Abstract
Purpose
Achievement of deep molecular response with a tyrosine kinase inhibitor in patients with chronic myeloid leukemia (CML) is required to attempt discontinuation of therapy in these patients. The current subanalysis from the Evaluating Nilotinib Efficacy and Safety in Clinical Trials as First-Line Treatment (ENEST1st) study evaluated whether age has an impact on the achievement of deeper molecular responses or safety with frontline nilotinib in patients with CML.
Methods
ENEST1st is an open-label, multicenter, single-arm, prospective study of nilotinib 300 mg twice daily in patients with newly diagnosed CML in chronic phase. The patients were stratified into the following 4 groups based on age: young (18–39 years), middle age (40–59 years), elderly (60–74 years), and old (≥75 years). The primary end point was the rate of molecular response 4 ([MR4] BCR–ABL1 ≤0.01% on the international scale) at 18 months from the initiation of nilotinib.
Results
Of the 1091 patients enrolled, 1089 were considered in the analysis, of whom, 23% (n = 243), 45% (n = 494), 27% (n = 300), and 5% (n = 52) were categorized as young, middle age, elderly, and old, respectively. At 18 months, the rates of MR4 were 33.9% (95% confidence interval [CI], 27.8–40.0%) in the young, 39.6% (95% CI, 35.3–44.0%) in the middle-aged, 40.5% (95% CI, 34.8–46.1%) in the elderly, and 35.4% (95% CI, 21.9–48.9%) in the old patients. Although the incidence of adverse events was slightly different, no new specific safety signals were observed across the 4 age groups.
Conclusions
This subanalysis of the ENEST1st study showed that age did not have a relevant impact on the deep molecular response rates associated with nilotinib therapy in newly diagnosed patients with CML and eventually on the eligibility of the patients to attempt treatment discontinuation.
Keywords: Chronic myeloid leukemia, Nilotinib, Frontline, Impact of age, Clinical trial, Molecular response
Introduction
Old age was considered as a negative prognostic factor for the treatment of chronic myeloid leukemia (CML) and predicted poor survival outcomes with earlier treatment regimens prior to the use of tyrosine kinase inhibitors (TKIs) (Kantarjian et al. 1987; Silver et al. 1999; Berger et al. 2003). With the advent of imatinib, the overall survival (OS) of patients with CML improved substantially (Kantarjian et al. 2006; Hochhaus et al. 2009). A number of studies have evaluated the effect of age with imatinib, which suggested that imatinib was able to nullify the negative effect of age on outcomes with CML therapy (Breccia et al. 2012; Proetel et al. 2014).
Second-generation TKIs, including nilotinib and dasatinib, were developed for the treatment of patients, who were resistant to or intolerant of imatinib. Nilotinib (Tasigna®) was first approved for patients with CML, who were resistant to or intolerant of imatinib, and subsequently for newly diagnosed patients with CML (Tasigna 2015). The pivotal study, Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients (ENESTnd), showed higher rates of major molecular response (MMR; BCR–ABL1 ≤0.1% on the International Scale [IS]) and lower rates of progression with two doses (300 and 400 mg) of nilotinib compared to imatinib (Hochhaus et al. 2016b). Recent data have shown that achievement of deeper molecular responses, molecular response 4 ([MR4] BCR–ABL1 IS ≤0.01%) and molecular response 4.5 ([MR4.5] BCR–ABL1 IS ≤0.0032%), results in better treatment outcomes in patients with CML (Falchi et al. 2013; Etienne et al. 2014; Hehlmann et al. 2014). Additionally, in studies investigating treatment-free remission (TFR) in patients with CML, the eligibility criteria are the achievement of stable deep molecular responses (Hughes et al. 2016; Saglio et al. 2016). The ENEST1st study was, therefore, conducted to evaluate the efficacy of nilotinib in achieving deeper molecular responses in a large patient population of newly diagnosed patients with CML who were BCR–ABL1 positive (Hochhaus et al. 2016a). The results from the study showed that a majority of the patients achieved MR4 by 24 months with progression-free survival (PFS) rate of almost 100%.
Studies evaluating the impact of age on the safety and efficacy of second-generation TKIs, including nilotinib, are limited. The limited data available on the effect of age only evaluated the response rates for broader categories like elderly patients with age greater than 60 or 65 and younger patients (le Coutre et al. 2009; Larson et al. 2011). The current subanalysis of the ENEST1st study evaluated the impact of age on the deep molecular response and safety with frontline nilotinib in four major categories of patients based on age.
Methods
Patients
The subanalysis included all patients enrolled in the multicenter ENEST1st study, which recruited patients from 26 European countries. The inclusion and exclusion criteria have been previously published (Hochhaus et al. 2016a). Briefly, male or female patients with Philadelphia chromosome (Ph) or BCR–ABL1 + CML in chronic phase (CML-CP), aged ≥18 years and were within 6 months of diagnosis of the disease, were enrolled. Patients were also required to have a World Health Organization performance status of ≤2. Patients who had prior treatment with hydroxyurea (>6 months) or imatinib (>3 months) were not included. Patients with ventricular-paced pacemaker, congenital long QT syndrome, QTcF >450 ms, myocardial infarction within the past 12 months, or other clinically significant heart disease were excluded. In addition, patients with impaired gastrointestinal function, concurrent uncontrolled medical conditions that would present unacceptable safety risks or compromise compliance with the protocol, or concomitant treatment with medications with the potential to prolong the QT interval or known to be strong inducers or inhibitors of cytochrome P450 3A4 are also excluded. Informed consent was obtained from each patient in writing before any study-specific procedures were performed.
Study design and treatment
The ENEST1st was a multicenter, single-arm study evaluating nilotinib at 300 mg twice daily (bid) in newly diagnosed and previously untreated patients. ENEST1st was registered in the EU Clinical Trials Registry (2009-017775-19) and ClinicalTrials.gov (NCT01061177). In this subanalysis, the patients were stratified to four subgroups according to age at the time of study entry. The 4 groups that were defined for the purpose of this study were young patients (18–39 years), middle-aged patients (40–59 years), elderly patients (60–74 years), and old patients (≥75 years). The primary end point was the rate of MR4 at 18 months from the initiation of nilotinib. The secondary end points included rates of MR4 and MR4.5 at various time points, OS, and PFS. Safety was evaluated throughout the study, and National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 for toxicity and adverse event (AE) reporting was used to report AEs. All patients were treated with an initial dose of nilotinib 300 mg bid for up to 24 months. Dose escalation was not allowed beyond 300 mg bid of nilotinib. Dose reductions were permitted for grade 3 or 4 hematologic AEs related to white blood cells or platelets and for clinically significant nonhematological AEs of severity greater than grade 2. The study protocol for the ENEST1st study did not require regular monitoring of lipid and glucose profiles.
