Abstract
Psychological stress has been associated with intestinal epithelial hyperpermeability, the basic process in various functional and organic bowel diseases. In the present study, we aimed to clarify the differences and underlining mechanisms in stress-induced barrier disruption in functionally and structurally distinct epitheliums, including the villus epithelium (VE) and follicle-associated epithelium (FAE), a specialized epithelium overlaid the domes of Peyer’s lymphoid follicles. Employing an Ussing Chamber system, the epithelial permeability was assessed in rats following water avoidance stress (WAS) in vivo and in mucosa tissues exposed to corticotropin-releasing factor (CRF) ex vivo. Decreased transepithelial resistance (TER) and increased paracellular and transcellular macromolecular permeability in colon, ileal VE and FAE had been observed in WAS rats and in CRF-exposed mucosa. Especially, the barrier dysfunction was more serious in the FAE. Moreover, WAS upregulated the expression of mast cell tryptase and protease-activated receptor-2 (PAR2), which positively correlated with epithelial conductance. Mast cell stabilizer cromolyn sodium obviously alleviated the barrier disruption induced by WAS in vivo and CRF in vitro. Serine protease inhibitor aprotinin and FUT-175, and selective PAR2 antagonist ENMD-1068 effectively inhibited the CRF-induced FAE hyperpermeability. Altogether, it concluded that the FAE was more susceptible to stress, and the mast cells and PAR2 signaling played crucial roles in this process.
Introduction
The human intestinal epithelium makes up the largest barrier, with a surface area up to 400 m2, separating the body from the luminal environment, such as toxins and antigens from food and microbes1, 2. Normally, only small amount of antigens could pass the intestinal barrier and play an important role in maintaining the immune function1. However, increased epithelial permeability may enhance the passage of antigens, facilitate the invasion of bacteria and lead to mucosal injury and inflammation. This process is the common pathophysiological basis of numerous organic and functional intestinal disorders such as inflammatory bowel disease and irritable bowel syndrome3.
Psychological stress is often suspected to adversely affect the intestinal barrier function2. It’s fact that, in gastrointestinal disorders, acute and persistent stress may induce/aggravate the gut dysfunctions and symptoms, impact the clinical remission and increase the risk of disease relapse4. The theory of brain-gut-axis has been well accepted which implicates a close relationship between the functions of the gut and the brain5. The bad emotions no doubt lead to negative sensation and mood of the gut. The mechanisms are complex and multifactorial, but at least including the neural and humoral pathways especially the autonomic nervous system and the hypothalamic-pituitary-adrenal axis6. Corticotropin releasing factor (CRF) is one of the most important stress hormones, produced in both the central nerve system and peripheral tissues, and has been shown to participate in stress-related gastrointestinal dysfunctions by acting on the CRF receptor type 1 and type 2 (CRF1 and CRF2)4. CRF could disrupt the intestinal barrier in multiple in vivo and in vitro researches2, 7. Several studies have suggested mast cells (MCs) as crucial effector cells of CRF in the gut, which express the CRF1 and CRF2 receptors and release multifunctional mediators such as proteases, histamine and cytokines upon activation7, 8. However, the precise mechanisms of stress-induced mast cell-mediated intestinal hyper-permeability remain poorly elucidated, particularly in functionally and structurally distinct regions of the gastrointestinal tract.
In most cases, assessment of intestinal epithelial permeability was focused on the villus epithelium (VE), but ignored the importance of follicle-associated epithelium (FAE)1, 9. The FAE is a specialized epithelium with distinct functional and structural features that overlays the domes of lymphoid follicles (Peyer’s patches) in the small intestine10, 11. The FAE typically contains numerous microfold cells which facilitates sampling and transporting luminal macromolecules and microorganisms to the underlying mucosal immune system10, 11. The epithelial permeability of the FAE obviously differs from the VE10, however, the differences in responses to psychological stress were rarely reported. Furthermore, systemic evaluation of the differences in stress-induced epithelial barrier disrupt among different bowel segments was required, since the intestinal barrier function varies in a segment-specific way12.
In this study, we aimed to assess the mechanisms and differences in stress-induced barrier dysfunction in structurally and functionally distinct epitheliums of the gut, including the colonic epithelium, ileal VE and FAE.
Results
Body weight, stool pellet, and visceral sensation
Chronic water avoidance stress (WAS) induced failure to thrive (Fig. 1A), increased stool pellet number during stress (Fig. 1B) and decreased pain threshold (Fig. 1C). Mast cell stabilizer cromolyn didn’t influence on the weight gain and defecation during stress, but effectively improved the pain threshold of WAS rats (Fig. 1A–C).
Figure 1.
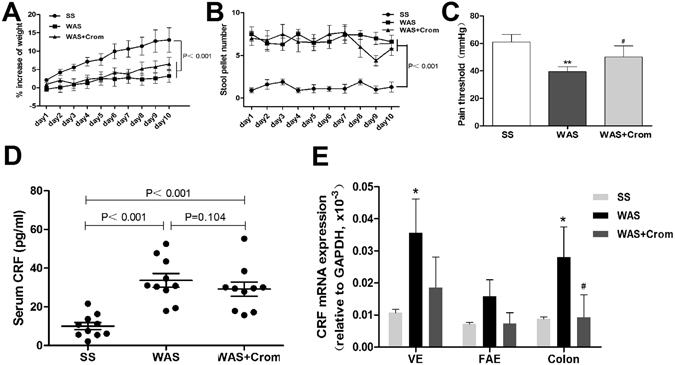
Effects of WAS on the body weight, stool pellet, visceral sensation, and CRF levels in serum and intestine. (A,B) WAS rats showed failure to thrive and stool pellet increasing compared with SS rats, mast cell stabilizer cromolyn didn’t influence on the weight gain and defecation during stress. (C) Decreased pain threshold in WAS rats and effectively improved in WAS + Crom rats. (D) WAS induced significant increase in serum CRF which didn’t inhibit by cromolyn. (E) mRNA expression of CRF in the colon, ileal VE and FAE enhanced during stress and partially reduced by cromolyn. N = 10 for each group. Data are displayed as the mean ± SD.
