Abstract
Positron emission tomography (PET) with fluorine-18-fluorodeoxyglucose (18F-FDG) can be applied to detect infection and inflammation. However, it was so far not known to what extent bacterial pathogens may contribute to the PET signal. Therefore, we investigated whether clinical isolates of frequently encountered bacterial pathogens take up 18F-FDG in vitro, and whether FDG inhibits bacterial growth as previously shown for 2-deoxy-glucose. 22 isolates of Gram-positive and Gram-negative bacterial pathogens implicated in fever and inflammation were incubated with 18F-FDG and uptake of 18F-FDG was assessed by gamma-counting and µPET imaging. Possible growth inhibition by FDG was assayed with Staphylococcus aureus and the Gram-positive model bacterium Bacillus subtilis. The results show that all tested isolates accumulated 18F-FDG actively. Further, 18F-FDG uptake was hampered in B. subtilis pts mutants impaired in glucose uptake. FDG inhibited growth of S. aureus and B. subtilis only to minor extents, and this effect was abrogated by pts mutations in B. subtilis. These observations imply that bacteria may contribute to the signals observed in FDG-PET infection imaging in vivo. Active bacterial FDG uptake is corroborated by the fact that the B. subtilis phosphotransferase system is needed for 18F-FDG uptake, while pts mutations protect against growth inhibition by FDG.
Subject terms: Bacteriology, Infectious-disease diagnostics, Bacterial infection
Introduction
Bacterial infections represent a major problem in hospital and community settings worldwide. In particular, due to increasingly ageing populations, higher rates of organ transplantations and expanding possibilities for chemotherapy, the numbers of immune-compromised patients who are at risk of bacterial infections are steadily increasing. Moreover, this problem is exacerbated by increased use of implanted biomaterials and medical devices, which are particularly susceptible to bacterial contaminations and infection1. Another major contributor to the problem of bacterial infections is the rapid increase of resistance to multiple antibiotics2. Infections caused by such multidrug-resistant microbes are difficult to treat and responsible for substantial patient morbidity and mortality2, 3.
The diagnosis of bacterial infections is nowadays mainly based on a combination of several specific and/or non-specific symptoms, systemic inflammation markers and the detection of pathogens by culturing, mass spectrometry, polymerase chain reaction (PCR) or serology. In the ideal situation, the causative agent is identified by culturing, but this takes time and is often challenging since sites of infection may be poorly accessible. Also, there is always a risk of contaminations by other bacteria not related to the actual infection resulting in false-positive culture outcomes. Since these challenges often lead to blind management of bacterial infections, there is an urgent need for diagnostic tools that allow real-time detection of infecting bacteria in the patient, for example by infection imaging1, 4, 5.
Although several promising approaches for the direct imaging of infecting bacteria have been proposed and explored, with the exception of 99mTechnetium-ciprofloxacin (99mTc-ciprofloxacin Infecton®)6, there are currently no bacteria-specific imaging approaches in clinical use1, 4. A clinically applied alternative is positron emission tomography (PET) with fluorine-18-fluorodeoxyglucose (18F-FDG), which can serve as a diagnostic tool for localizing infections, inflammatory conditions and unknown origins of fever7, 8. In this case, it is generally believed that the infection-specific signal originates from increased glucose uptake by cells involved in the inflammatory process, such as leukocytes, monocytes, lymphocytes, giant cells and macrophages9. Importantly, 18F-FDG-PET is safe, sensitive, easy and rapid to use3, 10–12, and it is potentially applicable in the monitoring of treatment13. Furthermore, the view that 18F-FDG-PET imaging enables the visualization of bacterial infections and evaluation of antimicrobial therapy has been confirmed in animal experiments and clinical studies14–18. On the other hand, diagnosing infections with 18F-FDG-PET still remains challenging due to the fact that, for instance, malignancies, sterile inflammation and physiological wound healing, may result in similarly high levels of 18F-FDG uptake as infections9, 15–17.
An intriguing question that has, so far, received little attention is whether infecting bacteria could potentially contribute to the signal derived in 18F-FDG-PET imaging. Specifically, it was not known whether different bacteria can accumulate 18F-FDG, and whether the uptake of FDG is potentially toxic for bacteria as is known to be the case for the structurally related glucose analog 2-deoxy-glucose (2-DG)19. The aims of this study were, therefore, to determine whether clinical isolates of different bacterial pathogens actively take up 18F-FDG in vitro, and whether this may lead to impaired bacterial growth.
Material and Methods
Bacteria
Clinical isolates of different bacterial pathogens were collected from the diagnostic laboratory of the Department of Medical Microbiology at the University Medical Center Groningen (UMCG; July-November 2015, 15 isolates derived from 15 different patients, Table 1). Identification of these bacterial isolates was performed using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Brüker, Daltonik GmbH, Leipzig, Germany)20. 7 bacterial strains from the American Type Culture Collection (ATCC) were also included in the analyses (Table 1). These different bacteria were chosen to cover the most commonly encountered bacterial pathogens at the UMCG. Bacteria were cultured on blood agar plates (5% sheep blood, Mediaproducts, Groningen, The Netherlands) for 1–3 days at 37 °C.
