Abstract
Malignant pleural mesothelioma (MPM) is an aggressive neoplasm with poor outcome. Novel radical radiation techniques using intensity modulated radiation therapy (IMRT) have become an important component of therapy in mesothelioma. Immunotherapy also provides new therapeutic options. However, how best to integrate immunotherapy with standard therapy such as radiation, chemotherapy and surgery remains unknown. A change of paradigm from adjuvant normofractionation to induction accelerated hypofractionated hemithoracic radiation could provide a platform to combine immunotherapy due to the potential benefit of short course high dose radiation on the immune system. Immunotherapy can also be combined with chemotherapy. Although chemotherapy is generally considered immunosuppressive, some chemotherapeutic agents do induce cell death that can be immunogenic and stimulate a specific immune response against the tumor. Immunotherapy could also be used in between cycles of chemotherapy to limit tumor cell repopulation and optimize the results of both treatments. The integration of immunotherapy into a multimodality approach is opening new avenue of treatment for mesothelioma.
Keywords: Malignant pleural mesothelioma (MPM), surgery, radiotherapy, chemotherapy, immunotherapy
Introduction
Malignant pleural mesothelioma (MPM) has been well-documented to be a long-term complication of asbestos exposure with an increasing incidence in industrialized countries (1). MPM has a dismal prognosis with a medium survival of 12 to 18 months after diagnosis (2). The efficacy of current therapies including surgery, chemotherapy and radiation is limited. Therefore, a large number of studies have focused on multimodality approaches for the treatment of this disease (3,4). Nowadays, combination of multimodality treatment with immunotherapy has been evaluated in pre-clinical studies and is entering clinical trials (5-9).
Standard of care in MPM
The standard of care for MPM treatment consists in chemotherapy with cisplatin and pemetrexed as first line therapy in patients who are not surgical candidates. Cisplatin-pemetrexed was associated with an improvement in survival of 2.8 months compared to cisplatin alone in a large multicenter randomized trial (10). More recently, a large randomized trial conducted in France with 448 patients demonstrated that the addition of bevacizumab to cisplatin-pemetrexed provided an additional benefit of 2.7 months over cisplatin-pemetrexed and this triplet combination is now used as a possible first-line treatment for unresectable MPM in appropriately selected patients (11). In patients with early stage disease, radical surgery including extended pleurectomy-decortication (EPD) and extrapleural pneumonectomy (EPP) has demonstrated to be associated with longer survival when combined with other treatment modality such as chemotherapy and/or radiation therapy (12). EPP and EPD have been shown to be of greater benefit in patients with epithelial subtypes of mesothelioma compared to non-epithelial subtypes.
Radiation therapy in MPM
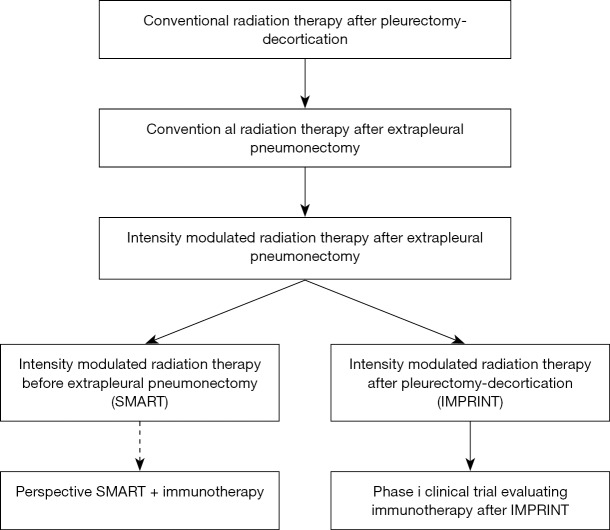
Radiation has been used for palliation, local prophylaxis of surgical port sites and for radical treatment. Radical radiation consists in radiation to the whole hemithorax to doses higher than 40 Gy (13,14). Radical radiation in mesothelioma has been reported for several decades, but it is only over the past 15 years with the introduction of intensity modulated radiation therapy (IMRT) that radical radiation has become an important modality to treat mesothelioma. Its role and optimal form of delivery is still in evolution. High dose hemithoracic IMRT was initially delivered after EPP (15,16). In recent years, experienced centers have expanded its application to hemithoracic IMRT after PD (IMPRINT) and to the preoperative setting with an accelerated course of hemithoracic radiation before EPP (SMART) (17,18). IMPRINT delivers 50.4 Gy of radiation to the entire hemithorax in 28 fractions. SMART consists in delivering 25 Gy in 5 fractions with a boost of 5 Gy to the area of gross disease detected on the CT scan or PET scan. A summary of the evolution of radical radiation for MPM is provided in Figure 1.