Serum and intestinal CRF levels
The serum levels of CRF increased in WAS rats relative to the SS rats, while cromolyn didn’t inhibit the serum CRF increase induced by stress (Fig. 1D). Similarly, the mRNA expression of CRF were enhanced significantly in ileal VE and colon (P < 0.01), and there was an increasing trend in ileal FAE (P = 0.061). Cromolyn may decrease the CRF expression in the intestine especially for the colonic mucosa (Fig. 1E).
Inflammatory scores
There were obvious intestinal inflammation in the mucosa of colon, ileum VE and FAE in WAS rats with increased lymphocytes infiltration and histological scores. Cromolyn treatment trended to improve the mucosal inflammation induced by WAS, especially in the ileal VE and FAE (Fig. 2A–L). Relative to colon, the WAS induced more serious inflammation in ileal VE. However, there were no significant differences between FAE with VE or colon (Fig. 2M).
Figure 2.
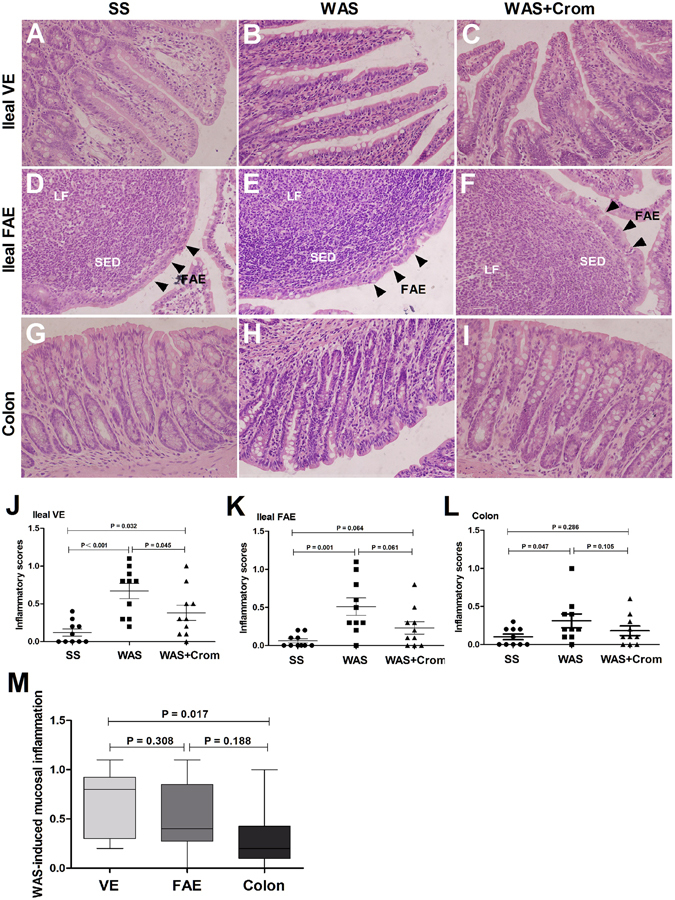
Effects of WAS on the inflammatory infiltration in the colon, ileal VE and FAE. (A–I) Typical hematoxylin-eosin staining of ileal VE, ileal FAE and colon form SS group, WAS group and WAS + Crom group, respectively. There were obviously inflammatory infiltration in WAS rats, and improved in WAS + Crom rats. (J–L) Histological scores in the colon, ileum VE and FAE were increased in WAS rats, and trended to be decreased by cromolyn treatment in WAS + Crom rats. (M) WAS induced more serious inflammation in ileal VE relative to colon. N = 10 for each group. Data are displayed as the mean ± SD.
Mast cell infiltration and tryptease/PAR2 expression
Mast cells, the tryptase+ cells, infiltration in mucosal tissues of ileal VE (Fig. 3A–C), ileal FAE (Fig. 3D–F) and colon (Fig. 3G–I) were increased in WAS rats, and decreased in cromolyn treated WAS rats. The expression of mast cell-derived tryptase in colon, ileum VE and FAE were obviously upregulated in WAS rats, and alleviated in those treated with cromolyn (Fig. 3J,K). The proteinase-activated receptor-2 (PAR2) was located on the epithelial layer of the colon, ileum VE and FAE. Higher expression of PRA2 was observed in WAS rats in colonic epithelium (Fig. 4A–C) as well as the ileum FAE and adjacent VE (Fig. 4D–F), which may be downregulated by cromolyn. It also was verified by the results of immunoblot, especially for the FAE (Fig. 4G,H). Furthermore, there were strongly positive relations between the levels of tryptase, as well as PAR2, and epithelial conductance in the colon, ileum VE and FAE, which is more obviously in FAE (Fig. 5).
Figure 3.
