TRUS 12 cores | Transrectal | 231 | NR | 3T | NR | T2, DWI, DCE | (I) Visual registration (150/231; 65%); (II) fusion (81/231; 35%) | 1 to 3 | (I) 52/150 (35%); (II) 39/81 (48%) |
NR | Gleason ≥3+4 | (I) 25/150 (17%); (II) 24/81 (30%) |
NR | MRI-fusion has a higher overall detection rate than visual registratiom | |
| Pepe et al. (47) | Prospective | Sequential | Negative DRE, rising PSA | Transperineal saturation biopsy (28 to 34 cores) | Transperineal (visual registration) + Transrectal (fusion) | 60 men with clinically significant cancer from a cohort of 200 men having repeat biopsy | Repeat saturation transperineal prostate biopsy | 3T | No | T2, DWI, DCE | TP Visual registration ((I) +TR fusion ((II) in all | 4 (for each technique) | NR | NR | Gleason ≥3+4 and/or >2 positive cores | (I) 56/60 (93%); (II) 40/60 (67%) |
NR | Transperineal visual registration biopsy has a higher detection rate than transrectal fusion biopsy of the anterior zone | |
| Puech et al. (55) | Prospective | Sequential | Rising PSA and suspicious lesion at MRI | TRUS 12 cores | Transrectal | 95 | 65/95 biopsy naïve; 30/95 prior negative biopsy | 1.5T | No | T2, DWI, DCE | Visual registration ((I) + fusion ((II) in 68 men (remaining 27 had 1 technique only) | 2 (for each technique) | 72/95 (76%) | On 68 men, with 79 MR imaging targets: (I) 37/79 (47%); (II) 42/79 (53%) | Cancer core length ≥3 mm or Gleason ≥3+4 | 65/95 (68%) | Possible only on 33/79 MR imaging targets: (I) 20/33 (61%); (II) 21/33 (64%) | No | |
| Valerio et al. (48) | Prospective | Sequential | Suspicious lesions at MRI | Transperineal template (22 to 41 cores) | Transperineal | 50 | 5/50 biopsy naïve; 5/50 prior negative biopsy; 40/50 prior positive biopsy (of which 8 post RT failure) |
1.5/3T | No | T2, DWI, DCE | Visual registration ((I) + fusion ((II) in all | (I) 3 to 6; (II) 3 to 5 | (I) 36/50 (72%); (II) 37/50 (74%) | (I) 41/79 (52%); (II)46/79 (59%) | Gleason score ≥4 and/or maximum cancer core length >4 mm | (I) 32/50 (64%); (II) 34/50 (68%) |
(I) 35/79 (44%); (II) 41/79 (52%) | No | |
| Wysock et al. (49) | Prospective | Sequential | Suspicious lesions at MRI | TRUS 12 cores | Transrectal | 125 | 67/125 biopsy naïve; 34/125 negative; 24/125 low-risk cancer |
3T | No | T2, DWI, DCE | Visual registration ((I) + fusion ((II) in all | 2 (for each technique) | (I) 40/125 (32%); (II) 45/125 (36%) | (I) 46/172 (27%); (II) 55/172 (32%) | Gleason ≥3+4 | (I) 24/125 (19%); (II) 29/125 (23%) |
(I) 26/172 (15%); (II) 35/172 (20%) | No | |
| Oderda et al. (50) | Retrospective | Randomized | Clinical suspicion and suspicious lesion at MRI | TRUS (variable number of cores) | Transrectal | 50 | (I) 14/25 biopsy naïve, 8/25 negative, 3/25 negative TURP; b) 15/25 biopsy naïve, 8/25 negative, 2/25 negative TURP | 1.5T | No | T2, DWI, DCE | (I) Visual registration (25 men); (II) elastic fusion (25 men) | (I) 2.6 (mean); (II) 3.6 (mean) | (I) 10/25 (40%); (II) 16/25 (63%) | (I) 7/26 (27%); (II) 16/27 (59%) | Gleason ≥3+3 and ≥2 positive cores | (I) 8/25 (32%); (II) 12/25 (48%) |
(I) 6/26 (23%); (II) 10/27 (37%) | Elastic fusion has a higher detection rate than visual registration | |
DRE, digital rectal examination; MRI, magnetic resonance imaging; TRUS, transrectal ultrasound; DWI, diffusion-weighted imaging; DCE, dynamic contrast enhanced; NR, not reported; TURP, transurethral resection of the prostate; RT, radiotherapy; PSA, prostate specific antigen; VR, visual registration; FB, fusion biopsy; TZ, transitional zone; TP, transperineal; TR, transrectal.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol 1989;142:71-4; discussion 74-5. [DOI] [PubMed] [Google Scholar]
- 2.European Association of Urology. Guidelines on Prostate Cancer. 2013. Available online: https://uroweb.org/wp-content/uploads/1607-Prostate-Cancer_LRV3.pdf
- 3.Bjurlin MA, Taneja SS., et al. Standards for prostate biopsy. Curr Opin Urol 2014;24:155-61. 10.1097/MOU.0000000000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin BS, Kang SH, Kim DY, et al. Pathological upgrading in prostate cancer patients eligible for active surveillance: Does prostate-specific antigen density matter? Korean J Urol 2015;56:624-9. 10.4111/kju.2015.56.9.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenkrantz AB, Verma S, Choyke P, et al. Prostate Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Patients with a Prior Negative Biopsy: A Consensus Statement by AUA and SAR. J Urol 2016;196:1613-8. 10.1016/j.juro.2016.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll PR, Parsons JK, Andriole G, et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Canc Netw 2016;14:509-19. 10.6004/jnccn.2016.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaziev G, Wadhwa K, Barrett T, et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int 2016;117:80-6. 10.1111/bju.12892 [DOI] [PubMed] [Google Scholar]
