Highlights
-
•
This is the first study to assess aPKCλ/ι expression in primary prostate cancer with metastatic disease.
-
•
A total of 43 patients with prostate cancer and its metastasis to the lymph node and/or bone were analyzed in this study.
-
•
We found no strong association between aPKCλ/ι expression and the prognosis of the patients.
Keywords: Prostate cancer, aPKC, Hormonal therapy
1. Introduction
Androgen deprivation is a major therapeutic option for the treatment of advanced/metastatic prostate cancer, however, most responders eventually develop resistance to this therapy. Second-line systemic treatments, including new types of androgen receptor signaling inhibitors, glucocorticoids, and cytotoxic agents, have been shown to have a survival benefit in patients with castration-resistant prostate cancer (CRPC); however, the efficacy of these drugs is often short-lived [1], [2], [3]. Thus, new therapeutic targets and clinical markers are urgently required.
The atypical protein kinase C λ/ι (aPKCλ/ι) is involved in several signal transduction pathways and the establishment of epithelial cell polarity [4]. Previous studies have suggested that the deregulation of aPKCλ/ι is associated with the pathogenesis and progression of various types of neoplasms [5], [6], [7]. Recently, the overexpression of aPKCλ/ι and its gene amplification have been found in lung and ovarian cancers [4], [8], [9], [10]. In addition, a higher aPKCλ/ι expression has been shown to correlate with poorer outcomes in patients with metastatic prostate cancer [11]. The present study performed immunohistochemical analyses of aPKCλ/ι in initially metastatic prostate cancer to reveal the impact of aPKCλ/ι expression on the prognosis in initially advanced prostate cancer.
2. Case presentation
A total of 43 patients with prostate cancer and associated metastasis to the lymph node and/or bone were analyzed in this study. This study was approved by the Yokohama City University Hospital Institutional Review Board and written informed consent was obtained from all enrolled patients. We performed immunohistochemistry in prostate biopsy specimens using a primary antibody raised against aPKCι (dilution 1:50, BD Biosciences, San Jose, CA, USA), as previously described [12]. The Kaplan-Meier product limit estimator was used to estimate the cancer-specific survival (CSS). The survival duration was defined as the time between the pathological diagnosis and death. The results were compared using a log-rank test. P values of <0.05 were considered to indicate statistical significance. We adhered to the PROCESS criteria for this study [13], [14].
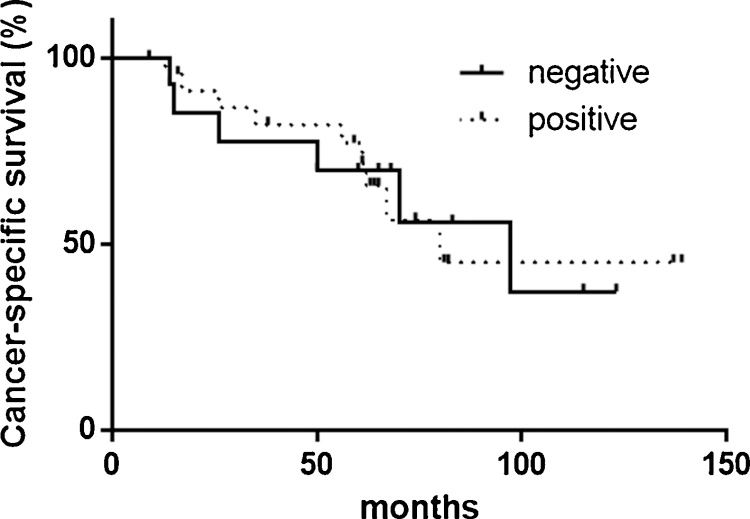
Positive signals for aPKC were detected in both the nuclei and cytoplasm of epithelial/carcinoma cells. Because of higher expression of aPKCλ/ι, we evaluated the nuclear expression in our analysis. Overall, aPKCλ/ιwas positive in 32 (74.4%) of 43 prostate cancer specimens. [Fig. 1] In 25 (78.1%) of 32 aPKCλ/ι-positive cases, similar levels of its expression were seen in non-neoplastic epithelial cells. There were no significant correlations between the aPKCλ/ι expression and CSS or in the clinicopathological features, including the Gleason score, pT stage, and the metastatic site. [Fig. 2] We previously reported that aPKCλ/ι was highly expressed in CRPCs in comparison to tumors that had no undergone androgen deprivation therapy [15], but the current staining did not reveal a significant correlation between the aPKCλ/ι expression and CSS.
Fig. 1.
Immunohistochemical staining of aPKCλ/ι. (a: Nuclear, b: Cytoplasmic, c: Nuclear and Cytoplasic, expression).
Fig. 2.
The CSS of patients with metastatic prostate cancer according to the aPKCλ/ι expression in primary tumors.
3. Discussion
This study is a first study to investigate the aPKCλ/ι expression in metastatic hormone sensitive prostate cancer. The current study is associated with a limitation regarding its small sample size. As a result, we could not definitively confirm the lack of any association between aPKCλ/ι expression in the initial biopsy specimens and the prognosis. aPKCλ/ι might contribute to tumor progression, such as the transition to CRPC rather than the aggressiveness of hormone-naive cancer. In summary, this is the first study to assess the aPKCλ/ι expression in primary prostate cancer with metastatic disease. We found no strong association between the aPKCλ/ι expression and the prognosis of these patients.