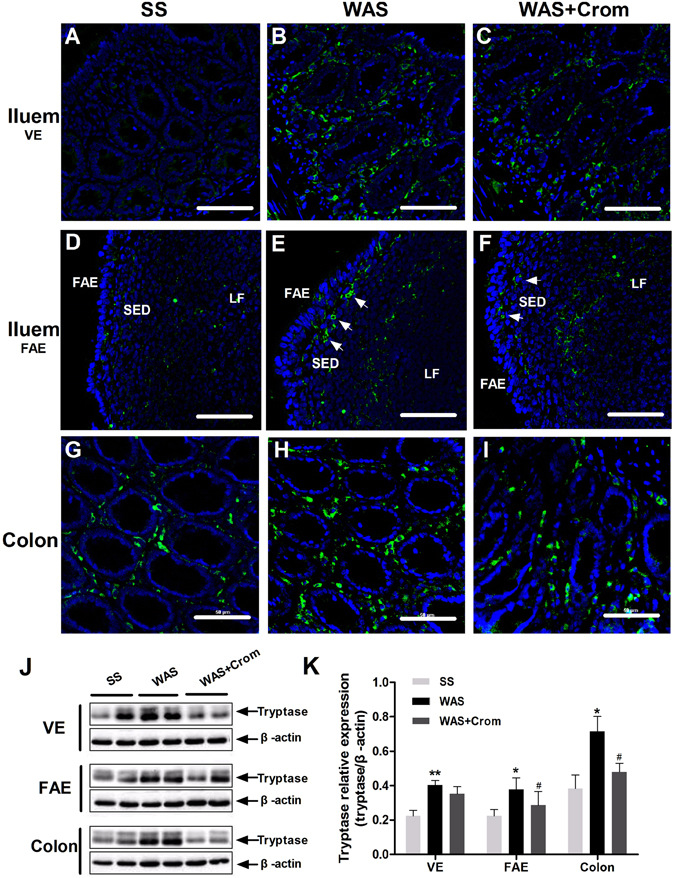
Effects of WAS on the expression of mast cell tryptase in the colon, ileal VE and FAE. (A–I) The tryptase+ cells increased in mucosal tissues of ileal VE, ileal FAE and colon in WAS rats, and decreased in cromolyn treated WAS rats. SED, subepithelial dome; LF, lymphoid follicles. Scale bars: 50 μm. (J,K) The expression of tryptase in colon, ileum VE and FAE were upregulated in WAS rats, and alleviated in WAS + Crom rats. N = 10 for each group. Data are displayed as the mean ± SD. *P < 0.05, **P < 0.01, compared with SS group; #P < 0.05, compared with WAS group. followed by IBS-D FSN stimulation (**P < 0.01). The gels were run under the same experimental conditions. Cropped blots are displayed and full-length blots are included in the supplementary information.
Figure 4.
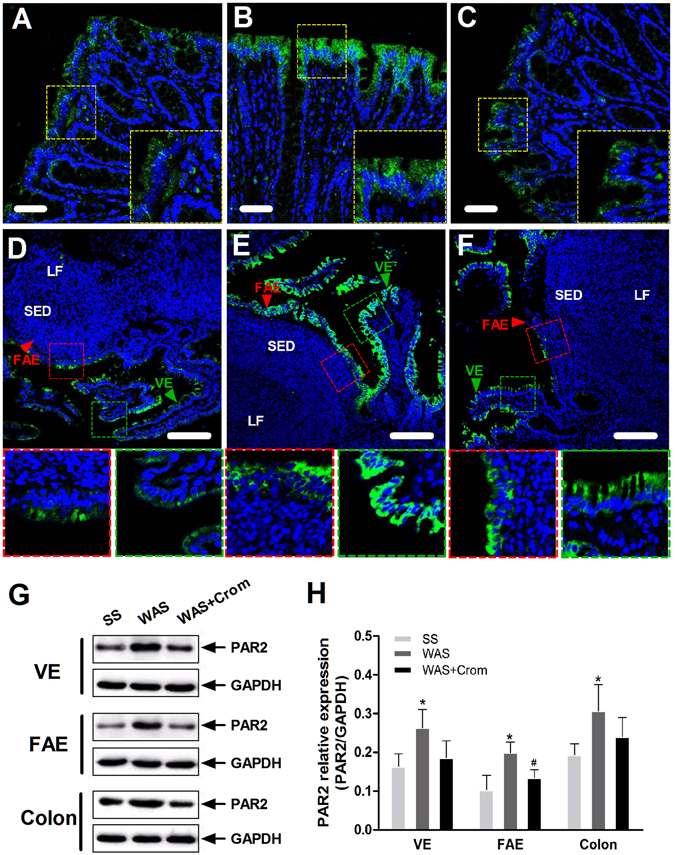
Effects of WAS on the expression of PAR2 receptor in the colon, ileal VE and FAE. (A–F) The PAR2 receptor was located on the epithelial layer of the colon, ileum FAE and adjacent VE, which upregulated in WAS rats, and may be downregulated by cromolyn. SED, subepithelial dome; LF, lymphoid follicles. Scale bars: 50 μm for (A–C) and 100 μm for (D–F). (G,H) Higher expression of PRA2 was observed in WAS rats in colonic epithelium as well as the ileum FAE and VE. Cromolyn treatment significantly reduced the levels of PAR2 in FAE. N = 10 for each group. Data are displayed as the mean ± SD. *P < 0.05, compared with SS group; #P < 0.05, compared with WAS group. The gels were run under the same experimental conditions. Cropped blots are presented and full-length blots are shown in the supplementary information.
Figure 5.
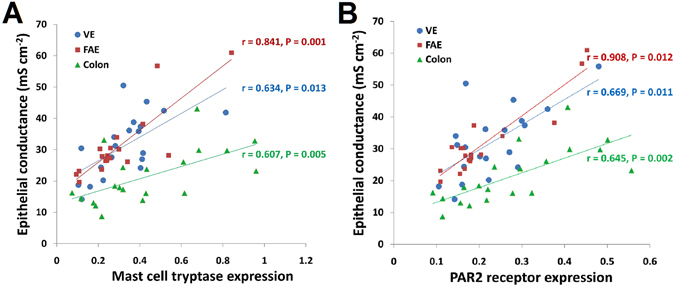
Positive correlations between mast cell tryptase/PAR2 and epithelial conductance in the colon, ileum VE and FAE. (A) Mast cell tryptase and (B) PAR2 receptor were positively correlated with epithelial conductance in the colon, ileum VE and FAE, which is more obviously in FAE. Data are displayed as the mean ± SD.
Stress-induced intestinal hyperpermeability was different in ileal VE, FAE and colonic epithelium
Stress induced significant decline of transepithelial resistance (TER) and increase of paracellular permeability to fluorescein-isothiocvanate (FITC)-dextran 4 kDa (FD4) in ileal VE, FAE and colon, which could be partially relieved by cromolyn (Fig. 6A,C). However, the transcellular permeability to fluorescein-isothiocvanate (FITC)-dextran 40 kDa (FD40) was enhanced obviously just in ileal FAE and blocked by mast cell stabilization (Fig. 6E). Importantly, the ileal FAE was the most influenced region by stress with higher decrease of TER and higher increase of FD4 and FD40 permeability. There was no significant difference between ileal VE and colon (Fig. 6B,D,F).
