Abstract
Background
Hair follicles are known to contain a well-characterized niche for adult stem cells: the bulge, which contains epithelial and melanocytic stem cells. Stem cells in the hair bulge, a clearly demarcated structure within the lower permanent portion of hair follicles, can generate the interfollicular epidermis, hair follicle structures, and sebaceous glands. The bulge epithelial stem cells can also reconstitute in an artificial in vivo system to a new hair follicle.
Methods
In this study, we have developed a new method to isolate human adult stem cells by mechanical centrifugation of punch biopsy from human hair follicles without culture condition. Here, we used human follicle stem cells (HFSCs), to improve the hair density in 11 patients (38 to 61 years old) affected by AGA in stage 3–5 as determined by the Norwood-Hamilton classification scale.
Results
The primary outcomes were microscopic identification and counting of HFSCs. The secondary outcomes were clinical preliminary results and safety and feasibility in HFSCs-treated scalp. Each scalp tissue suspension contained about 3,728.5±664.5 cells. The percentage of hair follicle-derived mesenchymal stem cells CD44+ [from dermal papilla (DP)] was about 5%+0.7% whereas the percentage of hair follicle epithelial stem cells CD200+ (from the bulge) was about 2.6%+0.3%. In total, 23 weeks after the last treatment with HFSCs mean hair count and hair density increases over baseline values. In particular, a 29%±5% increase in hair density for the treated area and less than a 1% increase in hair density for the placebo area.
Conclusions
We have shown that the isolated cells are capable to improve the hair density in patients affected by androgenetic alopecia (AGA). These cells appear to be located in the bulge area of human.
Keywords: Human hair follicles stem cells, hair follicle stem cells (HFSCs), stem cells in hair loss, alopecia, androgenetic alopecia (AGA), hair loss
Introduction
Eighty percent of Caucasian men experience some degree of androgenetic alopecia (AGA) before age 70 (1). Current legitimate treatments for AGA include finasteride, minoxidil, and hair transplantation (2). The role of platelet rich plasma has been demonstrated in recent reports (3,4).
In AGA, the follicle miniaturization is accompanied by a decrease of anagen, with an increase in the percentage of resting (telogen) hair follicles containing microscopic hairs in bald scalp (5). In addition to these intrinsic changes to the hair follicle, infiltrating lymphocytes and mast cells have been identified around the miniaturizing follicle (6), especially in the area of the stem cell-rich bulge area (7). In balding scalp, the number of hair follicle stem cells (HFSCs) remains intact, whereas the number of more actively proliferating progenitor cells markedly decreases (8). This suggests that balding scalp either lacks an activator or has an inhibitor of hair follicle growth.
Here, we used HFSCs, obtained by mechanical centrifugation of scalp’s punch biopsy, to improve the hair density in 11 patients (38 to 61 years old) affected by AGA.
The study protocol complied with the Declaration of Helsinki, the European regulations and all patients provided written informed consent before participating in the study.
Current regulations
In order to understand the sense of the current European regulations it is necessary to differentiate between “minimal manipulation” and advanced cell therapy performed by “extensive manipulation, which involves complex techniques of bioprocessing of therapeutic cells.
Reference is made to the Regulation n.1394/2007 of the European Parliament (EC) and of the Council 13 November 2007 on medicines for advanced therapies, where the definition of ‘bioprocess engineering products’ is given. Here it is specifically said that this definition excludes those products that contain, or are made exclusively of, cells and non-vital human or animal tissues and that do not have pharmacological, immunological or metabolic action. Included among the advanced therapy pharmaceutical products are those used for gene and somatic cell therapy [Directive 2001/83/(EC), European Community, Annex I]. Cells and tissues are to be considered products of bioprocess engineering if they undergo ‘considerable manipulation’.
The same regulation defines the difference between extensive and minimum manipulation, and lists, which are considered relevant, or not.
Manipulations that are not considered “bioprocess engineering” are: cutting, grinding, shaping, sterilization, centrifugation, soaking in antibiotic or antimicrobial solutions, sterilization, irradiation, separation, concentration or purification, filtration, lyophilisation, freezing, cryopreservation and nitrification.
