Abstract
For more than 70 years, the contention that high levels of testosterone or that the use of testosterone therapy (TTh) increases the development and progression of prostate cancer (PCa) has been widely accepted and practiced. Yet, the increasing and emerging evidence on testosterone research seems to challenge that contention. To review literature on the associations of endogenous and exogenous testosterone with decreased-, increased-, or null-risk of PCa, and to further evaluate only those studies that reported magnitude of associations from multivariable modeling as it minimizes confounding effects. We conducted a literature search to identify studies that investigated the association of endogenous total testosterone [continuous (per 1 unit increment and 5 nmol/L increment) and categorical (high vs. low)] and use of TTh with PCa events [1990–2016]. Emphasis was given to studies/analyses that reported magnitude of associations [odds ratio (OR), relative risk (RR) and hazard ratios (HRs)] from multivariable analyses to determine risk of PCa and their statistical significance. Most identified studies/analyses included observational and randomized placebo-controlled trials. This review was organized in three parts: (I) association of endogenous total testosterone (per 1 unit increment and 5 nmol/L increment) with PCa; (II) relationship of endogenous total testosterone (categorical high vs. low) with PCa; and (III) association of use of TTh with PCa in meta-analyses of randomized placebo-controlled trials. The first part included 31 observational studies [20 prospective (per 5 nmol/L increment) and 11 prospective and retrospective cohort studies (per 1 unit increment)]. None of the 20 prospective studies found a significant association between total testosterone (5 nmol/L increment) and increased- or decreased-risk of PCa. Two out of the 11 studies/analyses showed a significant decreased-risk of PCa for total testosterone per 1 unit increment, but also two other studies showed a significant increased-risk of PCa. Remaining studies reported null-risks values. Second part: eight of out of 25 studies reported an increased-risk of PCa for men with high levels of testosterone compared to low, but only four were statistically significant. However, 17 studies showed a decreased-risk of PCa after comparing high vs. low levels of testosterone, but 11 studies/analyses were statistically significant. Third part: two meta-analyses of randomized placebo-controlled trials (n=8 and n=11, each) that investigated use of TTh with PCa reported not significant decreased-risks of PCa. The contention that high levels of testosterone or that the use of TTh increases the risk of PCa doesn’t seem to be supported from the literature. Yet, we still need a study with the adequate power, follow-up data, epidemiological, pathological and clinical data that can support the safety and beneficial effects of high levels of endogenous testosterone or use of TTh in the natural history of PCa and in men’s health.
Keywords: Testosterone, treatment, endogenous, prostate, associations
Introduction
The relationship between high levels of endogenous testosterone or use of testosterone therapy (TTh) with risk of prostate cancer (PCa) remains one of the long-standing controversies in urological research since the past 70 years (1). Some of the contributing factors to this controversy is that PCa is still the second most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality among men worldwide (2,3). Although the five-year survival rate for PCa is extremely high (99%), advanced PCa has a markedly different prognosis with only a 34% five-year survival rate (4), making advanced PCa the clinically relevant outcome.
Another contributing factor has been the increasing interest about the positive and/or negative effects of testosterone in men’s health in the last decade. For instance, low levels of testosterone, or testosterone deficiency (TD), is a common medical condition affecting men, and it is characterized by having total testosterone levels <300 ng/dL (5-8). TD has been linked to a constellation of signs and symptoms that may include sexual dysfunction, decreased bone density and decreased body lean mass (5), as well as other chronic conditions such as cancer and cardiovascular diseases to name a few (9-20). Studies show that approximately 38.7% of men over 45-year-old (18) [24% among men 30–79 years old (8)] demonstrate TD. In the United States, reports show that there are approximately 2.4 million 40–69 years old men with TD (21,22). Yet, a greater concern is the projection that by 2025 approximately 6.5 million American men aged 30 to 80 will suffer from TD (8). In consequence, treatment of TD with TTh has increased dramatically as well; TTh prescriptions increased more than 3-fold, from 0.81% in 2001 to 2.9% in 2011 (23,24). Surprisingly, the controversy about testosterone and PCa is not about TD and PCa, or in conjunction with its treatment (TTh), and PCa, but mainly about the contention that high levels of testosterone and use of TTh play a role in the development or progression of PCa. Therefore, the main objectives were to review the literature on the associations of endogenous [continuous (per 1 unit increment and 5 nmol/L increment) and categorical (high vs. low)] and exogenous testosterone with decreased-, increased-, or null-risk of PCa; and to further evaluate only those studies that reported magnitude of associations from multivariable modeling, that will minimized confounding effects, and their statistical significance.
Criteria to define “decreased-risk”, “increased-risk”, and “null-risk” of PCa groups
In this review, we critically discuss previous and emerging literature on the association between continuous (per 1 unit increment and 5 nmol/L increment) and categorical (high vs. low) total testosterone and use of TTh with PCa from original observational research studies and meta-analyses of randomized-placebo controlled trials [1990–2016]. We mainly focused our search total testosterone due to a limited number of studies that reported free- or bioavailable-testosterone. The review is organized in three parts, where we report the association of (I) endogenous total testosterone [continuous (per 1 unit increment and 5 nmol/L increment)], (II) endogenous total testosterone (categorical high vs. low) and (III) use of TTh (meta-analyses) with decreased-, increased-, or null-risk of incident PCa cases. “Decreased-risk”, “increased-risk”, and “null-risk” of PCa groups were defined based on their magnitude of association, such as odd ratios (ORs), rate ratios or relative risk (RR), or hazard ratios (HRs), plus statistical significant values such as P value <0.05 or 95% confidence intervals (CIs) that does not include value of one. For instance, studies/analyses with “decreased-risk” had a magnitude of association (ORs, RRs, HRs) that is below 1; studies/analyses with “increased-risk” had ORs, RRs or HRs that are higher than 1; or “null-risk” if ORs, RRs and HRs is equal to 1 (or 0.999). These cutoff points were chosen arbitrarily, but magnitudes of association with values so close to the null (i.e., 0.999, 0.998, 1.01, 1.02) have a difficult clinical interpretation. In addition, we considered whether these magnitude of associations reached statistically significance (SS) or not (NS). For consistency, we only presented the magnitude of the association of PCa (aggressive and non-aggressive) for studies/analyses with continuous total testosterone (continuous increment of testosterone and 5 nmol/L increment) and those studies/analyses that categorized total testosterone (high vs. low levels of testosterone). Some studies may be repeated twice because they independently investigated association of testosterone with aggressive and non-aggressive PCa; the rationale for this latter statement stems from studies (25,26) that reported opposite values for low-grade and high-grade (i.e., low-grade OR =1.91 and high-grade OR =0.26) as reporting only one value will be misleading. Therefore, we referred to these as studies/analyses, and they will be counted independently. Studies that reported their findings by comparing low to high categorical groups of total testosterone, we took the reciprocal of the magnitude of the association to present the comparison of high to low categorical groups (this is value only for studies that reported ORs). In addition, we recalculated their 95% CIs to determine their statistical significance. Furthermore, only those studies that adjusted for PCa risk factors in multivariable models were included to minimize confounding effects.