Conflicts of interest
We declare no conflicts of interest.
Funding
KAKENHI grants (16K20152) from the Ministry of Education, Culture, Sports, Science and Technology of Japan were provided to T.K.
Ethical approval
Institutional review board of Yokohama City University Medical Center approved this study (D1507018).
Consent
We obtained written informed consent for publication. Institutional review board of Yokohama City University Medical Center approved this study (D1507018).
Author contribution
YY and TK wrote the manuscript.
YY YN HI IK HM performed the operation.
MY, HU wrote and checked the manuscript.
Guarantor
Takashi Kawahara.
Acknowledgement
This study was in part supported by the JST KAKENHI (No. 16K20152 for TK, 26460456 for YN, 26670709 for HI).
References
- 1.Singer E.A., Golijanin D.J., Miyamoto H., Messing E.M. Androgen deprivation therapy for prostate cancer. Expert Opin. Pharmacother. 2008;9(2):211–228. doi: 10.1517/14656566.9.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Crawford E.D., Higano C.S., Shore N.D., Hussain M., Petrylak D.P. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J. Urol. 2015;194(6):1537–1547. doi: 10.1016/j.juro.2015.06.106. [DOI] [PubMed] [Google Scholar]
- 3.Kawahara T., Miyoshi Y., Sekiguchi Z., Sano F., Hayashi N., Teranishi J., Misaki H., Noguchi K., Kubota Y., Uemura H. Risk factors for metastatic castration-resistant prostate cancer (CRPC) predict long-term treatment with docetaxel. PLoS One. 2012;7(10):e48186. doi: 10.1371/journal.pone.0048186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima Y., Akimoto K., Nagashima Y., Ishiguro H., Shirai S., Chishima T., Ichikawa Y., Ishikawa T., Sasaki T., Kubota Y. The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum. Pathol. 2008;39(6):824–831. doi: 10.1016/j.humpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa Y., Nagashima Y., Morioka K., Akimoto K., Kojima Y., Ishikawa T., Goto A., Kobayashi N., Watanabe K., Ota M. Colorectal laterally spreading tumors show characteristic expression of cell polarity factors, including atypical protein kinase C lambda/iota, E-cadherin, beta-catenin and basement membrane component. Oncol. Lett. 2014;8(3):977–984. doi: 10.3892/ol.2014.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields A.P., Frederick L.A., Regala R.P. Targeting the oncogenic protein kinase Ciota signalling pathway for the treatment of cancer. Biochem. Soc. Trans. 2007;35(Pt 5):996–1000. doi: 10.1042/BST0350996. [DOI] [PubMed] [Google Scholar]
- 7.Mizushima T., Asai-Sato M., Akimoto K., Nagashima Y., Taguri M., Sasaki K., Nakaya M.A., Asano R., Tokinaga A., Kiyono T. Aberrant expression of the cell polarity regulator aPKClambda/iota is associated with disease progression in cervical intraepithelial neoplasia (CIN): a possible marker for predicting CIN prognosis. Int. J. Gynecol. Pathol. 2016;35(2):106–117. doi: 10.1097/PGP.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 8.Regala R.P., Weems C., Jamieson L., Copland J.A., Thompson E.A., Fields A.P. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J. Biol. Chem. 2005;280(35):31109–31115. doi: 10.1074/jbc.M505402200. [DOI] [PubMed] [Google Scholar]
- 9.Regala R.P., Weems C., Jamieson L., Khoor A., Edell E.S., Lohse C.M., Fields A.P. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65(19):8905–8911. doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- 10.Eder A.M., Sui X., Rosen D.G., Nolden L.K., Cheng K.W., Lahad J.P., Kango-Singh M., Lu K.H., Warneke C.L., Atkinson E.N. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc. Natl. Acad. Sci. U. S. A. 2005;102(35):12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshihama Y., Izumisawa Y., Akimoto K., Satoh Y., Mizushima T., Satoh K., Chida K., Takagawa R., Akiyama H., Ichikawa Y. High expression of KIBRA in low atypical protein kinase C-expressing gastric cancer correlates with lymphatic invasion and poor prognosis. Cancer Sci. 2013;104(2):259–265. doi: 10.1111/cas.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawahara T., Kashiwagi E., Ide H., Li Y., Zheng Y., Ishiguro H., Miyamoto H. The role of NFATc1 in prostate cancer progression: cyclosporine A and tacrolimus inhibit cell proliferation, migration, and invasion. Prostate. 2015;75(6):573–584. doi: 10.1002/pros.22937. [DOI] [PubMed] [Google Scholar]
- 13.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P., Group S. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill D.P. Group P: preferred reporting of case series in surgery; the PROCESS guidelines. Int. J. Surg. 2016;36(Pt A):319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Ishiguro H., Akimoto K., Nagashima Y., Kojima Y., Sasaki T., Ishiguro-Imagawa Y., Nakaigawa N., Ohno S., Kubota Y., Uemura H. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]