Figure 1.
Evolution of radical hemithoracic radiation therapy in mesothelioma.
Immunotherapy in mesothelioma
Immunotherapy has opened new options in the treatment of mesothelioma. Clinical trials with dendritic cells (DC) vaccination and live-attenuated Listeria vaccine have shown encouraging results and are being considered for multicenter phase II trials (19,20). Several trials targeting mesothelin have also been conducted or are ongoing with antibody-based therapeutic agents or T-cell therapies (21).
Immune check point blockade are currently being tested in multiple clinical trials in mesothelioma. The results of immune check point inhibitors as a single agent have so far not been as encouraging as in other malignancies such as melanoma and lung cancer. Part of the limitations of these immune check point inhibitors may relate to the fact that the mutation rate in mesothelioma tumor has remained limited despite the long term exposure to a carcinogenic substance like asbestos (22). Hence, in contrast to lung cancer and melanoma, neoantigen formation in mesothelioma is relatively low. A recent large international clinical trial assessing the role of CLTA-4 blockade as single agent for second line therapy in mesothelioma was negative (23). In single arm clinical trials, however, there has been some encouraging response with inhibitors of the PD1/PD-L1 pathway in mesothelioma. Pembrolizumab, a PD-1 inhibitor, for instance has been associated with a 20% radiological response rate based on modified RECIST criteria (24).
Immunotherapy as a standalone therapy may not be the optimal treatment for mesothelioma and integration of immunotherapy with chemotherapy, radiation therapy and surgery will potentially be an important component to get the optimal benefit from these new treatments. Clinical trials assessing the role of PD-1 blockade in combination with chemotherapy or with radiation have recently been opened. The Canadian Cancer Trials Group is conducting a phase II randomized trial comparing patients treated with pembrolizumab-cisplatin-pemetrexed to patients treated with cisplatin-pemetrexed or with pembrolizumab alone. The trial is accruing very well and is expected to be completed in 2019. MD Anderson Cancer Center recently opened a phase I trial to assess potential toxicity when combining pembrolizumab to radiation therapy. Two groups are being evaluated, a radical group treated with hemithoracic radiation with the lung in place using IMRT or proton therapy after at least two cycles of chemotherapy and possibly PD, and a palliative group treated with palliative radiation using different hypofractionated doses.
Radiation and immunotherapy in mesothelioma
Radiation has traditionally been delivered with normofractionation using 1.8–2 Gy per fraction. Over the past 20 years, improvement in radiation technology has allowed the safe delivery of higher dose of radiation per fraction. Hypofractionated radiation with doses ranging between 3 and 8 Gy per fraction and ablative radiation with doses greater than 8 Gy are now routinely delivered in clinical practice. Hypofractionated and ablative radiation provides the advantage to be a shorter course of treatment compared to normofractionation. Increasing evidence also suggests that these short courses of high dose radiation are immunogenic and potentially provide an excellent platform to be combined with immunotherapy (25). Hypofractionated radiation could lead to an equilibrium between the proliferation of malignant cells and the immune-mediated tumor cell death triggered by radiation, thus keeping the tumor in a dormancy state until the immune system becomes exhausted (26). Hence, the addition of immune check point blockade in combination with hypofractionated radiation may have a synergistic effect tilting the balance from an equilibrium state towards tumor elimination.
Considering the encouraging results we have had with our SMART approach in mesothelioma using an accelerated course of hypofractionated radiation before surgery, we questioned whether the benefit was related to the immune activation generated by the short course of radiation (18,27). We therefore established animal models of mesothelioma in mice to assess the interaction of radiation and the immune system using a non-ablative short course of hypofractionated radiation such as 15 Gy in 3 fractions (9,27). When this type of radiation is delivered to the primary tumor, tumor-associated antigens (Ag) are released from dead tumor cells and then processed by antigen presenting cells (APC) such as DCs. APCs then traffic to the lymph nodes and present Ags to the naïve T cells which are activated and start to proliferate. A large number of activated T cells especially cytotoxic T cells (CTL) migrate and traffic to the tumor site where the tumor Ags are expressed, and exert cytolysis of tumor cells.