The study protocol was approved by the independent ethics committee (IEC) or institutional review board (IRB) for each center and was conducted according to the ethical principles of the Declaration of Helsinki.
Assessments
The molecular response rates assessed by the BCR–ABL1 transcript levels determined by multiplex polymerase chain reaction (PCR) at baseline and subsequently every 3 months by real-time quantitative PCR at a designated European Treatment Outcome Study (EUTOS) laboratory standardized to IS. Samples with a total of <10,000 ABL1 transcripts or <32,000 ABL1 transcripts were considered as not evaluable for MR4 or MR4.5, respectively. In the study, PFS was defined as the time from start of the study drug to the earliest progression to accelerated phase or blast crisis (AP/BC) or death from any cause, and OS was defined as the time from the start of the study drug to death from any cause.
Statistical analyses
The intention-to-treat (ITT) population and the safety sets consisted of all patients who received at least one dose of study drug and was used for demographics, baseline characteristics, efficacy analyses, and safety. For the evaluation of the molecular response, only those patients in the ITT population with typical BCR–ABL1 transcripts at screening, i.e., b3a2 and/or b2a2, were considered. For calculation of response rates “at” a designated time point, patients were considered responders only if an assessment at that time point showed achievement of the response. Response rates “by” a designated time point were calculated as cumulative response rates, counting all patients with a response detected at or before the specified time point as responders. All response rates were calculated as raw proportions. Rates of freedom from progression to AP/BC on treatment and OS were estimated using Kaplan–Meier product limit estimates according to ITT principles.
Results
Patients
In the ENEST1st study, from 2010 to 2012, 1164 patients were screened and 1091 patients were enrolled across 26 European countries in 307 sites (Fig. 1). Of the 1091 patients, 1089 patients who received ≥1 dose of nilotinib 300 mg bid were considered in the ITT analysis. Of the 1089 patients, 23% (n = 243), 45% (n = 494), 27% (n = 300), and 5% (n = 52) were categorized as young, middle age, elderly, and old, respectively, according to the defined criteria. Overall, the median age of the population was 53 years (range 18–91 years) and 59% were male. Except for the group with old patients, all groups had more males compared to females (Table 1). The young, middle-aged, and the elderly groups, respectively, had 34.6, 42.1, and 42.7% of female patients compared to 51.9% of females in the old group. Overall, the median time since diagnosis for the patients was 0.9 months (range <0.1–6.6 months) and was similar when evaluated by age group (Table 1). According to the Sokal risk score, overall, 377 patients (34.6%) were categorized as low risk, 408 (37.5%) as intermediate risk, and 197 (18.1%) as high risk; Sokal risk score could not be calculated for 107 patients (9.8%) due to missing information. More than 80% of patients (n = 900, 82.6%) were considered to be at low risk based on the EUTOS score and 8.6% (n = 94) at high risk, and information was missing for 8.7% (n = 95). In each of the age category, most of the patients were at low or intermediate risk based on the Sokal risk score and low risk based on the EUTOS risk score (Table 1). Based on the Sokal risk score, none of the patients in the old group were at low risk, while about half of the patients in the young (55.6%) and the middle age groups (41.1%) were at low risk. More than half of the patients in the elderly (53.0%) and in the old (69.2%) groups were at intermediate risk for Sokal score. Based on the EUTOS risk score, a slightly higher percentage of patients were at high risk in young age group (12.8%) compared to the other age categories. The percentages of eosinophils, basophils, and platelets were similar across the age groups, but the percentage of blasts was slightly higher, and the spleen size was comparatively bigger in the young patients than in the other age groups.
Fig. 1.
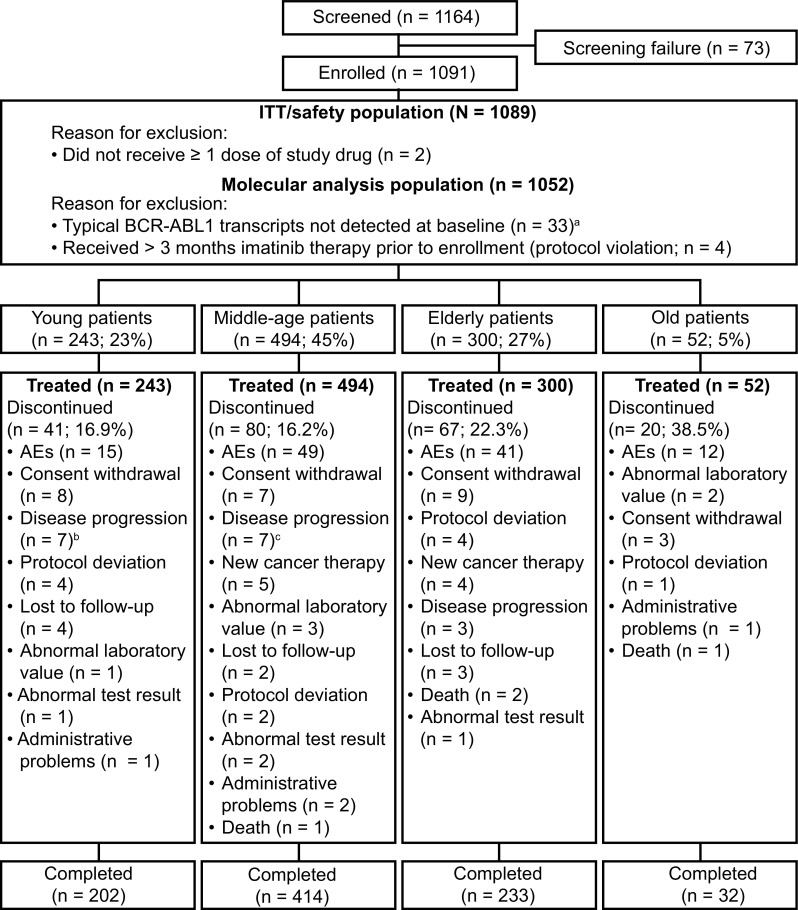
Patient disposition. aPatients not in the molecular analysis population were distributed in the age groups as follows: 9 patients in young, 18–39 years old, 10 patients in middle-aged, 40–59 years old, 10 patients in elderly, 60–74 years old, 4 patients in old, ≥75 years old, groups; btwo patients in the young group who discontinued due to progression to AP/BC are considered under disease progression; cfour patients in the middle-aged group who discontinued due to progression to AP/BC are considered under disease progression. ITT intention–to-treat, Ph philadelphia chromosome
Table 1.