Table 1.
Bacterial strains for 18F-FDG uptake experiments.
| Strain | Origin or American Type Culture Collection strain number | Date of isolation at UMCG |
|---|---|---|
| Citrobacter freundii | clinical isolate | July 2015 |
| clinical isolate | November 2015 | |
| Enterococcus faecalis | ATCC 29212 | — |
| clinical isolate | November 2015 | |
| Enterococcus faecium | clinical isolate | July 2015 |
| clinical isolate | November 2015 | |
| Escherichia coli | ATCC 25922 | — |
| clinical isolate | October 2015 | |
| Klebsiella pneumoniae | ATCC 700721 | — |
| clinical isolate | November 2015 | |
| Proteus mirabilis | ATCC 12453 | — |
| clinical isolate | October 2015 | |
| Pseudomonas aeruginosa | ATCC 27853 | — |
| clinical isolate | October 2015 | |
| Staphylococcus aureus | clinical isolate | July 2015 |
| clinical isolate | October 2015 | |
| Staphylococcus epidermidis | ATCC 12228 | — |
| clinical isolate | November 2015 | |
| Streptococcus pneumoniae | ATCC 49619 | — |
| clinical isolate | November 2015 | |
| Streptococcus pyogenes | clinical isolate | October 2015 |
| clinical isolate | November 2015 |
18F-FDG uptake studies
For 18F-FDG uptake studies, Streptococcus pneumoniae, Enterococcus faecalis and Enterococcus faecium were cultured in brain-heart infusion (BHI), while all other bacteria were cultured in tryptic soy broth (TSB). All bacteria were grown overnight at 37 °C under constant agitation (250 rpm), with the exception of S. pneumoniae which was grown in standing culture at 5% CO2. The next day, fresh cultures were prepared by dilution of overnight cultures in 9 mL TSB or BHI, and growth was continued up to an optical density (OD) of 1–5 McFarland units. Heat-killed Staphylococcus aureus and Escherichia coli were obtained by incubating the respective cultures for 30 min at 99 °C. To assess 18F-FDG uptake, the bacterial cultures were incubated with 5–10 megabecquerel (MBq) 18F-FDG dissolved in 0.5 mL NaCl (0.9%) at 37 °C for 5 min. Subsequently, 2 mL samples were ‘washed’ twice by centrifugation and resuspension of the bacterial pellets in phosphate buffered saline (PBS). To image 18F-FDG uptake, the bacteria were pelleted by centrifugation, and four Eppendorf tubes containing bacterial pellets were positioned at the center of the ring system of a micro-PET (µPET) Focus 220 scanner (Siemens Medical Solutions, TN, US) with the bacterial pellets in the field of view. Images were recorded in 30 min and analyzed with the AMIDE software package (Amide’s a Medical Imaging Data Examiner). To quantify 18F-FDG uptake, the bacterial pellets were placed in a calibrated gamma-counter (CompuGamma CS1282, LKB-Wallac, Turku, Finland). The radioactivity in each sample was measured, corrected for background and converted into kilobecquerel (kBq). Each 18F-FDG uptake experiment was performed thrice and all quantifications were executed in quadruple with two different clinical isolates per tested bacterial species. Numbers of colony forming units (CFUs) in different samples were determined in duplicate by resuspension of the bacterial pellets, serial dilution of the resuspended bacteria, and plating on blood agar containing 5% sheep blood. Only in case of Proteus mirabilis, MacConkey agar no.3 plates containing crystal violet were used (Mediaproducts, Groningen, The Netherlands). CFUs of bacteria not incubated with 18F-FDG were determined as a control to evaluate the effect of 18F-FDG on bacterial survival.
Time course analysis of 18F-FDG uptake
Clinical isolates of S. aureus and E. coli were grown overnight in TSB as indicated above, and the Bacillus subtilis type strain 168 (Table 2) was grown overnight in Lysogeny Broth (LB) at 37 °C under constant agitation (250 rpm). The next day, the bacteria were diluted 1:20 in 12 mL of the respective growth media and grown for ~2.5 h up to an OD of ~2 McFarland (37 °C, 250 rpm). Subsequently, 5–7 MBq 18F-FDG (dissolved in 0.5 mL of 0.9% NaCl) was added to the bacterial cultures. Cultures were incubated at 37 °C after the addition of 18F-FDG and culture aliquots of 2 mL were withdrawn at 0, 15, 30, 45 and 60 min. To stop 18F-FDG uptake, each culture aliquot was washed twice by centrifugation and resuspension of the bacterial pellets in PBS. Lastly, the bacteria were pelleted by centrifugation and 18F-FDG uptake in the pellets was measured with a calibrated CompuGamma CS1282 gamma-counter. CFUs were determined at t = 0, t = 30 and t = 60 min by resuspension of the pellets, serial dilution and plating on blood agar as described above. Heat-killed S. aureus and E. coli (30 min, 99 °C) served as negative controls. Uptake of 18F-FDG over time was evaluated in duplicate.
Table 2.