First part: association of endogenous total testosterone (continuous increment and 5 nmol/L increment) with decreased-, increased-, or null-risk of PCa
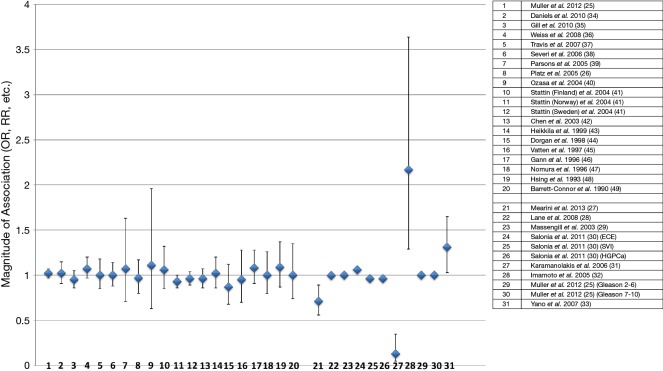
Thirty-one observational studies were identified that investigated the association of endogenous total testosterone with risk of PCa (aggressive and non-aggressive) (25-49). Twenty studies (25,26,34-49), out of the 31, were prospective and previously analyzed in a meta-analysis to determine association of continuous (5 nmol/L increment) endogenous total testosterone with risk of PCa (Figure 1 and Table 1) (50). The remaining 11 studies/analyses (25,27-33), which some may be repeated because they reported findings for both aggressive and non-aggressive PCa in opposite directions (25,26), were a combination of prospective and retrospective studies that focused on the association of continuous total testosterone (per 1 unit increment) with PCa.
Figure 1.
Serum testosterone (continuous and 5 nmol/L increments) in observational studies (prospective, retrospective) and its effects on PCa events (stage and grade). Studies/analyses only show multivariable analyses to minimize confounding effects. Magnitudes of association (ORs, RRs, etc.) and 95% CIs (25-49). PCa, prostate cancer; OR, odds ratio; RR, relative risk.
Table 1. Serum testosterone (continuous 5 nmol/L increments) in observational studies (prospective, retrospective) and its effects on PCa events (stage and grade). Only included studies that conducted multivariable analyses and reported magnitude of association (odds ratio, relative risk, etc.).
| Study author and year | Study design | Number of participants for independent studies | Follow-up (years or months) | Total or free testosterone (continuous increment 5 nmol/L, low levels vs. high levels) | Prostate cancer: total, grade and/or stage (n) | Results: only adjusted multivariable models | Statistically significant (SS: P<0.05) or not significant (NS) |
|---|---|---|---|---|---|---|---|
| Mearini et al. 2013 (27) | Prospective | 206 (103 PCa cases and 103 BPH) | Not applicable | Total testosterone, ng/mL | Total prostate cancer, n=103 | Continuous: OR =0.706; 95% CI =0.556–0.897 | SS (P=0.004) |
| Lane et al. 2008 (28) | Prospective study, radical prostatectomy | 455 | Median 14 (range 2–29 months) | Total testosterone, ng/dL | Gleason score pattern 4–5 at prostatectomy | Continuous: OR =0.998; 95% CI =0.997–1.000 | SS (P=0.048) |
| Massengill et al. 2003 (29) | Retrospective, radical prostatectomy | 879 | Mean 37.7 (months) | Total testosterone, ng/dL | Extraprostatic disease (pT3–T4)- cancer on any inked margin, any capsular penetration, or pelvic lymph node or seminal vesicle involvement, n=343 | Continuous: OR =0.999; 95% CI =0.998–1.000 | SS (P=0.0464) |
| Salonia et al. 2011 (30) | Prospective, radical prostatectomy | 673 | Not applicable | Total testosterone, ng/mL | Extracapsular extension (ECE), n=96 | Continuous: OR =1.06; 95% CI (not provided) | NS (P=0.26) |
| Seminal vesicle invasion (SVI), n=88 | Continuous: OR =0.96; 95% CI (not provided) | NS (P=0.47) | |||||
| High-grade prostate cancer (HGPCa) [defined with ECE, SVI, or Gleason grades (≥4+3)], n=153 | Continuous: OR =0.96; 95% CI (not provided) | NS (P=0.48) | |||||
| Karamanolakis et al. 2006 (31) | Prospective | 85 | Not applicable | Total testosterone, ng/mL | Incident prostate cancer, n=22 | Continuous: OR =0.13; 95% CI =0.05–0.35 | SS (P=0.0001) |
| Imamoto et al. 2005 (32) | Prospective, radical prostatectomy | 82 | Mean 20 (months) | Total testosterone, ng/mL | Extraprostatic disease (PT3-T4, N1), n= 24 | Continuous: HR =2.167; 95% CI =1.291–3.637) | SS (P=0.0034) |
| Muller et al. 2012 (25) | Prospective | 3,255 | Prostate biopsy at 24 and 48 (months) | Total testosterone, nmol/L | Gleason 2–6, n=819 | Continuous (untransformed): OR =1.0; 95% CI =0.99–1.03) | NS (P=0.59) |
| Gleason 7–10, n=228 | Continuous (untransformed): OR =1.00; 95% CI =0.98–1.03) | NS (P=0.72) | |||||
| Yano et al. 2007 (33) | Retrospective | 420 | Not applicable | Total testosterone, ng/mL | Positive prostate biopsy | Continuous: HR =1.31; 95% CI =1.03–1.65 | SS (0.02) |
| Muller et al. 2012† (25) | Prospective | 3,255 | 4 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=679) | Continuous (for 5 nmol/L): RR =1.02; 95% CI =0.97–1.07 | NS |
| Daniels et al. 2010† (34) | Prospective | 2,025 | 4.7 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=275) | Continuous (for 5 nmol/L): RR =1.02; 95% CI =0.91–1.15 | NS |