Baseline demographics
| Characteristics | Young (18–39 years) (n = 243) |
Middle age (40–59 years) (n = 494) |
Elderly (60–74 years) (n = 300) |
Old (≥75 years) (n = 52) |
|---|---|---|---|---|
| Median age (range), years | 32 (18–39) | 50 (40–59) | 66 (60–74) | 78 (75–91) |
| Male/female (%) | 159/84 (34.6%) | 286/208 (42.1%) | 172/128 (42.7%) | 25/27 (51.9%) |
| Time since initial diagnosis of CML (months); median (range) | 0.86 (0.07, 5.86) | 0.92 (0.07, 6.61) | 0.92 (0.03, 60.99) | 0.86 (0.07, 6.02) |
| Sokal score, n (%) | ||||
| High risk | 31 (12.8) | 84 (17.0) | 71 (23.7) | 11 (21.2) |
| Intermediate risk | 52 (21.4) | 161 (32.6) | 159 (53.0) | 36 (69.2) |
| Low risk | 135 (55.6) | 203 (41.1) | 39 (13.0) | – |
| Missing | 25 (10.3) | 46 (9.3) | 31 (10.3) | 5 (9.6) |
| EUTOS score, n (%) | ||||
| High risk | 31 (12.8) | 39 (7.9) | 22 (7.3) | 2 (3.8) |
| Low risk | 190 (78.2) | 412 (83.4) | 252 (84.0) | 46 (88.5) |
| Missing | 22 (9.1) | 43 (8.7) | 26 (8.7) | 4 (7.7) |
| Laboratory parameters | ||||
| Peripheral blasts %, mean ± SD (n) | 2.05 ± 3.03 (230) | 1.60 ± 2.33 (475) | 1.49 ± 2.25 (283) | 0.98 ± 1.04 (48) |
| Peripheral eosinophils %, mean ± SD (n) | 2.88 ± 3.37 (234) | 2.73 ± 2.64 (479) | 2.85 ± 3.14 (289) | 2.47 ± 2.37 (49) |
| Peripheral basophils %, mean ± SD (n) | 4.02 ± 3.75 (234) | 4.07 ± 3.83 (478) | 4.08 ± 3.95 (292) | 3.89 ± 3.72 (50) |
| Platelets (109/L), mean ± SD (n) | 446.47 ± 312.07 (240) | 464.08 ± 352.63 (487) | 463.28 ± 308.61 (297) | 423.69 ± 233.39 (51) |
| Spleen size, cm | 5.01 ± 6.19 (226) | 3.22 ± 4.90 (461) | 2.46 ± 4.13 (276) | 1.53 ± 3.06 (49) |
| Previous CML therapy, n (%) | ||||
| Imatinib ≤1 month | 17 (7) | 32 (6.5) | 16 (5.3) | 2 (3.8) |
| Imatinib >1–2 months | 13 (5.3) | 33 (6.7) | 24 (8.0) | 1 (1.9) |
| Imatinib >2–3 months | 16 (6.6) | 20 (4) | 10 (3.3) | 4 (7.7) |
CML chronic myeloid leukemia, EUTOS European Treatment Outcome Study
Of the 1089 patients enrolled, 881 (80.9%) completed 24 months of study treatment and 208 (19.1%) discontinued. Discontinuation rates were 16.9% in young patients, 16.2% in middle-aged patients, 22.3% in elderly, and 38.5% in old patients, which was the highest among the four age groups (Fig. 1). Across all age groups, the most common reason for discontinuation was AEs, accounting for 36.6, 61.2, 61.2, and 60% of all discontinuations in the young, middle-aged, elderly, and old patients, respectively. On the other hand, of the patients discontinued, 17.1, 8.1, 4.5%, and none discontinued due to disease progression or treatment failure in the young, middle-aged, elderly, and old age groups, respectively.
The overall median (range) duration of exposure was 722 days (5–821 days) and did not vary much across the four age groups studied (Table 2). The median dose intensity was around 600 mg/day across all groups. A total of 492 patients (45.2%) underwent dose change or interruptions. Dose reductions were comparatively less in old patients, while dose interruptions were more frequent in this group compared to the other groups (Table 2).
Table 2.