Laboratory strains used for growth in the presence of FDG.
| Strain | Genotype | References |
|---|---|---|
| S. aureus HG001 | rsbU repaired, tcaR | (33) |
| B. subtilis 168 | trpC2 | Laboratory Collection |
| B. subtilis QB5435 | trpC2, ptsG::cat, CmR | (34) |
| B. subtilis GP864 | trpC2 ptsI::EmR | (35) |
CmR, Chloramphenicol resistant; EmR, Erythromycin resistant.
Growth of S. aureus and B. subtilis in the presence of non-labeled FDG
To assess the toxicity of FDG, the laboratory strain S. aureus HG001 and different B. subtilis strains (Table 2) were grown overnight in TSB or LB, respectively. Where appropriate the media were supplemented with chloramphenicol or erythromycin at a concentration of 5 µg/mL. The overnight cultures were, in triplicate, diluted 1:50 in 100 µL fresh TSB or LB in 96-well microtiter plates, and the plates were incubated for 3 h at 37 °C with vigorous agitation (800 rpm). Lastly, each culture was again diluted 1:50 in 100 µL of the respective growth medium supplemented with 2 µg/mL, 20 µg/mL, 200 µg/mL or 2 mg/mL non-radioactive FDG, or without FDG. Cultures were incubated at 37 °C in a Biotek synergy 2 plate reader with shaking and the OD at 600 nm (OD600) was recorded at 10 min intervals. Each growth experiment was performed at least twice.
Statistical analyses
Kruskal Wallis and Mann-Whitney U tests were performed using IBM SPSS Statistics 23. P-values of 0.05 or less were considered significant.
Results
In vitro uptake of 18F-FDG
To evaluate whether bacterial pathogens are capable of active uptake of 18F-FDG, a range of different Gram-positive and Gram-negative bacteria including mostly clinical isolates were incubated with 18F-FDG, and uptake was determined with a calibrated gamma-counter. The 18F-FDG PET signals were quantified and the absorbed activity per CFU was determined as shown in Fig. 1(A and B). All bacterial isolates showed significant 18F-FDG uptake. No major differences were observed for most of the investigated Gram-positive and Gram-negative bacteria (p = 0.60; not shown). Nonetheless, statistically significant differences in 18F-FDG uptake were observed for different bacterial species (p < 0.01) as illustrated in Fig. 1. The Gram-positive bacterium Streptococcus pyogenes displayed overall the highest uptake activity per 107 CFUs (Fig. 1A and B). E. coli and Citrobacter freundii showed the highest 18F-FDG uptake amongst the tested Gram-negative species (Fig. 1B). In contrast, S. pneumoniae, E. faecium and P. mirabilis showed relatively low 18F-FDG uptake, but as shown for S. pneumoniae the 18F-FDG uptake was still significantly higher than the negative controls (p = 0.01; Fig. 1C). Importantly, other living bacteria, such as S. aureus and E. coli accumulated significantly higher 18F-FDG levels than the respective heat-killed bacteria, bacteria incubated in the absence of 18F-FDG, or medium controls (p = 0.004).
Figure 1.
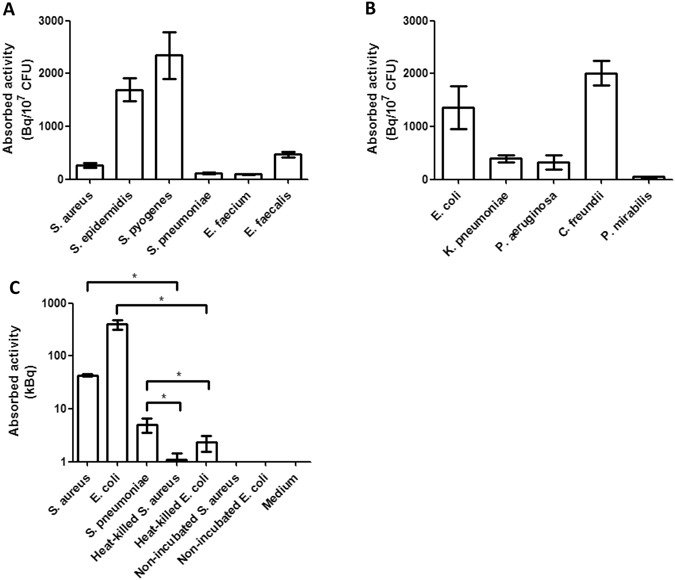
In vitro uptake of 18F-FDG by different bacteria. The mean uptake of 18F-FDG by clinical isolates of different Gram-positive (A) and Gram-negative (B) bacteria was expressed as absorbed activity in Bq/107 CFUs. (C) Total uptake in kBq of 18F-FDG by S. aureus, E. coli or S. pneumoniae in comparison to heat-killed bacteria, bacteria not incubated with 18F-FDG, and medium controls. The indicated standard deviations include the variations introduced by the use of at least two different isolates per tested species. Note that the detection limit for a bacterial species will lie between the nonspecifically absorbed activity measured for heat-killed bacteria, such as E. coli and S. aureus, and the lowest level of 18F-FDG uptake by the living bacteria, such as S. pneumoniae. This implies that under the tested conditions, the detection limit is ~2.4 kBq. *p ≤ 0.01. Values represent mean ± SEM (n = 8).
