Abstract
It is unclear whether there is an intermediate dark state between the S2 and S1 states of carotenoids. Previous two-dimensional electronic spectroscopy measurements support its existence and its involvement in the energy transfer from carotenoids to chlorophylls, but there is still considerable debate on the origin of this dark state and how it regulates the energy transfer process. Here we use ab initio calculations on excited-state dynamics and simulated two-dimensional electronic spectrum of carotenoids from purple bacteria to provide evidence supporting that the dark state may be assigned to a new Ag + state. Our calculations also indicate that groups on the conjugation backbone of carotenoids may substantially affect the excited-state levels and the energy transfer process. These results contribute to a better understanding of carotenoid excited states.
Carotenoids harvest energy from light and transfer it to chlorophylls during photosynthesis. Here, Feng et al. perform ab initio calculations on excited-state dynamics and simulated 2D electronic spectrum of carotenoids, supporting the existence of a new excited state in carotenoids.
Introduction
Carotenoids (Cars) take part in various processes in living organisms. In living animals and humans, they act as antioxidants preventing free radicals from destroying tissue cells1 and are relevant to the vision of retina2. In the photosynthesis of plants and microorganisms, Cars are responsible for harvesting light, transferring energy to chlorophylls (Chls), and protecting against excessive light by quenching excited states of Chls3, 4. Exploring the excited-state dynamic properties of Cars is fundamental to understand the mechanism of photosynthesis and is also helpful for developing artificial light-harvesting systems. Despite the studies for several decades, our knowledge on the electronic structure and excited-state properties of Cars, which determine the mechanism of energy decay in Cars and energy flow from Cars to Chls, is still limited. For example, the origin and nature of a dark state, which lies between the strongly one-photon allowed S2 (1Bu +) state and the forbidden S1 (2Ag −) state, has triggered intense research and has been under debate. This dark state would have a critical role in mediating the Car-to-Chl energy transfer process and the depopulation of the S2 state4–11. Discovery of this dark state would make the conventional carotenoid photophysics model S0→S2→S1→S0 (Fig. 1a), where S0 is the ground state 1Ag −, no longer accurate. Therefore, a new model may be required to account for the excited-state behavior of Cars4, 12, 13.
Fig. 1.
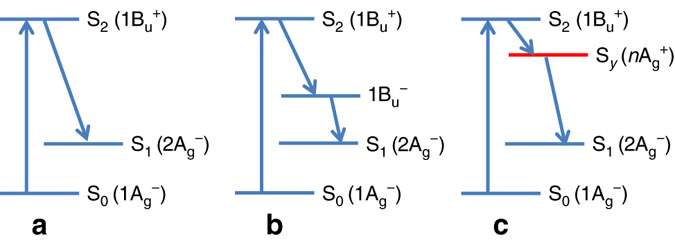
Photophysics model of carotenoids. The three-state model (a), the four-state model involving 1Bu − (b) and the new four-state model containing Sy (c) used to interpret the excited-state dynamics of carotenoids
A number of experimental and theoretical studies4, 8, 14, 15 have been carried out in the past 20 years to unravel this dark state. The 1Bu − state is the popular choice for the attribution of the dark state (Fig. 1b), and it also seems to be the only choice based on the present theory on the excited-state structure of polyenes, which are closely related to the Cars. However, this assignment is controversial due to the contradiction between the properties of the dark state measured experimentally and the behavior of the 1Bu − state determined from both experiments and theory4, 9. Especially, the conjugation-length dependence of the 1Bu − state is not in accord with that of the dark state as observed in the emission spectra and transient absorption spectra16–20. Recent two-dimensional electronic spectroscopy (2DES) and high-time resolution broadband pump-probe spectroscopy measurements on Cars spheroidene (N = 10), rhodopin glucoside (RG) (N = 11) and spirilloxanthin (N = 13) demonstrate clearly the decay of the S2 state to the dark state and the energy transfer channel from the dark state to the Qx state of Chls6, 10, 21, 22. From these experiments, emission energy of the dark state seems to lie a little bit (~0.1 eV) below that of the S2 state, irrespective of the conjugation length of Cars. Decrease of the 1Bu − energy with conjugation length is much steeper than this dark state. In addition, emission energy of the 1Bu − state might be smaller than 2 eV for Cars with N = 10–13 as predicted by Koyama et al.4, 18, according to which energy transfer to the Chls Qx state, whose absorption energy is ~2.1 eV, cannot realize. Thus, there may exist some other, unknown excited state in the vicinity of the S2 state.
Another important issue is how much the dark state is involved in the Car-to-Chl energy transfer. There is disagreement in the overall Car-to-Chl energy transfer efficiency between theoretical prediction and experimental measurement, e.g., 20 vs. 50–60% for Rhodopseudomonas (Rps.) acidophila 23, 24. As the spectral overlap of the dark state emission and Qx absorption is larger than that between the S2 and Qx states, the dark state, which is not considered in previous theoretical calculations, has been supposed to account for this disagreement6. However, some experiments demonstrate that the amount of energy transferred from dark states to Chls is minor4, 25, 26.
Here, we examine the excited-state dynamics of two Cars from purple bacteria and their Car-to-Chl energy transfer by virtue of many-body Green’s function theory and Förster–Dexter theory. 2D spectrum is also simulated by a density matrix-based dynamical method to compare with experiments. We provide evidence supporting a new dark state with Ag + symmetry, which is denoted by Sy, in Cars. Its absorption energy would be higher than the S2 state, whereas its emission energy would be lower. The Sy state is a singly excited state, in stark contrast to the well-known dark states 2Ag − and 1Bu − which are doubly excited states constituted by the two triplet excitons. The excited-state structure and the energy transfer appear to be highly sensitive to the methyl groups on the conjugated backbone of Cars.