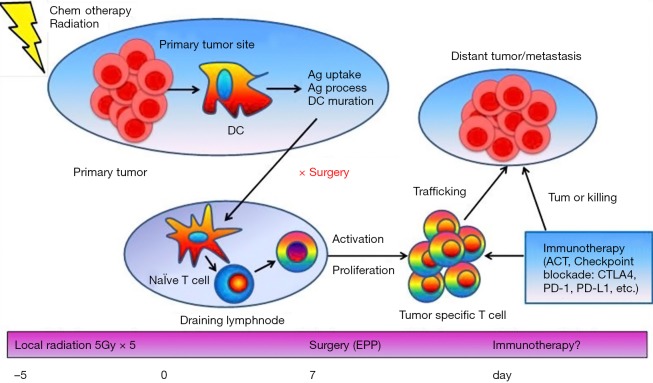
Assumingly, the process of activation of the immune system takes at least 7 days after the start of radiation. In our SMART protocol, radical surgery is performed an average 5 days after the end of radiation or at least 10 days after the start of radiation, suggesting that adequate time was provided to activate the immune system. The specific immune response generated by the preoperative radiation could therefore be an opportunity to add immune check point inhibitors before surgery to optimize the benefit of radiation and surgery (Figure 2).
Figure 2.
Abscopal effect induced by local radiation can be enhanced when combined with immune checkpoint inhibitors. When local radiation is delivered to the primary tumor, tumor-associated Ag are released from dead tumor cells and processed by APC such as DC. APCs then traffic to the lymph nodes and present Ags to naïve T cells which are activated and start to proliferate. A large number of activated T cells especially CTLs migrate and traffic to the tumor site and exert cytolysis of tumor cells. The SMART approach with an accelerated hypofractionated hemithoracic radiation followed by surgery could therefore provide an excellent platform to introduce immunotherapy in clinical practice. Ag, antigens; APC, antigen presenting cells; DC, dendritic cells; EPP, extrapleural pneumonectomy.
Using our mice model, we assess the impact of adding immune check point blockade to a short course of non-ablative radiation. The potential synergistic impact of this combined approach was studied on the primary tumor as well as distant tumor targeted through the abscopal effect. We previously demonstrated that blockade of immune suppressive CTLA-4 resulted in tumor growth delay when combined with chemotherapy in murine mesothelioma and thus used this compound with local radiation (7). We injected primary and secondary tumors into mice and treated the primary tumor only with local radiation. We observed that both the primary tumor and the secondary distant tumor had growth delayed. Local radiation resulted in more T cell infiltration into both tumors, including regulatory T cells (Treg) and CTLs. Interestingly, the proportion of Treg over effector T cells in both tumors was reversed after CTLA-4 blockade, while CD8 T cells were further activated. The expression of the immune-related genes was upregulated and cytokine production was significantly increased. Local radiation resulted in an increase of tumor-infiltrating T cells, while CTLA-4 blockade led to significant reduction of Tregs and increase of CTLs in both tumors. The abscopal effect was enhanced by targeting the immune checkpoints through modulation of T cell immune response in murine mesothelioma (9).
We then assessed the impact of local radiation on the immune system in combination with radical surgery in our mouse mesothelioma model to reproduce the SMART protocol (28). Blunt surgery and radical surgery were performed to analyze the short and long term impact of radiation and surgery. Our results showed that local radiation led to a specific immune activation against the tumor associated with significant upregulation of CD8+ T cells limiting the negative impact of an incomplete surgical resection. The same radiation protocol performed 7 days before radical surgery led to a long term antitumor immune protection that was primarily driven by CD4+ T cells. Radical surgery alone or in combination with local radiation completed 24 hours before radical surgery did not provide this vaccination effect. Combining this radiation protocol with CTLA-4 blockade provided better results than radiation alone. We concluded that a specific activation of the immune system against the tumor contributes to the benefit of accelerated hypofractionated radiation before surgery. The surgical removal of the primary tumor (and thus the site of neoantigen release) did not preclude the long term benefit of radiation. However, adequate timing between the radiation and surgery was important to ensure that the immune system had time to be activated. Hence, local radiation combined with surgery provides an excellent platform to introduce immune checkpoint blockades in the clinical setting. These experiments also suggest that a dose of 15 Gy of radiation may be sufficient to generate the immune benefit and that higher doses may not be necessary.