The extensive manipulation of cells and tissues is a process that may lead to cell activation and/or a stimulation of cell proliferation and these are also considered “extensively manipulated” cells that, although not specifically activated or stimulated to proliferate, are associated with biomaterials.
All cells that have undergone a manipulation of their genes are considered to be “extensively manipulated”.
According to reflection paper on classification of advanced therapy medicinal products draft agreed, 20 June 2014 EMA/CAT/600280/2010 Rev 1, Committe for Advanced Therapies (CAT), Line 10 “The same essential function for a cell population means that the cells when removed from their original environment in the human body are used to maintain the original function in the same anatomical or histological environment”, the authors resume that autologous use in one step surgery, minimal manipulation, monofunctional use “used for the same essential function in the recipient as in the donor”, manipulation with devices in aseptic conditions, are conditions that do not require Good Manufacturing Practices (GMP) rules for processing, Good Clinical Practices (GCP) for the clinical application and Ethical Committee approval.
Methods
Patients
This study enrolled male patients who displayed AGA in stage 3–5 as determined by the Norwood-Hamilton classification scale. Additional exclusion factors were set based on systemic and local criteria. Specifically, systemic criteria for exclusion included evidence of sepsis, immunosuppression and cancer, as well as use of pharmacological therapeutics targeting AGA (i.e., finasteride, dutasteride, or antiandrogens) in the previous 12 months. Localized exclusion criteria included use of topical treatments for AGA (i.e., minoxidil, prostaglandin analogs, retinoids, or corticosteroids) in the previous 12 months and withdrawal of informed consent.