Results
Excitation energy of Sy
Extensive computational researches for the dark state in Cars have been performed using various first-principle methods8, 14, 27–33. However, they mainly focus on the controversial 1Bu − state. With many-body Green’s function theory, we study the excited-state dynamics of RG (N = 11) in Rps. acidophila (Fig. 2) and spirilloxanthin (N = 13) in Rhodospirillum rubrum. The calculated absorption energy of the S2 state for RG and spirilloxanthin are 2.65 and 2.42 eV. In experiments, the S2 energy is 2.48 eV for RG in methanol and 2.36 eV for spirilloxanthin in n-hexane4. In solution and the protein environment, absorption spectra of Cars redshift owing to the polarizability of the medium4, 22, 34, 35. If taking this into account, our calculations agree well with experiments. We also prove that putting some protein fragments near Cars has limited effects on the excitation energies of Cars (Supplementary Fig. 2). Excitation energies of the S1 and 1Bu − states for RG (spirilloxanthin) are calculated to be 1.87 and 2.61 eV (1.68 and 2.32 eV), respectively, employing the scheme proposed by Tavan and Schulten36. Above S2 by 0.55 eV for RG and 0.52 eV for spirilloxanthin, our results predict a new state (Sy) of the Ag + symmetry. It is optically forbidden from the ground state and must be indiscernible in experimental optical absorption spectra. The Sy state is a singly excited state, with the wave function represented dominantly by the transitions HOMO−1 → LUMO and HOMO → LUMO+1 (Figs. 3, 4; Supplementary Fig. 3 and Supplementary Note 2). The high-level quantum chemistry approach EOM-CCSD can also get this state and a similar S2–Sy energy gap (Supplementary Table 2). The transition dipole moment of the Sy state is 0.7 and 1.6 Debye for RG and spirilloxanthin, respectively, much smaller than that of the S2 state (20.3 and 28.6 Debye) and comparable to the dark state measured in experiments18. Moreover, the Sy state remains optically forbidden when twisting the conjugated backbone of Cars from all-trans to cis configurations (Supplementary Fig. 4 and Supplementary Table 3).
Fig. 2.
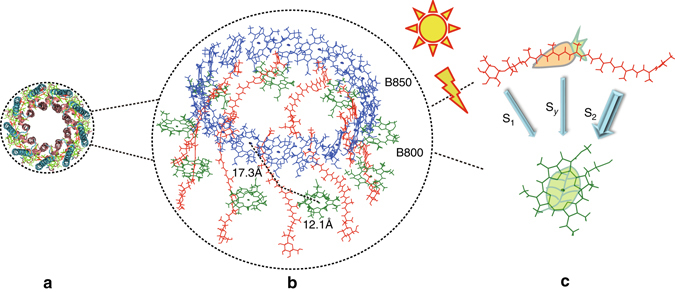
Structure of Rps. acidophila. a Top view of the structure of light-harvesting complex II in Rps. acidophila. b Enlarged view of carotenoids and chlorophylls in a. For a better distinction, carotenoids, the nine B800 chlorophylls, and the eighteen B850 chlorophylls are depicted in red, green, and blue, respectively in b. After these antenna pigments absorb light, carotenoids transfer energy to chlorophylls B800 and B850, mainly through the S2 state, partly through the Sy and S1 states as illustrated in c
Fig. 3.
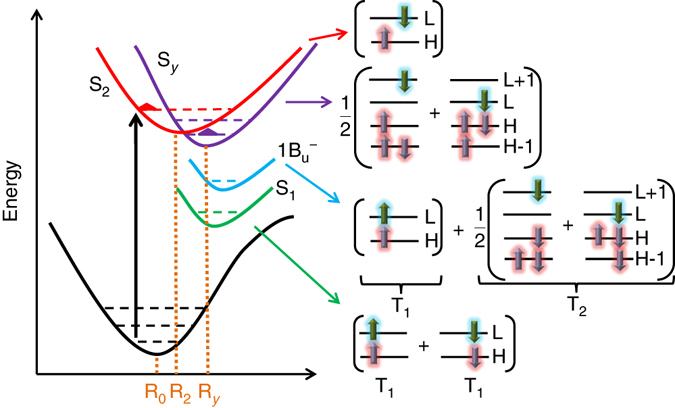
Scheme of the potential energy curves for carotenoids with N ≥ 9 (left) and the compositions of excited states (right). R0, R2, and Ry represent geometries at the potential minima of states S0, S2, and Sy, respectively. H and L represent the highest occupied and the lowest unoccupied molecular orbitals, respectively. Arrows on the orbital illustrate the population and the spins of electrons in this orbital. S2 and Sy are singly excited states. S1 (formed by two T1) and 1Bu − (formed by T1 and T2) are doubly excited states. T1 and T2 are the lowest and the second lowest triplet exciton, respectively
Fig. 4.
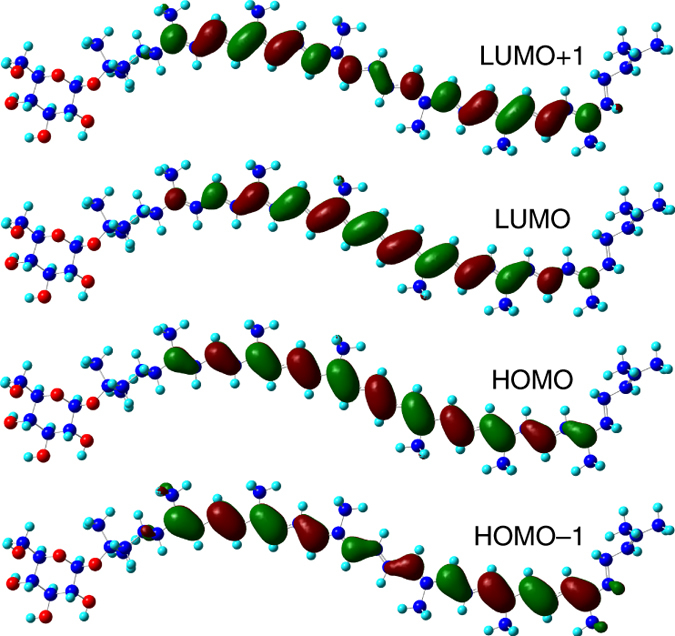
Molecular orbitals of rhodopsin glucoside. The wave function isosurfaces for the highest two occupied and the lowest two unoccupied orbitals of rhodopsin glucoside. C, O, and H atoms are shown in blue, red, and cyan, respectively
Emission energy of Sy
After optical absorption, Cars first relax in the S2 state. From the ground-state geometry (R0 in Fig. 3) to the excited-state minimum geometry of the S2 state (R2 in Fig. 3), Stokes shift of the S2 state is 0.18 eV for both RG and spirilloxanthin. This is in accord with the shift of 0.15 eV for RG measured experimentally4, 23. In this process, the Sy state downshifts by 0.66 eV for RG and 0.68 eV for spirilloxanthin, respectively (Fig. 3). Now, at the potential minimum of the S2 state, the energy of the Sy state is just a little bit higher (0.07 eV for RG and 0.02 eV for spirilloxanthin) than the S2 state. If extrapolating further along the R0→R2 reaction coordinate, the energy of the Sy state falls by an additional 0.1 eV (Fig. 3). The emission energy of the Sy state (at Ry in Fig. 3) is thus lower than that of the S2 state (see also Supplementary Figs. 8 and 9; Supplementary Note 4). Although the crossing point between the S2 and Sy states is not in the R0→R2 region, nonadiabatic transition from S2 to Sy can happen with the aid of vibration. The Sy state may also have a role in tuning the relaxation to lower states like S1 (Fig. 1c). The emission energy of the Sy state for RG and spirilloxanthin is above the absorption energy of the chlorophyll Qx state, making the Car-to-Chl energy transfer via the Sy state realizable.
