Abstract
Evidence shows that Black women diagnosed with breast cancer are substantially less likely to undergo BRCA testing and other multipanel genetic testing compared to White women, despite having a higher incidence of early‐age onset breast cancer and triple‐negative breast cancer (TNBC). Our study identifies predictors of BRCA testing among Black women treated for breast cancer and examines differences between BRCA testers and nontesters. We conducted an analysis of 945 Black women ages 18–64 diagnosed with localized or regional‐stage invasive breast cancer in Pennsylvania and Florida between 2007 and 2009. Logistic regression was used to identify predictors of BRCA 1/2 testing. Few (27%) (n = 252) of the participants reported having BRCA testing. In the multivariate analysis, we found that perceived benefits of BRCA testing (predisposing factor) ([OR], 1.16; 95% CI: 1.11–1.21; P < 0.001), income (enabling factor) ([OR], 2.10; 95% CI: 1.16–3.80; p = 0.014), and BRCA mutation risk category (need factor) ([OR], 3.78; 95% CI: 2.31–6.19; P < 0.001) predicted BRCA testing. These results suggest that interventions to reduce disparities in BRCA testing should focus on identifying patients with high risk of mutation, increasing patient understanding of the benefits of BRCA testing, and removing financial and other administrative barriers to genetic testing.
Keywords: Black women, BRCA 1/2 testing, breast cancer, cancer prevention, genetics
Introduction
Racial disparities in BRCA1 and BRCA2 genetic testing persist despite clinical availability of testing for mutations over the past 20 years 1, 2. While rates of genetic testing among women diagnosed with breast cancer appear to be increasing 3, Black women affected with breast cancer are substantially less likely to undergo BRCA1/2 genetic testing compared to White women with the disease 2. This racial disparity is concerning as Black women have a higher incidence of early‐age onset breast cancer before age 50 (33% vs. 21.9%) 4; are twice as likely to be diagnosed with triple‐negative breast cancer (TNBC) (22 vs.11%) 5, an aggressive form of breast cancer that has been associated with a BRCA1 gene mutation 6; and have a 42% higher mortality rate from breast cancer compared to White women 7. As recent studies have documented a high prevalence of BRCA and other high penetrance gene mutations among Black women with breast cancer 8, 9, 10, there is a critical need to increase uptake of genetic testing among this population to improve personalized cancer care and to reduce cancer risk.
Germline mutations in BRCA1 and BRCA2 tumor suppressor genes are associated with an increased risk of breast and ovarian cancers 6. BRCA1 mutation carriers have a 55–65% risk and BRCA2 carriers have a 45% risk of developing breast cancer by age 70 11. BRCA1 mutation carriers have a 39% risk and BRCA2 carriers have a 11–17% risk of developing ovarian cancer by age 70 11. Genetic testing has implications for precision prevention, as a woman who has inherited a BRCA1 or BRCA2 mutation can reduce her cancer risk through risk‐reducing surgeries 12, and also receive enhanced screening to promote early detection 13. There are also implications for cancer survivors long after treatment and for their at‐risk relatives who can benefit from knowing their hereditary cancer risk 14, 15.
The National Comprehensive Cancer Network (NCCN) guidelines recommend BRCA1/2 testing in women diagnosed with breast cancer at age 45 and younger and those diagnosed with triple‐negative breast cancer at age 60 and younger 16. However, a recent population‐based study of over 3000 breast cancer patients found significant racial disparities in BRCA testing between Black and White patients that were driven by patient and healthcare‐provider‐related factors 2. The difference in use of BRCA testing was not eliminated after adjustment for mutation risk, sociodemographic and clinical factors, and attitudes about BRCA testing. The authors also found that Black women were less likely to report a recommendation from their physician to have BRCA testing even after adjustment for mutation risk. In this current analysis, we focus on patient‐level factors to identify predictors of BRCA testing among Black women with breast cancer. We also examine differences between Black women who had BRCA testing and those who did not. Our study is guided by the Andersen Behavioral Model of Health Care Utilization, shown in Figure 1 17. This theoretical framework posits that an individual's use of a particular healthcare service is a function of predisposing, enabling, and need factors 18.
Figure 1.