AGA diagnoses were established on the basis of a detailed medical history (i.e., screening for drugs linked to hair loss), clinical examination, and trichoscopic features (i.e., >20% variability in hair diameter between affected and unaffected areas). Patients were clinically diagnosed with AGA upon presentation of an increase in miniaturized terminal hair and/or a reduced number of hairs on physical examination and phototrichograms, along with negative hair pull tests. Laboratory tests were performed to exclude alternative causes of hair loss, such as poor nutrition, anemia, thyroid dysfunction, and syphilis. Urinalysis was used to detect levels of 17-idrocorticosteroid, 17-ketosteroid, dehydroepiandrosterone, free cortisol, pregnanetriol, and testosterone in all participants. Finally, circulating levels of cortisol, dihydrotestosterone, DHEA, D4-androstenedione, 17-hydroxyprogesterone, 3-α-diol glucuronide, prolactin, and gonadotropins were measured on all participants.
Human autologous hair follicle suspension procedure and preparation
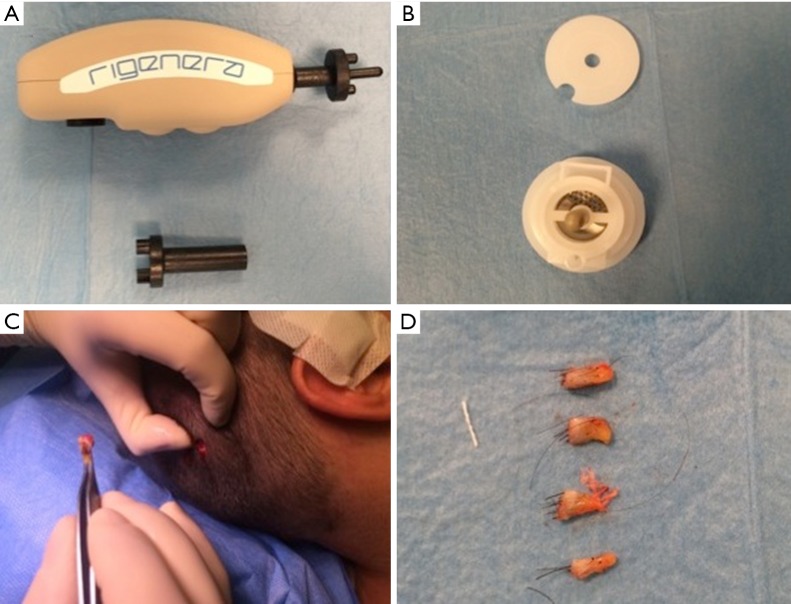
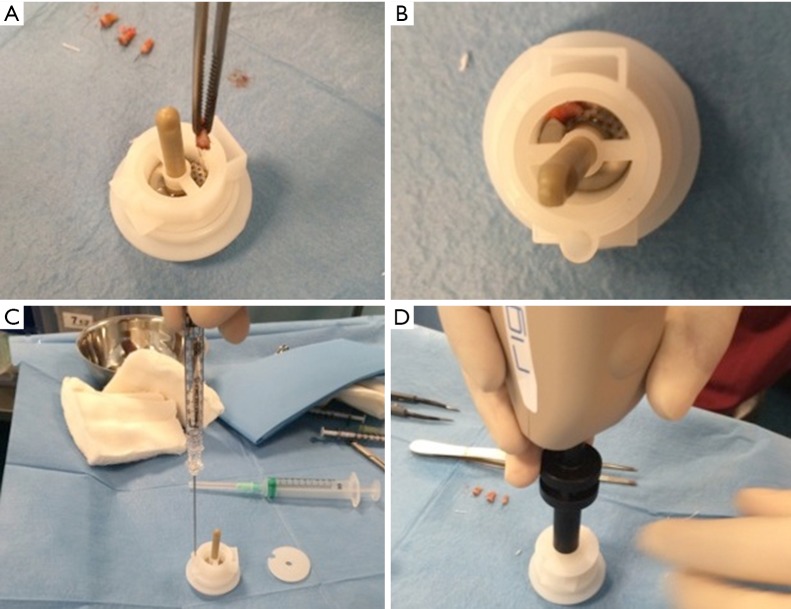
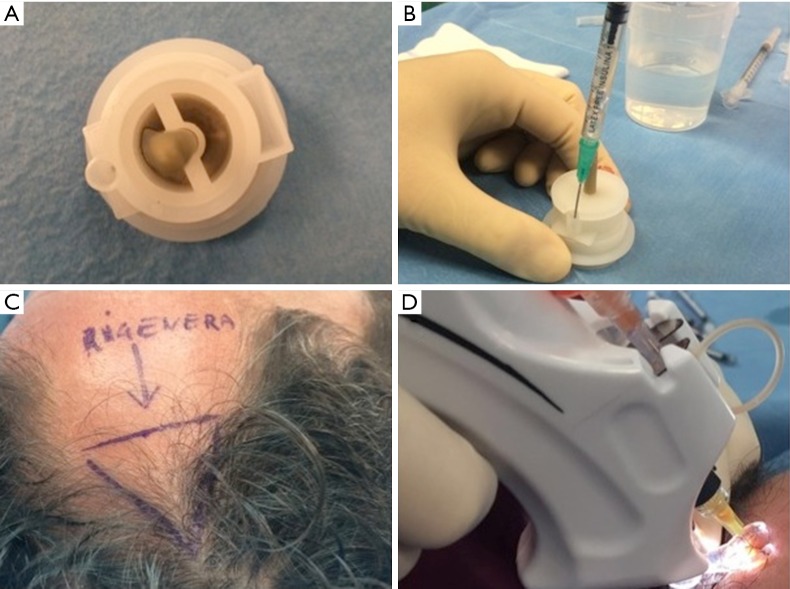
Autologous suspension of HFSCs for immediate clinical use were prepared using an innovative medical device called Rigeneracons (CE certified class I, HBW srl; Turin, Italy) (Figure 1A,B). After the extraction of the scalp tissues during punch biopsy (Figure 1C), the authors cut the scalp tissues into the strips (2 mm × 2 mm) (Figure 1D) eliminating the excess adipose tissue. The strips were gently collected and disaggregated under sterile conditions (vertical laminar flow hood) by Rigeneracons (Figure 2A,B) in 1.2 mL of physiologic solution [NaCl 0,9% (mE/mL: Na+ 0.154; Cl− 0.154); mOsm/L 308, pH 4.5–7.0] (Figure 2C). After 60 seconds of centrifugation at 80 RPM per minute (Figure 2D), the cell suspension was collected from the system (Figure 3A,B) and mechanically infiltrated into the scalp of the patients affected by AGA (Figure 3C,D). In addition, the cell suspension obtained was cultured and subsequently characterized by cytospin and immunocytochemistry to identify the HFSCs.