From the R0 to R2 geometries, the bond length alternation, i.e., the difference between the average lengths of C–C and C=C bonds, is reduced. As the S2 state is an ionic excited state, whereas the Sy state can be considered to be a covalent-like one due to its weak transition dipole moment, the electron-hole binding energy (E b) in the S2 state should be much more influenced by structural variation than that in the Sy state. We do find that from R0 to R2, E b in the Sy state remains constant while that in the S2 state reduces by ∼0.5 eV. From R0 to R2, the gap between the unoccupied and occupied molecular orbitals (E gap) narrows by ∼0.7 eV. The excitation energy, which equals E gap−E b in physics, thus decreases faster for the Sy state than the S2 state from R0 to R2 (Fig. 5c). Energies of the S1 and 1Bu − states are also found to exhibit much higher sensitivity on the bond length alternation than the S2 state8, 36.
Fig. 5.
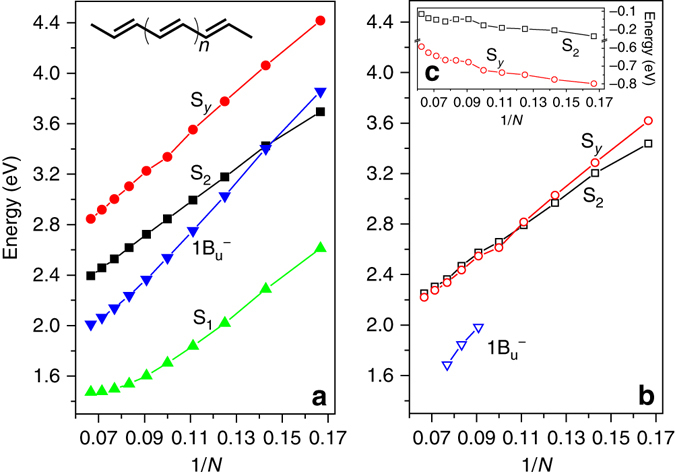
Dependence of excitation energies of polyenes on the conjugation length N. a At the ground-state geometry. b At the S2 state potential minimum geometry. c The energy shifts of the S2 and Sy states from the ground-state geometry to the S2 state potential minimum geometry. Energies of 1Bu − in b are taken from the experimental fluorescence spectra4
Dependence of the Sy emission energy on conjugation length
Dependence of the emission energy of the dark state on the conjugation length of Cars is a crucial factor to determine the attribution of the dark state4. Polyenes are ideal models to investigate this issue. We examine a series of polyenes H3C–(C2H2)N–CH3 with N = 6–15 (Fig. 5). Although the absorption energy of the Sy state varies faster with the conjugation length than that of the S2 state (Fig. 5a), the S2–Sy energy gap at the potential minimum of the S2 state remains at ∼0.03 eV for N ≥ 10 with the Sy below S2 (Fig. 5b). Taking into account the additional 0.1 eV downshift of the Sy state to its own potential minimum as discussed above, the emission energy of the Sy state is lower than that of the S2 state for N ≥ 9 and the gap between them remains at ∼ 0.1 eV. This agrees well with the transient absorption studies that the dark state lies below the S2 state for Cars with N ≥ 911, and also 2DES measurements that the potential minimum of the dark state is 0.1 eV below that of the S2 state for N = 10, 11, and 136, 10, 21.
2D spectrum of Rps. acidophila
In a recent study, Scholes and coworkers utilized the broadband 2DES to investigate carotenoid dark states in purple bacteria6. The 2DES is a four-wave mixing technique that is extremely sensitive to electronic coherence and energy relaxation dynamics37, 38. To verify our model, we calculate theoretical 2D spectrum of Rps. acidophila based on a model Hamiltonian that is consistent with our ab initio calculations. Note that the original 2D experiment is carried out using a broadband pulse that overlaps with the very red edge of the S2 band and the blue edge of the Qx band in a sample of Rps. acidophila. The experimentally observed 2D S2 peak at ~535 nm (2.34 eV) is dependent of the excitation laser spectrum, and is also in good agreement with the calculated S2 transition energy at the S2 minimum. This excitation energy corresponds to a highly displaced geometry along the bond length-alternation coordinate. In this geometry, the four-state model with the Sy state energy lower than that of the S2 state applies, therefore, we place the Sy energy minimum at 0.1 eV below the S2 energy minimum in our 2D simulation, in accordance with our calculations for RG. Figure 6 shows the simulated 2D spectrum at a delay time of 200 fs, and the simulated spectrum is in agreement with the experimental spectrum6. Specifically, the diagonal peaks, S2/Sy cross-peak, and pronounced S2/S1 excited-state absorption peak that splits into two by the lower-diagonal S2/Sy cross-peak are all correctly reproduced in our model simulations. Therefore, our theoretical calculations are consistent with the 2D experiments.
Fig. 6.
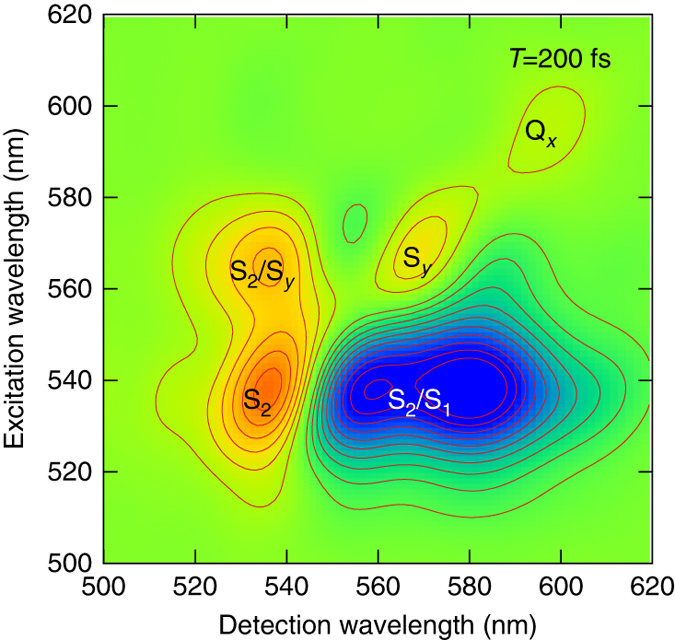
Simulated two-dimensional (2D) electronic spectrum of Rps. acidophila at the population time T = 200 fs. The diagonal peaks are symbolized by the corresponding state; the cross-peaks indicate that energy is transferred from the state represented by the first symbol to the acceptor state represented by the second symbol
Influence of groups on the excitation energies of Cars
Comparing RG with the N = 11 polyene and spirilloxanthin with the N = 13 polyene, the Sy−S2 energy gap in the Cars is the same as that in the polyene. However, the S2−1Bu − and S2−S1 energy gaps in the polyene are about 0.3 eV wider than those in the Cars of the same conjugation length. The structural difference between Cars and polyenes is reflected in two respects: (i) the conjugated backbone is symmetrical in polyenes but distorted in Cars, (ii) there are groups, e.g., methyl groups, on the conjugated backbone in Cars but not in polyenes. Through changing the shape of the polyene and substituting some hydrogen atoms on it by groups (Supplementary Fig. 5), we find that groups affect substantially the energy gap between the S2 state and the doubly excited states S1 and 1Bu −, whereas the gap between the S2 and Sy states is independent of any modification to the conjugated backbone (Supplementary Table 4). This implies that energies of the S1 and 1Bu − states, and thus the competition between the S2→S1 decay and the Car-to-Chl energy transfer might be tuned by modifying the groups attached to the conjugated backbone.