Drug exposure
| Young (n = 243) | Middle age (n = 494) | Elderly (n = 300) | Old (n = 52) | |
|---|---|---|---|---|
| Duration of exposure, days (median, range) | 722.00 (46–821) | 724 (5–793) | 724 (1–798) | 709 (4–780) |
| Time of treatment, days (median, range) | 728 (46–960) | 728 (5–888) | 728 (1–817) | 718 (4–780) |
| Average daily dose, mg/day, (mean ± SD) | 583.1 ± 50.7 | 587.0 ± 46.4 | 577.6 ± 66.4 | 576.5 ± 64.9 |
| Dose intensity, mg/day (median, range) | 600 (257.8–620.5) | 600 (144.4–689.0) | 600 (173.9–625.7) | 598.5 (300.0–600.0) |
| Dose reductions, n (%) | 71 (29.2%) | 146 (29.6%) | 90 (30.0%) | 14 (26.9%) |
| 1 Dose reduction | 32 (13.2) | 76 (15.4) | 46 (15.3) | 6 (11.5) |
| 2 Dose reductions | 15 (6.2) | 30 (6.1) | 20 (6.7) | 5 (9.6) |
| >2 Dose reductions | 24 (9.9) | 40 (8.1) | 24 (8) | 3 (5.8) |
| Dose interruptions, n (%) | 84 (34.6%) | 168 (34.0%) | 113 (37.7%) | 22 (42.3%) |
| 1 Dose interruption | 39 (16.1) | 95 (19.2) | 57 (19.0) | 14 (26.9) |
| 2 Dose interruptions | 20 (8.2) | 44 (8.9) | 21 (7.0) | 4 (7.7) |
| >2 Dose interruptions | 25 (10.3) | 29 (5.9) | 35 (11.7) | 4 (7.7) |
Molecular response rates
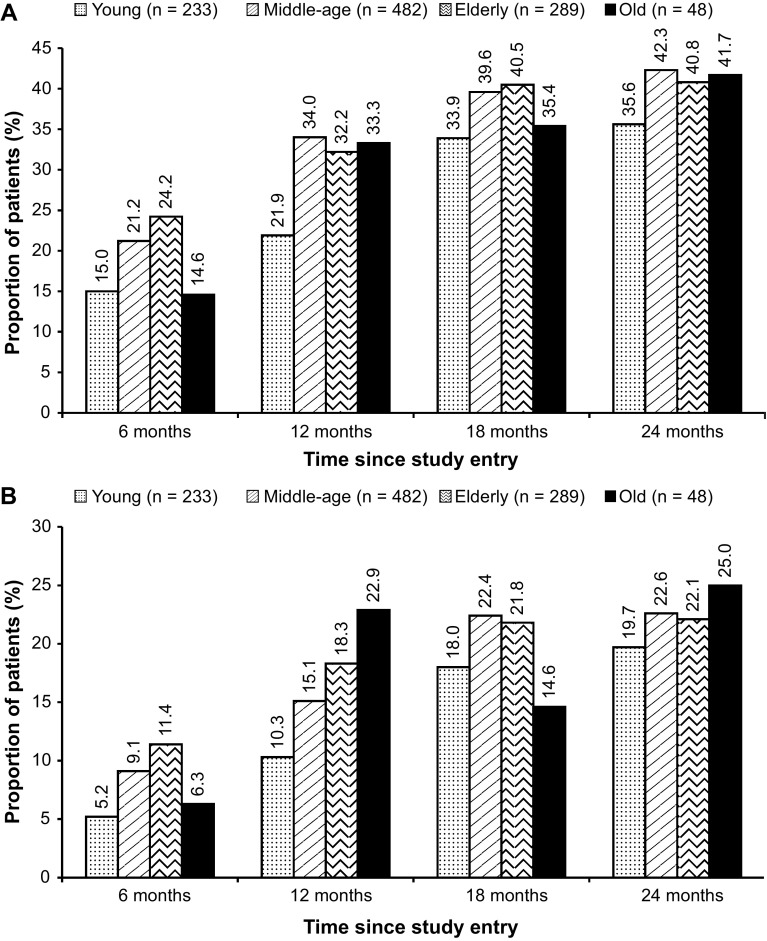
Among the 1082 patients, 1052 were considered for the evaluation of the molecular response rates. Overall, the rate of MR4 at 18 months was 38.4% (95% CI, 35.5–41.3%; n = 404). At 18 months, the rates of MR4 were 33.9% (95% CI, 27.8–40.0%) in the young adult patients, 39.6% (95% CI, 35.3–44.0%) in the middle-aged patients, 40.5% (95% CI, 34.8–46.1%) in the elderly patients, and 35.4% (95% CI, 21.9–48.9%) in the old patients (Fig. 2a). The rates of MR4.5 at 18 months were 18% (95% CI, 13.1–23.0%), 22.4% (95% CI, 18.7–26.1%), 21.8% (95% CI, 17.0–26.6%), and 14.6% (95% CI, 4.6–24.6%) in the young adults, middle-aged, elderly, and old patients, respectively. The MR4 and MR4.5 rates at 6, 12, and 24 months are presented in Fig. 2. In the overall population, cumulative rates of MR4 and MR4.5 by 24 months were 55.2% (n = 581) and 38.6% (n = 406), respectively. By 24 months, the rates of MR4 among patients were 50.2% (95% CI, 43.8–56.6%; n = 117) in the young age group, 57.1% (95% CI, 52.6–61.5%; n = 275) in the middle-aged group, and 57.4% (95% CI, 51.7–63.1%; n = 166) and 47.9% (95% CI, 33.8–62.0%; n = 23) in the elderly and the old patients, respectively. The rates of MR4.5 in the young adults, middle-aged, elderly, and old patients were 35.6% (95% CI, 29.5–41.8%; n = 83), 39.4% (95% CI, 35.1–43.8%; n = 190), 39.8% (95% CI, 34.1–45.4%; n = 115), and 37.5% (95% CI, 23.8–51.2%; n = 18) by 24 months, respectively. The rates of MR4 based on the Sokal risk score are presented in Table 3. Similar rates of MR4 were seen across the age groups, except for the young adult patients with intermediate risk and old patients with high risk, which showed considerably lower rates of MR4; 14 of 49 patients (28.6%) in the young group with intermediate risk and 2 of 10 patients (20.0%) in the old group with high risk achieved MR4.
Fig. 2.
Molecular response rates in the molecular analysis population (n = 1052). a Rates of MR4 at 6, 12, 18, and 24 months by age group. MR 4 molecular response 4 (MR4; BCR–ABL1 IS ≤0.01), IS international scale. b Rates of MR4.5 at 6, 12, 18, and 24 months by age group. MR 4.5 molecular response 4.5 (MR4.5; BCR–ABL1 IS ≤0.0032%), IS international scale
Table 3.
Effect of Sokal risk on the MR4 by age group
| MR4 by 24 months by Sokal risk group, % | Young (n = 233) | Middle age (n = 482) | Elderly (n = 289) | Old (n = 48) |
|---|---|---|---|---|
| Low | 56.9 | 62.3 | 63.2 | – |
| Intermediate | 28.6 | 54.1 | 58.4 | 51.5 |
| High | 44.8 | 46.4 | 54.4 | 20.3 |
MR 4 molecular response 4 (BCR–ABL1 IS ≤0.01%), IS international scale
Overall survival and progression-free survival
Overall, for the patients included in the ITT set, the estimated OS at 12 months was 99.6% (95% CI, 99.0–99.9%) and at 24 months was 98.9% (95% CI, 98.0–99.4%). There were 18 deaths reported in this study, of which 14 occurred more than 28 days after the last dose of the study drug. The four deaths occurring within 28 days of the last dose of the study drug or the 24-month evaluation included death due to pulmonary embolism, aortic valve stenosis, thrombocytopenia, and pneumonia (one patient each). None of the four deaths were suspected to be related to the study drug. The 14 deaths occurring after 28 days of the last dose of the study drug or the 24-month evaluation included death due to secondary cancer (three patients), sepsis (two patients), unknown cause (two patients), progression of CML, pneumonia, cardiac failure, cerebral infarction, ischemic stroke, complications during a stem cell transplant, and suicide (one patient, each).