To evaluate whether bacterial 18F-FDG uptake can also be detected by µPET imaging, the 18F-FDG uptake by several Gram-negative and Gram-positive clinical isolates was qualitatively assessed with a µPET Focus 220 scanner. Indeed, the resulting µPET images clearly show 18F-FDG uptake by the tested bacteria (Fig. 2). S. pneumoniae showed the least uptake activity, which might relate to the high autolytic activity of this bacterium21. As expected, little if any 18F-FDG uptake was detectable for heat-killed S. aureus and E. coli, as was the case for non-incubated bacteria and medium controls. Notably, there were some apparent differences in the relative signals observed in Figs 1 and 2 for, respectively, the E. faecium and E. faecalis samples, and for the S. aureus and S. epidermidis samples. This may relate to the fact that counting with the calibrated gamma-counter as in Fig. 1 provides quantitative data on 18F-FDG uptake, whereas the µPET scans as in Fig. 2 give semi-quantitative information on 18F-FDG uptake. For example, there may have been some variation in the distribution of the pelleted bacteria in the samples that were imaged by µPET scans. Furthermore, the observed variations may have been due, to some extent, to differing numbers of metabolically active bacteria in the samples respectively used for Fig. 1 and 2.
Figure 2.
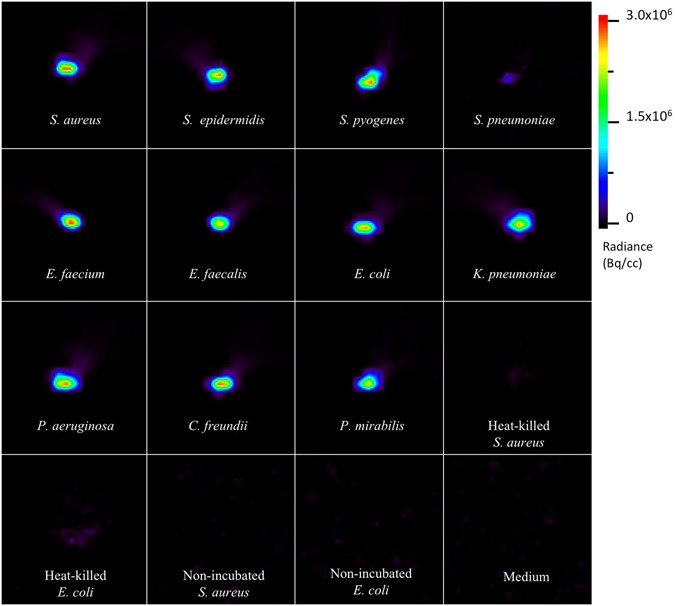
µPET images of 18F-FDG uptake by bacterial isolates. Different clinical bacterial isolates were grown to an OD of 1–5 McFarland units in 9 mL of growth medium, pelleted and resuspended in the same volume at 15 min before the incubation with 0.5 ml of 5–10 MBq 18F-FDG for 5 min. Heat-killed S. aureus or E. coli were obtained by incubating the respective cultures for 30 min at 99 °C. As negative controls, S. aureus or E. coli cells incubated without added 18F-FDG, or with fresh medium were used. All samples were washed twice in PBS and washed bacterial pellets corresponding to 2 mL culture samples were imaged for 30 min with a µPET scanner for a qualitative evaluation of the bacterial 18F-FDG uptake. The scale bar indicates the color map range of Radiance in Bq per cubic centimeter (cc).
To approximate the time needed for uptake of 18F-FDG, time course experiments were performed with S. aureus and E. coli, two bacterial species showing significant differences in 18F-FDG uptake (Fig. 1). The results presented in Fig. 3 show indeed significant differences in the rates of uptake by both species, E. coli reaching saturation almost instantaneously while 18F-FDG uptake by S. aureus was relatively slow. Within 60 min of incubation, E. coli absorbed significantly more 18F-FDG than S. aureus (p < 0.001), while E. coli and S. aureus both showed higher signals than the respective heat-killed controls (p < 0.01).
Figure 3.
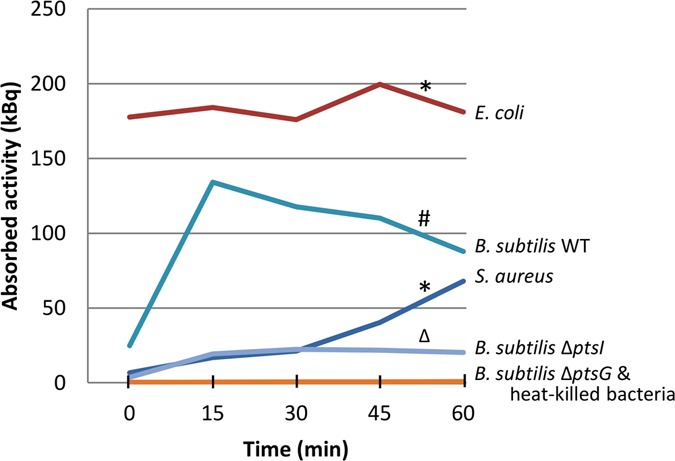
Time course analysis of in vitro uptake of 18F-FDG. Mean 18F-FDG uptake was determined over a period of 60 min for clinical isolates of S. aureus (red) and E. coli (dark blue), and for the B. subtilis type strain 168 (light blue) and its ptsG (orange) or ptsI mutant (purple) derivatives. Heat-killed bacteria (orange) served as negative controls. Samples were withdrawn at the indicated time points to assess the 18F-FDG uptake. *p < 0.01 vs. heat-killed controls; #p < 0.001 vs. both mutants; Δp < 0.001 vs. the ΔptsG mutant.