Energy transfer from Cars to Chls
Contribution of the dark state to the Car-to-Chl energy transfer is still an open question. It is recently proposed that the dark state-mediated energy transfer rate is of the same magnitude as the S2-mediated one6, 21. This is in contradiction with previous work4, 25, 26. The nonadiabatic transition from the S2 to Sy states is always present for longer Cars according to discussions in the previous sections. The Sy state must be involved in the Car-to-Chl energy transfer. We thus further investigate the energy transfer capability of the Sy state, which is important for understanding the energy transfer mechanism in photosynthesis and resolving the controversies in this respect.
We calculate the energy transfer in the RG-B850 and RG-B800 pairs in Rps. acidophila as linked by dashed lines in Fig. 2b. Energy flow in these two kinds of pairs has been supposed to dominate the Car-to-Chl energy transfer24. Figure 7a shows the calculated absorption spectrum of the RG-B800 pairs, which is comparable to the experimental spectrum of LH2 complex in Rps. acidophila strain 100503, 6. Positions of the Qx and Qy peaks deviate from the experimental ones by <0.1 eV. A charge-transfer state, with the electron excited from the Car to the Chl, appears between the S2 and Qx states, which can result in the formation of Car radicals as detected in experiments4. The rate of energy transfer is evaluated via k = (2π/ℏ)|V DA|2 J DA, where V DA and J DA are the electronic coupling strength and the spectral overlap between donor (D) and acceptor (A) transitions, respectively. V DA is calculated within the framework of many-body Green’s function theory.
Fig. 7.
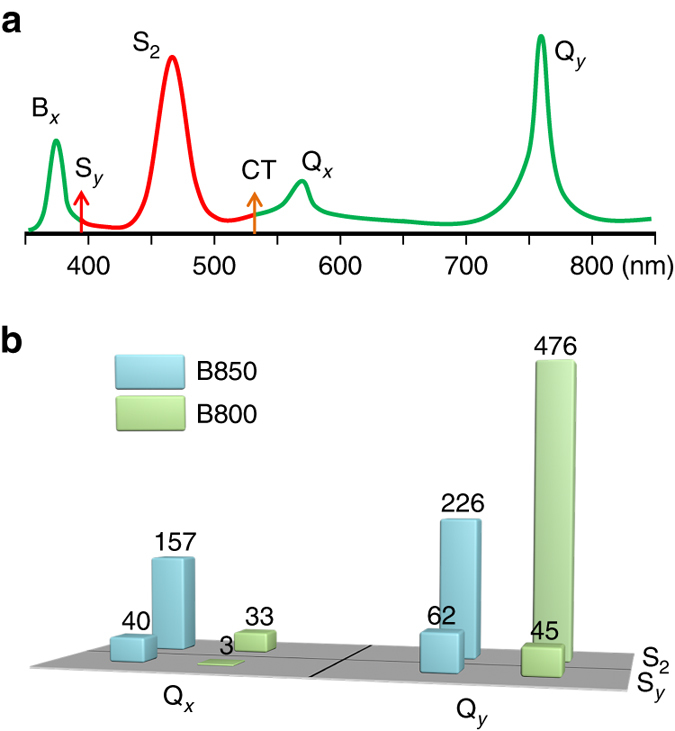
Absorption spectrum and electronic coupling strength for a carotenoid–chlorophyll complex. a Calculated absorption spectrum of the rhodopin glucoside-B800 complex as linked by the dashed line in Fig. 2b. CT denotes the charge-transfer state. Oscillator strengths of the Sy and CT states are very weak. Here arrows are used to indicate their positions. b Electronic coupling strengths (in cm−1) for the energy transfer passways from the S2 and Sy states to the Qx and Qy states in the rhodopin glucoside-B850 and rhodopin glucoside-B800 pairs as linked by dashed lines in Fig. 2b. Distances between the centers of corresponding rhodopin glucoside and B850/B800 molecules are given in Fig. 2b
We use the spectral overlap data from Krueger et al.24 and Ostroumov et al.21, where J DA(S2−Qx) = 13J DA(S2−Qy) and J DA(S2−Qx) = J DA(Sy−Qx). Figure 7b compares the electronic coupling strength for each energy transfer passway from Car to Chl. We find that the energy transfer rate of the S2-B800 Qy channel is much higher than expected theoretically before24 and can reach 70% of that of the S2-B850 Qx channel which has been considered to be the dominating channel. This branching ratio is in good agreement with the experiment39, supporting the experimental observations that both Qx and Qy have the role of energy acceptors and the amount of energy transferred to B800 is comparable to that to B8504, 39–42. The Sy-mediated energy transfer is predominated by the Sy-B850 Qx channel. The overall Sy-mediated energy transfer rate is one order of magnitude smaller than that of the S2-mediated one. Thus, although the dark state participates in the energy transfer process, the Car-to-Chl energy transfer is still governed by the S2 state (see Fig. 2c for the energy flow).