| Gill et al. 2010† (35) | Prospective | 1,388 | 1.9 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=452) | Continuous (for 5 nmol/L): RR =0.95; 95% CI =0.86–1.05 | NS |
| Weiss et al. 2008†(36) | Prospective | 1,614 | 13 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=727) | Continuous (for 5 nmol/L): RR =1.07; 95% CI =0.97–1.20 | NS |
| Travis et al. 2007† (37) | Prospective | 1,066 | 3.4 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=533) | Continuous (for 5 nmol/L): RR =1.00; 95% CI =0.85–1.18 | NS |
| Severi et al. 2006† (38) | Prospective | 2,377 | 8.7 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=518) | Continuous (for 5 nmol/L): RR =1.00; 95% CI =0.88–1.14 | NS |
| Parsons et al. 2005† (39) | Prospective | 794 | 18.5 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=88) | Continuous (for 5 nmol/L): RR =1.07; 95% CI =0.71–1.63 | NS |
| Platz et al. 2005† (26) | Prospective | 896 | 5 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=448) | Continuous (for 5 nmol/L): RR = 0.97; 95% CI =0.80–1.17 | NS |
| Ozasa et al. 2004† (40) | Prospective | 141 | 10 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=40) | Continuous (for 5 nmol/L): RR =1.11; 95% CI =0.63–1.96 | NS |
| Stattin (Finland) et al. 2004† (41) | Prospective | 375 | 10.8 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=84) | Continuous (for 5 nmol/L): RR =1.06; 95% CI =0.85–1.32 | NS |
| Stattin (Norway) et al. 2004† (41) | Prospective | 2,133 | 16.4 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=534) | Continuous (for 5 nmol/L): RR =0.93; 95% CI =0.86–1.00 | NS |
| Stattin (Sweden) et al. 2004† (41) | Prospective | 423 | 3.5 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=86) | Continuous (for 5 nmol/L): RR =0.96; 95% CI =0.89–1.04 | NS |
| Chen et al. 2003† (42) | Prospective | 600 | 3 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=300) | Continuous (for 5 nmol/L): RR =0.96; 95% CI =0.86–1.07 | NS |
| Heikkilä et al. 1999† (43) | Prospective | 466 | 24 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=166) | Continuous (for 5 nmol/L): RR =1.02; 95% CI =0.86–1.20 | NS |
| Dorgan et al. 1998† (44) | Prospective | 344 | 4.1 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=166) | Continuous (for 5 nmol/L): RR =0.87; 95% CI =0.68–1.12 | NS |
| Vatten et al. 1997† (45) | Prospective | 239 | 10 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=59) | Continuous (for 5 nmol/L): RR =0.95; 95% CI =0.70–1.28 | NS |
| Gann et al. 1996† (46) | Prospective | 612 | 6.3 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=222) | Continuous (for 5 nmol/L): RR =1.08; 95% CI =0.91–1.28 | NS |
| Nomura et al. 1996† (47) | Prospective | 282 | 22 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=141) | Continuous (for 5 nmol/L): RR =1.00; 95% CI =0.80–1.26 | NS |
| Hsing et al. 1993† (48) | Prospective | 196 | 13 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=98) | Continuous (for 5 nmol/L): RR =1.09; 95% CI =0.87–1.37 | NS |
| Barrett-Connor et al. 1990† (49) | Prospective | 1,001 | 14 (years) | Total testosterone, nmol/L (increase of 5 nmol/L) | Incident prostate cancer (n=57) | Continuous (for 5 nmol/L): RR =1.00; 95% CI =0.74–1.35 | NS |
†, previously meta-analyzed in Boyle et al. 2016 (50).
None of the twenty prospective studies reported a statistical significant association between continuous [5 nmol/L increment] total testosterone and increased- or decreased-risk of PCa [Figure 1 (numbers 1–20) and Table 1]. In fact, these 20 studies were used for analyses in a meta-analysis (50) that reported a summary RR of 0.99, which also did not reach statistical significance (95% CI =0.96–1.02).
Interestingly, two (27,31) of the 11 remaining studies/analyses, which were a combination of prospective and retrospective studies, showed a significant decreased-risks of PCa for continuous increment of total testosterone [OR =0.706; 95% CI =0.556–0.897 (27) and OR =0.13; 95% CI =0.05–0.35 (31)] [Figure 1 (numbers 21 and 27) and Table 1]. However, there were also two other studies (32,33) that showed significant increased-risks of PCa for continuous increment of total testosterone [HR =2.167; 95% CI =1.291–3.637 (32) and HR =1.31; 95% CI =1.03–1.65 (33)] [Figure 1 (numbers 28 and 31) and Table 1]. The remaining studies reported not significant and “null-risks” values for PCa [Figure 1 (numbers 22, 23, 24, 25, 26,29, 30)] (25,28-30).