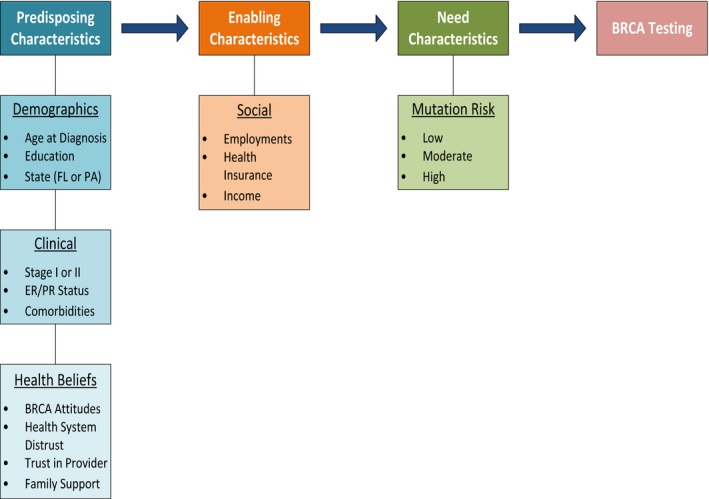
Andersen behavioral model of healthcare utilization.
Methods
Study design
We conducted an analysis of 945 Black women ages 18–64 diagnosed with localized or regional‐stage invasive breast cancer in Pennsylvania (PA) and Florida (FL) between January 1, 2007 and December 31, 2009 who were enrolled in a population‐based study. Eligibility criteria, methods of patient recruitment, and study design of this study have been previously described 2. Briefly, participants were surveyed by mail 24–36 months after cancer diagnosis with additional telephone recruitment efforts made for Black nonresponders up to 48 months after diagnosis. Women diagnosed before the age of 65 were included, to enrich the sample of women who would be appropriate candidates for genetic testing based on the NCCN Clinical Practice Guidelines 19.
The overall response rate was 61% (58% for Black women and 62% among White women) 20. Patient response rate was calculated using American Association for Public Opinion Research (AAPOR) guidelines, definition 4, which adjusts the response rate based on the estimated proportion of cases of unknown eligibility that are actually eligible 21. The responses were categorized: complete, refusal, nonrespondent, and ineligible, which included deceased respondents, those with incorrect contact information, and those with language barriers, or who reported not having been diagnosed with breast cancer. As shown in Figure 2, of the total 3737 Black patients sent a mailed survey, 1389 were ineligible, 1027 completed and returned the survey, 210 refused to participate, and 1111 were nonresponders. This resulted in the reported response rate of 58% using AAPOR definition 4. Of the 1027 respondents, participants were excluded from the current analysis if their self‐reported race was not Black (N = 5), or they were diagnosed with stage 3 or 4 breast cancer or unknown stage at diagnosis (N = 77). These exclusions resulted in the final study population of 945. This study was approved by the institutional review boards at the University of Pennsylvania, the Pennsylvania (PA) State Cancer Registry, Florida (FL) State Cancer Registry, and Massachusetts General Hospital (MGH).
Figure 2.
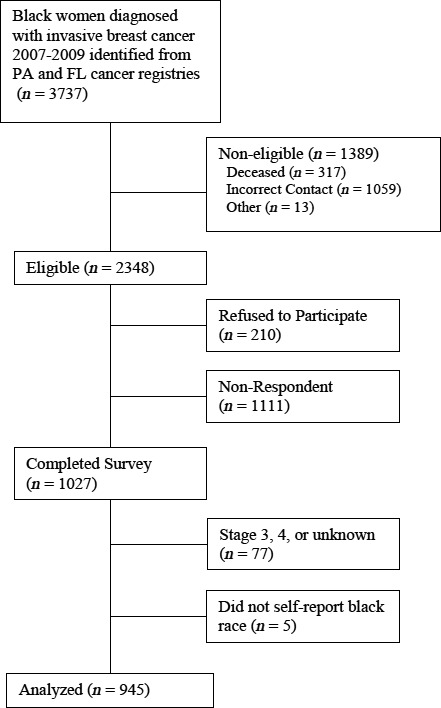
Survey responses.
Measures
Sociodemographic and clinical factors
Participants completed a written survey including, but not limited to, items on patient demographics, personal and family cancer history, attitudes about BRCA testing, and use of genetic testing. BRCA1/2 testing was assessed with a single item that provided the following explanation, “BRCA testing: this is a blood test (not a test on the tumor) that looks for genetic mutations in the BRCA1 and BRCA2 gene. Results can tell if a woman is at increased risk for developing ovarian cancer and a second breast cancer. Results can also help determine if relatives are at risk for these cancers.” Participants were asked if they completed BRCA testing. Education, employment, income, health insurance type, and comorbidities were assessed using previously established survey items. Age, stage at diagnosis, and receptor status were determined from FL and PA cancer registry data files.
BRCA mutation risk category
Risk of carrying a BRCA1/2 mutation was categorized into mutually exclusive categories based on high, moderate, and low risk as shown in Table 1. We used age at diagnosis, family history, and Ashkenazi Jewish heritage to categorize risk based upon 2007 NCCN guidelines, which recommended genetic testing for women diagnosed with breast cancer at age 40 and younger 19.