Overall, there were six progression events, three patients each on treatment progressed to AP and BC, though none of them died on the study. Of the six events, two were observed in the young group and four in the middle-aged group. By 24 months, the estimated rate of freedom from progression to AP/BC on treatment was 99.1% (95% CI, 97.7–99.7%) in middle-aged and 99.2% (95% CI, 96.6–99.8%) in young patients, while it was 100% in both the elderly and old patients.
Adverse events
The overall safety results of the ENEST1st study are presented in Hochhaus et al. (2016a). The most common nonhematological AEs included rash, pruritus, headache, abdominal pain, fatigue, nausea, alopecia, and nasopharyngitis (Hochhaus et al. 2016a). Although the incidence of AEs was slightly different, no new specific safety signals were observed across the four age groups (Table 4). The most frequent nonhematological AEs were rash in young (21.4%) and the middle-aged (24.9%), and pruritus (18.3%) and nausea (19.2%) in elderly and old patients, respectively. Among hematological AEs, thrombocytopenia was the most frequent AE that was observed in young (14.8%), middle-aged (9.3%), and elderly patients (9.3%), while in old patients anemia (15.4%) was the more common hematological AE (Table 5). The common biochemical laboratory abnormalities included increase in bilirubin, increase in alanine aminotransferase (ALT), decrease in phosphate, increase in lipase, and arterial hypertension (Table 6). Among the age groups, arterial hypertension was less frequent in young patients (2.9%) compared to the other age groups (middle-aged, 6.3%; elderly, 7.3%; old, 9.6%); while increases in bilirubin, ALT, and aspartate aminotransferase (AST) were less frequent or absent in the old population. Frequency of grade 3 or 4 abnormalities was relatively less for nonhematological AEs compared to the hematological AEs. Of the total incidence of thrombocytopenia and neutropenia, more than 50% were of grade 3 or 4 except in the old patients, in whom it was only 1.9%. Overall, the incidence of grade 3 or 4 AEs was less frequent in old patients.
Table 4.
Most frequent all grades (≥10% in any group) nonhematological adverse events (AEs)
| AEs | Young (n = 243), n (%) | Middle age (n = 494), n (%) | Elderly (n = 300), n (%) | Old (n = 52), n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Abdominal pain | 33 (13.6) | 1 (0.4) | 74 (15.0) | 5 (1.0) | 45 (15.0) | 2 (0.7) | 8 (15.4) | – |
| Rash | 52 (21.4) | 1 (0.4) | 123 (24.9) | 1 (0.2) | 52 (17.3) | 1 (0.3) | 6 (11.5) | – |
| Pruritus | 33 (13.6) | 1 (0.4) | 83 (16.8) | 1 (0.2) | 55 (18.3) | 1 (0.3) | 9 (17.3) | – |
| Fatigue | 31 (12.8) | 1 (0.4) | 76 (15.4) | 4 (0.8) | 41 (13.7) | 1 (0.3) | 3 (5.8) | – |
| Headache | 51 (21.0) | 5 (2.1) | 86 (17.4) | 3 (0.6) | 25 (8.3) | – | 4 (7.7) | – |
| Nausea | 19 (7.8) | 2 (0.8) | 59 (11.9) | – | 35 (11.7) | 3 (1.0) | 10 (19.2) | – |
| Nasopharyngitis | 36 (14.8) | – | 44 (8.9) | – | 31 (10.3) | – | 2 (3.8) | – |
| Alopecia | 25 (10.3) | 1 (0.4) | 59 (11.9) | – | 24 (8.0) | – | 7 (13.5) | – |
| Dry skin | 17 (7.0) | – | 43 (8.7) | – | 30 (10.0) | – | 3 (5.8) | – |
| Myalgia | 26 (10.7) | 1 (0.4) | 52 (10.5) | – | 19 (6.3) | 1 (0.3) | 2 (3.8) | – |
| Muscle spasm | 13 (5.3) | – | 48 (9.7) | – | 30 (10.0) | – | 2 (3.8) | – |
| Diarrhea | 16 (6.6) | - | 39 (7.9) | 1 (0.2) | 35 (11.7) | 1 (0.3) | 4 (7.7) | – |
Table 5.
Most frequent hematologic adverse events (AEs) (≥5% in any group) of interest
| AEs | Young, n (%) | Middle age, n (%) | Elderly, n (%) | Old, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grade 3–4 | All grades | Grade 3–4 | All grades | Grade 3–4 | All grades | Grade 3–4 | |
| Thrombocytopenia | 36 (14.8) | 20 (8.2) | 46 (9.3) | 28 (5.6) | 28 (9.3) | 17 (5.7) | 3 (5.8) | 1 (1.9) |
| Anemia | 13 (5.3) | 4 (1.6) | 28 (5.7) | 5 (1.0) | 18 (6.0) | 7 (2.3) | 8 (15.4) | 1 (1.9) |
| Neutropenia | 14 (5.8) | 12 (4.9) | 20 (4.0) | 11 (2.2) | 7 (2.3) | 6 (2.0) | 2 (3.8) | 1 (1.9) |
| Leukopenia | 7 (2.9) | 1 (0.4) | 13 (2.6) | 4 (0.8) | 5 (1.7) | 3 (1.0) | – | – |
| Lymphopenia | 2 (0.8) | – | 1 (0.2) | – | 1 (0.3) | – | – | – |
Table 6.