Figure 6.
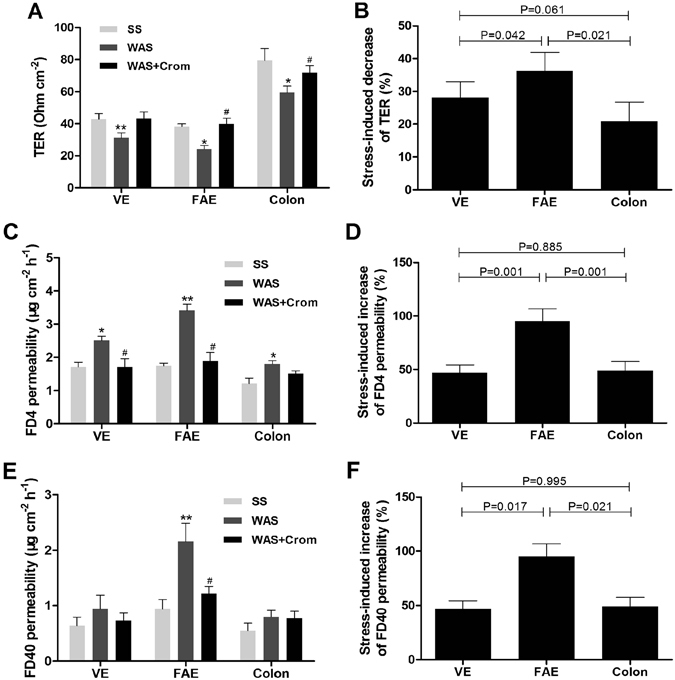
Stress-induced intestinal hyperpermeability was different in ileal VE, FAE and colonic epithelium. (A,C,E) WAS induced significant decline of TER and increase of paracellular FD4 permeability in ileal VE, FAE and colon, and FD40 permeability in ileal FAE, which could be partially blocked by cromolyn. (B,D,F) The ileal FAE was more sensitive to stress with higher decrease of TER and higher increase of FD4 and FD40 permeability relative to the ileal VE and colon. N = 10 for each group. Data are displayed as the mean ± SD. *P < 0.05, **P < 0.01, compared with SS group; #P < 0.05, compared with WAS group.
Differences in CRF-induced increase of permeability in VE, FAE and colonic epithelium
CRF exposure mimicked the changes in epithelial permeability induced by stress, including TER decreasing and increased permeability to FD4 in ileal VE, FAE and colon, as well as hyperpermeability to FD40 in ileal FAE and VE (Fig. 7A,C,E). Similarly, the barrier disruption, such as TER decreasing and permeability increasing, was trend to be more serious in the ileal FAE relative to the ileal VE and colon, but most of them had no statistical significance (Fig. 7B,D,F).
Figure 7.
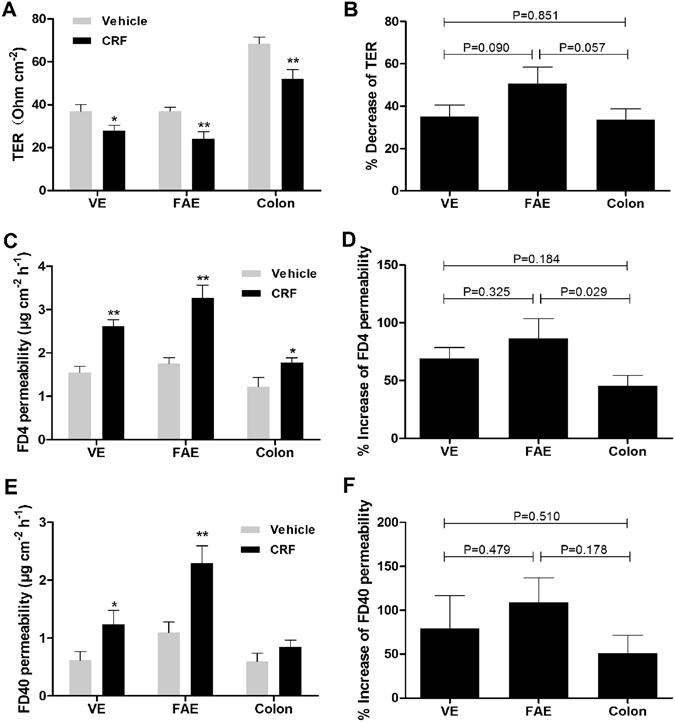
Differences in CRF-induced increase of permeability in ileal VE, FAE and colonic epithelium. (A,C,E) CRF exposure disrupted the barrier function of the ileal VE, FAE and colon, including TER decreasing and increased permeability to FD4 and FD40. (B,D,F) The TER decreasing and FD4 and FD40 permeability increasing were trend to be more serious in the ileal FAE relative to the ileal VE and colon, but part of them had no statistical significance. N = 12 for each group. Data are displayed as the mean ± SD. *P < 0.05, **P < 0.01, compared with vehicle.
Mast cells and PAR2 mediate FAE barrier disruption by CRF exposure in vitro
The mechanisms underlined the FAE heperpermeability induced by stress were further studied. CRF exposure led to notable decrease of TER in the FAE in a time-dependent manner which could be markedly inhibited by cromolyn and partially blocked by protease inhibiter aprotinin and FUT-175 (Fig. 8A). The heperpermeability to FD4 and FD40 induced by CRF were also alleviated when pretreated with cromolyn as well as aprotinin, FUT-175 and ENMD-1068. Moreover, mast cell activation by compound 48/80 resulted in the FAE heperpermeability to FD4 and FD40, and entirely blocked by mast cell stabilization. Selective PAR-2 activation by SLIGRL-NH2 significantly increased both the FD4 and FD40 permeability of FAE (Fig. 8B–E).
Figure 8.
