Abstract
Though difficult to achieve, complete resection of malignant pleural mesothelioma is paramount to improving patient survival. Surgeons have traditionally been limited to using visual inspection and manual palpation to locate and remove cancerous tissue. However, intraoperative molecular imaging (IMI) is a promising new technology in surgery. Molecular imaging utilizes a fluorescent tracer that selectively accumulates in cancer cells. An imaging device is then used to detect and augment the fluorescent signal emitted from the fluorescent cancer cells. Our group and others have demonstrated that molecular imaging with either indocyanine green (ICG) or a folate receptor alpha (FRα) targeted fluorophore can accurately identify a number of intrathoracic malignancies. Early studies of intraoperative imaging have suggested its efficacy for malignant pleural mesothelioma. In a murine model of mesothelioma, intraoperative imaging was found to have sensitivity of 87% and specificity of 83%. In a pilot human study, eight patients with biopsy-proven epithelial malignant pleural mesothelioma were administered 5 mg/kg of intravenous ICG injection 24 h prior to resection. The following day, a near-infrared (NIR) imaging device was used to detect tumor fluorescence intraoperatively. After what was believed to be complete tumor excision, the wound bed was re-imaged for residual fluorescence indicative of retained tumor. When residual fluorescence was detected, additional tissue was resected, if feasible, and specimens were sent for pathologic correlation. In all cases, intraoperative fluorescence localized to mesothelioma deposits which were confirmed on final pathology. Following resection, fluorescence was confirmed ex vivo with a mean tumor-to-background ratio (TBR) of 3.2 (IQR: 2.9–3.4). It is hoped that this technology will improve outcomes for mesothelioma patients by allowing for a more complete oncologic resection.
Keywords: Intraoperative imaging, mesothelioma, surgical oncology
The cancer surgeon’s challenge
Local recurrence has long been the Achilles heel of cancer surgery, with multiple studies demonstrating the importance of achieving a complete (R0) resection in improving 5-year survival rates (1). Thus, a successful tumor resection requires removal of all gross disease to achieve disease clearance as well as removal of all small tumor nodules. Complete (R0) resection is particularly difficult to achieve in surgery for malignant pleural mesothelioma due to the technical difficulty of pleurectomy/decortication and extrapleural pneumonectomy as well as the insidious nature of the cancer itself (2). Surgeons have traditionally been limited to two tools—visual inspection and manual palpation—to achieve disease clearance. Despite the difficulty in obtaining an R0 resection, it is paramount to improve the 5-year patient survival. In fact, many studies have shown that resection margin positivity is an independent predictor of worse survival (2,3).
Intraoperative molecular imaging (IMI): a new tool in the surgeon’s armamentarium
One potential solution to the challenge of intraoperative identification of small tumor deposits is molecular targeted imaging with a fluorescent contrast agent that demarcates cancerous cells and improves tumor clearance (4-7). Targeted molecular imaging has several advantages over conventional imaging technologies. First, it does not require ionizing radiation and is thus safe for the patient. Additionally, the technology is intuitive and visual, making it easy to understand without advanced training. Finally, intraoperative imaging is rapid, making it easy to visualize large tissue surfaces in real time without disrupting the flow of operations.
This technology requires the development of two interrelated components: (I) a targeted fluorescent tracer that accumulates rapidly and selectively in the tissue of interest and (II) an imaging modality to detect and quantify the contrast agent in vivo (5). A variety of fluorescent contrast agents have been used for targeting tumors during surgeries for both benign and malignant disease. Contrast agents that emit light within the visible color spectrum have the advantage that specialized equipment for detection is not necessary; however, tissue penetration is very limited due to tissue scatter and blood absorption (8). Those agents that emit light in the near-infrared (NIR) spectrum, however, are not subject to the same degree of tissue scatter and thus can achieve increased depth of penetration. The primary drawback is that NIR imaging requires a specialized imaging device for displaying the emitted signal.