Table 1.
BRCA1/2 Mutation risk categories
High risk – Women were categorized as high mutation risk if they met any of the five criteria below:
|
Moderate risk – Women were categorized as moderate mutation risk if they did not meet high‐risk criteria and met either of the two criteria below:
|
Low risk – Women were categorized as low mutation risk if they did not meet criteria for high or moderate risk, that is,
|
Attitudes about BRCA 1/2 testing
Attitudes about BRCA testing were measured using a multidimensional scale that included four items about the potential benefits of testing, one item about the cost of testing as a barrier, and three items about the potential adverse effects. Participants rated their level of agreement with each item on a five‐point Likert scale. First, benefits of testing were assessed with the following four items: (1) BRCA 1/2 testing would help my family members manage their cancer risk; (2) My BRCA 1/2 test results would help me manage my cancer risk; (3) My BRCA 1/2 test results would help my doctor manage my cancer risk; and (4) Testing negative for a BRCA mutation would be reassuring about my cancer risk. Second, participants were asked the following item related to cost of testing, BRCA 1/2 testing is too expensive for me to afford. Third, adverse effects of testing were assessed with the following three items: (1) BRCA 1/2 testing would lead to problems in my family; (2) Testing positive for a BRCA mutation would lead to problems with my job; and (3) Testing positive for BRCA mutation would lead to problems with my health or life insurance. The scale was developed through extensive qualitative research with women considering genetic testing that included identification of themes and item generation. The scale was validated through an expert panel of genetic counselors and cancer genetics specialists as well as prior studies 22, 23, 24, 25, 26 showing variation across patient characteristics hypothesized to be associated with testing attitudes and through the scale's association with testing use in prior studies. The scale has been shown to have good reliability (Cronbach's Alpha = 0.75) 22, 23. Higher scores indicate more positive attitudes regarding testing. To further explore the role of attitudes toward BRCA testing on test utilization among Black women, we examined the subscale scores for benefits (Cronbach's Alpha = 0.91), costs (single item), and concerns (0.76) in a sensitivity analysis.
Healthcare system distrust
We assessed healthcare system distrust using a nine‐item scale that includes two primary domains, one that assessed values congruence (values of the healthcare system such as honesty, motives, and equity) and the other assessed technical competence of the healthcare system (Cronbach's Alpha = 0.85) 27, 28, 29, 30. Higher scores generated indicated greater distrust of the healthcare system.
Family support
Participants were also asked about family support using four items from a shorter version of the Perceived Social Support from Family Measure (Cronbach's Alpha = 0.73) 31, 32. Higher scores indicated greater family support.
Trust in provider
Trust in provider was measured using seven items from the Trust in Physician scale and measures different aspects of a trusting physician relationship from a patient's perspective (Cronbach's Alpha = 0.81) 33, 34, 35, 36. Our survey asked participants to report which doctor they are thinking about when answering the questions related to trust. Fifty‐one percent of patients referred to their oncologist, 25% referred to their surgeon, 12% referred to another type of doctor, and 12% did not specify the type of physician. Responses ranged from “strongly disagree” (one point) to “strongly agree” (five points). All items were summed; higher scores indicated more trust in provider.
Statistical analysis
Comparisons were made between women who reported having BRCA testing and those who did not use two‐sample t‐tests for continuous variables and chi‐squared tests for categorical variables. We used descriptive statistics to characterize the study sample. Patient characteristics included age at diagnosis, stage at diagnosis (I or II) 37, ER/PR receptor status (negative, positive, unknown), education (high school or less, college, graduate school, unknown), income (<$30,000, $30,000–70,000, >$70,000, unknown), health insurance type (Medicaid, Medicare, self‐pay, employer based), state (Pennsylvania, Florida), and number of comorbidities (0, 1, 2, or more). We also compared BRCA testers with nontesters based on BRCA attitude scale (continuous), healthcare system distrust scale (continuous), family support scale (continuous), trust in healthcare provider scale (continuous), and risk group (low, moderate, high). In a multivariate analysis, we assessed the association between predisposing (education, comorbidities, BRCA attitude, trust in provider); enabling (insurance type and income); and need factors (BRCA mutation risk categories) and BRCA 1/2 testing using logistic regression. Model fit was assessed using the Hosmer–Lemeshow Goodness‐of‐Fit Test. We also performed sensitivity analyses excluding women in the low mutation risk group and assessing associations of BRCA attitude subscales with BRCA testing. A two‐sided P < 0.05 was used as the statistical significance level. Statistical analyses were performed using STATA/IC version 14, (College Station, TX).