- 8.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. 10.1007/s00330-011-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barentsz JO, Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur Urol 2016;69:41-9. 10.1016/j.eururo.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013;63:125-40. 10.1016/j.eururo.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Wegelin O, van Melick HH, Hooft L, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol 2017;71:517-31. 10.1016/j.eururo.2016.07.041 [DOI] [PubMed] [Google Scholar]
- 12.Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015;33:17.e1-7. 10.1016/j.urolonc.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 13.Muthigi A, George AK, Sidana A, et al. Missing the Mark: Prostate Cancer Upgrading by Systematic Biopsy over Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Biopsy. J Urol 2017;197:327-34. 10.1016/j.juro.2016.08.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol 2012;61:177-84. 10.1016/j.eururo.2011.08.042 [DOI] [PubMed] [Google Scholar]
- 15.Kirkham AP, Haslam P, Keanie JY, et al. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin Radiol 2013;68:1016-23. 10.1016/j.crad.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 16.Ahmed HU, Kirkham A, Arya M, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol 2009;6:197-206. 10.1038/nrclinonc.2009.18 [DOI] [PubMed] [Google Scholar]
- 17.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 2011;108:E171-8. 10.1111/j.1464-410X.2011.10112.x [DOI] [PubMed] [Google Scholar]
- 18.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 2013;189:860-6. 10.1016/j.juro.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013;64:544-52. 10.1016/j.eururo.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 20.Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol 2014;192:648-58. 10.1016/j.juro.2014.03.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park BK, Park JW, Park SY, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol 2011;197:W876-81. 10.2214/AJR.11.6829 [DOI] [PubMed] [Google Scholar]
- 22.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186:1281-5. 10.1016/j.juro.2011.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kongnyuy M, George AK, Rastinehad AR, et al. Magnetic Resonance Imaging-Ultrasound Fusion-Guided Prostate Biopsy: Review of Technology, Techniques, and Outcomes. Curr Urol Rep 2016;17:32. 10.1007/s11934-016-0589-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64:713-9. 10.1016/j.eururo.2013.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 2013;189:86-91. 10.1016/j.juro.2012.08.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013;190:1721-7. 10.1016/j.juro.2013.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fütterer JJ, Barentsz JO. MRI-guided and robotic-assisted prostate biopsy. Curr Opin Urol 2012;22:316-9. 10.1097/MOU.0b013e328354833c [DOI] [PubMed] [Google Scholar]
- 28.Overduin CG, Fütterer JJ, Barentsz JO. MRI-guided biopsy for prostate cancer detection: a systematic review of current clinical results. Curr Urol Rep 2013;14:209-13. 10.1007/s11934-013-0323-z [DOI] [PubMed] [Google Scholar]
- 29.Radtke JP, Teber D, Hohenfellner M, et al. The current and future role of magnetic resonance imaging in prostate cancer detection and management. Transl Androl Urol 2015;4:326-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994;271:368-74. 10.1001/jama.1994.03510290050036 [DOI] [PubMed] [Google Scholar]
- 31.D'Amico AV, Whittington R, Schultz D, et al. Outcome based staging for clinically localized adenocarcinoma of the prostate. J Urol 1997;158:1422-6. 10.1016/S0022-5347(01)64230-7 [DOI] [PubMed] [Google Scholar]
- 32.Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71:933-8. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed HU, Hu Y, Carter T, Arumainayagam N, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol 2011;186:458-64. 10.1016/j.juro.2011.03.147 [DOI] [PubMed] [Google Scholar]
- 34.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66:22-9. 10.1016/j.eururo.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 35.Porpiglia F, DE, Luca S, Passera R, et al. Multiparametric-Magnetic Resonance/Ultrasound Fusion Targeted Prostate Biopsy Improves Agreement Between Biopsy and Radical Prostatectomy Gleason Score. Anticancer Res 2016;36:4833-9. 10.21873/anticanres.11045 [DOI] [PubMed] [Google Scholar]