Potential mechanism of 18F-FDG uptake
In order to assess the possible mechanism of 18F-FDG uptake, we applied the Gram-positive model bacterium B. subtilis for which mutations in the phosphotransferase system (PTS) that facilitates glucose uptake are available22. Indeed, B. subtilis ptsI and ptsG deletion strains displayed a strongly impaired uptake of 18F-FDG compared to the parental wild-type strain B. subtilis 168 (p < 0.001; Fig. 3). Of note, the ptsI deletion mutant showed a somewhat higher uptake than the ptsG deletion mutant (p = < 0.001), which is in line with the fact that PtsG is the major glucose transporter23, while PtsI is involved in the phosphorylation of internalized glucose making its uptake by PtsG irreversible24. These findings show that, at least in B. subtilis, 18F-FDG is taken up via the PTS system.
FDG-impaired bacterial growth
To test whether FDG could be toxic for bacteria growth experiments were performed with S. aureus HG001 and B. subtilis 168, where the growth media were supplemented with increasing concentrations of FDG up to 2 mg/mL. As shown in Fig. 4A, S. aureus HG001 reached somewhat lowered OD600 values in the stationary phase when the medium contained 200 µg/mL FDG or more. Similarly, B. subtilis reached a lowered optical density in the stationary phase when cultured in the presence of 2 mg/mL FDG (Fig. 4B). This indicates that FDG is mildly toxic for bacteria, such as S. aureus and B. subtilis, at high concentrations. To verify this, the B. subtilis ptsI and ptsG deletion mutants were also cultured in the presence of FDG. The results show that both mutations significantly diminished the growth impairment caused by 2 mg/mL FDG (Fig. 4C and D). Together, these findings show that active FDG uptake may be toxic for bacteria, but only at very high concentrations that will not be reached during 18F-FDG-PET imaging in vivo.
Figure 4.
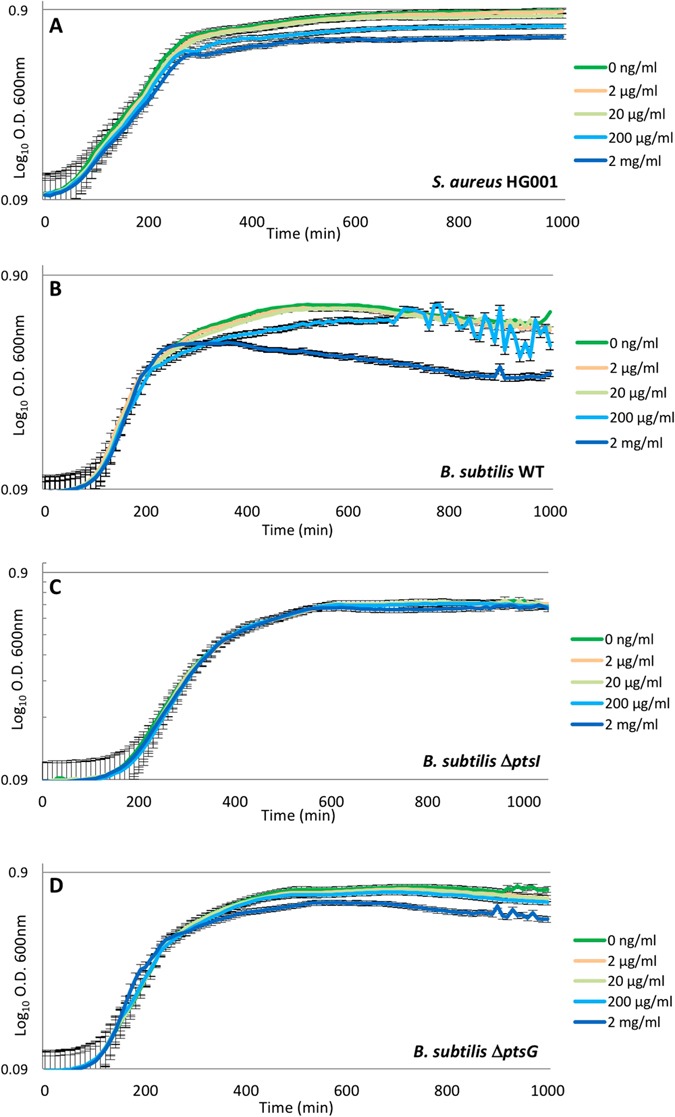
Growth of S. aureus and B. subtilis in the presence of non-labeled FDG. S. aureus HG001 was grown in TSB (A), and the B. subtilis strains 168 (WT), ΔptsI and ΔptsG were grown in LB (B,C and D) with increasing concentrations of non-radiolabeled FDG as indicated in color code. OD600 readings were recorded every 10 min.