Results
Participant characteristics
A total of 945 Black women ages 18–64 diagnosed with localized or regional‐stage invasive breast cancer were included in this study. Overall, 27% (n = 252) of the women reported having BRCA testing and 73% (n = 693) did not have BRCA testing. Among women who had BRCA testing, 49.2% (n = 124) had a high BRCA mutation risk; 34.9% (n = 88) had a moderate risk; and 15.9% (n = 40) had a low mutation risk. Of those who reported not having BRCA testing, 22.2% (139) had a high BRCA mutation risk; 39.5% (n = 247) had a moderate risk; and 38.2% (n = 239) had a low mutation risk. Sample characteristics of BRCA testers and nontesters are presented in Table 2 and is organized using predisposing, enabling, and need factors based on the theoretical framework. In an unadjusted analysis, predisposing characteristics revealed that Black women who reported having BRCA testing were younger (mean age 47.2 vs. 53.2 years, P < 0.001); more likely to have completed graduate education (21.6 vs. 11.5, P < 0.001); had more positive attitudes about BRCA testing (28.4 vs. 25.5, P < 0.001); were less likely to have two or more comorbidities (17.1 vs. 20.1, P = 0.013); and had higher levels of trust in their healthcare providers (28.9 vs. 28.1, P = 0.014). Additionally, women who received testing were more likely to agree with statements of the benefits of BRCA testing (P < 0.001). There were no significant differences in agreement with BRCA testing being too expensive, or concerns about BRCA testing between women who did and did not receive testing. Among women who completed testing, enabling factors were being employed for wages (58.7 vs. 42.3, P < 0.001), having employer‐based health insurance (47.6 vs. 35.8 P < 0.001); and having an annual income level of >$70,000 (31.1 vs. 16.1, P < 0.001). After aggregating risk factors based on need for genetic testing, women with a high mutation risk (49.2 vs. 22.2, P < 0.001) were more likely to report having BRCA testing compared to those with lower mutation risk.
Table 2.
Predisposing, enabling, and need characteristics of breast cancer survivors
| Characteristics | BRCA testing (YES) (n = 252) No. % | BRCA testing (NO) (n = 693) No.% | P‐value | ||
|---|---|---|---|---|---|
| Predisposing factors | |||||
| State | |||||
| FL | 149 | 59.1 | 374 | 53.97 | 0.158 |
| PA | 103 | 40.9 | 319 | 46.03 | |
| Stage | |||||
| 1 | 120 | 47.6 | 334 | 48.2 | 0.875 |
| 2 | 132 | 52.4 | 359 | 51.8 | |
| Age at diagnosis, mean, SD | 47.2 ± 8.1 | 53.2 ± 7.4 | <0.001 | ||
| Education | |||||
| ≤High school | 63 | 25.2 | 258 | 38.0 | <0.001 |
| Any college | 133 | 53.2 | 343 | 50.5 | |
| Graduate school | 54 | 21.6 | 78 | 11.5 | |
| ER/PR status | |||||
| Negative | 88 | 34.9 | 193 | 27.9 | 0.105 |
| Positive | 149 | 59.1 | 458 | 66.1 | |
| Unknown | 15 | 6.0 | 42 | 6.1 | |
| Comorbidities | |||||
| 0 | 152 | 60.3 | 344 | 49.6 | 0.013 |
| 1 | 57 | 22.6 | 210 | 30.3 | |
| 2+ | 43 | 17.1 | 139 | 20.1 | |
| BRCA attitudes scale | 28.4 ± 4.1 | 25.5 ± 4.0 | <0.001 | ||
| BRCA benefits (family members) | |||||
| Strongly agree or agree | 204 | 80.95 | 377 | 54.40 | <0.001 |
| BRCA benefits (help me manage cancer risk) | |||||
| Strongly agree or agree | 191 | 75.79 | 339 | 48.92 | <0.001 |
| BRCA benefits (help my doctor manage cancer risk) | |||||
| Strongly agree or agree | 203 | 80.56 | 348 | 50.22 | <0.001 |
| BRCA benefits (reassure me about cancer risk) | |||||
| Strongly agree or agree | 172 | 68.25 | 346 | 49.93 | <0.001 |
| BRCA cost (too expensive for me) | |||||
| Strongly agree or agree | 73 | 28.97 | 218 | 31.46 | 0.464 |