Figure 1.
Rigenera procedure phase 1 (punch biopsy and cutting of scale tissues). (A) Rigenera Securdrill Device; (B) Rigeneracons kit; (C) the extraction of the scalp tissues during punch biopsy; (D) the authors cut the scalp tissues into the strips (2 mm × 2 mm).
Figure 2.
Rigenera procedure phase 2 (positioning of scalp tissue in Rigeneracons and centrifugation). (A) The strips collected into Rigeneracons; (B) detail of Rigeneracons containing one strip; (C) the addition of 1.2 mL of physiologic solution; (D) centrifugation at 80 RPM with Rigenera Securdrill device for 60 seconds.
Figure 3.
Rigenera procedure phase 3 (cell suspension contained in Rigeneracons and infiltration). (A) The cell suspension obtained by the system contained in Rigeneracons; (B) harvesting of cell suspension; (C) the selected area of the scalp treated; (D) mechanical and controlled infiltration performed by Ultim Gun.
The aim was to disaggregate a small piece of scalp tissue and opportunely select a cell population with a size of 50 µm.
Human autologous hair follicle suspension protocol and injection
For each patient, the scalp affected by hair loss was divided into four areas (frontal, parietal, vertex, and occipital); local anesthesia was not injected in the treated areas. Interfollicular HFSCs injections (0.2 mL·cm2) were administered to select areas of the scalp at a depth of 5 mm using an Ultim gun (Anti-Aging Medical Systems, Montrodat, France) equipped with a 30-gauge (Figure 3D), 1 mL Luer lock syringe in two sessions spaced 60 days apart.
In patients with hair loss localized to the frontal and parietal regions, HFSCs injections were delivered exclusively to the frontal scalp while placebo injections (i.e., physiological saline) were injected in the parietal regions. Likewise, for patients with hair loss limited to the parietal and vertex regions, HFSCs was injected in the parietal region, and placebo was injected in the vertex region of the scalp. Equivalent numbers of autologous HFSCs and placebo injections were made.
Assessment of hair growth and clinical evaluation
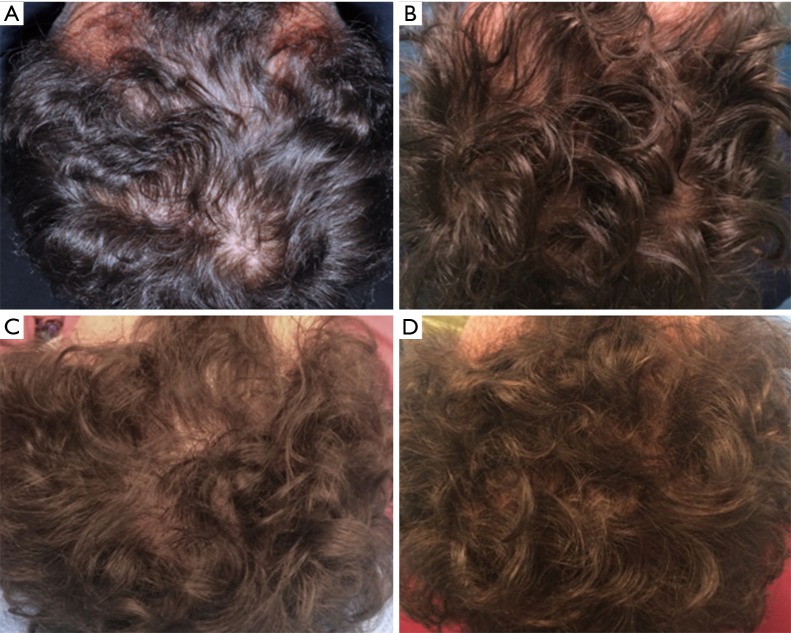
All patients were evaluated in four stages: T0, beginning of study (Figure 4A); T1 in 3 weeks (Figure 4B); T2, in 9 weeks (Figure 4C); T3, in 16weeks and T4 in 23 weeks after the last treatment (Figure 4D). The hair growth evaluated after the last treatment was compared by photography with the baseline evaluation made before treatments and between the HFSCs treatment area and the control area, which received placebo injections. Photographs of the areas of a sample scalp treated with HFSCs are shown in Figures 3C,5A. The effects of HFSCs and placebo treatments on hair growth were assessed in all patients with the help of global photography (Figure 5B), physician’s and patient’s global assessment scale. In all patients, two translational areas of hair loss, one at the border of the treatment half and a second along the border of the placebo half, were demarcated with a semi-permanent tattoo.