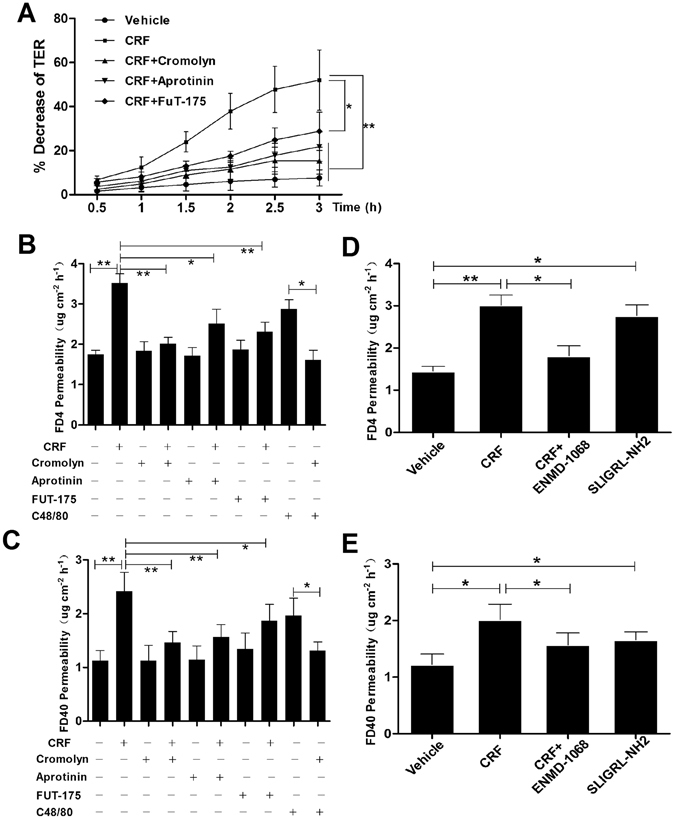
Mast cells stabilization and protease/PAR2 inhibition improved the FAE barrier disruption under CRF exposure in vitro. (A) CRF exposure led to notable decrease of TER in the FAE in a time-dependent manner, and markedly blocked by mast cell stabilizer cromolyn, protease inhibiter aprotinin and PAR2 antagonist FUT-175. (B,C) CRF significantly increased the FD4 and FD40 permeability, which may alleviate by cromolyn as well as aprotinin, FUT-175 and ENMD-1068. Compound 48/80, a mast cell activator, induced FAE heperpermeability to FD4 and FD40, and entirely blocked by mast cell stabilization. (D,E) Selective PAR-2 activation by SLIGRL-NH2 significantly increased both the FD4 and FD40 permeability of FAE. N = 12 for each group. Data are displayed as the mean ± SD. *P < 0.05, **P < 0.01.
Discussion
In this study, for the first time, we mapped the different susceptibility of functionally distinct epitheliums to stress-induced intestinal injury, which indicated that the FAE was more sensitive to stress-exposure. Mast cells as important effector cells in response to stress may participate in the process of epithelial hyper-permeability. Especially, we demonstrated that inhibition of mast cells and blocking the protease/PAR2 signaling obviously improved the barrier dysfunction in FAE.
Stress induced mild mucosal inflammation and visceral hypersensitivity in the rat model, with increased intraepithelial lymphocytes infiltration and reduced pain threshold to colorectal distention. It also has been similarly reported elsewhere8, 13. The negative effect of stress on intestinal barrier function may be one of the plausible mechanisms in triggering inflammation and pain13, 14. Indeed, as we shown, psychological stress lead to significant increase in both ileal and colonic epithelial permeability, such as decreased transepithelial resistance and elevated paracellular (FD4) and transcellular (FD40) macromolecular permeability. Meanwhile, levels of CRF, the key stress hormone, raised in serum and intestinal tissues under stress. CRF mainly expresses in the central nervous system, but also can be released locally by enterochromaffin cells, immune cells, regional sensory and sympathetic nerves in peripheral tissues15–18. Central and peripheral administration of exogenous CRF have been shown to mimic the stress-incuced intestinal dysfunctions including increasing ion and water secretion, mucus release and epithelial permeability17. Our in vitro observation also showed that CRF obviously disrupted the mucosal permeability in ileum and colon. It further verified that stress affect the intestinal barrier function involving the CRF pathway.
More importantly, we focused on the potentially different susceptibility of functionally distinct epithelium to stress-induced barrier disruption, including the ileal and colonic epithelium, and the VE and FAE. This idea was based on the fact that the structure and function of mucosal barrier differ in distinct segments and regions of gastrointestinal tract12, 19. On the whole, the ileal FAE showed a more significant elevation in paracellular and transcellular permeability compared with the ileal villous epithelium and colonic epithelium in rats exposed to stress in vivo and in mucosal tissues treated with CRF in vitro. However, there was no marked difference between ileal villous epithelium and colonic epithelium. It indicated that the FAE was more sensitive to stress.
It is reasonable that the FAE is more vulnerable to stress-induced barrier dysfunction, although it has not been addressed. Firstly, the FAE overlays the Peyer’s patches in the small intestine and typically contain microfold cell10; in addition, unlike the villous epithelium, it has less abundant goblet cells and different absorptive cells with absence of secretory component expression10. Therefore, the structure of FAE is entirely different from the VE. Secondly, the FAE is an important route for antigen sampling that differs from the normal VE20. It has been suggested to be associated with the unique biochemical properties of microfold cells, namely the highly efficient sampling of antigens20, 21. All these differences may form the basis of susceptibility of FAE to injury. More direct evidence showed that the first observable signs of Crohn’s disease are microscopic erosions originating from the FAE1, 11, 22–24. Although the FAE only account for a very small proportion of the whole intestinal epithelium10, it may be the key factor in regulating the mucosal and even systemic immune. These findings strengthened the hypothesis that the FAE might be the site of pathological changes onset in response to psychological stress. Consequently, more concerns should be paid to the FAE in permeability increasing and barrier dysfunction in conditions associated with stress including inflammatory and functional bowel diseases.