| BRCA concerns (family problems) | |||||
| Strongly agree or agree | 22 | 8.73 | 53 | 7.65 | 0.586 |
| BRCA concerns (problems with my job) | |||||
| Strongly agree or agree | 19 | 7.54 | 49 | 7.07 | 0.805 |
| BRCA concerns (problems with my health insurance or life insurance) | |||||
| Strongly agree or agree | 56 | 22.22 | 131 | 18.90 | 0.257 |
| Healthcare system distrust scale | 26.0 ± 6.0 | 25.6 ± 5.9 | 0.398 | ||
| Trust in provider scale | 28.9 ± 4.3 | 28.1 ± 4.5 | 0.014 | ||
| Family support scale | 16.1 ± 3.6 | 16.0 ± 3.5 | 0.598 | ||
| Enabling factors | |||||
| Employment | |||||
| Not employed | 104 | 41.3 | 400 | 57.7 | <0.001 |
| Employed for wages | 148 | 58.7 | 293 | 42.3 | |
| Health insurance type | |||||
| Employer based | 120 | 47.6 | 248 | 35.8 | <0.001 |
| Medicaid | 15 | 6.8 | 100 | 14.4 | |
| Medicare | 29 | 11.5 | 122 | 17.6 | |
| Self‐pay | 53 | 21.0 | 106 | 15.3 | |
| Other/Missing | 33 | 13.1 | 117 | 16.9 | |
| Income | |||||
| <30K | 73 | 30.3 | 302 | 49.8 | <0.001 |
| 30–70K | 93 | 38.6 | 207 | 34.1 | |
| >70K | 75 | 31.1 | 98 | 16.1 | |
| Need factors | |||||
| Risk category | |||||
| High risk | 124 | 49.2 | 139 | 22.2 | <0.001 |
| Moderate risk | 88 | 34.9 | 247 | 39.5 | |
| Low risk | 40 | 15.9 | 239 | 38.2 | |
Boldface indicates statistical significance (p<.05 and p<0.01).
Predictors of BRCA testing
In a multivariate logistic regression model of the factors that predicted BRCA testing (Table 3), only attitudes about BRCA testing, income, and BRCA mutation risk predicted BRCA testing. Women with more positive attitudes about BRCA testing had a significantly higher odds of testing ([OR], 1.16; 95% CI: 1.11–1.21; P < 0.001). In addition, women with an income of $70,000 and higher were twice as likely to have testing compared to women with a lower income ([OR], 2.10; 95% CI: 1.16–3.80; P = 0.014). BRCA mutation risk category was associated with testing and women with a high mutation risk were nearly four times likely to have testing compared to low‐risk women ([OR], 3.78; 95% CI: 2.31–6.19; P < 0.001). Education level, comorbidities, insurance type, and trust in provider were not significantly associated with BRCA testing after multivariate adjustment. Healthcare system distrust was not significantly associated with BRCA testing in the univariate or multivariate analyses. The Hosmer–Lemeshow goodness‐of‐fit test indicated that our model fit the data well (P = 0.487). We performed sensitivity analysis excluding the low mutation risk group, and the associations of predictors with BRCA testing were similar (data not shown). Additionally, we performed sensitivity analysis using the BRCA attitudes subscales of benefits, costs, and concerns (Table 3). We found that greater agreement with the benefits of BRCA testing was strongly associated with testing (OR = 2.28; 95% CI: 1.78–2.93; P < 0.001). Greater agreement that BRCA testing was too expensive was associated with significantly lower odds of testing (OR = 0.73; 95% CI: 0.62–0.86; P < 0.001). Cost of BRCA testing was a significant predictor of testing when entered as a continuous variable in the multivariate adjusted model, despite the fact that it did not reach statistical significance in the unadjusted analysis in Table 2, where agreement was dichotomized into only two categories. Concerns that BRCA testing would lead to problems with family, job, or insurance were not significantly associated with testing use. Including the BRCA attitudes subscales rather than the full scale did not meaningfully change the associations of other variables with BRCA testing (data not shown).
Table 3.