Second part: relationship of endogenous total testosterone (categorical high vs. low) with decreased-, increased-, or null-risk of PCa
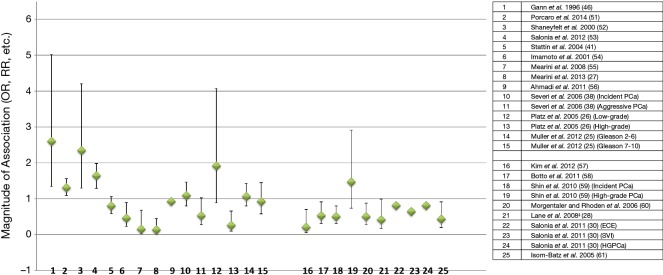
In this section, we identified 25 studies/analyses that investigated endogenous total testosterone in categorical format high vs. low levels (Figure 2 and Table 2) (25-28,30,38,41,46,51-61). This was a combination of prospective, retrospective, nested-control studies and one meta-analysis of two nested-control studies (52). There were some studies that originally reported low to high levels of testosterone [Figure 2 (numbers 16 to 25) and Table 2 (28,30,57-61)], but we converted these comparisons to high to low and recalculated the 95% CI’s for statistical significance purposes. This conversion was conducted to maintain consistency among all studies about their comparison groups (high vs. low) and because the contention is that high levels of testosterone increase risk of PCa. For the meta-analyses [Figure 2 (number 3) and Table 2] of the two nested-control studies, we only provided the summary OR as the direction of the measure of association of both independent studies is similar to the meta-analyses (52).
Figure 2.
Serum testosterone (categorical: high vs. low levels) in observational studies (prospective, retrospective, case-control) and its effects on PCa events. Studies/analyses only show multivariable analyses to minimize confounding effects. Previous studies comparing low vs. high levels of testosterone were inverted to compare high vs. low levels of testosterone and 95% CIs were recalculated (25-28,30,38,41,46,51-61). PCa, prostate cancer.
Table 2. Serum testosterone (categorical: high vs. low levels) in observational studies (prospective, retrospective, case-control) and its effects on PCa events (stage and grade). Only included studies that conducted multivariable analyses and reported magnitude of association (odds ratio, relative risk, etc.). Previous studies comparing low vs. high levels of testosterone were inverted to compare high vs. low levels of testosterone and 95% CIs recalculated.
| Study author and year | Study design | Number of participants for independent studies | Follow-up (years) | Total or free testosterone [continuous increment (high levels vs. low levels)] | Prostate cancer: total, grade and/or stage (n) | Results: only adjusted multivariable models | Statistically significant (SS: P<0.05) or not significant (NS) |
|---|---|---|---|---|---|---|---|
| Gann et al. 1996 (46) | Prospective, nested case-control study | 612 | Mean 18 (range 12–48) | Total testosterone, ng/mL | Incident prostate cancer, n=222) | High quartile (7.02 median) vs. low quartile (2.99); median: OR =2.60; 95% CI =1.34–5.02 | SS (P for trend =0.004) |
| Porcaro et al. 2014 (51) | Retrospective, radical prostatectomy | 220 | Not applicable | Total testosterone, ng/mL (15.5 nmol/L converted to ng/mL) | Gleason score (high-grade tumor-pathology Gleason ≥8.0) | ≥4.47 vs. <4.47: OR =1.31; 95% CI =1.09–1.56 | SS |
| Shaneyfelt et al. 2000 (52) | Meta-analysis (2 nested case—control studies) | 808 (320 cases/488 control) | 12 years and 6.3 | Total testosterone | Incident prostate cancer, n=320 | High quartile vs. low quartile: OR =2.34; 95% CI =1.30–4.20 | SS |
| Salonia et al. 2012 (53) | Retrospective, radical prostatectomy | 724 | Not applicable | Total Testosterone, ng/mL | High- or very high-risk (tumors stage, ≥T3a; Gleason score, 8–10; and serum PSA level, >20 ng/mL) of recurrence | >10th–<90th percentile: OR =0.64; 95% CI =0.54–0.80 | SS (<0.01) |
| 90th vs. 10th of percentile: OR =1.64; 95% CI =1.29–1.98 | SS (<0.01) | ||||||
| Stattin et al. 2004 (41) | Nested case-control study | 2,242 | Not applicable | Total testosterone, nmol/L | Total prostate cancer, n=708 | High quintile vs. low quintile: OR =0.80; 95% CI =0.59–1.06 | NS (P for trend =0.05) |
| Free testosterone, nmol/L | High quintile vs. low quintile: OR =0.82; 95% CI =0.60–1.14 | NS (P for trend =0.44) | |||||
| Imamoto et al. 2001 (54) | Retrospective | 130 | Mean 6.3 years | Total testosterone, ng/mL | Progression-free survival-metastatic prostate cancer (stage D2) | High vs. low (≥ 4.9 vs. <4.9 ng/mL): HR =0.46; 95% CI =0.23–0.90 | SS (P=0.03) |
| Mearini et al. 2008 (55) | Prospective | 65 | Median 13 months | Total testosterone, ng/mL | Incident total prostate cancer | >2.4 vs. ≤2.4: OR =0.147; 95% CI =0.03–0.68 | SS (P=0.014) |
| Mearini et al. 2013 (27) | Prospective | 206 (103 PCa cases and 103 BPH) | Not applicable | Total testosterone, ng/mL | Total prostate cancer, n=103 | ≥2.4 vs. <2.4 ng/mL: OR =0.134; 95% CI =0.039–0.453 | SS (P=0.001) |
| Ahmadi et al. 2011 (56) | Hospital-based case-control study | 511 | Not applicable | Total testosterone, nmol/L | Incident total prostate cancer, n=194 | >13.5 vs. ≤13.5: OR =0.92; 95% CI =0.89–0.96 | SS (P for trend <0.001) |
| Calculated free testosterone, nmol/L | >160.8 vs. ≤ 160.8: OR =0.99; 95% CI =0.98–0.99 | SS (P for trend =004) | |||||