The mechanisms underlying permeability increasing has been widely studied in small intestinal and colonic epithelium under stress, including various neuroimmunological and neuroendocrine mechanisms25, 26. Particularly, MCs may play an important role in the prosess7, 8, 26. We verified that MCs degranulation, induced by compound 48/80, could disrupt the barrier of the FAE. However, very few researches addressed the possible relationship between MCs and stress induced FAE hyper-permeability. In our preliminary study, we demonstrated that there were increased tryptaes+-MCs and elevated expression of tryptase in parallel with its receptor PAR2 in FAE of the WAS rats, moreover, the tryptase and PAR2 level was positively related with the epithelial conductance of the FAE. Intraperitoneal injection with cromolyn, a MCs stabilizer, significantly improved the barrier function and upregulated the pain threshold. This further indicated that MCs activation involves in the process of stress-induced barrier disruption of the FAE, similarly to the VE2, 7, 8.
Mast cell act as a key effector in stress-induced intestinal epithelial barrier dysfunction7, 27. CRF is the important stress factor for the activation of MCs since both the 2 types of CRF receptors express on MCs4, 28. In vivo and in vitro administration of CRF resulted in MCs degranulating which could be prevented by blocking CRF receptors2, 7. Additionally, MCs, often found close to nerve fibers29, may also be directly activated by the nervous systems during stress and be independent with central and local CRF6, 30. It suggested that MCs could be a potential therapeutic target for barrier disruption of the FAE under stress. Our further in vitro investigation showed that MCs stabilization, protease inhibition and PAR2 blocking effectively eliminated the FAE hyper-permeability induced by CRF exposure. These findings are in line with previous evidences that PAR2 participated in the intestinal mucosal inflammation31. Tryptase may directly acted on the epithelial PAR2 and rearranged the tight junction proteins and perijunctional F-actin and eventually resulted in paracellular leakage and inflammation of the gut18. PAR2 activation may also promote the release of pro-inflammatory peptides such as calcitonin gene-related peptide and substance P, and lead to secretion of TNFα and IFNγ which in turn increase paracellular peameability and trigger intestinal inflammation2, 18. Then we tend to believe that elevated MCs and PAR2 signaling may also play a significant role in the FAE hyper-permeability under stress conditions.
In summary, stress-induced intestinal permeability increasing and barrier dysfunction was more sensitive and serious in the FAE than ileal villous epithelium and colonic epithelium. The FAE may be the initial site of lesion formation and depravation happened in the gut in conditions related to stress thus it need more attentions. MCs and the protease/PAR2 signaling played important roles in this process and promised to be potential therapeutic targets in preventing the stress-induced barrier disruption of the FAE.
Methods
Animals
Male Sprague-Dawley rats (aged 6 weeks, weighed 180–200 g; Experimental Animal Center, HUST, Wuhan, China) were used. Rats were housed under specific pathogen-free conditions with 12/12-hour light/dark cycle, temperature maintained at 23 °C and ad libitum access to standard rat chow and tap water. Before the following experiments, the rats were allowed to acclimatize to the new environment for one-week and get familiar to the experimenter. All experimental procedures were performed in accordance with the ethical guidelines of the International Association for the Study of Pain (IASP) and the Animal Management Rules of the Chinese Ministry of Health (Document No. 55, 2001), and approved by and the Animal Care and Use Committee, Union Hospital, Tongji Medical College, HUST, China (Approval ID 2013025).
Water avoidance stress, sham stress and groups
Rats were randomly assigned to the sham stress group (SS, 12 rats), the water avoidance stress group (WAS, 12 rats) and the WAS + cromolyn sodium group (WAS + Crom, 12 rats). The rats in WAS + Crom group were injected with mast cell stabilizer cromolyn sodium (25 mg/kg) intraperitoneally 30 minutes before stress every day32, 33. As control, the SS group and WAS group were injected with equal amounts of physiological saline. The protocol of WAS and SS was described elsewhere previously34. That is, each rat was placed on a platform (8-cm length × 6-cm width) affixed at the center of a plastic cage (48-cm length × 48-cm width), which was filled with 25 °C warm water (about 1 cm below the platform), for 1 hour daily for 10 consecutive days. For the SS group, rats were placed on the same devices but without water in the cages. All the experiments were performed at 9–11 AM every day. The body weight was recorded each day before the WAS and SS, and the total number of stool pellets was counted during the 1-hour WAS or SS. After the 10-day stress, rats were anaesthetized by 2% pentobarbital sodium, then the serum and intestinal tissues including ileum VE, ileum FAE and colon were obtained and used for measurements. Visceral sensation was assessed before execution by abdominal withdrawal reflex (AWR) scores to colorectal distention described elsewhere by Al-Chaer et al.35. Inflammatory scores were graded according to the infiltration of intraepithelial lymphocyte and interstitial edema blindly by 2 investigators with a modified scale previously described by Al-Chaer et al. via H&E staining35, 36.
Enzyme-linked immunosorbent assay for serum CRF
Serum samples were collected. CRF levels were detected through an enzyme linked immunosorbent assay (ELISA) kit (R&D Systems, USA) following the manufacturer’s instructions. Serum CRF was quantitated against a CRF standard curve.
Real-time quantitative PCR analysis for intestinal CRF
The enterogenous CRF was assessed via mRNA expression in the intestine by means of real-time quantitative PCR (RT-qPCR). Total RNA in VE, FAE and colonic tissues were extracted using the Trizols Reagent (Invitrogen, Life Technologies). Complementary DNA was synthesized using the PrimeScript™ RT Master Mix Kit (TaKaRa) and then used for real-time quantitative PCR with a QuantiTect SYBR Green PCR Kit (QIAGEN) on a ROCHE LightCycler® 480 System. Primer sequences used for amplification were as follows: CRF forward 5′-GAAGAGAAAGGGGAAAGGCAAAGA-3′ and reserve 5′-GCGGTGAGGGGCGTGGAGTT-3′ (NM_031019); GAPDH forward 5′-ACCACAGTCCATGCCATCAC-3′ and reserve 5′-TCCACCACCCTGTTGCTGTA-3′ (NM_001289745). The relative expression of mRNA species was quantified by 2−ΔΔCT method.