Logistic regression model predicting BRCA 1/2 testing adjusted for risk group, insurance type, income, education, comorbidities, BRCA attitude, and physician trust
| Variables | OR | 95% CI | P‐value |
|---|---|---|---|
| Predisposing factors | |||
| Education | |||
| ≤High School | Ref | ||
| College | 1.10 | 0.70–1.73 | 0.673 |
| Graduate School | 1.44 | 0.77–2.67 | 0.252 |
| Missing | 2.31 | 0.30–18.03 | 0.425 |
| Comorbidities | |||
| 0 | Ref | ||
| 1 | 0.80 | 0.52–1.23 | 0.313 |
| 2+ | 1.06 | 0.62–1.81 | 0.843 |
| BRCA attitudes scale | 1.16 | 1.11–1.21 | <0.001 |
| BRCA attitudes scale componentsa | |||
| Benefits | 2.28 | 1.78–2.93 | <0.001 |
| Costs | 0.73 | 0.62–0.86 | <0.001 |
| Concerns | 0.85 | 0.68–1.07 | 0.166 |
| Trust in provider scale | 0.99 | 0.95–1.03 | 0.684 |
| Enabling factors | |||
| Insurance type | |||
| Employer based | Ref | ||
| Medicaid | 0.50 | 0.23–1.09 | 0.081 |
| Medicare | 0.66 | 0.35–1.24 | 0.195 |
| Self‐pay | 1.36 | 0.84–2.19 | 0.210 |
| Other/Missing | 0.66 | 0.35–1.25 | 0.202 |
| Income | |||
| <30K | Ref | ||
| 30–70K | 1.54 | 0.93–2.53 | 0.092 |
| >70K | 2.10 | 1.16–3.80 | 0.014 |
| Missing | 0.88 | 0.37–2.10 | 0.767 |
| Need factors | |||
| BRCA mutation risk categories | |||
| Low risk | Ref | ||
| Moderate risk | 1.53 | 0.93–2.51 | 0.094 |
| High risk | 3.78 | 2.31–6.19 | <0.001 |
Logistic regression model was rerun with BRCA attitude scale components entered into the model, rather than the full scale. Odds ratios for other variables were not meaningfully changed in this model.
Boldface indicates statistical significance (p<.05 and p<0.01).
Discussion
This study is, to the best of our knowledge, the largest population‐based study that comprehensively examined predictors of BRCA1/2 genetic testing among Black women with breast cancer. Our study found that few (27%) Black women with breast cancer reported having BRCA testing. For participants who reported not having BRCA testing, 22% had a high mutation risk but did not receive genetic testing suggesting an unmet need. These findings indicate a critical need for healthcare providers to assess mutation probability of women with breast cancer and order genetic counseling and testing when appropriate. There is an urgent need for a greater number of Black women to receive genetic testing as they suffer disproportionately from breast cancer and experience higher rates of variants of unknown significance (VUS) compared to Caucasian populations 38.
Interestingly, after categorizing our variables using the Andersen Behavioral Model of Health Care Utilization theoretical framework and conducting a multivariate adjustment, we found that perceived attitudes about BRCA testing (predisposing factor), income (enabling factor), and BRCA mutation risk category (need factor) remained significant predictors of BRCA testing. When we examined subscales of attitudes toward BRCA testing, we found that perceived benefits of BRCA testing was positively associated with testing, while women who agreed that BRCA testing was too expensive had lower odds of testing. Concerns about problems raised by testing for family members, employment, and insurance were not associated with BRCA testing. In contrast, prior studies found that Black women expressed greater concern about the risks associated with genetic testing and genetic discrimination compared to women of other races 39, 40. One possible explanation for our finding is that Black women's awareness of BRCA testing and its benefits could be increasing, diminishing concerns about risks of testing. Based on our results, educating Black women about the benefits of BRCA testing is important and can lead to more positive attitudes and greater uptake of BRCA testing among this population. In one recent study among 1536 breast cancer patients, Jagsi et al. 41 found that Black and Hispanic women who had a strong desire for testing were more likely to report an unmet need for discussion about testing with a healthcare provider compared to White breast cancer patients. These findings suggest the need for healthcare providers to engage in discussions about cancer risk and the need for genetic testing with Black women who are diagnosed with breast cancer who meet the criteria for genetic testing. Existing educational and psychosocial resources offered by Facing our Risk of Cancer Empowered (FORCE), BrightPink Organization, Bring Your Brave Campaign and Know: BRCA Tool, which were created by the Centers for Disease Control and Prevention (CDC), can be used to increase awareness of BRCA and other gene tests among this population. Our study did not assess participation in genetic counseling, which has been shown to have positive psychosocial outcomes on patients 42.
Consistent with previous studies 43, 44, 45, we found that higher income was a predictor of BRCA testing for this population and concerns about cost were associated with lower odds of testing. Similarly, in a recent analysis of a national sample of 3628 individuals whose clinicians ordered a comprehensive BRCA testing, most were White, college educated, with higher incomes 44. Another recent study examined factors associated with BRCA testing among 440 Black breast cancer patients and found that healthcare provider referral, private health insurance, and household income greater than $35,000 were associated with genetic counseling and testing 45. Similarly, Jones et al. 43 found that among 340 young Black breast cancer survivors, income, education, and lack of access to healthcare services due to high out of pocket costs predicted BRCA testing. While the cost of genetic testing has substantially decreased, racial and socioeconomic disparities in the use of testing still exist 4, 46. Black women diagnosed with breast cancer with lower incomes may experience cost‐related barriers to having genetic testing. Most genetic testing companies now offer financial assistance programs to assist patients with economic barriers; healthcare providers can explore these options to assist patients in need.