Chemotherapy and immunotherapy in mesothelioma
Neglected issue of cancer cell repopulation between cycles of chemotherapy
Chemotherapy is widely used to treat patients with cancers. Chemotherapy is typically given every 3 weeks for 3–6 cycles in total, and expected to kill proliferating tumor cells in the S-phase of the cell cycle. However, normal cells such as bone marrow and gastro-intestinal mucosa cells that proliferate rapidly are also killed, leading to systemic toxicity. The intervals between cycles of chemotherapy allow normal cells to recover. However, surviving cancer cells also have a chance to re-proliferate, a phenomenon known as repopulation (26). Cancer cell repopulation used to be a neglected factor, but now has been recognized as a major cause of drug resistance (29). Specific inhibitors targeting this process have been studied in a wide variety of malignancies and obtained inspiring results (30-32). Cancer stem cells may be a predominant component in the process of cancer cell repopulation during the intervals of chemotherapy treatments (Figure 3).
Figure 3.
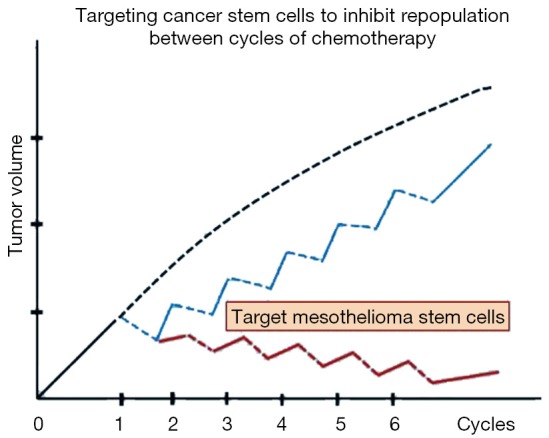
Mesothelioma stem cells (MSC) could be a key target to inhibit repopulation between cycles of chemotherapy. Tumor growth delay can be achieved with chemotherapy, however, tumor grows back rapidly due to cancer cell repopulation between cycles of chemotherapy (blue curve). This hypothesis model assumes that targeting MSC would be able to eliminate cancer cell repopulation during the intervals of chemotherapy (red curve).
Based on a large clinical trial completed in 2003, the first line of chemotherapy for MPM has become cisplatin and pemetrexed, which was approved by the Food and Drug Administration (FDA). The clinical benefit is that survival time was prolonged by 2.8 months compared with cisplatin alone (11). Hence, cancer cell repopulation might be a potential strategy to improve the efficacy of chemotherapy by specifically inhibiting this process. Tumor repopulation is a phenomenon that has not been well studied in MPM; it is often overlooked due to current customized experimental study strategies. Therefore, new therapeutic options to abrogate tumor repopulation will provide new avenues to improve chemotherapeutic response and clinical outcome (33).
The impact of chemotherapy on the immune system
For a long time, the belief was that chemotherapy was immunosuppressive by killing immune cells thus having a deleterious effect on immunity to fight the cancer (34,35). However, some chemotherapeutic agents such as taxanes (paclitaxel and docetaxel) have been demonstrated to be immunostimulatory rather than immunosuppressive against tumors, suggesting that other mechanisms than inhibition of cell division may be at play (35,36). In support of these experimental observations, some studies have indicated that advanced breast cancer patients responded to treatment with paclitaxel or docetaxel through an increase of interferon (IFN)-γ, IL-2, IL-6, GM-CSF cytokine levels and enhancement of natural killer (NK) and lymphokine-activated killer (LAK) cell activity in peripheral blood (37).
Cyclophosphamide as an alkylating agent has been used clinically for several decades. There is also considerable experience using this drug as an immunosuppressive agent for the treatment of autoimmune diseases. Hence, interestingly, besides its antiproliferative effects, cyclophosphamide has a paradoxal immunomodulatory effects depending on the dosage used. Indeed, high-dose cyclophosphamide can lead to complete eradication of haematopoietic cells, while low dose is able to selectively suppress Tregs only. Hence, low dose cyclophosphamide can be used to counteract immunosuppression in cancer. However, recently a study suggested that cyclophosphamide has an even more complex interaction with the immune system by increasing the number of myeloid-derived suppressor cells (MDSC) (38).