With regard to imaging devices, NIR cameras originally designed for imaging large animals have been adapted to humans in prototype imaging systems (9,10). These systems require two light sources—one for white light and one for NIR fluorescence—that illuminate the surgical field. White light reflected from the surgical field is collected by a motorized zoom lens and directed to a color video camera. Normally, there is virtually no NIR fluorescence from the surgical field. However, when an exogenous NIR fluorophore is introduced, the invisible near infrared fluorescent light is emitted to a NIR camera. Both cameras acquire their images, permitting simultaneous visualization of anatomy (white light) and function (NIR light) (8). Although many commercial grade fluorescent imaging systems are available, we have demonstrated that a small portable interchangeable imager of fluorescence is much less expensive and still effective for detecting fluorescent tumors (11).
Preclinical studies
Several groups have demonstrated the efficacy of IMI for the detection of intrathoracic and extrathoracic malignancies (7,12-19). Our group has investigated two different tracers for the intraoperative detection of pulmonary nodules and occult metastases—indocyanine green (ICG) and a folate receptor alpha (FRα) targeted fluorophore. All studies discussed hereafter received approval from the University of Pennsylvania Institutional Review Board and, where applicable, the Institutional Animal Care and Use Committee.
ICG was the first fluorescent dye tested for the IMI of lung nodules. ICG is a non-targeted NIR contrast agent with excitation and emission wavelengths of approximately 785 and 800 nm, respectively. ICG localizes to solid tumors due to the enhanced permeability and retention (EPR) effect. Though ICG cannot extravasate into normal tissue, it is able to leak from the defective capillaries and wide fenestrations of neovasculature in tumor tissue. Several groups have demonstrated in human patients that ICG can be used to detect hepatocellular carcinoma (HCC) and colorectal cancer liver metastases (12,14).
We demonstrated in preclinical studies in mice and canines that fluorescent NIR imaging with ICG can both delineate tumor margins and identify residual disease in a surgical wound that is not visible to the naked eye (20). First, we developed a murine model to simulate local resection and facilitate identification of retained disease. Eighty percent of flank tumors were removed and the animals were then examined by a blinded, experienced surgeon using visual inspection and finger palpation. Mice with residual tumor identified by these methods were excluded and the remaining mice were imaged with ICG. This technique identified 85% of residual cancers. In a follow-up experiment, mice following partial resections of flank tumors without residual disease identified by traditional methods (i.e., visual inspection and finger palpation) were collected. Twenty-two such mice were closed and used as controls whereas another 22 mice underwent additional surgery to resect disease with ICG guidance. All of the controls developed recurrent flank tumors within 1 week, while 20 out of 22 mice that had image-guided surgery using ICG had no tumor recurrence at 1 week (20).
In a second proof-of-principle study, ICG imaging was used in canines with spontaneously occurring sarcomas (21). The technology was successful in 14 out of 15 animals, demonstrating strong tumor fluorescence. The tumor that was weakly fluorescent turned out to be a myxosarcoma on final pathology. This particular histologic subtype tends to be composed of loosely arranged stellate or spindle-shaped cells separated by mucinous stroma rather than solid tumor parenchyma. Following removal of the primary canine tumors, the resection beds were examined by both the primary surgeon and assistant who assessed them for residual fluorescence. Ten wound beds were not deemed to be significantly fluorescent and biopsies confirmed that there was no residual tumor on final pathology. Five canines had highly fluorescent resection beds, and positive margins were confirmed in 4 of 5 of these animals with the fifth animal showing inflammation on final pathology.
As the above results suggest, ICG’s mechanism of tumor localization via the EPR effect lacks a degree of tumor specificity, in that it does not discriminate well between tumor cells and peritumoral inflammation. In light of this deficiency, other receptor-targeted contrast agents have been developed to enhance the specificity of the technology. Our group has tested a contrast agent (EC17) to target FRα based on the principle that pulmonary adenocarcinomas highly overexpress this receptor.
Early studies of intraoperative imaging have suggested its efficacy for malignant pleural mesothelioma. In a preliminary study of ICG molecular imaging, we have shown that intraoperative imaging can enhance mesothelioma resection using a murine surgical model of MPM (22). Eighty mice were injected intravenously with ICG, underwent partial resection, and then randomized to surgery with intraoperative imaging versus surgery without imaging. Surgeons identified incomplete resections in 25% of mice randomized to surgery with IMI versus in 7.5% of mice undergoing surgery alone. Residual tumor deposits ranged from 0.4 to 1.9 mm, with mean TBR of residual disease of 2.8 (IQR 2.5–3.6). Sensitivity and specificity of molecular imaging was 87% and 83%, respectively.