Immunoflorescence staining
The intestinal tissues were fixed with 4% paraformaledehyde, embedded in paraffin and cut into slice. After routinely dewaxing and hydration, the slices were conducted antigen heat retrieval in citrate buffer (0.01 M, pH 6.0), and then blocked with 10% donkey serum (containing 0.3% Triton X-100) for 45 min at room temperature. Sections were incubated with the following primary antibodies overnight at 4 °C: mouse monoclonal anti-mast cell tryptase antibody (1:500, ab2378, Abcam) or rabbit monoclonal anti-PAR2 antibody (1:400, ab180953, Abcam). After washing with phosphate buffer saline (PBS) for 5 min * 3 times, sections were respectively stained with donkey anti-mouse or rabbit Alexa Fluor 488 ((IgG H&L) secondary antibodies (1:300, Invitrogen) for 60 min at room temperature, and then washed with PBS for 5 min * 3 times. Finally, DAPI (1 μg/ml, Beyotime Biotech, China) were used for nuclei staining. Images were viewed and captured using a confocal laser scanning microscope (Nikon, Japan) with excitation wavelength appropriate for Alexa Fluor (488 nm or 594 nm), and analyzed using NIS Elements Viewer Software (Nikon, Japan).
Western blot analysis
The intestinal tissues were homogenized in a RIPA extraction buffer with a protease inhibitor cocktail (Roche) for 30 min. Proteins were quantified via a BCA protein assay kit (Beyotime Biotechnology, China), and then prepared in Laemmli buffer and boiled in water bath. Equal amounts of tissue lysates (50 mg) were loaded and separated on10% SDS-PAGE gels with constant voltage of 100 V for 100 min, and transferred to PVDF membranes with constant current of 300 mA for 80 min in ice bath. Membranes were subsequently blocked in 8% (w/v) non-fat dried milk for 1 h at room temperature, then incubated overnight at 4 °C with specific antibodies against tryptase (Abcam, ab109226, 1:2000), PAR2 (Abcam, ab154835, 1:1000), β-actin(Abbkine, A01010, 1:2000) and GAPDH (Abbkine, A01020, 1:2000). After fully washing, the membranes were probed with HRP-conjugated goat anti-rabbit or mouse antibody (1:5000, Pierce, USA) for 1 h at room temperature. Then, the protein bands were developed in the SuperSignal West Pico Substrate (Pierce, USA) and quantified by the FluorChem FC3 software (ProteinSimple, USA).
Ussing chamber experiments and mucosal-to-serosal fluxes of macromolecules
The ileal VE, ileal FAE and colon from each group were stripped from the seromuscular layer in ice-bathed and oxygenated Krebs’ buffer. The mucosal tissues or patches were mounted on sliders with a circular hole (opening area 0.25 cm2) in the center, and covered this entire area of the hole which maintains the exposed area of 0.25 cm2. Then, the sliders were installed on U-type chambers for Ussing Chamber System, and the tissues were bathed in 37 °C oxygenated Krebs’ solution (5 ml on each side of the U-type chamber). The serosal buffer contained glucose as an energy source, while the mucosal buffer replaced by equivalent mannitol for osmotically balancing. The spontaneous potential difference (PD) was monitored via agar-salt bridges connected to calomel electrodes, and the appropriate short-circuit current (Isc) was added via Ag-AgCl electrodes to maintain a zero PD through an automatic voltage clamp (World Precision Instruments, USA). The TER and conductance was calculated from the spontaneous PD and Isc by means of Ohm’s law. After a 20-min equilibration period, the TER was recorded. Meanwhile, 1 mg/ml FD4 (or FD40), which represented the paracellular (or transcellular) macromolecular permeability, was added to the mucosal side of the U-type chambers, and sampled in the serosal side at 30-min intervals over a 3-h period. The FD4 (or FD40) intensity of each sample was measured by a Fluorescence Microplate Reader (BioTek Instruments, USA). The mucosal-to-serosal FD4 (or FD40) flux was determined by the increase of concentration of FD4 (or FD40) in the serosal side within 3 hours. Our preliminary results showed that tissues maintained alive for at least 4 hours with consistent transepithelial PD and Isc response to 10 mM forskolin adding to the mucosal chamber.
Furthermore, intestinal tissues from normal SD rats were used, mounted on Ussing Chambers and equilibrate for 20 min to reach a stabilized TER and Isc. Cromolyn (10−4 M), a mast cell stabilizer; aprotinin (10−5 M) or FUT-175 (10−5 M), the serine protease inhibitor were added to the serosal chamber 30 min prior to 0.25 μM CRF or 10 μg/ml compound 48/80 exposure. In order to clarify the role of PAR2 in the FAE barrier disruption, additional experiments with both specific PAR-2 agonists SLIGRL-NH2 (1 mM) and antagonist ENMD-1068 (5 mM) were further conducted. The TER, FD4 and FD40 flux were measured over a 3-h period as previously described.
Chemicals and solutions
The Kreb’s solution contained (in mM, pH 7.2–7.4): 119 Na+, 4.7 K+, 1.2 H2PO4 −, 25 HCO3 −, 1.2 Mg2+, 2.5 Ca2+ and 11.1 Glucose, and all the reagents were from Sinopharm Chemical Reagent Co., Ltd, China. Human/rat corticotropin releasing factor (CRF) was acquired from R&D Systems Inc., MN, USA. Cromolyn sodium, compound 48/80, aprotinin, nafamostat mesylate (FUT-175), ENMD-1068, SLIGRL-NH2, FD4 and FD40 were obtained from Sigma-Aldrich Co., LLC, USA.