| Severi et al. 2006 (38) | Case-Cohort design | 2,383 | Mean 8.7 years | Total testosterone, nmol/L | Incident total prostate cancer, n=614 | 4th quartile vs. 1st quartile: HR =1.09; 95% CI =0.80–1.47 | NS (P for trend =0.9) |
| Total testosterone, nmol/L | Aggressive prostate cancer (Gleason score >7 or poorly differentiated, stage T4 or N+ (positive lymph node) or M+ (distant metastases), n=88 | 4th quartile vs. 1st quartile: HR =0.53; 95% CI =0.28–1.03 | SS (P for trend =0.03) | ||||
| Platz et al. 2005 (26) | Nested case-control design | 920 | Not applicable | Total testosterone, ng/mL | Low-grade prostate cancer (Gleason sum <7) | 4th quartile vs. 1st quartile: OR =1.91; 95% CI =0.89–4.07 | SS (P for trend =0.02) |
| High-grade prostate cancer (Gleason sum ≥7) | 4th quartile vs. 1st quartile: OR =0.26; 95% CI =0.10–0.66 | SS (P for trend =0.01) | |||||
| Muller et al. 2012 (25) | Prospective | 3,255 | Prostate biopsy at 24 and 48 | Total testosterone, nmol/L | Gleason 2–6, n= 819 | Fifth quintile (20.6–45.7 nmol/L) vs. first quintile (2.7–10.3 nmol/L): OR =1.06; 95% CI =0.80–1.43) | NS (P=0.64) |
| Gleason 7–10, n= 228 | Fifth quintile (20.6-45.7 nmol/L) vs. first quintile (2.7–10.3 nmol/L): OR =0.92; 95% CI =0.58–1.45) | NS (P=0.70) | |||||
| Kim et al. 2012‡ (57) | Retrospective, radical prostatectomy | 60 | Mean 18 (range 12–48) | Total testosterone, ng/mL | Extraprostatic invasion | ≥3 vs. <3 ng/mL: OR =0.20; 95% CI=0.057–0.706 | SS |
| Botto et al. 2011‡ (58) | Prospective, radical prostatectomy | 431 | Not applicable | Total testosterone, ng/mL | Predominant Gleason Pattern 4 (Gleason score 7=4+3, 8=4+4, and 9=4+5), n=132 | ≥3 vs. <3 ng/mL: OR =0.53; 95% CI =0.31–0.91 | SS |
| Shin et al. 2010‡ (59) | Prospective, prostate biopsy | 568 | Not applicable | Total testosterone, ng/mL | Total prostate cancer (incidence), n=194 | ≥3.85 vs. <3.85 ng/mL: OR =0.50; 95% CI =0.31–0.80 | SS |
| High grade prostate cancer (Gleason score ≥7), n=18 | ≥3.85 vs. <3.85 ng/mL: OR =1.47; 95% CI =0.74–2.91 | NS | |||||
| Morgentaler and Rhoden et al. 2006‡ (60) | Retrospective, hypogonadism | 345 | Not applicable | Total testosterone, ng/dL | Total prostate cancer, n=52 | Total testosterone >250 vs. ≤250 ng/dL: OR =0.50; 95% CI =0.27–0.88 | SS |
| Lane et al. 2008‡ (28) | Prospective study, radical prostatectomy | 455 | Median 14 (range 2–29) | Total testosterone, ng/dL | Gleason score pattern 4–5 at prostatectomy | ≥220 vs. <220 ng/dL: OR =0.41; 95% CI = 0.18–0.99 | SS |
| Salonia et al. 2011‡ (30) | Prospective, radical prostatectomy | 673 | Not applicable | Total testosterone, ng/mL | Extracapsular extension (ECE) | ≥3 vs. <3 ng/mL: OR =0.81; 95% CI (not provided) | NS |
| Seminal vesicle invasion (SVI) | OR =0.64; 95% CI (not provided) | NS | |||||
| HGPCa | OR =0.81; 95% CI (not provided) | NS | |||||
| Isom-Batz et al. 2005‡ (61) | Retrospective, radical prostatectomy | 326 | Median 36 (range 4–133) | Total testosterone, ng/mL | Pathological stage (organ confined, focal or extracapsular extension, invasion into the seminal vesicles or nodal metastasis), n=245 | ≥ 300 vs. <300 ng/mL: OR =0.43; 95% CI =0.20–0.91 | SS |
‡, low to high levels of testosterone comparison groups were inverted to compare “high to low” testosterone levels, and 95% CIs recalculated. ECE, extracapsular extension; SVI, seminal vesicle invasion; HGPCa, high-grade prostate cancer.
Eight out of 25 studies/analyses reported an increased-risk of PCa for men with high levels of testosterone compared to low [Figure 2 (numbers 1–4, 10, 12, 14, 19)] (25,26,38,46,51-53,59), but only four were statistically significant (numbers 1–4) (46,51-53). The remaining 17 studies/analyses showed a decreased-risk of PCa after comparing high vs. low levels of testosterone [Figure 2 (numbers 5–9,11, 13, 15, 16, 17, 18, 19 and 20–25) and Table 2] (25-28,30,38,41,54-61), but only 11 were statistically significant [Figure 2 (numbers 6–9,13, 16, 17, 18, 20, 21, 25) and Table 2] (26,27,54-61).
Third part: association of use of TTh with decreased-, increased-, or null-risk of PCa in meta-analyses of randomized placebo-controlled trials
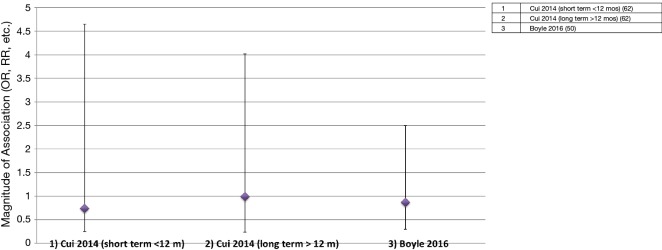
As of to date, two meta-analyses of randomized placebo-controlled trials that investigated use of TTh with risk of PCa have been conducted (Figure 3 and Table 3) (50,62). The first meta-analyses (62) consisted of 8 trials, and they were separated in two groups: first group (n=5 trials) consisted of men who used TTh for less than 12 months and the second group (n=3 trials) consisted of men who used TTh between 12 and 36 months. Findings from the first group reported a non-significant reduced-risk of PCa (summary OR<12 months =0.74; 95% CI =0.25–4.65). However, results from the second group were considered null-risk of PCa (summary OR12-36 months =0.99; 95% CI =0.24–4.02), possibly due to the few number of trials (3 trials) and small sample size of PCa cases (n=6). The most recent meta-analyses (50), which included 11 trials, reported a reduced-risk of PCa for men who used TTh for less than 12 months, but it was not statistically significant (summary OR<12 months =0.87; 95% CI =0.30–2.5).