Most frequent (≥5% in any group) laboratory abnormalities
| Laboratory abnormalities | Young, n (%) | Middle age, n (%) | Elderly, n (%) | Old, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| All grade | Grade 3 or 4 | All grade | Grade 3 or 4 | All grade | Grade 3 or 4 | All grade | Grade 3 or 4 | |
| Total bilirubin ↑ | 25 (10.3) | 5 (2.1) | 36 (7.3) | 5 (1.0) | 17 (5.7) | 4 (1.3) | 2 (3.8) | |
| ALT ↑ | 27 (11.1) | 4 (1.6) | 49 (9.9) | 9 (1.8) | 9 (3.0) | 2 (0.7) | 1 (1.9) | 1 (1.9) |
| AST ↑ | 14 (5.8) | 1 (0.4) | 26 (5.3) | 4 (0.8) | 11 (3.7) | 1 (0.3) | – | |
| Phosphate ↓ | 13 (5.3) | 6 (2.5) | 48 (9.7) | 15 (3.0) | 15 (5.0) | 3 (1.0) | 1 (1.9) | |
| Lipase ↑ | 7 (2.9) | 3 (1.2) | 37 (7.5) | 16 (3.2) | 30 (10) | 18 (6.0) | 3 (5.8) | 1 (1.9) |
| Hypertension | 7 (2.9) | 31 (6.3) | 4 (0.8) | 22 (7.3) | 6 (2.0) | 5 (9.6) | 2 (3.8) | |
ALT alanine aminotransferase, AST aspartate aminotransferase
The cardiovascular AEs by age group are presented in Table 7. By Fisher’s exact test, there was a significant difference (P ≤0.0001) in the incidence of cardiovascular events (CVEs) overall, across the age groups, with very low incidence in the young patients compared to the other age groups (Table 7). Among the CVEs, the incidence of ischemic heart disease was significantly different across age groups (P <0.0002), while peripheral arterial occlusive disease and ischemic cerebrovascular event did not differ significantly across age groups. It was not mandatory to monitor the lipid profile and glucose routinely as per the protocol. However, AEs related to hypercholesterolemia (3.0%), hyperglycemia (3.3%), and diabetes mellitus (1.2%) were reported earlier for the overall population (Hochhaus et al. 2016a).
Table 7.
Cardiovascular adverse events (AEs) by age group
| Cardiovascular AEs | Young (n = 243) | Adult (n = 494) | Elderly (n = 300) | Old (n = 52) | P value by Fisher’s exact test* |
|---|---|---|---|---|---|
| Cardiovascular events | 2 (0.8%) | 26 (5.3%) | 30 (10%) | 7 (13.5%) | <0.0001 |
| Ischemic heart disease | 1 (0.4%) | 14 (2.8%) | 17 (5.7%) | 5 (9.6%) | 0.0002 |
| Peripheral arterial occlusive disease | 1 (0.4%) | 9 (1.8%) | 9 (3.0%) | 1 (1.9%) | 0.12 |
| Ischemic cerebrovascular event | 0 | 4 (0.8%) | 4 (1.3%) | 1 (1.9%) | 0.19 |
*P values provided are nominal, post hoc, and for descriptive purpose only; no multiplicity adjustments were made
Discussion
The ENEST1st study evaluated deep molecular response with nilotinib in newly diagnosed patients with CML (Hochhaus et al. 2016a). This subanalysis of the ENEST1st study was conducted to assess the impact of age on deep molecular response and AEs with frontline nilotinib. The ENEST1st study had shown that among the patients analyzed for molecular response, 38.4% achieved MR4 at 18 months and 55.2% achieved MR4 by 24 months. When categorized into 4 age groups comprising young, middle age, elderly, and old patients, molecular response rates across the groups were consistent with the overall population. To the best of our knowledge, this subanalysis is the first to compare the safety and efficacy of frontline nilotinib across the four different age groups.
In the earlier studies with busulfan, hydroxyurea, and allogeneic stem cell transplantation, older age was considered to negatively impact the response and survival outcomes (Silver et al. 1999; Berger et al. 2003) and indicated poor prognosis in patients with CML (Kantarjian et al. 1987). However, later studies evaluating the impact of age on response rates with imatinib have been conflicting. In a study by Rosti et al., in patients with CML in late CP, lower rates of complete hematologic response (CHR) and complete cytogenetic response (CCyR) were observed in patients >65 years compared to younger patients, though OS was the same (Rosti et al. 2007). Gugliotta et al. in 2011, however, suggested no impact of age on the response rates upon treatment with imatinib (Gugliotta et al. 2011). However, it should be noted that there was a slight difference in the disease stage of the patients in the two studies. The first study included patients in late chronic phase who were resistant to interferon alpha, treated with imatinib, while the second study included patients in early chronic phase treated with frontline imatinib. Earlier in a large study of more than 700 patients at the MD Anderson center, no differences in the CCyR and OS were observed between patients over 60 years and younger patients (Cortes et al. 2003).
A few studies have evaluated the impact of age on the safety and efficacy of second-generation TKIs in elderly. In the phase 2 study, in imatinib-resistant/intolerant patients who were treated with nilotinib, more patients <65 years of age achieved major cytogenetic response (MCyR; 63%) and CCyR (44%), compared with patients ≥65 years (MCyR, 48%; CCyR, 38%) (Lipton et al. 2008). The estimated OS rates at 12 months were higher for patients <65 years (97%) compared with patients ≥65 years (91%) (Lipton et al. 2008; le Coutre et al. 2009). However, in the ENACT study, which evaluated the response rates for patients ≥60 years, rate of CHR was comparable to that of the overall population (le Coutre et al. 2009). In the pivotal phase 3 ENESTnd study, MMR rates by 24 months for patients aged ≥65 years and those <65 years were similar for those treated with 300 mg but was slightly lower for the older patients (61%) on 400 mg of nilotinib bid, compared to younger patients (67%) (Larson et al. 2011). A subanalysis in the pivotal DASISION trial, which compared frontline imatinib with dasatinib, was one of the very few trials that compared the response rates in three different age groups comprising patients <46 years, patients aged between 46 years and 55 years, and those aged >65 years (Khoury et al. 2010). The response rates did not vary substantially with CCyR rates in the three groups ranging between 78 and 88% and MMR rates from 45 to 50%.
A number of studies have demonstrated that deep molecular response with TKIs resulted in better outcomes in patients with CML (Falchi et al. 2013). In the ENESTcmr study, patients who could not achieve molecular response with imatinib could achieve it after switching to nilotinib; while in the ENESTnd study more patients achieved MR4 and MR4.5 with nilotinib compared to imatinib (Hughes et al. 2014; Hochhaus et al. 2016b). The ENEST1st study conducted in a large patient population of more than 1000 patients further confirmed the efficacy of frontline nilotinib with a better response than in the ENESTnd study, in which 39 and 25% achieved MR4 and MR4.5 by 24 months, compared to 56 and 38% in the ENEST1st study, respectively (Hochhaus et al. 2016a).