Discussion
Ever since the discovery of the intermediate dark state between the S2 and S1 states in Cars by Cerullo et al. in 2002, its role in photosynthesis becomes increasingly emphasized5. On the basis of the conventional theoretical model for the electronic structures of polyenes developed by Tavan and Schulten36, the 1Bu − state has long been regarded as the candidate for the dark state. The strongly dependence of the S2–1Bu − energy gap on the conjugation length make this assignment questionable4, 9. The Sy state of the Ag + symmetry has a more moderate conjugation length dependence than the 1Bu − state. More importantly, variation of the Sy emission energy with respect to the conjugation length is parallel to the S2 state, and their energy gap is kept at a small value (~ 0.1 eV) for Cars with N > 9. This is consistent with the experimental findings, such as those in 2DES, that the emission energy gap between the dark state and the S2 state is about 300 cm−1 and independent of the conjugation length4, 6, 10, 21. This small energy gap ensures the extremely fast (~10 fs) internal conversion from the S2 state to the dark state as observed experimentally4, 5, 11. The agreement between our theoretical 2D spectrum (Fig. 6) and the experimental data lends support to our model with a new Ag + state below S2 at a large bond-length alternation. Interestingly, the experimental 2D study resolves the diagonal peak due to the dark state. Furthermore, based on ultrafast-transient absorption and transient-grating experiments, some groups have suggested that the dark-state is due to a double-minima structure on the S2 potential energy surface, not a distinct electronic state43, 44. This double-minima model hypothesizes that the lowest one of the S2 potential surface minima exists at the conformation where the carotenoid is twisted from the all-trans isomer by ~90° with respect to one C=C bond of the conjugated polyene backbone. This may be true for molecules with a short conjugated backbone such as the protonated Schiff bases45, which is also demonstrated in our previous theoretical work46. Nevertheless, this does not seem to be the case for molecules with a long conjugated backbone (Supplementary Fig. 10). The new state described in the present article, with strong energy shift along the bond length alternation coordinate that crossovers in energy with the S2 state (Fig. 3), does exhibit a double-well like feature that may explain the spectral shifts observed in the recent experiments. Noticeably, the theoretical 2D spectrum based on our model correctly describes the S2/Sy cross-peak above the diagonal in the experiment, whereas in the double-minima model one should expect to see extensive spectral diffusion and an elongated S2 peak along the detection wavelength (below the diagonal), which is inconsistent with the 2D experimental data. In addition, the clear diagonal Sy peak is also not explained by the double-minima model. On the basis of the above analysis, a carotenoid photophysics four-state model is given in Fig. 1c, which involves the S1, Sy, and S2 excited states.
One important motive to study the dark state in experiments is to solve the obvious distinction in the estimated Car-to-Chl energy transfer efficiency between previous theoretical calculations (20%) and experimental measurements (50–60%)6, 21, 24. Above we have illustrated that the portion of energy transferred via the Sy state is minor based on the assumption J DA(S2−Qx) = J DA(Sy−Qx) proposed by Ostroumov et al.21, 27. Even if J DA(Sy−Qx) > J DA(S2−Qx), considering the smaller energy gap between the Sy and Qx states than that between the S2 and Qx states, the contribution of the Sy state cannot fill the gap between theoretical calculations and experiments. The electronic coupling strengths we calculate by many-body Green’s function theory are 1.5 times stronger than those from previous theoretical calculations by the transition density-cube approach24. We suggest that the disagreement in energy transfer efficiency between previous theoretical work and experiments may be due not only to the absence of the dark state in the theoretical model, but also to the underestimation of electronic coupling strengths in previous calculations.
In conclusion, our study provides evidence for a new state Sy of the Ag + symmetry in Cars, thus contributing to a better understanding of carotenoid excited states. Future experiments would be required to test the accuracy of our calculations.
Methods
Ground-state geometry
Density-functional theory (DFT) with the Coulomb-attenuating method variant of the Becke 3-parameter-Lee-Yang-Parr (CAM-B3LYP) exchange-correlation functional47 is used to optimize geometries of Cars and Chls by the Gaussian 09 program48. CAM-B3LYP has been shown to give more reasonable structures than other functionals14, 49.
Excitation energy
Many-body Green’s function theory, which includes the combination of GW method and Bethe–Salpeter equation (BSE)50, 51, is applied to compute the excitation energies with a Gaussian orbital based GW-BSE package52, 53. Calculations are performed at the level of full BSE, i.e., considering the mixing between resonant and antiresonant transitions, as Tamm–Dancoff approximation can cause large errors for organic molecules54–56. This scheme has been applied for electronic excitations in many organic systems54, 57–59. The S1 and 1Bu − states bear doubly excited character, involving two coupled triplet excitations. Their excitation energies cannot be obtained from BSE directly as BSE can only deal with one electron–hole pair excitation. Here, we estimate their energies according to Tavan and Schulten’s theory36, i.e., E(S1) = 2E(T1) and , where T1 and T2 are the lowest two triplet states of Cars and are computed via GW-BSE. This approach possesses high accuracy as proved by Tavan and Schulten. In the Supplementary Note 1, Supplementary Fig. 1, and Supplementary Table 1, a detailed discussion on the accuracy of our strategy to predict the S1 and 1Bu − energies is presented.
Potential minimum at the excited state
Equilibrium structure of Cars in the S2 state, which originates from the HOMO → LUMO transition (point R2 in Fig. 3) is optimized by the constrained density-functional theory (CDFT) where occupations in HOMO and LUMO are fixed at 1 during structural relaxation60. As the Sy state is composed predominantly by transitions HOMO-1 → LUMO and HOMO → LUMO+1 with equal weight, its potential minimum cannot be predicted by structural optimization on its own energy surface using CDFT. We locate the potential minimum of the Sy state (point Ry in Fig. 3) approximately by extrapolating along the R0 → R2 reaction coordinate of the S2 state. We validate the reasonability of the Sy potential minimum predicted by this scheme through relaxing Cars in the Sy state with excited-state forces from BSE. BSE forces are computed by the approach proposed by Ismail-Beigi and Louie with the exception that we use finite difference method to evaluate derivatives of single-particle energies and wave functions with respect to nuclear positions61, 62. This technique to compute BSE forces has been well tested to give consistent results with those from Ismail-Beigi and Louie on the excited-state structures of CO and NH3 molecules61 (see Supplementary Note 3, Supplementary Table 5, and Supplementary Figs. 6 and 7 for details of the theory and test of the accuracy).
Theoretical 2D spectrum
We utilize a density matrix-based dynamical method to simulate 2D spectrum of Rps. acidophila. This method accounts for full density-matrix dynamics and bath memory effects in the simulated 2D spectrum, and details of the theory are described in refs 63, 64. The model adopted for the Rps. acidophila system consists of five carotenoid states (S0, S1, Sy, S2, and Sn) and one chlorophyll state (Qx). The transition energies are set at 16,900, 17,810, 18,620, and 17,500 cm−1 for Qx, Sy, S2, and S1 → Sn, respectively. This model places the Sy energy at 0.1 eV below the S2 state to describe carotenoid electronic states at a geometry highly displaced along the bond length-alternation coordinate, in accordance with our calculations for RG. The dynamics are described by a Lindblad master equation including five population relaxation terms: = 0.3 ps, = 1 ps, =2 ps, =3 ps, =1 ps. The line broadenings are described by Gaussian static disorders with σ = 450 cm−1, and couplings to a super-Ohmic bath with a spectral density . The coupling strength γ 0 and cut-off ω c are set at 0.6 and 850 cm−1, respectively. Note that in this work, we aim to demonstrate that our model produces 2D spectrum that is consistent with experimental results, therefore we do not perform full fittings to the experimental spectra, and the bath and dynamical parameters used in our model are only set tentatively. Additional calculations that provide nonadiabatic couplings and explore the potential surfaces more completely are required to fully describe the experimental 2D data.