Figure 3.
Meta-analyses of randomized controlled trials (RCTs) that investigated the effect of TTh on PCa events (stage and grade). Studies/analyses only show multivariable analyses to minimize confounding effects. Magnitudes of association (ORs, RRs, etc.) and 95% CIs (50,62). TTh, testosterone therapy; PCa, prostate cancer; OR, odds ratio; RR, relative risk.
Table 3. Meta-analyses of randomized controlled trials (RCTs) that investigated the effect of TTh on PCa events (stage and grade). Only included studies that conducted multivariable analyses and reported magnitude of association (odds ratio, relative risk, etc.).
| Study author and year | Study design | Number of studies/trials | Number of participants (n) | TTh/placebo (n) | Prostate cancer: total, grade and/or stage (n) | Results: only multivariable models | Statistically significant (SS: P<0.05) or not significant (NS) |
|---|---|---|---|---|---|---|---|
| Cui et al. 2014 (62) | Randomized controlled trials: short term (<12 months) | 5 | 1,168 | 778/390 | Incident prostate cancer, n=12 | Summary: OR =0.74; 95% CI =0.25–4.65 | NS |
| Randomized controlled trials: long term (12–36 months) | 3 | 379 | 191/188 | Incident prostate cancer, n=6 | Summary: OR =0.99; 95% CI =0.24–4.02 | NS | |
| Boyle et al. 2016 (50) | Randomized controlled trials (most of the trials <12 months) | 11 | 2,013 | 1,114/899 | Incident prostate cancer, n=20 | Summary: OR =0.87; 95% CI =0.30–2.5 | NS |
Discussion
The contention that high levels of testosterone or that the use of TTh increases the risk of PCa doesn’t seem to be supported with the present body of literature, which were adjusted for potential PCa risk factors. A significant number of observational studies (prospective and retrospective) that investigated the association of endogenous (continuous per 1 unit increment or 5 ng/mL increment) total testosterone with PCa were not statistically significant. Most of these studies reported magnitudes of association that were null. On the other hand, those observational studies that categorized endogenous total testosterone (high vs. low) and investigated its relationship with PCa seemed to show a different picture; there were more studies/analyses reporting a reduced-risk of PCa (17 out of 25) than increased-risk of PCa (8 out of 25) among men with high levels of total testosterone compared to those with low levels. Most of these reduced-risks findings were statistically significant (11 out of 17) than the increased-risk of PCa ones (4 out of 8). Meta-analyses of randomized placebo-controlled trials that investigated use of TTh and its association with PCa were more consistent on reporting magnitude of associations of reduced-risks of PCa, but they did not reach statistical significance.
Although the contention of high levels of testosterone is challenged by the body of literature presented, we can’t conclude or promote that there is “zero” risk by using TTh or increasing levels of testosterone among PCa patients. It is important to note that any kind of epidemiologic study design experience different limitations such as sample size/power (mainly in randomized trials), no randomization (i.e., observational studies), no or limited follow-up data, different methodology on data collection and different definitions of exposures and outcomes to conduct pooled-analysis. In addition, comparison among studies is challenging as some studies define high vs. low levels of testosterone with different cutoff points or develop different criteria to define aggressive and non-aggressive PCa. Therefore, there is a need further studies with the adequate power, follow-up data, epidemiological and clinicopathological data that can provide a better explanation about the safety and protective effects of high levels of endogenous testosterone or use of TTh in the natural history of PCa and in men’s health.
Furthermore, it seems that the long-standing controversy between endogenous/exogenous testosterone and PCa will remain for some time. First, the evolution of PCa research shows that advanced PCa is the most clinical relevant outcome (4). However, there are few studies, observational and randomized controlled trials, focusing on advanced PCa and endogenous/exogenous testosterone. In addition, there are significant racial disparities of PCa showing African-American men with the highest incident and mortality rates (63,64); yet, there are limited number of studies investigating the relationship between endogenous/exogenous testosterone and PCa among African-American men (65-67). These can be relevant research topics that should be addressed in future studies.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9-12. 10.1016/S0022-5347(05)64820-3 [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079-92. 10.1016/j.eururo.2012.02.054 [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 4.Stanford JL, Stephenson RA, Coyle LM, et al. Prostate cancer trends 1973 - 1995. Bethesda, MD; 1999. NIH Pub. No 99-4543. [Google Scholar]
- 5.Khera M, Broderick GA, Carson CC, 3rd, et al. Adult-Onset Hypogonadism. Mayo Clin Proc 2016;91:908-26. 10.1016/j.mayocp.2016.04.022 [DOI] [PubMed] [Google Scholar]
- 6.Khera M, Adaikan G, Buvat J, et al. Diagnosis and treatment of testosterone deficiency: Recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J Sex Med 2016;13:1787-804. 10.1016/j.jsxm.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Travison TG, Araujo AB, O'Donnell AB, et al. A population-level decline in serum testosterone levels in american men. J Clin Endocrinol Metab 2007;92:196-202. 10.1210/jc.2006-1375 [DOI] [PubMed] [Google Scholar]
- 8.Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241-7. 10.1210/jc.2007-1245 [DOI] [PubMed] [Google Scholar]
- 9.Platz EA, Kawachi I, Rimm EB, et al. Plasma steroid hormones, surgery for benign prostatic hyperplasia, and severe lower urinary tract symptoms. Prostate Cancer Prostatic Dis 1999;2:285-9. 10.1038/sj.pcan.4500380 [DOI] [PubMed] [Google Scholar]