MDSC plays a critical role in cancer immune evasion by inhibiting adaptive and innate immunity. Therefore, MDSC is considered an obstacle for the successful cancer immunotherapy. Interestingly, Alizadeh et al. (39) demonstrated that doxorubicin selectively eliminated MDSC in the spleen, blood, and tumor beds in a murine mammary cancer model. On the contrary, the frequency of CD4, CD8 T cells and NK cells were significantly increased after doxorubicin treatment. Importantly, the cytolytic enzymes perforin and granzyme B and cytokine IFN-γ produced by NK and CTL were increased as well. The results indicate that doxorubicin can be used as a cytotoxic drug and an immunostimulatory agent, through selectively inhibiting MDSC thus facilitating CTL activity (38).
In another study, an interesting phenomenon was observed with doxorubicin treatment resulting in increased CD86 expression on B cells and increased CD4+ T cell activation in the presence of superantigen, an effect that could be blocked by CD86 antibody. In addition, doxorubicin resulted in a decrease of the anti-inflammatory cytokines IL-10 and TNF-α. Specific chemotherapy agents can thus be beneficial on the immune system in cancers and could be particularly interesting in combination with immunotherapy (39,40).
Combination of chemo-immunotherapy to inhibit cancer cell repopulation
Immune modulation approaches are becoming promising strategies to inhibit cancer cell repopulation during courses of chemotherapy (41,42). As previously demonstrated, CD4+ CD25+ Tregs can promote tumor growth. Using an intrathoracic murine model of malignant mesothelioma, we provided evidence suggesting that Treg blockade could enhance survival when combined with pemetrexed in established tumor (6). AC29 murine mesothelioma cells were injected into the right pleural cavity of CBA mice for tumor development. Four days after the tumor injection, tumor-bearing mice were then treated with pemetrexed alone, Treg blockade alone, or a combination of pemetrexed and Treg blockade. We observed a synergistic antitumor effect of Treg blockade combined with pemetrexed resulting in prolonged survival. The combination of Treg blockade and pemetrexed was associated with decreased tumor-infiltrating Tregs, increased IL-2 production, DC maturation, and increased IFN-γ in tumor-infiltrating CD8+ T cells when compared with mice treated with pemetrexed alone or Treg blockade alone. The survival benefit was abrogated if anti-CD8 mAb was administered simultaneously. Likewise, the survival benefit resulting from the combined Treg blockade with pemetrexed was not observed when immunodeficient mice were used. Therefore, this study suggests that Treg blockade combined with pemetrexed can suppress mesothelioma growth in established tumor in vivo through an immune-mediated process. This study also validated a new intrathoracic tumor model of pleural effusion to explore the role of antitumor immunity in murine mesothelioma (6).
In further studies, systemic depletion of Treg with monoclonal antibody against CD25 was carried out in a murine mesothelioma AC29 subcutaneous model (41). Tumor growth delay was achieved by cisplatin followed by the CD25 antibody PC61 or cyclophosphamide. The BrdU labeling index indicated that tumor cell repopulation between weekly cycles of cisplatin treatment was significantly inhibited by PC61. The CD4+ CD25+ Foxp3+ Tregs in tumor and lymphoid organs were almost completely depleted, whereas the CD4+ or CD8+ T cells did not change. PC61 after chemotherapy resulted in an increase of gene expression of IFN-γ, granzyme B, perforin, and IP-10, thus leading to tumor cell lysis in cytotoxic lymphocyte assay. Nevertheless, cell killing induced by cyclophosphamide combined with cisplatin was due to cytotoxicity rather than specific immune response. Treg depletion between cycles of chemotherapy could improve the outcome of mesothelioma (41,42).