Energy transfer
The electronic energy transfer rate, k, is calculated via k = (2π/ℏ)|V DA|2 J DA according to the Förster–Dexter theory. The electronic coupling strength V DA is computed within the framework of many-body Green’s function theory via
| 1 |
where X D/A is the BSE exciton wave function for donor (D) and acceptor (A), ω0 the energy transferred, S DA the overlap matrix between donor and acceptor orbitals. K x and K d are the exchange and direct terms of the BSE electron–hole interaction kernel. Electronic coupling arising from these two terms resemble the Förster and Dexter coupling, respectively65. V DA computed by Eq. (1) consists excellently with that by the high-level quantum chemistry approach CASSCF66.
Data availability
All data supporting the findings of this study are available from the corresponding author on request.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants Nos. 21433006, 21573131, 21173130, and 21603056) and the Natural Science Foundation of Shandong Province (Grant No. JQ201603). Y.-C.C. thanks the Ministry of Science and Technology, Taiwan (Grant No. NSC 105-2113-M-002-012), National Taiwan University (Grant No. 103R891305), and Center for Quantum Science and Engineering (Subproject: 103R891401) for financial support. Computational resources have been provided by the National Supercomputing Centers in Jinan.
Author contributions
Y.M. designed the calculations and J.F. carried out most of the calculations. C.-W.T. and Y.-C.C. performed the calculation of 2D spectra. T.C., X.L., and H.Y. took part in the optimization of configurations. Y.M. and M.R. performed coding on the many-body Green’s function theory and Förster–Dexter theory. Y.M., J.F., and Y.-C.C. wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-00120-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997;418:91–97. doi: 10.1016/S0014-5793(97)01355-0. [DOI] [PubMed] [Google Scholar]
- 2.Nolan J, Meagher K, Kashani S, Beatty S. What is meso-zeaxanthin, and where does it come from? Eye. 2013;27:899–905. doi: 10.1038/eye.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cogdell RJ, Gall A, Köhler J. The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes. Q. Rev. Biophys. 2006;39:227–324. doi: 10.1017/S0033583506004434. [DOI] [PubMed] [Google Scholar]
- 4.Polívka T, Sundström V. Ultrafast dynamics of carotenoid excited states−from solution to natural and artificial systems. Chem. Rev. 2004;104:2021–2072. doi: 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]
- 5.Cerullo G, et al. Photosynthetic light harvesting by carotenoids: detection of an intermediate excited state. Science. 2002;298:2395–2398. doi: 10.1126/science.1074685. [DOI] [PubMed] [Google Scholar]
- 6.Ostroumov EE, Mulvaney RM, Cogdell RJ, Scholes GD. Broadband 2D electronic spectroscopy reveals a carotenoid dark state in purple bacteria. Science. 2013;340:52–56. doi: 10.1126/science.1230106. [DOI] [PubMed] [Google Scholar]
- 7.Gradinaru CC, et al. An unusual pathway of excitation energy deactivation in carotenoids: singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc. Natl Acad. Sci. 2001;98:2364–2369. doi: 10.1073/pnas.051501298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostroumov E, Müller MG, Marian CM, Kleinschmidt M, Holzwarth AR. Electronic coherence provides a direct proof for energy-level crossing in photoexcited lutein and β-carotene. Phys. Rev. Lett. 2009;103:108302. doi: 10.1103/PhysRevLett.103.108302. [DOI] [PubMed] [Google Scholar]
- 9.Polívka T, Sundström V. Dark excited states of carotenoids: consensus and controversy. Chem. Phys. Lett. 2009;477:1–11. doi: 10.1016/j.cplett.2009.06.011. [DOI] [Google Scholar]
- 10.Maiuri M, et al. Ultra-broadband 2D electronic spectroscopy of carotenoid-bacteriochlorophyll interactions in the LH1 complex of a purple bacterium. J. Chem. Phys. 2015;142:212433. doi: 10.1063/1.4919056. [DOI] [PubMed] [Google Scholar]
- 11.Polli D, et al. Conjugation length dependence of internal conversion in carotenoids: role of the intermediate state. Phys. Rev. Lett. 2004;93:163002. doi: 10.1103/PhysRevLett.93.163002. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto H, et al. The very early events following photoexcitation of carotenoids. Arch. Biochem. Biophys. 2004;430:61–69. doi: 10.1016/j.abb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Koyama Y, Rondonuwu FS, Fujii R, Watanabe Y. Light-harvesting function of carotenoids in photo-synthesis: the roles of the newly found 11Bu− state. Biopolymers. 2004;74:2–18. doi: 10.1002/bip.20034. [DOI] [PubMed] [Google Scholar]
- 14.Coccia E, Varsano D, Guidoni L. Ab initio geometry and bright excitation of carotenoids: quantum monte carlo and many body green’s function theory calculations on peridinin. J. Chem. Theory Comput. 2014;10:501–506. doi: 10.1021/ct400943a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knecht S, Marian CM, Kongsted J, Mennucci B. On the photophysics of carotenoids: a multireference DFT study of peridinin. J. Phys. Chem. B. 2013;117:13808–13815. doi: 10.1021/jp4078739. [DOI] [PubMed] [Google Scholar]
- 16.Sashima T, Koyama Y, Yamada T, Hashimoto H. The 1Bu+, 1Bu−, and 2Ag− energies of crystalline lycopene, β-carotene, and mini-9-β-carotene as determined by resonance-raman excitation profiles: dependence of the 1Bu− state energy on the conjugation length. J. Phys. Chem. B. 2000;104:5011–5019. doi: 10.1021/jp994185b. [DOI] [Google Scholar]
- 17.Furuichi K, Sashima T, Koyama Y. The first detection of the 3Ag− state in carotenoids using resonance-Raman excitation profiles. Chem. Phys. Lett. 2002;356:547–555. doi: 10.1016/S0009-2614(02)00412-8. [DOI] [Google Scholar]