- 10.Rohrmann S, Nelson WG, Rifai N, et al. Serum sex steroid hormones and lower urinary tract symptoms in third national health and nutrition examination survey (NHANES III). Urology 2007;69:708-13. 10.1016/j.urology.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 2008;159:507-14. 10.1530/EJE-08-0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: A review. Ther Clin Risk Manag 2009;5:427-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansone A, Romanelli F, Gianfrilli D, et al. Endocrine evaluation of erectile dysfunction. Endocrine 2014;46:423-30. 10.1007/s12020-014-0254-6 [DOI] [PubMed] [Google Scholar]
- 14.Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: A review of the continuing controversy. J Urol 2015;193:403-13. 10.1016/j.juro.2014.07.123 [DOI] [PubMed] [Google Scholar]
- 15.Samplaski MK, Loai Y, Wong K, et al. Testosterone use in the male infertility population: Prescribing patterns and effects on semen and hormonal parameters. Fertil Steril 2014;101:64-9. 10.1016/j.fertnstert.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Lackner JE, Mark I, Schatzl G, et al. Hypogonadism and androgen deficiency symptoms in testicular cancer survivors. Urology 2007;69:754-8. 10.1016/j.urology.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 17.O'Carrigan B, Fournier M, Olver IN, et al. Testosterone deficiency and quality of life in australasian testicular cancer survivors: A prospective cohort study. Intern Med J 2014;44:813-7. 10.1111/imj.12500 [DOI] [PubMed] [Google Scholar]
- 18.Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: The HIM study. Int J Clin Pract 2006;60:762-9. 10.1111/j.1742-1241.2006.00992.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloner RA, Carson C, 3rd, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol 2016;67:545-57. 10.1016/j.jacc.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 20.Platz EA. Low testosterone and risk of premature death in older men: Analytical and preanalytical issues in measuring circulating testosterone. Clin Chem 2008;54:1110-2. 10.1373/clinchem.2008.104901 [DOI] [PubMed] [Google Scholar]
- 21.Malik RD, Lapin B, Wang CE, et al. Are we testing appropriately for low testosterone?: Characterization of tested men and compliance with current guidelines. J Sex Med 2015;12:66-75. 10.1111/jsm.12730 [DOI] [PubMed] [Google Scholar]
- 22.Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: Estimates from the massachusetts male aging study. J Clin Endocrinol Metab 2004;89:5920-6. 10.1210/jc.2003-031719 [DOI] [PubMed] [Google Scholar]
- 23.Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the united states, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. 10.1001/jamainternmed.2013.6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the united kingdom and the united states, 2000 to 2011. J Clin Endocrinol Metab 2014;99:835-42. 10.1210/jc.2013-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller RL, Gerber L, Moreira DM, Andriole G, et al. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by dutasteride of prostate cancer events trial. Eur Urol 2012;62:757-64. 10.1016/j.eururo.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 26.Platz EA, Leitzmann MF, Rifai N, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev 2005;14:1262-9. 10.1158/1055-9965.EPI-04-0371 [DOI] [PubMed] [Google Scholar]
- 27.Mearini L, Zucchi A, Nunzi E, et al. Low serum testosterone levels are predictive of prostate cancer. World J Urol 2013;31:247-52. 10.1007/s00345-011-0793-x [DOI] [PubMed] [Google Scholar]
- 28.Lane BR, Stephenson AJ, Magi-Galluzzi C, et al. Low testosterone and risk of biochemical recurrence and poorly differentiated prostate cancer at radical prostatectomy. Urology 2008;72:1240-5. 10.1016/j.urology.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 29.Massengill JC, Sun L, Moul JW, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol 2003;169:1670-5. 10.1097/01.ju.0000062674.43964.d0 [DOI] [PubMed] [Google Scholar]
- 30.Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011;117:3953-62. 10.1002/cncr.25985 [DOI] [PubMed] [Google Scholar]
- 31.Karamanolakis D, Lambou T, Bogdanos J, et al. Serum testosterone: A potentially adjunct screening test for the assessment of the risk of prostate cancer among men with modestly elevated PSA values (> or =3.0 and <10.0 ng/ml). Anticancer Res 2006;26:3159-66. [PubMed] [Google Scholar]
- 32.Imamoto T, Suzuki H, Fukasawa S, et al. Pretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomy. Eur Urol 2005;47:308-12. 10.1016/j.eururo.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 33.Yano M, Imamoto T, Suzuki H, et al. The clinical potential of pretreatment serum testosterone level to improve the efficiency of prostate cancer screening. Eur Urol 2007;51:375-80. 10.1016/j.eururo.2006.08.047 [DOI] [PubMed] [Google Scholar]
- 34.Daniels NA, Nielson CM, Hoffman AR, et al. Osteoporotic Fractures In Men (MrOS) Study Group. Sex hormones and the risk of incident prostate cancer. Urology 2010;76:1034-40. 10.1016/j.urology.2010.01.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill JK, Wilkens LR, Pollak MN, et al. Androgens, growth factors, and risk of prostate cancer: The multiethnic cohort. Prostate 2010;70:906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss JM, Huang WY, Rinaldi S, et al. Endogenous sex hormones and the risk of prostate cancer: A prospective study. Int J Cancer 2008;122:2345-50. 10.1002/ijc.23326 [DOI] [PubMed] [Google Scholar]
- 37.Travis RC, Key TJ, Allen NE, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the european prospective investigation into cancer and nutrition. Int J Cancer 2007;121:1331-8. 10.1002/ijc.22814 [DOI] [PubMed] [Google Scholar]
- 38.Severi G, Morris HA, MacInnis RJ, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15:86-91. 10.1158/1055-9965.EPI-05-0633 [DOI] [PubMed] [Google Scholar]