Most studies, including the ENESTnd that evaluated the impact of age, have categorized patients broadly into elderly patients who were older than 60 or 65 years and those who were younger. The classification made in this analysis, with subgroups ranging from 15 to 20 years each, would enable us to identify any differences, which may be lost due to a broader classification. The current study did not show any major difference between the age groups analyzed, though comparatively weaker responses were seen for younger patients compared to the older patients. The younger patients in particular had a higher percentage of blasts and spleen size compared to the older patients as has also been reported in other studies (Pemmaraju et al. 2012; Kalmanti et al. 2014; Castagnetti et al. 2015). Somewhat similar approach has earlier been seen with the CML IV study with >1500 patients and the GIMEMA CML working group studies with >2500 patients, which were large studies and also evaluated the impact of age in patients with CML treated with TKIs. In these studies (Kalmanti et al. 2014; Castagnetti et al. 2015), in which the patients were classified into 3 to 4 categories of age, as in the current study, it was seen that younger patients with CML typically present with a more expanded disease (Pemmaraju et al. 2012; Kalmanti et al. 2014; Castagnetti et al. 2015) and a higher incidence of hematologic toxicity as also seen here (Pemmaraju et al. 2012). In a study by Kalmanti et al., poor prognostic indicators in younger patients did not seem to affect their response to frontline imatinib (Kalmanti et al. 2014). However, according to Pemmaraju et al. young adults showed comparatively lower response rates to frontline TKIs compared to older patients, though transformation-free survival, and the OS remained similar (Pemmaraju et al. 2012). Further analysis in this age group might be needed. Since the current study enrolled newly diagnosed patients according to protocol inclusion and exclusion criteria, the elderly and the old patients could have been healthier; hence, better efficacy and tolerability to some drug- or disease-related AEs were seen compared to young adults. The responses could also reflect a possible selection bias of the investigators in recruiting younger patients into the clinical trials compared to population-based registries as is also reported by other authors (Rohrbacher et al. 2009).
Recently, the therapeutic landscape of CML is moving toward the goal of achieving TFR, and one of the major criteria for patients to attempt to discontinue treatment is a sustained molecular response (Hughes and Ross 2016). Many studies investigating the predictors of successful TFR have also evaluated age as a potential predictor (Lee et al. 2016; Etienne et al. 2017). The ENEST1st study evaluated deep molecular response with frontline nilotinib, which potentially indicates that the population may be eligible to attempt TFR. The impact on age, if any, on attaining deep molecular response may indicate potential differences in the age of the population, which can attempt TFR. However, since no significant differences were observed in the response rates with the different age groups, an impact of age on the patients attempting TFR or on the outcome of TFR may not be likely. This is further indicated in the studies, in which age was not found to be a predictor for outcomes of TFR (Lee et al. 2016).
Overall, safety was consistent with the known profile of nilotinib. The overall safety signals for the ENEST1st study were similar to those of the ENESTnd, though at a lower frequency than ENESTnd suggesting better management of the disease in this study (Hochhaus et al. 2016a). Although the distribution of some of the AEs differed across age groups, safety signals specific for a particular age group could not be identified except for CVEs, which were significantly less for the young patients. These data highlight the need for appropriate monitoring for relevant risk factors in all patients receiving nilotinib therapy with immediate appropriate intervention when needed, especially if a CVE occurs. (Rea et al. 2015; Castagnetti et al. 2016).
Even though the study enrolled a large patient population with sufficient number of patients in each group, the subanalysis was not designed to formally test the difference across the subgroups. In the ENEST1st study, monitoring of glucose and lipid was not mandatory and the overall frequency of these AEs and also their differences with age, if any, could not be ascertained and were probably underestimated. The current classification used in the study does not conform to any standard classification of age, e.g., the US census bureau or World Health Organization, and was done to introduce additional categories, though arbitrarily, to identify differences in population if any.
The ENEST1st study had shown high molecular response rates with approximately 55.2% of the patients achieving MR4 by 24 months. This subanalysis of the ENEST1st study showed that age had minimal impact on the deep molecular response rates associated with nilotinib therapy in newly diagnosed patients with CML and eventually on the eligibility of the patients to attempt TFR. This together with almost 100% freedom from progression by 24 months in any of the age groups further demonstrated the efficacy of frontline nilotinib. Although the main causes of discontinuation were similar across the young, middle-aged, elderly, and old patients, the distribution varied slightly across the age groups. Understanding the variations in disease characteristics and AEs with TKI therapy with respect to patient age may help improve CML therapy. Especially in older patients with a higher proportion of comorbidities, a more flexible dosing scheme may be warranted to increase tolerability while maintaining the deep molecular responses.
Acknowledgements
The authors wish to thank all physicians, study coordinators, and documentation assistants for their active participation and cooperation. In addition to the authors of this report, members of the Scientific Study Management Committee included Philippe Rousselot, MD, and David Marin, MD; members of the data management committee included Nelson Spector, MD, PhD, and Armand Keating, MD, FRCP. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. The authors thank Amrita Dutta, PhD, Novartis Healthcare Pvt Ltd., Hyderabad, for medical editorial assistance.
Funding
The study was sponsored by Novartis Pharmaceuticals Corporation.