Discussion
The current study shows for the first time that clinical isolates of many major Gram-positive and Gram-negative bacterial pathogens can actively take up 18F-FDG. This is consistent with the previous finding of Weinstein et al.25, who reported that type strains of E. coli, Klebsiella pneumoniae and S. aureus can take up this compound. The combined findings imply that bacterial pathogens may contribute to the signal observed in 18F-FDG PET imaging of bacterial infections. Furthermore, the observed 18F-FDG uptake was, at least in B. subtilis, shown to be facilitated by the PTS system for glucose uptake. Lastly, our present observations show that FDG may be toxic for bacteria but only at very high doses that exceed the physiological levels during 18F-FDG PET imaging about 100- to 1000-fold.
Notably, of all presently tested bacterial species, S. pyogenes displayed the highest uptake of 18F-FDG. This bacterium is notorious for causing necrotizing fasciitis, a rapidly progressive infection of deep layers of the skin and subcutaneous tissues, which requires immediate antibiotic therapy and aggressive surgery26. The high uptake levels of 18F-FDG suggest that S. pyogenes may have been the metabolically most active bacterium under the tested conditions. All other bacteria showed lower 18F-FDG uptake in vitro. In view of the fulminant pathology of invasive S. pyogenes infections, it is conceivable that this bacterium displays also a high metabolic activity in vivo.
In line with the finding that 18F-FDG is actively taken up by the investigated bacteria, the absence of the glucose transporter PtsG abrogated FDG uptake by B. subtilis. The absence of PtsI, also known as Enzyme I of the PTS system22, had a strong but less drastic negative effect on 18F-FDG uptake suggesting that its contribution to FDG uptake can to some extent be bypassed. Further, our present results show that FDG is toxic for S. aureus and B. subtilis, but only at high concentrations that are not reached during 18F-FDG PET imaging in vivo. Yet, the observed toxicity of FDG is reminiscent of the toxic effects of 2-DG. In the latter case, the PTS system recognizes 2-DG and phosphorylates it into 2-DG-6-phosphate, which is subsequently dephosphorylated by hexose-6-phosphatase. This cycle of 2-DG phosphorylation and de-phosphorylation depletes the bacterial cell of phosphoenolpyruvate and eventually adenosine triphosphate (ATP)27. Thus, 2-DG has the ability to de-energize bacteria. This may also be the case for FDG, which would explain the observed growth inhibition at high FDG concentrations. Of note, the growth of B. subtilis ptsG or ptsI deletion mutants was much less affected by FDG than growth of the parental strain, which is consistent with the reduced uptake of 18F-FDG.
Currently, there is ample evidence that 18F-FDG PET is a very useful diagnostic tool for many infectious indications, including fever of unknown origin, endocarditis, spondylodiscitis, and vascular graft infections8, 11, 12. This approach can help in defining the location and extent of infection, thereby providing guidance for biopsy and for therapy follow-up. In general, the detection limit in the imaging of infections by PET will depend on the sensitivity and resolution of the PET camera, on the volume of the bacterial lesion, on the surrounding tissue, and on the target-to-normal tissue (T/N) ratios of radioactivity at sites of infection. Our present study shows that 18F-FDG uptake by infecting bacteria potentially contributes to the overall signal detected by PET imaging of bacterial infections. However, it remains difficult to approximate the contribution of the bacterial 18F-FDG uptake to the total 18F-FDG uptake signal measured during in vivo PET imaging of an infection, because the bacterial contribution to the total signal will vary depending on the bacterial species that causes the infection, on the metabolic state of the infecting bacteria, and on the total number of infecting bacteria. Moreover, the inflammatory status of the infected tissue will impact on the total signal, especially because white blood cell migration may vary depending on the extent of inflammation, and also because inflammation may lead to increased blood flow and vascular permeability. Furthermore, the total signal is likely to depend on the particular body site that is infected. Altogether, this means that, in practice, the relative bacterial contribution to the total 18F-FDG signal will be different for individual patients and for different types of infections. For example, chronic infections can be visualized with 18F-FDG, but the signal contributed by the bacteria themselves may be lower than in acute infections if their metabolic activity is lower, which would result in lower uptake of 18F-FDG. In such cases, an option to improve the bacterial signal could be to start imaging at later time points after tracer injection. Clearly, the conditions in vivo are likely to be different compared to the conditions applied in our in vitro study. It is therefore presently not possible to make reliable calculations of the detection limit for infections in vivo.