Human studies with intraoperative imaging
Our work with intraoperative imaging has focused on both lung cancer and mesothelioma. In the initial proof of principle clinical trial of EC17, 30 patients with an indeterminate pulmonary nodule were intravenously administered the folate receptor-targeted contrast agent (23). Intraoperatively, patients underwent an “optical biopsy” in which their lesion was graded fluorescent or non-fluorescent to determine if the nodule was a primary pulmonary adenocarcinoma. Standard frozen section pathology and immunohistochemical staining on permanent sections were then performed as the gold standard for validation of molecular imaging. Nineteen of the 30 patients had fluorescent nodules, all of which were confirmed on final pathology to be primary adenocarcinomas. There were no false positive or false negative results by molecular imaging, whereas frozen section improperly diagnosed one lesion as benign leading to improper surgical management of that patient. Additionally, molecular imaging required 2.4 min for mean time to diagnosis compared to nearly 30 min for frozen section. Thus, this paper demonstrated that molecular imaging can rapidly and accurately diagnose pulmonary adenocarcinomas, and is a valuable adjunct to standard pathology.
Finally, we demonstrated that EC17 can identify small tumor deposits at resection margins in a murine model and in a series of three human patients (24). In the mouse study, we used a cohort of mice status post partial resection with residual tumor intentionally left at the surgical margins. Without intraoperative imaging, a surgeon was able to detect residual tumor in 18 out of 60 mice with positive wound bed margins by visual inspection and finger palpation alone (30%) and falsely identified four mice as having positive margins. A second investigator who could utilize intraoperative imaging with EC17 in addition to visual inspection and finger palpation identified the same 18 mice with positive wound bed margins plus an additional 30 mice with residual tumors (80% accuracy). Thus, it was calculated that fluorescent imaging was 80% sensitive and 100% specific for positive margins while visual inspection and manual palpation was only 30% sensitive and 90% specific. A follow-up series of three human patients with biopsy proven lung adenocarcinoma showed sharp demarcation between tumor and normal tissue and no residual fluorescence in the tumor bed or at the specimen margins following resection.
A later human study by our group demonstrated that ICG can be successfully utilized in the identification of pulmonary nodules (25). In 18 human patients undergoing resection of a pulmonary nodule, ICG based molecular imaging successfully identified 14 of 18 primary nodules. Interestingly, the technology also discovered that an additional 5 subcentimeter cancerous nodules that were not identified on conventional preoperative imaging. ICG imaging demonstrated remarkable accuracy and tissue penetration, identifying nodules as small as 0.2 cm and as deep as 1.3 cm below the tissue surface. Among the primary nodules not identified by ICG, one was a metastatic melanoma and another a pulmonary embolus on final pathology. Both lesions lack epithelial cells, supporting the theory that the EPR effect drives ICG localization into tumor tissue.