Figure 4.
A smoker 45-year-old male patient affected by androgenetic alopecia classified AGA 3 according to Nordwood-Hamilton scale. (A) Preoperative situation at T0 of the scalp with hair loss localized to the parietal, temporal and frontal areas; (B) postoperative situation of the scalp at T1 after 3 weeks from the last treatment; (C) postoperative situation of the scalp at T2, after 9 weeks; (D) postoperative situation of the scalp at T4 after 23 weeks later the last treatment with increase of hair density.
Figure 5.
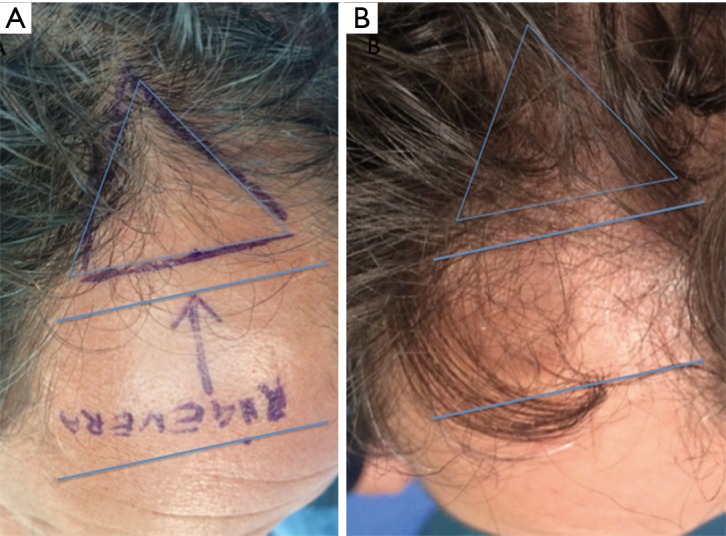
Detail of temporal right area of male patient affected by androgenetic alopecia classified AGA 3 according to Nordwood-Hamilton scale. (A) Preoperative situation at T0 of the scalp with hair loss localized to the right temporal and frontal area identified by blue lines; (B) postoperative situation of the scalp in the same area at T4 after 23 weeks later the last treatment with increase of hair density.
Cytospin and immunocytochemistry procedures
Eleven samples of HFSCs suspension were analyzed in the Anatomic Pathology Institute of Tor Vergata University. Scalp tissue suspensions, fixed with 4% paraformaldehyde, were characterized for mesenchymal and epithelial stem cells markers, such as CD44 (9) and CD200 (10), respectively. After cell adhesion on a glass slide by cytospin, immunocytochemistry was performed with specific primary antibodies (CD44 sc-9960, 1:10; CD200 ab203887, 1:100).
Results
The primary outcomes were microscopic identification and counting of HFSCs. The secondary outcomes were clinical preliminary results and safety and feasibility in HFSCs-treated scalp.