Lastly, with 18F-FDG PET it is not possible to differentiate between sterile inflammation and infection. Also, 18F-FDG PET will neither tell us whether bacteria are involved in an infection, nor which bacteria are involved. Specific bacteria-targeted PET tracers are therefore desirable. For example, these could include labeled antibiotics like 18F- or 99mTc-ciprofloxacin (Infecton®)6, 28–31. A very promising alternative for 18F-FDG may be 2-[18 F]-fluorodeoxysorbitol (18F-FDS), since sorbitol is a sugar alcohol that is mainly metabolized by the Gram-negative Enterobacteriaceae5, 25. Another attractive approach appears to be the detection of bacteria with 6-[18F]-fluoromaltose (18F-FM)32, because maltose and maltodextrins are metabolizable by all classes of bacteria, in contrast to mammalian cells. Thus, 18F-FM may allow the distinction between bacterial infection and other causes of inflammation. On this basis it is well conceivable that the combined use of 18F-FDS and 18F-FM will facilitate the detection of a wide spectrum of bacteria causing infections. With the aid of both substrates, it should not only be possible to specifically image infecting bacteria over mammalian cells, but also to discriminate infecting Gram-positive and Gram-negative bacteria. The latter would then guide effective antimicrobial therapy. Yet, despite these evident advantages, no clinical studies on infection imaging with 18F-FDS and 18F-FM have thus far been reported.
Conclusion
To date, 18F-FDG is one of the few tracers that may be clinically applied in infection imaging. We now show that this tracer can be taken up by a wide range of commonly encountered bacterial pathogens. We therefore hypothesize that bacterial pathogens may also take up 18F-FDG in vivo, thereby contributing to the FDG-PET signal observed in infection imaging. Future experiments are needed to determine the proportion of 18F-FDG that is taken up by infecting bacteria as compared to the 18F-FDG taken up by inflammatory cells. This will unveil the significance of our present findings in the clinical context.
Acknowledgements
We thank Lisanne Braams and Janine Doorduin for technical assistance during the experiments, and Jörg Stülke for providing the B. subtilis ptsG and ptsI deletion mutants. R.A.S. was supported by a fellowship from Conacyt. J.M.v.D. acknowledges funding from the European Commission, the University of Groningen and the University Medical Center Groningen in the context of the H2020-MSCA-COFUND-2015 Grant Agreements 713482 and 713660. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
M.H., J.W.A.S., R.A.S., A.W.J.M.G., G.M.v.D., J.M.v.D., R.H.J.A.S., and M.v.O. conceived and designed the experiments. M.H., J.W.A.S., and R.A.S. performed the experiments. M.H., R.A.S., J.R.d.J., J.M.v.D., and M.v.O. analyzed the data. H.H.B., G.L., P.H.E., A.W.J.M.G., J.M.v.D., and R.H.J.A.S. contributed reagents, materials and analysis tools. M.H., R.A.S., H.H.B., A.W.J.M.G., G.M.v.D., J.M.v.D., R.H.J.A.S., and M.v.O. wrote the manuscript. All authors have reviewed and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Riemer H. J. A. Slart and Marleen van Oosten contributed equally to this work.
Change history
7/17/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.van Oosten M, et al. Targeted imaging of bacterial infections: advances, hurdles and hopes. FEMS Microbiol Rev. 2015;39:892–916. doi: 10.1093/femsre/fuv029. [DOI] [PubMed] [Google Scholar]
- 2.Laxminarayan R, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Di. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 3.Love C, Palestro CJ. Radionuclide imaging of infection. J Nucl Med Technol. 2004;32:47–57. [PubMed] [Google Scholar]
- 4.Heuker M, et al. Preclinical studies and prospective clinical applications for bacteria-targeted imaging: the future is bright. Clin Transl Imaging. 2016;4:253–264. doi: 10.1007/s40336-016-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordonez AA, et al. A systematic approach for developing bacteria-specific imaging tracers. J Nucl Med. 2017;58:144–150. doi: 10.2967/jnumed.116.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britton KE, et al. Imaging bacterial infection with 99mTc-ciprofloxacin (Infecton®) J Clin Pathol. 2002;55:817–823. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 8.Besson FL, et al. Contribution of (18)F-FDG PET in the diagnostic assessment of fever of unknown origin (FUO): a stratification-based meta-analysis. Eur J Nucl Med Mol Imaging. 2016;43:1887–1895. doi: 10.1007/s00259-016-3377-6. [DOI] [PubMed] [Google Scholar]
- 9.Glaudemans AW, Israel O, Slart RH. Pitfalls and limitations of radionuclide and hybrid imaging in infection and inflammation. Semin Nucl Med. 2015;45:500–512. doi: 10.1053/j.semnuclmed.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Glaudemans, A. W. et al. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 623036, doi:10.1155/2013/623036 (2013). [DOI] [PMC free article] [PubMed]
- 11.Signore A, Glaudemans AW. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann Nucl Med. 2011;25:681–700. doi: 10.1007/s12149-011-0521-z. [DOI] [PubMed] [Google Scholar]