Data expression and statistical analysis
The data were expressed as the mean ± standard deviation. The normality of the data, as well as the homogeneity of variance, was tested. Accordingly, the parametric one-way analysis of variance (ANOVA) or non-parametric Kruskal-Wallis tests was used for data analyzing, and the least significant difference (LSD) test or Dunnett’s T3 test was used for multiple comparisons. The correlation between tryptase/PAR2 expression and epithelial conductance was analyzed by means of Pearson correlation analysis. P-values less than 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (NSFC), China (No: 81570486, 81330014, 81070300).
Author Contributions
L.Z. designed and performed the experiments, analyzed the data, drafted the manuscript; J.S. supported writing of the manuscript and data evaluation; T.B. conducted the electrophysiological experiments and analyzed the data; W.Q. provided technical and material supports; X.H.H. designed and supervised the study, and obtained fundings.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05064-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Keita AV, Söderholm JD. Barrier dysfunction and bacterial uptake in the follicle-associated epithelium of ileal Crohn’s disease. Ann. N. Y. Acad. Sci. 2012;1258:125–134. doi: 10.1111/j.1749-6632.2012.06502.x. [DOI] [PubMed] [Google Scholar]
- 2.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivinus-Nébot M, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 4.Saunders PR, et al. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig. Dis. Sci. 2002;47:208–215. doi: 10.1023/A:1013204612762. [DOI] [PubMed] [Google Scholar]
- 5.Al Omran Y, Aziz Q. The brain-gut axis in health and disease. Adv. Exp. Med. Biol. 2014;817:135–153. doi: 10.1007/978-1-4939-0897-4_6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Song J, Hou X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. Motil. 2016;22:181–192. doi: 10.5056/jnm15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanuytsel T, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 8.Vicario M, et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav. Immun. 2010;24:1166–1175. doi: 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez de Medina F, Romero-Calvo I, Mascaraque C, Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014;20:2394–2404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 10.Allan CH, Trier JS. Structure and permeability differ in subepithelial villus and Peyer’s patch follicle capillaries. Gastroenterology. 1991;100:1172–1179. doi: 10.1016/0016-5085(91)90766-E. [DOI] [PubMed] [Google Scholar]
- 11.Keita AV, et al. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol. Motil. 2013;25:e406–417. doi: 10.1111/nmo.12127. [DOI] [PubMed] [Google Scholar]
- 12.Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J. Comp. Physiol. B. 2010;180:591–598. doi: 10.1007/s00360-009-0440-7. [DOI] [PubMed] [Google Scholar]
- 13.Miquel S, et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016;6:19399. doi: 10.1038/srep19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theodorou V, Ait Belgnaoui A, Agostini S, Eutamene H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes. 2014;5:430–436. doi: 10.4161/gmic.29796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Wijngaard RM, Klooker TK, de Jonge WJ, Boeckxstaens GE. Peripheral relays in stress-induced activation of visceral afferents in the gut. Auton Neurosci. 2010;153:99–105. doi: 10.1016/j.autneu.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Koido S, et al. Production of corticotropin-releasing factor and urocortin from human monocyte-derived dendritic cells is stimulated by commensal bacteria in intestine. World J. Gastroenterol. 2014;20:14420–14429. doi: 10.3748/wjg.v20.i39.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodiño-Janeiro BK, et al. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J. Neurogastroenterol. Motil. 2015;21:33–50. doi: 10.5056/jnm14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong CJ, et al. Mast cell activation is involved in stress-induced epithelial barrier dysfunction in the esophagus. J. Dig. Dis. 2015;16:186–196. doi: 10.1111/1751-2980.12226. [DOI] [PubMed] [Google Scholar]
- 19.Novosad VL, Richards JL, Phillips NA, King MA, Clanton TL. Regional susceptibility to stress-induced intestinal injury in the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G418–426. doi: 10.1152/ajpgi.00166.2013. [DOI] [PubMed] [Google Scholar]
- 20.Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Bennett KM, et al. Induction of Colonic M Cells during Intestinal Inflammation. Am. J. Pathol. 2016;186:1166–1179. doi: 10.1016/j.ajpath.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velin AK, Ericson AC, Braaf Y, Wallon C, Söderholm JD. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut. 2004;53:494–500. doi: 10.1136/gut.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders DS. Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease. J. Clin. Pathol. 2005;58:568–572. doi: 10.1136/jcp.2004.021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gullberg E, Söderholm JD. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann. N. Y. Acad. Sci. 2006;1072:218–232. doi: 10.1196/annals.1326.028. [DOI] [PubMed] [Google Scholar]
- 25.Keita AV, Söderholm JD, Ericson AC. Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterol. Motil. 2010;22:770–778. doi: 10.1111/j.1365-2982.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- 26.Santos J, et al. Stress neuropeptides evoke epithelial responses via mast cell activation in the rat colon. Psychoneuroendocrinology. 2008;33:1248–1256. doi: 10.1016/j.psyneuen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Kelly JR, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J. Physiol. Pharmacol. 2009;60:33–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim. Biophys. Acta. 2012;1822:85–92. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 31.Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin. Immunopathol. 2012;34:133–149. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 32.Cordeiro PG, et al. Prevention of ischemia-reperfusion injury in a rat skin flap model: the role of mast cells, cromolyn sodium, and histamine receptor blockade. Plast. Reconstr. Surg. 2000;105:654–659. doi: 10.1097/00006534-200002000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Hei ZQ, et al. Pretreatment of cromolyn sodium prior to reperfusion attenuates early reperfusion injury after the small intestine ischemia in rats. World J. Gastroenterol. 2007;13:5139–5146. doi: 10.3748/wjg.v13.i38.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil DW, et al. Role of sympathetic nervous system in rat model of chronic visceral pain. Neurogastroenterol. Motil. 2016;28:423–431. doi: 10.1111/nmo.12742. [DOI] [PubMed] [Google Scholar]
- 35.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 36.Song J, et al. Mast Cell-dependent Mesenteric Afferent Activation by Mucosal Supernatant From Different Bowel Segments of Guinea Pigs With Post-infectious Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2015;21:236–246. doi: 10.5056/jnm14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