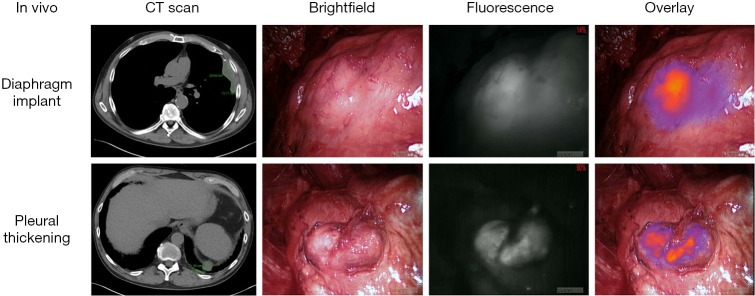
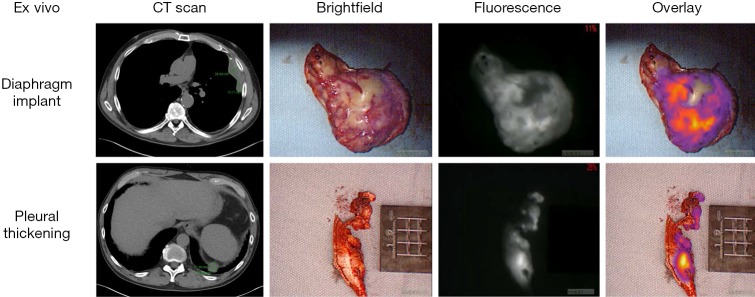
In the pilot human study, eight patients with biopsy-proven epithelial malignant pleural mesothelioma were administered 5 mg/kg of intravenous ICG injection 24 h prior to resection. All patients tolerated ICG injection without evidence of toxicity. The following day, a NIR imaging device was used to detect tumor fluorescence intraoperatively (Figure 1). After what was believed to be complete tumor excision, the wound bed was re-imaged for residual fluorescence indicative of retained tumor. When residual fluorescence was detected, additional tissue was resected, if feasible, and specimens were sent for pathologic correlation. In all cases, intraoperative fluorescence localized to mesothelioma deposits, which were confirmed on final pathology. Following resection, fluorescence was confirmed ex vivo (Figure 2) with mean TBR of 3.2 (IQR: 2.9–3.4). The number of additional resected specimens following wound bed imaging ranged from one to four per patient (mean 1.8) with an average size of 0.9 cm (range: 0.3–2.2 cm). These tumor specimens were frequently found in difficult-to-reach anatomical locations, including the costophrenic sulcus and directly beneath the thoracotomy site. These results are the basis of an ongoing phase I human trial and a further trial is planned with FRα-targeted agents, given the known FRα overexpression of malignant mesothelioma (26).
Figure 1.
Intraoperative NIR imaging of two mesothelioma deposits in a representative patient from the pilot human study. Preoperative CT scan is shown at left, followed by conventional thoracoscopic view in vivo, fluorescence imaging, and overlay of fluorescence imaging onto the brightfield view.
Figure 2.
Back table ex vivo specimens resected from the same patient as described in Figure 1. Preoperative CT scan is shown at left, followed by brightfield imaging, fluorescence imaging, and overlay of fluorescence imaging onto the brightfield view.
Summary and future directions
IMI is a new tool with a variety of applications to the general field of surgical oncology and more specifically to thoracic malignancies, including malignant pleural mesothelioma. Our group has demonstrated that ICG, a non-specific contrast agent, can accurately identify lung cancers of various subtypes. Furthermore, preliminary results suggest that ICG is effective in identifying mesothelioma in humans and a murine model. Additionally, we have shown that a FRα-targeted fluorescent tracer can identify pulmonary adenocarcinomas with greater than 90% specificity. Early studies have shown similar efficacy in identifying pulmonary metastases, positive surgical margins, and lymph node positivity. The application of this tracer to mesothelioma is the subject of ongoing inquiry by our laboratory.
The potential of this work is far-reaching. In the future, additional targeted contrast agents can be developed for other cancer subtypes to expand the clinical utility of molecular imaging to the entire field of surgical oncology. Ultimately, it is hoped that this technology will improve outcomes for cancer patients by providing a rapid, minimally invasive diagnosis, decreasing total operative time, and allowing for a more complete oncologic resection.