- 18.Fujii R, et al. Fluorescence spectroscopy of all-trans-anhydrorhodovibrin and spirilloxanthin: detection of the 1Bu− fluorescence. J. Phys. Chem. A. 2001;105:5348–5355. doi: 10.1021/jp010150b. [DOI] [Google Scholar]
- 19.Onaka K, et al. The state energy and the displacements of the potential minima of the 2Ag− state in all-trans-β-carotene as determined by fluorescence spectroscopy. Chem. Phys. Lett. 1999;315:75–81. doi: 10.1016/S0009-2614(99)01212-9. [DOI] [Google Scholar]
- 20.Fujii R, Inaba T, Watanabe Y, Koyama Y, Zhang J-P. Two different pathways of internal conversion in carotenoids depending on the length of the conjugated chain. Chem. Phys. Lett. 2003;369:165–172. doi: 10.1016/S0009-2614(02)01999-1. [DOI] [Google Scholar]
- 21.Ostroumov EE, Mulvaney RM, Anna JM, Cogdell RJ, Scholes GD. Energy transfer pathways in light-harvesting complexes of purple bacteria as revealed by global kinetic analysis of two-dimensional transient spectra. J. Phys. Chem. B. 2013;117:11349–11362. doi: 10.1021/jp403028x. [DOI] [PubMed] [Google Scholar]
- 22.Maiuri M, et al. Solvent-dependent activation of intermediate excited states in the energy relaxation pathways of spheroidene. Phys. Chem. Chem. Phys. 2012;14:6312–6319. doi: 10.1039/c2cp23585d. [DOI] [PubMed] [Google Scholar]
- 23.Krueger BP, Scholes GD, Jimenez R, Fleming GR. Electronic excitation transfer from carotenoid to bacteriochlorophyll in the purple bacterium Rhodopseudomonas acidophila. J. Phys. Chem. B. 1998;102:2284–2292. doi: 10.1021/jp973062t. [DOI] [Google Scholar]
- 24.Krueger BP, Scholes GD, Fleming GR. Calculation of couplings and energy-transfer pathways between the pigments of LH2 by the ab initio transition density cube method. J. Phys. Chem. B. 1998;102:5378–5386. doi: 10.1021/jp9811171. [DOI] [Google Scholar]
- 25.Wohlleben W, Buckup T, Herek JL, Cogdell RJ, Motzkus M. Multichannel carotenoid deactivation in photosynthetic light harvesting as identified by an evolutionary target analysis. Biophys. J. 2003;85:442–450. doi: 10.1016/S0006-3495(03)74489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damjanović A, Ritz T, Schulten K. Energy transfer between carotenoids and bacteriochlorophylls in light-harvesting complex II of purple bacteria. Phys. Rev. E. 1999;59:3293. doi: 10.1103/PhysRevE.59.3293. [DOI] [Google Scholar]
- 27.Dolan PM, Miller D, Cogdell RJ, Birge RR, Frank HA. Linear dichroism and the transition dipole moment orientation of the carotenoid in the LH2 antenna complex in membranes of Rhodopseudomonas acidophila strain 10050. J. Phys. Chem. B. 2001;105:12134–12142. doi: 10.1021/jp010271b. [DOI] [Google Scholar]
- 28.Ghosh D, Hachmann J, Yanai T, Chan GK-L. Orbital optimization in the density matrix renormalization group, with applications to polyenes and β-carotene. J. Chem. Phys. 2008;128:144117. doi: 10.1063/1.2883976. [DOI] [PubMed] [Google Scholar]
- 29.Kleinschmidt M, Marian CM, Waletzke M, Grimme S. Parallel multireference configuration interaction calculations on mini-β-carotenes and β-carotene. J. Chem. Phys. 2009;130:044708. doi: 10.1063/1.3062842. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Tavan P. Electronic excitations in long polyenes revisited. J. Chem. Phys. 2012;136:124309. doi: 10.1063/1.3696880. [DOI] [PubMed] [Google Scholar]
- 31.Enriquez MM, et al. The intramolecular charge transfer state in carbonyl-containing polyenes and carotenoids. J. Phys. Chem. B. 2010;114:12416–12426. doi: 10.1021/jp106113h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magdaong NM, et al. Excited state properties of a short π-electron conjugated peridinin analogue. Chem. Phys. Lett. 2014;593:132–139. doi: 10.1016/j.cplett.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magdaong NM, et al. High efficiency light harvesting by carotenoids in the LH2 complex from photosynthetic bacteria: unique adaptation to growth under low-light conditions. J. Phys. Chem. B. 2014;118:11172–11189. doi: 10.1021/jp5070984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson PO, Gillbro T, Ferguson L, Cogdell RJ. Absorption spectral shifts of carotenoids related to medium polarizability. Photochem. Photobiol. 1991;54:353–360. doi: 10.1111/j.1751-1097.1991.tb02027.x. [DOI] [Google Scholar]
- 35.Macpherson AN, Gillbro T. Solvent dependence of the ultrafast S2−S1 internal conversion rate of β-carotene. J. Phys. Chem. A. 1998;102:5049–5058. doi: 10.1021/jp980979z. [DOI] [Google Scholar]
- 36.Tavan P, Schulten K. Electronic excitations in finite and infinite polyenes. Phys. Rev. B. 1987;36:4337–4358. doi: 10.1103/PhysRevB.36.4337. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Y-C, Fleming GR. Dynamics of light harvesting in photosynthesis. Annu. Rev. Phys. Chem. 2009;60:241–262. doi: 10.1146/annurev.physchem.040808.090259. [DOI] [PubMed] [Google Scholar]
- 38.Ginsberg NS, Cheng Y-C, Fleming GR. Two-dimensional electronic spectroscopy of molecular aggregates. Acc. Chem. Res. 2009;42:1352–1363. doi: 10.1021/ar9001075. [DOI] [PubMed] [Google Scholar]
- 39.Macpherson AN, Arellano JB, Fraser NJ, Cogdell RJ, Gillbro T. Efficient energy transfer from the carotenoid S2 state in a photosynthetic light-harvesting complex. Biophys. J. 2001;80:923–930. doi: 10.1016/S0006-3495(01)76071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto H, Ogura M, Nakabayashi T, Tasumi M. Sub-picosecond excited-state dynamics of a carotenoid (spirilloxanthin) in the light-harvesting systems of Chromatium vinosum: relaxation process from the optically allowed S2 state. Chem. Phys. 1998;236:309–318. doi: 10.1016/S0301-0104(98)00207-9. [DOI] [Google Scholar]
- 41.Hörvin Billsten H, et al. Dynamics of energy transfer from lycopene to bacteriochlorophyll in genetically-modified LH2 complexes of rhodobacter sphaeroides. Biochem. 2002;41:4127–4136. doi: 10.1021/bi011741v. [DOI] [PubMed] [Google Scholar]