Cancer immunotherapy has shown promising results when combined with chemotherapy. Blocking CTLA-4 signaling by monoclonal antibody between cycles of chemotherapy may inhibit cancer cell repopulation and enhance the antitumoral immune reaction, thus improve the efficacy of chemotherapy in mesothelioma. The impact of CTLA-4 blockade on the early stage of tumor development was evaluated in a subcutaneous murine mesothelioma model (41). CTLA-4 blocking antibody was administered following each cycle of chemotherapy, and monotherapy was included as controls. Antitumor effect was evaluated by tumor growth delay and survival of the animals. Tumor cell repopulation was quantified by bromodeoxyuridine incorporation and Ki67 by immunohistochemistry and/or flow cytometry. In vitro cell killing was determined by classic chromium-released assay, and reverse transcription PCR (RT-PCR) was carried out to determine the gene expression of associated cytokines. Anti-CTLA-4 monoclonal antibody was able to inhibit tumor growth at early stage of tumor development. Antitumor effect was achieved by administration of CTLA-4 blockade between cycles of chemotherapy. Tumor cell repopulation during the intervals of cisplatin was inhibited by CTLA-4 blockade. Anti-CTLA-4 therapy gave rise to an increased number of CD4 and CD8 T cells infiltrating the tumor. RT-PCR showed that the gene expression of interleukin IL-2, IFN-γ, granzyme B, and perforin increased in the tumor milieu. Blockade of CTLA-4 signaling showed effective anticancer effect, correlating with inhibiting cancer cell repopulation between cycles of chemotherapy and upregulating tumor-infiltrating T lymphocytes, cytokines, and cytolytic enzymes in a murine mesothelioma model (7,41).
We also found that CD1d-restricted natural killer T (NKT) cells provided activity against cancer by producing IFN-γ (42). To elucidate the antitumor effect of invariant NKT (iNKT) cells in the tumor microenvironment we used the intrathoracic murine MPM model that we previously developed to provide pleural effusion as a good surrogate of the tumor microenvironment. We found that the number of iNKT cells increased dramatically in the pleural effusion after intrathoracic tumor cell injection at an earlier phase compared with accumulation of CD8 T cells. These iNKT cells showed increased expression of CD25 and increased ratio of cells positive for IFN-γ intracellular staining. iNKT cells sorted from pleural effusion of tumor burden mice produced larger amount of IFN-γ compared with naive mice. Mice pretreated in vivo with anti-CD1d-blocking Ab showed increased amount of pleural effusion and decreased ratio of total and effector-type CD8 T cells as well as decreased intracellular IFN-γ expression of CD8 T-cell in the pleural effusion. In vivo administration of α-galactosylceramide (α-GalCer, α-GC), a specific activator of NKT cells, led to prolonged survival associated with less pleural effusion and increased ratio of IFN-γ-positive iNKT cells and CD8 T cells in the pleural effusion. Therefore, this study suggests that iNKT cells accumulating in the tumor microenvironment play an antitumor effect by producing IFN-γ and enhancing subsequent CD8 T-cell response. Furthermore, in vivo administration of α-GalCer could suppress mesothelioma growth by activating iNKT cells (8).
We studied the impact of NKT cell activation by α-GC on cancer cell repopulation during chemotherapy in murine mesothelioma. The number of NKT cells was found to be increased during the development of murine mesothelioma. NKT cells specifically recognize α-GC through CD1d resulting in their activation and expansion. Tumor-bearing mice were treated with chemotherapy once weekly, and α-GC was followed after each cycle of chemotherapy. Anti-tumor effect was evaluated on wild-type (WT) and CD1d knockout (CD1dKO) mice. Cancer cell proliferation and apoptosis were evaluated by Ki67 and TUNEL immunohistochemistry. CD4(+) and CD8(+) T cell proportion and activation in tumor, spleen, draining lymph node and peripheral blood were determined by flow cytometry, and gene expression of activated T cell-related cytokines was quantified by RT-PCR. NKT cells were identified by CD1d-α-GC-tetramer staining. In WT mice, tumor growth delay was achieved by cisplatin (Cis), and this effect was improved in combination with α-GC, but α-GC alone had little effect. Cancer cell proliferation during chemotherapy was significantly inhibited by α-GC, while cancer cell death was significantly upregulated. α-GC following chemotherapy resulted in NKT cell expansion and an increase of IFN-γ production in the draining lymph node, blood and spleen. Gene expression of immune-associated cytokines was upregulated. Strikingly, the percentage of inducible T cell co-stimulator positive CD4 T cells, Th17/Tc17 cells increased in splenocytes. In CD1d KO mice, however, Cis alone was less effective and Cis + α-GC provided no additional benefit over Cis alone. α-GC alone had minimal effect in both mice. In conclusion, NKT activation between cycles of chemotherapy could improve the outcome of mesothelioma treatment (42).