- 12.Bruggink JL, et al. Accuracy of FDG-PET-CT in the diagnostic work-up of vascular prosthetic graft infection. Eur J Vasc Endovasc Surg. 2010;40:348–354. doi: 10.1016/j.ejvs.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–516. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido V, et al. In vivo monitoring of Staphylococcus aureus biofilm infections and antimicrobial therapy by [18F]fluoro-deoxyglucose-microPET in a mouse model. Antimicrob Agents Chemother. 2014;58:6660–6667. doi: 10.1128/AAC.03138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odekerken JC, Brans BT, Welting TJ, Walenkamp GH. (18)F-FDG microPET imaging differentiates between septic and aseptic wound healing after orthopedic implant placement: a longitudinal study of an implant osteomyelitis in the rabbit tibia. Acta Orthop. 2014;85:305–313. doi: 10.3109/17453674.2014.900894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odekerken JC, Walenkamp GH, Brans BT, Welting TJ, Arts JJ. The longitudinal assessment of osteomyelitis development by molecular imaging in a rabbit model. Biomed Res Int. 2014;2014:424–652. doi: 10.1155/2014/424652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keidar Z, Primisashvili N, Leiderman M, Nitecki S, Israel O. 18F-FDG uptake in noninfected prosthetic vascular grafts: incidence, patterns, and changes over time. J Nucl Med. 2014;55:392–395. doi: 10.2967/jnumed.113.128173. [DOI] [PubMed] [Google Scholar]
- 18.Keidar Z, Engel A, Hoffman A, Israel O, Nitecki S. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J Nucl Med. 2007;48:1230–1236. doi: 10.2967/jnumed.107.040253. [DOI] [PubMed] [Google Scholar]
- 19.Russell JB, Wells JE. The ability of 2-deoxyglucose to promote the lysis of Streptococcus bovis JB1 via a mechanism involving cell wall stability. Curr Microbiol. 1997;35:299–304. doi: 10.1007/s002849900258. [DOI] [PubMed] [Google Scholar]
- 20.Veloo ACM, Elgersma PE, Friedrich AW, Nagy E, van Winkelhoff AJ. The influence of incubation time, sample preparation and exposure to oxygen on the quality of the MALDI-TOF MS spectrum of anaerobic bacteria. Clin Microbiol Infect. 2014;20:1091–1097. doi: 10.1111/1469-0691.12644. [DOI] [PubMed] [Google Scholar]
- 21.Martner A, Skovbjerg S, Paton JC, Wold AE. Streptococcus pneumoniae autolysis prevents phagocytosis and production of phagocyte-activating cytokines. Infect Immun. 2009;77:3826–3837. doi: 10.1128/IAI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotrba P, Inui M, Yukawa H. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J Biosci Bioeng. 2001;92:502–517. doi: 10.1016/S1389-1723(01)80308-X. [DOI] [PubMed] [Google Scholar]
- 23.Gonzy-Treboul G, de Waard JH, Zagorec M, Postma PW. The glucose permease of the phosphotransferase system of Bacillus subtilis: evidence for IIGlc and IIIGlc domains. Mol Microbiol. 1991;5:1241–1249. doi: 10.1111/j.1365-2958.1991.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 24.Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein, E. A. et al. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med. 6, 259ra146, doi:10.1126/scitranslmed.3009815 (2014). [DOI] [PMC free article] [PubMed]
- 26.Darenberg J, et al. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis. 2007;45:450–458. doi: 10.1086/519936. [DOI] [PubMed] [Google Scholar]
- 27.Russell JB, Cook GM. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev. 1995;59:48–62. doi: 10.1128/mr.59.1.48-62.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarda L, et al. Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J Nucl Med. 2003;44:920–926. [PubMed] [Google Scholar]
- 29.Siaens RH, Rennen HJ, Boerman OC, Dierckx R, Slegers G. Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J Nucl Med. 2004;45:2088–2094. [PubMed] [Google Scholar]
- 30.Dumarey N, Blocklet D, Appelboom T, Tant L, Schoutens A. Infection is not specific for bacterial osteo-articular infective pathology. Eur J Nucl Med Mol Imaging. 2002;29:530–535. doi: 10.1007/s00259-001-0749-2. [DOI] [PubMed] [Google Scholar]
- 31.Langer O, et al. In vitro and in vivo evaluation of [18F]ciprofloxacin for the imaging of bacterial infections with PET. Eur J Nucl Med Mol Imaging. 2004;32:143–150. doi: 10.1007/s00259-004-1646-2. [DOI] [PubMed] [Google Scholar]
- 32.Gowrishankar G, et al. Investigation of 6-[18F]-fluoromaltose as a novel PET tracer for imaging bacterial infection. PLoS One. 2014;9:e107951. doi: 10.1371/journal.pone.0107951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbert S, et al. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun. 2010;78:2877–2889. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stülke J, et al. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol Microbiol. 1997;25:65–78. doi: 10.1046/j.1365-2958.1997.4351797.x. [DOI] [PubMed] [Google Scholar]
- 35.Singh K, Schmalisch MH, Stülke J, Görke B. Carbon catabolite repression in Bacillus subtilis: Quantitative analysis of repression exerted by different carbon sources. J Bacteriol. 2008;190:7275–7284. doi: 10.1128/JB.00848-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