Acknowledgements
Funding: We acknowledge that this work was partially funded by an NIH Program Project P01 CA087971.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Aliperti LA, Predina JD, Vachani A, et al. Local and systemic recurrence is the Achilles heel of cancer sur-gery. Ann Surg Oncol 2011;18:603-7. 10.1245/s10434-010-1442-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buduhan G, Menon S, Aye R, et al. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 2009;88:870-5; discussion 876. 10.1016/j.athoracsur.2009.05.036 [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: re-sults in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. 10.1016/S0022-5223(99)70469-1 [DOI] [PubMed] [Google Scholar]
- 4.Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. Annu Rev Med 2010;61:359-73. 10.1146/annurev.med.60.052907.094936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singhal S. The future of surgical oncology: Image-Guided cancer surgery. JAMA Surg 2016;151:184-5. 10.1001/jamasurg.2015.3604 [DOI] [PubMed] [Google Scholar]
- 6.Vahrmeijer AL, Hutteman M, van der Vorst JR, et al. Image-guided cancer surgery using near-infrared fluo-rescence. Nat Rev Clin Oncol 2013;10:507-18. 10.1038/nrclinonc.2013.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondal SB, Gao S, Zhu N, et al. Real-time fluorescence image-guided oncologic surgery. Adv Cancer Res 2014;124:171-211. 10.1016/B978-0-12-411638-2.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol 2013;58:R37-61. 10.1088/0031-9155/58/11/R37 [DOI] [PubMed] [Google Scholar]
- 9.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging 2010;9:237-55. [PMC free article] [PubMed] [Google Scholar]
- 10.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence im-aging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 2009;16:2943-52. 10.1245/s10434-009-0594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okusanya OT, Madajewski B, Segal E, et al. Small portable interchangeable imager of fluorescence for fluo-rescence guided surgery and research. Technol Cancer Res Treat 2015;14:213-20. 10.7785/tcrt.2012.500400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizawa T, Fukushima N, Shibahara JA, et al. Real-Time identification of liver cancers by using indocya-nine green fluorescent imaging. Cancer 2009;115:2491-504. 10.1002/cncr.24291 [DOI] [PubMed] [Google Scholar]
- 13.Keereweer S, Mieog JS, Mol IM, et al. Detection of oral squamous cell carcinoma and cervical lymph node metastasis using activatable near-infrared fluorescence agents. Arch Otolaryngol Head Neck Surg 2011;137:609-15. 10.1001/archoto.2011.89 [DOI] [PubMed] [Google Scholar]
- 14.van der Vorst JR, Schaafsma BE, Hutteman M, et al. Near-infrared fluorescence-guided resection of colo-rectal liver metastases. Cancer 2013;119:3411-8. 10.1002/cncr.28203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating JJ, Nims S, Venegas O, et al. Intraoperative imaging identifies thymoma margins following neoad-juvant chemotherapy. Oncotarget 2016;7:3059-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating J, Newton A, Venegas O, et al. Near-Infrared intraoperative molecular imaging can locate metasta-ses to the lung. Ann Thorac Surg 2017;103:390-8. 10.1016/j.athoracsur.2016.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt D, Okusanya O, Judy R, et al. Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation. PLoS One 2014;9:e103342. 10.1371/journal.pone.0103342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating J, Singhal S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin Thorac Cardiovasc Surg 2016;28:127-36. 10.1053/j.semtcvs.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi S, Lomnes SJ, Laurence RG, et al. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging 2005;4:172-81. [DOI] [PubMed] [Google Scholar]
- 20.Madajewski B, Judy BF, Mouchli A, et al. Intraoperative near-infrared imaging of surgical wounds after tu-mor resections can detect residual disease. Clin Cancer Res 2012;18:5741-51. 10.1158/1078-0432.CCR-12-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt D, Parthasarathy AB, Okusanya O, et al. Intraoperative near-infrared fluorescence imaging and spec-troscopy identifies residual tumor cells in wounds. J Biomed Opt 2015;20:76002. 10.1117/1.JBO.20.7.076002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armato SG, 3rd, Blyth KG, Keating JJ, et al. Imaging in pleural mesothelioma: A review of the 13th Interna-tional Conference of the International Mesothelioma Interest Group. Lung Cancer 2016;101:48-58. 10.1016/j.lungcan.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy GT, Okusanya OT, Keating JJ, et al. The optical biopsy: a novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann Surg 2015;262:602-9. 10.1097/SLA.0000000000001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keating JJ, Okusanya OT, De Jesus E, et al. Intraoperative molecular imaging of lung adenocarcinoma can identify residual tumor cells at the surgical margins. Mol Imaging Biol 2016;18:209-18. 10.1007/s11307-015-0878-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nod-ules. Ann Thorac Surg 2014;98:1223-30. 10.1016/j.athoracsur.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueno R, Appasani K, Mercer H, et al. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;121:225-33. 10.1067/mtc.2001.111176 [DOI] [PubMed] [Google Scholar]