- 39.Parsons JK, Carter HB, Platz EA, et al. Serum testosterone and the risk of prostate cancer: Potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev 2005;14:2257-60. 10.1158/1055-9965.EPI-04-0715 [DOI] [PubMed] [Google Scholar]
- 40.Ozasa K, Nakao M, Watanabe Y, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among japanese men. Cancer Sci 2004;95:65-71. 10.1111/j.1349-7006.2004.tb03172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stattin P, Lumme S, Tenkanen L, et al. High levels of circulating testosterone are not associated with increased prostate cancer risk: A pooled prospective study. Int J Cancer 2004;108:418-24. 10.1002/ijc.11572 [DOI] [PubMed] [Google Scholar]
- 42.Chen C, Weiss NS, Stanczyk FZ, et al. Endogenous sex hormones and prostate cancer risk: A case-control study nested within the carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev 2003;12:1410-6. [PubMed] [Google Scholar]
- 43.Heikkilä R, Aho K, Heliovaara M, et al. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: A longitudinal study. Cancer 1999;86:312-5. [DOI] [PubMed] [Google Scholar]
- 44.Dorgan JF, Albanes D, Virtamo J, et al. Relationships of serum androgens and estrogens to prostate cancer risk: Results from a prospective study in finland. Cancer Epidemiol Biomarkers Prev 1998;7:1069-74. [PubMed] [Google Scholar]
- 45.Vatten LJ, Ursin G, Ross RK, et al. Androgens in serum and the risk of prostate cancer: A nested case-control study from the janus serum bank in norway. Cancer Epidemiol Biomarkers Prev 1997;6:967-9. [PubMed] [Google Scholar]
- 46.Gann PH, Hennekens CH, Ma J, et al. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst 1996;88:1118-26. 10.1093/jnci/88.16.1118 [DOI] [PubMed] [Google Scholar]
- 47.Nomura AM, Stemmermann GN, Chyou PH, et al. Serum androgens and prostate cancer. Cancer Epidemiol Biomarkers Prev 1996;5:621-5. [PubMed] [Google Scholar]
- 48.Hsing AW, Comstock GW. Serological precursors of cancer: Serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol Biomarkers Prev 1993;2:27-32. [PubMed] [Google Scholar]
- 49.Barrett-Connor E, Garland C, McPhillips JB, et al. A prospective, population-based study of androstenedione, estrogens, and prostatic cancer. Cancer Res 1990;50:169-73. [PubMed] [Google Scholar]
- 50.Boyle P, Koechlin A, Bota M, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: A meta-analysis. BJU Int 2016;118:731-41. 10.1111/bju.13417 [DOI] [PubMed] [Google Scholar]
- 51.Porcaro AB, Petrozziello A, Ghimenton C, et al. Associations of pretreatment serum total testosterone measurements with pathology-detected gleason score cancer. Urol Int 2014;93:269-78. 10.1159/000354621 [DOI] [PubMed] [Google Scholar]
- 52.Shaneyfelt T, Husein R, Bubley G, et al. Hormonal predictors of prostate cancer: A meta-analysis. J Clin Oncol 2000;18:847-53. 10.1200/JCO.2000.18.4.847 [DOI] [PubMed] [Google Scholar]
- 53.Salonia A, Abdollah F, Capitanio U, et al. Serum sex steroids depict a nonlinear u-shaped association with high-risk prostate cancer at radical prostatectomy. Clin Cancer Res 2012;18:3648-57. 10.1158/1078-0432.CCR-11-2799 [DOI] [PubMed] [Google Scholar]
- 54.Imamoto T, Suzuki H, Akakura K, et al. Pretreatment serum level of testosterone as a prognostic factor in japanese men with hormonally treated stage D2 prostate cancer. Endocr J 2001;48:573-8. 10.1507/endocrj.48.573 [DOI] [PubMed] [Google Scholar]
- 55.Mearini L, Costantini E, Zucchi A, et al. Testosterone levels in benign prostatic hypertrophy and prostate cancer. Urol Int 2008;80:134-40. 10.1159/000112602 [DOI] [PubMed] [Google Scholar]
- 56.Ahmadi H, Allameh F, Baradaran N, et al. Circulating sex hormones play no role in the association between sexual activity and the risk of prostate cancer. J Sex Med 2011;8:905-13. 10.1111/j.1743-6109.2010.02115.x [DOI] [PubMed] [Google Scholar]
- 57.Kim HJ, Kim BH, Park CH, et al. Usefulness of preoperative serum testosterone as a predictor of extraprostatic extension and biochemical recurrence. Korean J Urol 2012;53:9-13. 10.4111/kju.2012.53.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Botto H, Neuzillet Y, Lebret T, et al. High incidence of predominant gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011;186:1400-5. 10.1016/j.juro.2011.05.082 [DOI] [PubMed] [Google Scholar]
- 59.Shin BS, Hwang EC, Im CM, et al. Is a decreased serum testosterone level a risk factor for prostate cancer? A cohort study of korean men. Korean J Urol 2010;51:819-23. 10.4111/kju.2010.51.12.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology 2006;68:1263-7. 10.1016/j.urology.2006.08.1058 [DOI] [PubMed] [Google Scholar]
- 61.Isom-Batz G, Bianco FJ, Jr, Kattan MW, et al. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol 2005;173:1935-7. 10.1097/01.ju.0000158040.33531.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2014;17:132-43. 10.1038/pcan.2013.60 [DOI] [PubMed] [Google Scholar]
- 63.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for hispanics/latinos, 2015. CA Cancer J Clin 2015;65:457-80. 10.3322/caac.21314 [DOI] [PubMed] [Google Scholar]
- 64.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 65.Wallington SF, Luta G, Noone AM, et al. Assessing the awareness of and willingness to participate in cancer clinical trials among immigrant latinos. J Community Health 2012;37:335-43. 10.1007/s10900-011-9450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooks SE, Muller CY, Robinson W, et al. Increasing minority enrollment onto clinical trials: Practical strategies and challenges emerge from the NRG oncology accrual workshop. J Oncol Pract 2015;11:486-90. 10.1200/JOP.2015.005934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgentaler A. Controversies and advances with testosterone therapy: A 40-year perspective. Urology 2016;89:27-32. 10.1016/j.urology.2015.11.034 [DOI] [PubMed] [Google Scholar]