Tumor-associated macrophage might be critical target to control mesothelioma genicity
We developed an RN5 murine mesothelioma in Nf2 heterozygous mice that were exposed to asbestos fibers (43). We observed that the number of macrophages and mesothelial precursor cells increased in parallel in peritoneal lavage. This phenomenon was confirmed by injecting RN5 cells ip into mice. More interestingly, when macrophages were depleted, the population of mesothelial precursors significantly decreased as well. The number of tumor spheroids in the peritoneal lavage reduced compared with untreated mice, suggesting that macrophages may play critical roles in mesothelioma tumorigenesis in this model (unpublished data).
A recent study also showed evidence that M1 macrophages secrete pro-inflammatory cytokines and express CD40 and CD80, while suppressive M2 macrophages secrete anti-inflammatory cytokines and express CD206 and CX3CR1 during mesothelioma progression and during chemo-immunotherapy. Jackaman et al. (44) showed in an in vitro study that mesothelioma-conditioned media generated CD206− CX3CR1+ MCP-1+ TGF-β+ macrophages that induced T cell proliferation but prevented T cell IFN-γ production. In vivo studies showed that inoculation of macrophages with mesothelioma cells together led to faster tumor growth, and depleting macrophages using anti-F4/80 antibody induced tumor regression. IL-2/agonist anti-CD40 antibody polarized macrophages into M1 phenotype coinciding with tumor regression thus leading to a therapeutic target for the generation of antitumor immunity (44).
Concluding remarks
Chemotherapy is the standard of care for patients with advanced MPM. Innovative approaches with radical pleural IMRT (IMPRINT) and accelerated IMRT followed by radical surgery (SMART) have been shown to be safe and feasible in the treatment of MPM and provide new therapeutic options in the treatment of this disease. We have shown in a mice model that short course of high dose non-ablative radiation could promote an anti-tumor immune response. This approach could thus provide a valuable platform for immunotherapy in clinical practice. Meanwhile, integration of chemotherapy and immunotherapy with immune checkpoint inhibitors could lead to better outcomes.
Acknowledgements
This work was funded by Princess Margaret Cancer Research Foundation (PMCRF) and Canadian Mesothelioma Foundation (CMF).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379-87. 10.2486/indhealth.45.379 [DOI] [PubMed] [Google Scholar]
- 2.Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417-27. 10.1056/NEJMoa1115050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. 10.1186/s13014-015-0575-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Schil PE, Opitz I, Weder W, et al. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J 2014;44:754-64. 10.1183/09031936.00207213 [DOI] [PubMed] [Google Scholar]
- 5.Anraku M, Tagawa T, Wu L, et al. Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine malignant mesothelioma. J Immunol 2010;185:956-66. 10.4049/jimmunol.0900437 [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Yun Z, Tagawa T, et al. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther 2012;11:1809-19. 10.1158/1535-7163.MCT-11-1014 [DOI] [PubMed] [Google Scholar]
- 7.Tagawa T, Wu L, Anraku M, et al. Antitumor impact of interferon-γ producing CD1d-restricted NKT cells in murine malignant mesothelioma. J Immunother 2013;36:391-9. 10.1097/CJI.0b013e3182a801f2 [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Wu MO, De la Maza L, et al. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015;6:12468-80. 10.18632/oncotarget.3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. 10.1158/1078-0432.CCR-16-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 11.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. 10.1016/S0140-6736(15)01238-6 [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72. 10.1586/ers.10.35 [DOI] [PubMed] [Google Scholar]
- 13.Yajnik S, Rosenzweig KE, Mychalczak B, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2003;56:1319-26. 10.1016/S0360-3016(03)00287-6 [DOI] [PubMed] [Google Scholar]
- 14.Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006;1:289-95. 10.1016/S1556-0864(15)31583-5 [DOI] [PubMed] [Google Scholar]
- 15.Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83. 10.1016/j.ijrobp.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel PR, Yoo S, Broadwater G, et al. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:362-8. 10.1016/j.ijrobp.2011.11.057 [DOI] [PubMed] [Google Scholar]
- 17.Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. 10.1200/JCO.2016.67.2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the "SMART" approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. 10.1097/JTO.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen R, Hegmans JP, Maat AP, et al. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am J Respir Crit Care Med 2016;193:1023-31. 10.1164/rccm.201508-1573OC [DOI] [PubMed] [Google Scholar]
- 20.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18:858-68. 10.1158/1078-0432.CCR-11-2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan R, Thomas A, Alewine C, et al. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J Clin Oncol 2016;34:4171-9. 10.1200/JCO.2016.68.3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407-16. 10.1038/ng.3520 [DOI] [PubMed] [Google Scholar]
- 23.Kindler HL, Scherpereel A, Calabrò L, et al. Tremelimumab as second- or third-line treatment of unresectable malignant mesothelioma (MM): results from the global, double-blind, placebo controlled DETERMINE study. J Clin Oncol 2016;34:abstr8502.