BRCA mutation risk category, created using age at diagnosis, family history, and Ashkenazi Jewish heritage based on the NCCN 2007 clinical practice guidelines, strongly predicted BRCA testing in this study. As expected, Black women with a higher mutation risk based on age at diagnosis and family history were more likely to report having testing. The 2007 NCCN guidelines recommend genetic testing for women diagnosed at age 40 and younger; in our sample, approximately 30% of the Black women diagnosed with breast cancer were categorized as having a high BRCA mutation risk. The NCCN guidelines has been recently updated and testing is now recommended for women with a personal history of breast cancer diagnosed at age 45 and younger and individual's diagnosed with TNBC at age 60 and younger 47, potentially making more Black women eligible for testing. Despite these existing guidelines, previous studies have found that Black women diagnosed with breast cancer are less likely to receive a physician recommendation for testing compared to White breast cancer patients 2, 41, 43, 44. The role of healthcare providers in identifying appropriate individuals for testing and ensuring that genetic testing is completed to identify patients with a hereditary cancer syndrome should be emphasized.
Historically, healthcare system distrust among African Americans has contributed to lower utilization of healthcare services and lower participation in research studies 48, 49. In the context of genetic testing, Armstrong et al. 50 found that the effect of healthcare system distrust on the likelihood of testing did not differ by race (Black vs. White women) after an adjustment analysis. However, individuals who were less willing to undergo genetic testing with insurance disclosure had high values distrust and individuals less willing to undergo genetic testing from specialist had higher competence distrust. In another study, Sheppard et al. 51 found that among 100 Black women with a cancer risk, those with higher levels of medical mistrust reported lower participation in genetic counseling and testing. In our study, we found that trust in healthcare provider and healthcare system distrust were not independent predictors of BRCA testing in our study. One possible explanation is that while women may distrust the healthcare system, in general, they may have higher levels of trust in their particular provider, which results in women following through with testing that the provider recommends. While this current analysis focused on identifying patient‐level factors that are predictors of BRCA testing and did not include healthcare provider recommendation as a variable, the parent study, conducted by McCarthy et al. 2, examined racial differences in BRCA testing between Black and White breast cancer patients and found that having a provider recommendation for BRCA testing was a strong predictor of testing.
Testing for mutations in cancer predisposing genes, such as BRCA1/2 and other high penetrance genes, has now become the standard of care for breast cancer patients with a personal and family history indicative of mutation risk 52. The numerous benefits of genetic testing, including precision medicine and precision prevention, provide compelling reasons to increase access to testing for all who will benefit 53, 54, 55. As stated in the Cancer Moonshot Blue Ribbon Panel Report, identifying individuals with a hereditary cancer syndrome is a national priority as it will allow for genetic counseling that is evidenced‐based and would promote cancer prevention and early detection leading to improved health outcomes 56. If current racial disparities in genetic testing persist among women who are diagnosed with breast cancer, the benefits and advances in genetics and precision medicine may not reach minority populations, ultimately widening the disparities gap. Additionally, rates of variants of unknown significance (VUS) are higher among Black women compared to those of European ancestry due to less testing experience 55. While most VUS are benign, some will be deleterious but still cannot be used for clinical decision making 52. The identification of Black women with a breast cancer susceptibility gene will advance our understanding of the genetic influences of breast cancer in this population, thus leading to greater cancer prevention efforts.
Evidence‐based interventions are needed to engage high‐risk Black women in having genetic testing. This study has identified relevant factors that could be the target of a theoretically based intervention. Randomized controlled trails of studies to engage Black women in cancer risk assessment and to improve uptake of genetic testing for hereditary cancer syndromes are lacking. Few promising interventions exist that have included samples of Black women with a HBOC risk or exclusively among Black women 57, 58. Mays et al. 57 evaluated the efficacy of BreastCARE intervention for women and their primary care providers. Women were randomized to BreastCARE, a tablet‐based risk assessment tool that provides tailored print out of a risk report for patients and their providers or control group that received risk assessment by telephone. The total sample (N = 1235) included 24% Hispanic and 22% Black women. The authors found that BreastCARE increased discussions of family cancer history, personal breast cancer risk, and genetic counseling/testing. Apple et al. 58 conducted a quality improvement project to determine the effectiveness of nurse navigators on conducting education and screening for hereditary breast and ovarian cancer syndrome and found that identifying at‐risk individuals at the time of breast biopsy impacted surgical management of patients with a hereditary risk. Joseph et al. 59 conducted a pilot randomized trial to compare two approaches for engaging 38 high‐risk low‐income women in free genetic counseling. The sample included 39% Hispanic and 13% Black women. In the first intervention arm, genetic counseling assistants contacted the participants to offer them an appointment for genetic counseling. The second intervention arm, women were mailed a printed brochure about cancer risk and the risk assessment program and were also offered a genetic counseling appointment. These existing interventions are promising in addressing the lower utilization of genetic testing among high‐risk Black women, future studies should focus on further developing and testing existing innovative interventions or programs to engage this population in having genetic testing to make informed decisions about cancer risk reduction.