Microscopic identification and counting of HFSCs
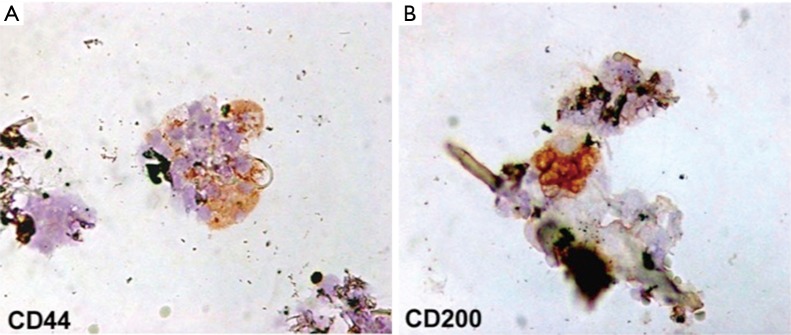
Each scalp tissue suspension contained about 3,728.5±664.5 cells. The percentage of hair follicle-derived mesenchymal stem cells CD44+ [from dermal papilla (DP)] was about 5%+0.7% (Figure 6A) whereas the percentage of hair follicle epithelial stem cells CD200+ (from the bulge) was about 2.6%+0.3% (Figure 6B). Positive cells were counted in the total area under a light microscope at 400× magnification (Eclipse E600, Nikon, Japan) and microphotographs captured by DXM1200F Digital camera (Nikon) using ACT-1 software (Nikon). The remaining cells were mainly represented by S100+ dermal fibroblasts (>85%) and epidermal cells (epithelial cells and melanocytes <10%) recognizable by their characteristic morphological aspects (data not shown).
Figure 6.
Immunophenotypic characterization of hair follicle stem cells in human scalp tissue suspension. Immmunocytochemistry for CD44 and CD200 stem cell markers. (A) hair follicle-derived mesenchymal stem cells; (B) hair follicle epithelial stem cells. Original magnification 400×.
Clinical results
In total, 23 weeks after the last treatment with HFSCs mean hair count and hair density increases (Figure 4D) over baseline values (Figure 4A). In particular, a 29%±5% increase in hair density for the treated area and less than a 1% increase in hair density for the placebo area. At the baseline, no statistical differences in hair count or hair density existed between the HFSCs treatment area and control area of the scalp.
In this preliminary report, we showed the clinical effect of the injection of scalp tissue suspension. However, we hypothesize that stem cells can improve the formation of new follicles, but this hypothesis must be demonstrated in a following study.
Discussion
The reconstitution of a fully organized and functional hair follicle from dissociated cells propagated under defined tissue culture conditions is a challenge still pending in tissue engineering (11).
It is then of great interest to find different strategies aiming to regenerate or neogenerate the hair follicle under conditions proper of an adult individual. Based upon current knowledge on the epithelial and dermal cells and their interactions during the embryonic hair generation and adult hair cycling, many researchers have tried to obtain mature hair follicles using different strategies and approaches depending on the causes of hair loss (11).
In this preliminary study, the authors have developed a new method to isolate human adult stem cells by mechanical centrifugation of punch biopsy from human hair follicles without culture condition, and they reported for the first time, up to our knowledge, the counting of these cells and the preliminary results obtained by the human follicle stem cells injections in the scalp of patients affected by AGA, improving hair density.
In particular, the authors reported the percentage of hair follicle-derived mesenchymal stem cells CD44+, from DP, and the percentage of hair follicle epithelial stem cells CD200+, from the bulge.
The authors, now, feel the necessity discuss as follow, current advances in the different experimental strategies to regenerate or neogenerate hair follicles, with emphasis on those involving neogenesis of hair follicles in adult individuals using isolated cells and tissue engineering. Most of these experiments were performed using rodent cells, particularly from embryonic or newborn origin. However, no successful strategy to generate human hair follicles from adult cells has yet been reported. Perhaps the most important challenge is to provide three-dimensional culture conditions mimicking the structure of living tissue. Improving culture conditions that allow the expansion of specific cells while protecting their inductive properties, as well as methods for selecting populations of epithelial stem cells, should give us the necessary tools to overcome the difficulties that constrain human hair follicle neogenesis (11).
These cells appear to be located in the bulge area of human hair follicles. Hair follicles are known to contain a well-characterized niche for adult stem cells: the bulge, which contains epithelial and melanocytic stem cells (12). Stem cells in the hair bulge, a clearly demarcated structure within the lower permanent portion of hair follicles, can generate the interfollicular epidermis, hair follicle structures, and sebaceous glands (7,13). The bulge epithelial stem cells can also reconstitute in an artificial in vivo system to a new hair follicle (14,15).