Compliance with ethical standards
Conflict of interest
FJ Giles reports consultancy from Novartis. D Rea reports honoraria from Novartis, BMS, Incyte, and Pfizer. G Rosti reports fees for speaker’s bureau from Novartis, BMS, Incyte, and Pfizer. NCP Cross reports fees for speaker’s bureau, advocacy board, and honoraria from Novartis. JL Steegmann reports research support from Ariad, BMS, Novartis, and Pfizer; honoraria from Idem, and fees for advisory board from Idem. L Griskevicius reports research grant from Novartis. P le Coutre reports honoraria from Novartis, BMS, Pfizer, and Ariad. DCoriu reports honoraria and fees for advisory board from Novartis, Pfizer, and BMS. GJ Ossenkoppele GJ reports consultancy from Novartis, Celgene, J&J, and Seattle SG, and research grant from Amgen, Johnson &Johnson, and Celgene. FX Mahon reports honoraria from BMS, Novartis, Pfizer, and Ariad, and research grant from Novartis. S Saussele reports honoraria from BMS, Novartis, Pfizer, and Incyte, and research grant from Novartis and BMS. P Koskenvesa reports consultancy from Pfizer, Novartis, and Incyte. TH Brümmendorf reports research grant from Novartis and Pfizer, and fees for advisory board from Novartis, BMS, Pfizer, and Ariad. F Castagnetti reports consultancy and honoraria from Novartis, BMS, Incyte, and Pfizer. A Hochhaus reports research funding from Novartis, Pfizer, Ariad, and BMS. B Vincenzi and J Haenig are employees of Novartis. L Petrov, A Hellmann, and G Gastl have no conflicts to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Berger U, et al. Chronic myeloid leukemia in the elderly: long-term results from randomized trials with interferon alpha. Leukemia. 2003;17:1820–1826. doi: 10.1038/sj.leu.2403042. [DOI] [PubMed] [Google Scholar]
- Breccia M, Tiribelli M, Alimena G. Tyrosine kinase inhibitors for elderly chronic myeloid leukemia patients: a systematic review of efficacy and safety data. Crit Rev Oncol Hematol. 2012;84:93–100. doi: 10.1016/j.critrevonc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Castagnetti F, et al. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol. 2015;26:185–192. doi: 10.1093/annonc/mdu490. [DOI] [PubMed] [Google Scholar]
- Castagnetti F, et al. Nilotinib 300mg twice daily: an academic single-arm study of newly diagnosed chronic phase chronic myeloid leukemia patients. Haematologica. 2016;101:1200–1207. doi: 10.3324/haematol.2016.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, et al. Effects of age on prognosis with imatinib mesylate therapy for patients with Philadelphia chromosome-positive chronic myelogenous leukemia. Cancer. 2003;98:1105–1113. doi: 10.1002/cncr.11629. [DOI] [PubMed] [Google Scholar]
- Etienne G, et al. Achieving deeper molecular response is associated with a better clinical outcome in chronic myeloid leukemia patients on imatinib front-line therapy. Haematologica. 2014;99:458–464. doi: 10.3324/haematol.2013.095158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne G, et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- Falchi L, et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am J Hematol. 2013;88:1024–1029. doi: 10.1002/ajh.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliotta G, et al. Frontline imatinib treatment of chronic myeloid leukemia: no impact of age on outcome, a survey by the GIMEMA CML working party. Blood. 2011;117:5591–5599. doi: 10.1182/blood-2010-12-324228. [DOI] [PubMed] [Google Scholar]
- Hehlmann R, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–423. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia. 2016;30:57–64. doi: 10.1038/leu.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhaus A, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23. doi: 10.1182/blood-2016-01-694265. [DOI] [PubMed] [Google Scholar]
- Hughes TP, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124:729–736. doi: 10.1182/blood-2013-12-544015. [DOI] [PubMed] [Google Scholar]
- Hughes TP, et al. Results from enestop: treatment-free remission (Tfr) following switch to nilotinib (Nil) in patients (Pts) with chronic myeloid leukemia in chronic phase (Cml-Cp) Haematologica. 2016;101:65–65. [Google Scholar]
- Kalmanti L, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol. 2014;93:71–80. doi: 10.1007/s00277-013-1937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, Keating MJ, McCredie KB, Walters R, Talpaz M, Smith TL, Freireich EJ. Old age: a sign of poor prognosis in patients with chronic myelogenous leukemia. South Med J. 1987;80:1228–1232. doi: 10.1097/00007611-198710000-00007. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- Khoury HJ, et al. Safety and efficacy of dasatinib (DAS) vs. imatinib (IM) by baseline comorbidity in patients with chronic myeloid leukemia in chronic phase (CML-CP): analysis of the DASISION trial. Blood. 2010;116:1401–1401. [Google Scholar]
- Larson RA, et al. Nilotinib shows safety and efficacy in older patients (≥65 years) with newly diagnosed chronic myeloid leukemia in chronic phase comparable with that in younger patients with chronic myeloid leukemia in chronic phase: results from ENESTnd. Blood. 2011;118:1608–1609. doi: 10.1182/blood-2011-08-369017. [DOI] [Google Scholar]
- le Coutre PD, et al. Efficacy and safety of nilotinib in elderly patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML) in chronic phase (CP): a sub-analysis of the ENACT (expanding nilotinib access in clinical trials) study. Blood. 2009;114:1272–1273. [Google Scholar]
- Lee SE, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101:717–723. doi: 10.3324/haematol.2015.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JH, le Coutre PD, Wang J, Yang M, Szczudlo T, Giles F. Nilotinib in elderly chronic myeloid leukemia patients in chronic phase (CML-CP) with imatinib resistance or intolerance: efficacy and safety analysis. Blood. 2008;112:1110–1110. [Google Scholar]
- Pemmaraju N, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97:1029–1035. doi: 10.3324/haematol.2011.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetel U, et al. Older patients with chronic myeloid leukemia (≥65 years) profit more from higher imatinib doses than younger patients: a subanalysis of the randomized CML-Study IV. Ann Hematol. 2014;93:1167–1176. doi: 10.1007/s00277-014-2041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea D, et al. Usefulness of the 2012 European CVD risk assessment model to identify patients at high risk of cardiovascular events during nilotinib therapy in chronic myeloid leukemia. Leukemia. 2015;29:1206–1209. doi: 10.1038/leu.2014.342. [DOI] [PubMed] [Google Scholar]
- Rohrbacher M, et al. Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia. 2009;23:602–604. doi: 10.1038/leu.2008.245. [DOI] [PubMed] [Google Scholar]
- Rosti G, et al. Impact of age on the outcome of patients with chronic myeloid leukemia in late chronic phase: results of a phase II study of the GIMEMA CML working party. Haematologica. 2007;92:101–105. doi: 10.3324/haematol.10239. [DOI] [PubMed] [Google Scholar]
- Saglio G, et al. Results from ENESTfreedom: treatment-free remission (Tfr) following frontline nilotinib (Nil) in patients (Pts) with chronic myeloid leukemia in chronic phase (Cml-Cp) Haematologica. 2016;101:240–241. doi: 10.3324/haematol.2016.143818. [DOI] [Google Scholar]
- Silver RT, et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood. 1999;94:1517–1536. [PubMed] [Google Scholar]
- Tasigna [package insert] (2015) Novartis Pharmaceuticals Corporation, NJ, East Hanover