- 42.Papagiannakis E, et al. Light harvesting by carotenoids incorporated into the B850 light-harvesting complex from rhodobacter sphaeroides R-26.1: excited-state relaxation, ultrafast triplet formation, and energy transfer to bacteriochlorophyll. J. Phys. Chem. B. 2003;107:5642–5649. doi: 10.1021/jp027174i. [DOI] [Google Scholar]
- 43.Ghosh S, et al. Femtosecond heterodyne transient-grating studies of nonradiative decay of the S2 (11Bu+) state of β-carotene: contributions from dark intermediates and double-quantum coherences. J. Phys. Chem. B. 2015;119:14905–14924. doi: 10.1021/acs.jpcb.5b09405. [DOI] [PubMed] [Google Scholar]
- 44.De Weerd FL, Van Stokkum IH, Van Grondelle R. Subpicosecond dynamics in the excited state absorption of all-trans-β-carotene. Chem. Phys. Lett. 2002;354:38–43. doi: 10.1016/S0009-2614(02)00095-7. [DOI] [Google Scholar]
- 45.Liebel M, et al. Direct observation of the coherent nuclear response after the absorption of a photon. Phys. Rev. Lett. 2014;112:238301. doi: 10.1103/PhysRevLett.112.238301. [DOI] [PubMed] [Google Scholar]
- 46.Kaczmarski MS, Ma Y, Rohlfing M. Diabatic states of a photoexcited retinal chromophore from ab initio many-body perturbation theory. Phys. Rev. B. 2010;81:115433. doi: 10.1103/PhysRevB.81.115433. [DOI] [Google Scholar]
- 47.Yanai T, Tew DP, Handy NC. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP) Chem. Phys. Lett. 2004;393:51–57. doi: 10.1016/j.cplett.2004.06.011. [DOI] [Google Scholar]
- 48.Frisch MJ, et al. Gaussian 09, Revision B.01. Wallingford, CT: Gaussian, Inc; 2010. [Google Scholar]
- 49.Jacquemin D, Adamo C. Bond length alternation of conjugated oligomers: wave function and DFT benchmarks. J. Chem. Theory Comput. 2011;7:369–376. doi: 10.1021/ct1006532. [DOI] [PubMed] [Google Scholar]
- 50.Hybertsen MS, Louie SG. Electron correlation in semiconductors and insulators: band gaps and quasiparticle energies. Phys. Rev. B. 1986;34:5390. doi: 10.1103/PhysRevB.34.5390. [DOI] [PubMed] [Google Scholar]
- 51.Onida G, Reining L, Rubio A. Electronic excitations: density-functional versus many-body Green’s-function approaches. Rev. Mod. Phys. 2002;74:601–659. doi: 10.1103/RevModPhys.74.601. [DOI] [Google Scholar]
- 52.Rohlfing M, Krüger P, Pollmann J. Efficient scheme for GW quasiparticle band-structure calculations with applications to bulk Si and to the Si(001)-(2×1) surface. Phys. Rev. B. 1995;52:1905–1917. doi: 10.1103/PhysRevB.52.1905. [DOI] [PubMed] [Google Scholar]
- 53.Rohlfing M, Louie SG. Electron-hole excitations and optical spectra from first principles. Phys. Rev. B. 2000;62:4927–4944. doi: 10.1103/PhysRevB.62.4927. [DOI] [Google Scholar]
- 54.Ma Y, Rohlfing M, Molteni C. Modeling the excited states of biological chromophores within many-body green’s function theory. J. Chem. Theory Comput. 2010;6:257–265. doi: 10.1021/ct900528h. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y, Rohlfing M, Molteni C. Excited states of biological chromophores studied using many-body perturbation theory: effects of resonant-antiresonant coupling and dynamical screening. Phys. Rev. B. 2009;80:241405. doi: 10.1103/PhysRevB.80.241405. [DOI] [Google Scholar]
- 56.Grüning M, Marini A, Gonze X. Exciton-plasmon states in nanoscale materials: breakdown of the Tamm−Dancoff approximation. Nano Lett. 2009;9:2820–2824. doi: 10.1021/nl803717g. [DOI] [PubMed] [Google Scholar]
- 57.Yin H, Ma Y, Mu J, Liu C, Rohlfing M. Charge-transfer excited states in aqueous DNA: insights from many-body Green’s function theory. Phys. Rev. Lett. 2014;112:228301. doi: 10.1103/PhysRevLett.112.228301. [DOI] [PubMed] [Google Scholar]
- 58.Blase X, Attaccalite C, Olevano V. First-principles GW calculations for fullerenes, porphyrins, phtalocyanine, and other molecules of interest for organic photovoltaic applications. Phys. Rev. B. 2011;83:115103. doi: 10.1103/PhysRevB.83.115103. [DOI] [Google Scholar]
- 59.Faber C, Attaccalite C, Olevano V, Runge E, Blase X. First-principles GW calculations for DNA and RNA nucleobases. Phys. Rev. B. 2011;83:115123. doi: 10.1103/PhysRevB.83.115123. [DOI] [Google Scholar]
- 60.Artacho E, et al. Structural relaxations in electronically excited poly(para-phenylene) Phys. Rev. Lett. 2004;93:116401. doi: 10.1103/PhysRevLett.93.116401. [DOI] [PubMed] [Google Scholar]
- 61.Ismail-Beigi S, Louie SG. Excited-state forces within a first-principles Green’s function formalism. Phys. Rev. Lett. 2003;90:076401. doi: 10.1103/PhysRevLett.90.076401. [DOI] [PubMed] [Google Scholar]
- 62.Ismail-Beigi S, Louie SG. Self-trapped excitons in silicon dioxide: mechanism and properties. Phys. Rev. Lett. 2005;95:156401. doi: 10.1103/PhysRevLett.95.156401. [DOI] [PubMed] [Google Scholar]
- 63.Cheng Y-C, Engel GS, Fleming GR. Elucidation of population and coherence dynamics using cross-peaks in two-dimensional electronic spectroscopy. Chem. Phys. 2007;341:285–295. doi: 10.1016/j.chemphys.2007.07.049. [DOI] [Google Scholar]
- 64.Cheng Y-C, Fleming GR. Coherence quantum beats in two-dimensional electronic spectroscopy. J. Phys. Chem. A. 2008;112:4254–4260. doi: 10.1021/jp7107889. [DOI] [PubMed] [Google Scholar]
- 65.Hsu C-P, Fleming GR, Head-Gordon M, Head-Gordon T. Excitation energy transfer in condensed media. J. Chem. Phys. 2001;114:3065–3072. doi: 10.1063/1.1338531. [DOI] [Google Scholar]
- 66.Muñoz-Losa A, Curutchet C, Galván IF, Mennucci B. Quantum mechanical methods applied to excitation energy transfer: a comparative analysis on excitation energies and electronic couplings. J. Chem. Phys. 2008;129:034104. doi: 10.1063/1.2953716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available from the corresponding author on request.