The study published by Yu et al. (12) showed for the first time that human hair follicles also contain a stem cell population that can be differentiated into neuron, smooth muscle cell, and melanocyte lineages in induction medium. In addition, their data demonstrate that Oct4-positive cells are present in human skin, and most of them are located in the hair follicles in vivo. Oct4 belongs to the family of POU-domain transcription factors that are normally expressed in pluripotent cells of the developing embryo and mediate pluripotency (16).
It is possible that these Oct4-positive cells in the hair follicles are related to these pluripotent stem cells that can perceivably give rise to follicular melanoblasts, Merkel cells, and other cells. These stem cells might generate diverse cell types during tissue renewal or repair in response to environmental cues.
More research is warranted to further characterize these stem cells in the hair follicles. The hair bulge is a stem cell niche, which can be highlighted by K15 staining. Again, Yu et al. (12) demonstrated that most of the Oct4-positive cells in human skin are located in the areas highlighted by K15 staining in vivo, suggesting that these stem cells are located in the bulge area, an area that provides a unique differentiation-restricted environment for adult stem cells. In conclusion, their data indicate that human hair follicles contain multipotent stem cells other than epithelial and melanocytic stem cells, and these cells are located in the bulge area. These cells show promising plasticity in ex vivo and in vitro conditions, making them potential candidates for cell engineering and cell replacement therapies.
Human scalp tissues are easily accessible, and the fact that hair spheres can be generated from autologous adult tissue makes it an attractive source for individualized cell-based therapies.
Each mature hair follicle is a regenerating system, which physiologically undergoes cycles of growth (anagen), regression (catagen), and rest (telogen) numerous times in adult life (17). In catagen, HFSCs are maintained in the bulge. Then, the resting follicle re-enters anagen (regeneration) when proper molecular signals are provided. During late telogen to early anagen transition, signals from the DP stimulate the hair germ and quiescent bulge stem cells to become activated (18). Many paracrine factors are involved in this crosstalk at different hair cycle stages and some signaling pathways have been implicated (19-21). In anagen, stem cells in the bulge give rise to hair germs, then the transient amplifying cells in the matrix of the new follicle proliferate rapidly to form a new hair filament (22).
However, the cell dynamics in this process is less clear than in the physiological renewal and further studies are required to understand this process.
When the cellular niches are completely lost, it is necessary to generate a completely new hair follicle in a process called hair follicle neogenesis.
Based on the knowledge on the epithelial and dermal cells, and their interactions, during the embryonic hair generation and adult hair cycling, different experimental approaches have been designed to regenerate hair follicles or generate new ones by the neogenesis process. These hair regeneration and neogenesis attempts can be classified into four categories: (I) reversion of pathological intra- and/or extra-follicular environment, for instance AGA; (II) regeneration of complete hair follicles from the recombination of hair follicle parts; (III) neogenesis of hair follicles from isolated cells; and (IV) neogenesis of hair follicles by tissue engineering.
Regeneration of hair follicles was also observed in humans (23) when dermal sheath tissue was used, which was sufficient to regenerate also the DP structure. After implantation, the whisker DP was capable of inducing hair follicle regeneration retaining the information to determine hair fiber type and follicle size (24).
Grafting of dermal-inductive tissue was limited by the fact that it was not possible to generate more hair follicles than the obtained from the donor tissues. To overcome this limitation different approaches and experimental models using freshly or cultured isolated cells from both dermal and dermal/epidermal origin were tested. Most of them involved neonatal and embryonic murine cells.
In recent study published in 2015 by Balañá et al. (11) the authors prepared in a laboratory a dermal-epidermal skin substitute by seeding an acellular dermal matrix with cultured hair follicle epithelial stem cells and dermal papillar cells (DPCs), both obtained from adult human scalp. These constructs were grafted into a full-thickness wound generated on nude mice skin. In fourteen days, histological structures reminiscent of many different stages of embryonic hair follicle development were observed in the grafted area. These structures showed concentric cellular layers of human origin, and expressed k6hf, a keratin present in epithelial cells of the companion layer. Although the presence of fully mature hair follicles was not observed, these results showed that both epithelial and dermal cultured cells from adult human scalp in a dermal scaffold were able to produce in vivo structures that recapitulate embryonic hair development.