- 24.Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. 10.1016/S1470-2045(17)30169-9 [DOI] [PubMed] [Google Scholar]
- 25.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 26.Brade AM, Tannock IF. Scheduling of radiation and chemotherapy for limited-stage small-cell lung cancer: repopulation as a cause of treatment failure? J Clin Oncol 2006;24:1020-2. 10.1200/JCO.2005.04.9676 [DOI] [PubMed] [Google Scholar]
- 27.de Perrot M, Dong Z, Bradbury P, et al. Impact of tumour thickness on survival after radical radiation and surgery in malignant pleural mesothelioma. Eur Respir J 2017;49. pii: 1601428. 10.1183/13993003.01428-2016 [DOI] [PubMed] [Google Scholar]
- 28.De la Maza L, Wu M, Wu L, et al. In situ vaccination after accelerated hypofractionated radiation and surgery in a mesothelioma mouse model. Clin Cancer Res 2017. [Epub ahead of print]. 10.1158/1078-0432.CCR-17-0438 [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Tannock IF. Repopulation in murine breast tumors during and after sequential treatments with cyclophosphamide and 5-fluorouracil. Cancer Res 2003;63:2134-8. [PubMed] [Google Scholar]
- 30.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 2005;5:516-25. 10.1038/nrc1650 [DOI] [PubMed] [Google Scholar]
- 31.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science 2006;312:1171-5. 10.1126/science.1125950 [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res 2005;65:2825-31. 10.1158/0008-5472.CAN-04-3137 [DOI] [PubMed] [Google Scholar]
- 33.Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015;517:209-13. 10.1038/nature14034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan KS. Molecular Pathways: Targeting Cancer Stem Cells Awakened by Chemotherapy to Abrogate Tumor Repopulation. Clin Cancer Res 2016;22:802-6. 10.1158/1078-0432.CCR-15-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Lou X, Jin L, et al. Necrosis, and then stress induced necrosis-like cell death, but not apoptosis, should be the preferred cell death mode for chemotherapy: clearance of a few misconceptions. Oncoscience 2014;1:407-22. 10.18632/oncoscience.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237-51. 10.1038/nrc3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsavaris N, Kosmas C, Vadiaka M, et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer 2002;87:21-7. 10.1038/sj.bjc.6600347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 2016;78:661-71. 10.1007/s00280-016-3152-1 [DOI] [PubMed] [Google Scholar]
- 39.Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 2014;74:104-18. 10.1158/0008-5472.CAN-13-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zirakzadeh AA, Kinn J, Krantz D, et al. Doxorubicin enhances the capacity of B cells to activate T cells in urothelial urinary bladder cancer. Clin Immunol 2017;176:63-70. 10.1016/j.clim.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Yun Z, Tagawa T, et al. Tumor cell repopulation between cycles of chemotherapy is inhibited by regulatory T-cell depletion in a murine mesothelioma model. J Thorac Oncol 2011;6:1578-86. 10.1097/JTO.0b013e3182208ee0 [DOI] [PubMed] [Google Scholar]
- 42.Wu L, Yun Z, Tagawa T, et al. Activation of CD1d-restricted natural killer T cells can inhibit cancer cell proliferation during chemotherapy by promoting the immune responses in murine mesothelioma. Cancer Immunol Immunother 2014;63:1285-96. 10.1007/s00262-014-1597-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blum W, Pecze L, Felley-Bosco E, et al. Establishment of immortalized murine mesothelial cells and a novel mesothelioma cell line. In Vitro Cell Dev Biol Anim 2015;51:714-21. 10.1007/s11626-015-9885-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackaman C, Yeoh TL, Acuil ML, et al. Murine mesothelioma induces locally-proliferating IL-10(+)TNF-α(+)CD206(-)CX3CR1(+) M3 macrophages that can be selectively depleted by chemotherapy or immunotherapy. Oncoimmunology 2016;5:e1173299. 10.1080/2162402X.2016.1173299 [DOI] [PMC free article] [PubMed] [Google Scholar]