- 36.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 2012;188:1732-8. 10.1016/j.juro.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe Y, Terai A, Araki T, et al. Detection and localization of prostate cancer with the targeted biopsy strategy based on ADC map: a prospective large-scale cohort study. J Magn Reson Imaging 2012;35:1414-21. 10.1002/jmri.23587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyagawa T, Ishikawa S, Kimura T, et al. Real-time Virtual Sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol 2010;17:855-60. 10.1111/j.1442-2042.2010.02612.x [DOI] [PubMed] [Google Scholar]
- 39.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol 2011;186:2214-20. 10.1016/j.juro.2011.07.102 [DOI] [PubMed] [Google Scholar]
- 40.Muthigi A, Sidana A, George AK, et al. Midline lesions of the prostate: role of MRI/TRUS fusion biopsy and implications in Gleason risk stratification. Int Urol Nephrol 2016;48:1445-52. 10.1007/s11255-016-1336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chelluri R, Kilchevsky A, George AK, et al. Prostate Cancer Diagnosis on Repeat Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsy of Benign Lesions: Recommendations for Repeat Sampling. J Urol 2016;196:62-7. 10.1016/j.juro.2016.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cool DW, Romagnoli C, Izawa JI, et al. Comparison of prostate MRI-3D transrectal ultrasound fusion biopsy for first-time and repeat biopsy patients with previous atypical small acinar proliferation. Can Urol Assoc J 2016;10:342-8. 10.5489/cuaj.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schimmöller L, Quentin M, Blondin D, et al. Targeted MRI-guided prostate biopsy: are two biopsy cores per MRI-lesion required? Eur Radiol 2016;26:3858-64. 10.1007/s00330-016-4266-x [DOI] [PubMed] [Google Scholar]
- 44.Felker ER, Lee-Felker SA, Feller J, et al. In-bore magnetic resonance-guided transrectal biopsy for the detection of clinically significant prostate cancer. Abdom Radiol (NY) 2016;41:954-62. 10.1007/s00261-016-0750-7 [DOI] [PubMed] [Google Scholar]
- 45.Lee DJ, Recabal P, Sjoberg DD, et al. Comparative Effectiveness of Targeted Prostate Biopsy Using Magnetic Resonance Imaging Ultrasound Fusion Software and Visual Targeting: a Prospective Study. J Urol 2016;196:697-702. 10.1016/j.juro.2016.03.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberlin DT, Casalino DD, Miller FH, et al. Diagnostic Value of Guided Biopsies: Fusion and Cognitive-registration Magnetic Resonance Imaging Versus Conventional Ultrasound Biopsy of the Prostate. Urology 2016;92:75-9. 10.1016/j.urology.2016.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepe P, Garufi A, Priolo G, et al. Transperineal Versus Transrectal MRI/TRUS Fusion Targeted Biopsy: Detection Rate of Clinically Significant Prostate Cancer. Clin Genitourin Cancer 2017;15:e33-6. 10.1016/j.clgc.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 48.Valerio M, McCartan N, Freeman A, et al. Visually directed vs. software-based targeted biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urol Oncol 2015;33:424.e9-16. 10.1016/j.urolonc.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 49.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 2014;66:343-51. 10.1016/j.eururo.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 50.Oderda M, Faletti R, Battisti G, et al. Prostate Cancer Detection Rate with Koelis Fusion Biopsies versus Cognitive Biopsies: A Comparative Study. Urol Int 2016;97:230-7. 10.1159/000445524 [DOI] [PubMed] [Google Scholar]
- 51.Arsov C, Rabenalt R, Blondin D, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol 2015;68:713-20. 10.1016/j.eururo.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 52.Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol 2013;189:493-9. 10.1016/j.juro.2012.08.195 [DOI] [PubMed] [Google Scholar]
- 53.Mouraviev V, Verma S, Kalyanaraman B, et al. The feasibility of multiparametric magnetic resonance imaging for targeted biopsy using novel navigation systems to detect early stage prostate cancer: the preliminary experience. J Endourol 2013;27:820-5. 10.1089/end.2012.0215 [DOI] [PubMed] [Google Scholar]
- 54.Cool DW, Zhang X, Romagnoli C, et al. Evaluation of MRI-TRUS fusion versus cognitive registration accuracy for MRI-targeted, TRUS-guided prostate biopsy. AJR Am J Roentgenol 2015;204:83-91. 10.2214/AJR.14.12681 [DOI] [PubMed] [Google Scholar]
- 55.Puech P, Rouvière O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology 2013;268:461-9. 10.1148/radiol.13121501 [DOI] [PubMed] [Google Scholar]
- 56.Wegelin O, van Melick HHE, Somford DM, et al. The Future Trial: Fusion Target Biopsy of the Prostate Using Real-Time Ultrasound and MR Images. A Multicenter RCT on Target Biopsy Techniques in the Diagnosis of Prostate Cancer. J Clin Trials 2015;5:248 10.4172/2167-0870.1000248 [DOI] [Google Scholar]