The analysis of all these studies could lead to the conclusion that hair follicle neogenesis using human epithelial and dermal cells is a very difficult task that could requires special culture conditions, somehow recreating the normal or embryonic skin environment, and the use of embryonic or neonatal cells.
Really, in more of 50 years, great progress was reported, starting from early 60s, to arrive now to april 2017, in which, contrary to what appeared to emerge from previous studies, we have reported the last clinical advancement in the possibility to use human follicle stem cells obtained by mechanical centrifugation, respecting the European rules, without culture or use of enzymes, for AGA treatment.
Conclusions
Our preliminary data suggest that the injection of HFSCs preparations has a positive therapeutic effect on male androgenic alopecia without major side effects. Therefore, the authors recommend future study and clinical trials incorporate more data about the use of HFSCs.
Acknowledgements
None.
Ethical Statement: The Ethical Committee approval is not required and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Alsantali A, Shapiro J. Androgens and hair loss. Curr Opin Endocrinol Diabetes Obes 2009;16:246-53. 10.1097/MED.0b013e32832b100a [DOI] [PubMed] [Google Scholar]
- 2.Price VH. Treatment of hair loss. N Engl J Med 1999;341:964-73. 10.1056/NEJM199909233411307 [DOI] [PubMed] [Google Scholar]
- 3.Gentile P, Garcovich S, Bielli A, et al. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl Med 2015;4:1317-23. 10.5966/sctm.2015-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentile P, Cole JP, Cole MA, et al. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int J Mol Sci 2017;18(2). pii: E408. 10.3390/ijms18020408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med 1999;341:491-7. 10.1056/NEJM199908123410706 [DOI] [PubMed] [Google Scholar]
- 6.Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: Implications for pathogenesis. Br J Dermatol 1992;127:239-46. 10.1111/j.1365-2133.1992.tb00121.x [DOI] [PubMed] [Google Scholar]
- 7.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilo-sebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990;61:1329-37. 10.1016/0092-8674(90)90696-C [DOI] [PubMed] [Google Scholar]
- 8.Garza LA, Yang CC, Zhao T, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest 2011;121:613-22. 10.1172/JCI44478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Wang Y, Gao Y, et al. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int J Mol Med 2013;31:913-21. [DOI] [PubMed] [Google Scholar]
- 10.Ohyama M, Terunuma A, Tock CL, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 2006;116:249-60. 10.1172/JCI26043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balañá ME, Charreau HE, Leirós GJ. Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J Stem Cells 2015;7:711-27. 10.4252/wjsc.v7.i4.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Fang D, Kumar SM, et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol 2006;168:1879-88. 10.2353/ajpath.2006.051170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science 2004;303:359-63. 10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 2004;22:411-7. 10.1038/nbt950 [DOI] [PubMed] [Google Scholar]
- 15.Taylor G, Lehrer MS, Jensen PJ, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000;102:451-61. 10.1016/S0092-8674(00)00050-7 [DOI] [PubMed] [Google Scholar]
- 16.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells 2001;19:271-8. 10.1634/stemcells.19-4-271 [DOI] [PubMed] [Google Scholar]
- 17.Alonso L, Fuchs E. The hair cycle. J Cell Sci 2006;119:391-3. 10.1242/jcs.02793 [DOI] [PubMed] [Google Scholar]
- 18.Greco V, Chen T, Rendl M. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009;4:155-69. 10.1016/j.stem.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanpain C, Lowry WE, Geoghegan A, et al. Self- renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004;118:635-48. 10.1016/j.cell.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 20.Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc 2003;8:46-55. 10.1046/j.1523-1747.2003.12171.x [DOI] [PubMed] [Google Scholar]
- 21.Roh C, Tao Q, Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics 2004;19:207-17. 10.1152/physiolgenomics.00134.2004 [DOI] [PubMed] [Google Scholar]
- 22.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011;144:92-105. 10.1016/j.cell.2010.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, et al. Trans-gender induction of hair follicles. Nature 1999;402:33-4. 10.1038/46938 [DOI] [PubMed] [Google Scholar]
- 24.Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa- type fibres are specified. Development 1992;115:1103-9. [DOI] [PubMed] [Google Scholar]