The strengths of our publication are its large population‐based design and its focus on hereditary cancer risk among Black women diagnosed with breast cancer, an understudied population. Our study expands the literature as it addresses a critical barrier to progress in the field, the underutilization of genetic testing among Black women with breast cancer. Our findings present relevant factors that can be the target of a program to improve utilization of genetic testing for breast cancer risk among this population. Until the racial disparity of genetic testing is addressed, the benefits of genetic testing will not be realized for all Americans. Our study has some limitations. As breast cancer patients were surveyed several years after their cancer diagnosis and them undergoing genetic testing, we cannot establish causality between factors such as BRCA attitudes and genetic testing. We were also unable to obtain information from each participant's medical records on TNBC tumor subtype and did not include this as a variable, which we recognize as a limitation given the high incidence of TNBC among Black women. In addition, the results of BRCA testing were not ascertained at the time of the study. We focused on self‐reported use of BRCA testing and although the research team found that patient report of BRCA 1/2 testing had high positive and negative predictive values (91 and 96%, respectively) with several medical records at the Penn Medical System, we were unable to confirm testing for all participants. Although the cancer registries in FL and PA were comprised of diverse samples, we cannot be sure that our results are generalizable to other states with similar demographic patterns. Additionally, we included only Stage I and II breast cancers, and therefore our results may not be generalizable to women diagnosed with late‐stage disease.
In conclusion, Black women suffer disproportionately from breast cancer and should have genetic testing when appropriate. Our study shows that multiple factors influence uptake of BRCA testing among Black women diagnosed with breast cancer, such as perceived benefits of BRCA testing, income level, and BRCA mutation risk. Interventions to reduce disparities in BRCA testing should focus on identifying patients with high risk of mutation, increasing patient understanding of the benefits of BRCA testing, and removing financial and other administrative barriers to genetic testing. Women diagnosed with breast cancer, specifically those with early‐age onset breast cancer and TNBC are at‐risk for harboring a cancer predisposition gene. Therefore, it is critical that healthcare providers recognize these red flags, assess mutation probability among Black women with breast cancer, and order genetic counseling and testing when appropriate.
Conflict of Interest
None declared.
Cancer Medicine 2017; 6(7):1787–1798
References
- 1. Randall, T. C. , and Armstrong K.. 2016. Health care disparities in hereditary ovarian cancer: are we reaching the underserved population? Curr. Treat. Options Oncol. 17:39 https://doi.org/10.1007/s11864-016-0417-1. [DOI] [PubMed] [Google Scholar]
- 2. McCarthy, A. M. , Bristol M., Domchek S. M., Groeneveld P. W., Kim Y., Motanya U. N. 2016. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J. Clin. Oncol. 34:2610–2618. https://doi.org/10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katz, S. J. , Kurian A. W., and Morrow M.. 2015. Treatment decision making and genetic testing for breast cancer: mainstreaming mutations. JAMA 314:997–998. [DOI] [PubMed] [Google Scholar]
- 4. Daly, B. , and Olopade O. I.. 2015. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J. Clin. 65:221–238. [DOI] [PubMed] [Google Scholar]
- 5. DeSantis, C. E. , Fedewa S. A., Goding Sauer A., Kramer J. L., Smith R. A., Jemal A.. 2015. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J. Clin. 66:(1)31–42. https://doi.org/10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 6. Foulkes, W. D. 2008. Inherited susceptibility to common cancers. N. Engl. J. Med. 359:2143–2153. [DOI] [PubMed] [Google Scholar]
- 7. DeSantis, C. E. , Siegel R. L., Sauer A. G., Miller K. D., Fedewa S. A., Alcaraz K. I., et al. 2016. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 66:290–308. [DOI] [PubMed] [Google Scholar]
- 8. Churpek, J. E. , Walsh T., Zheng Y., Moton Z., Thornton A. M., Lee M. K., et al. 2015. Inherited predisposition to breast cancer among African American women. Breast Cancer Res. Treat. 149:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lynce, F. , Smith K. L., Stein J., DeMarco T., Wang Y., Wang H., et al. 2015. Deleterious BRCA1/2 mutations in an urban population of Black women. Breast Cancer Res. Treat. 153:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pal, T. , Bonner D., Cragun D., Monteiro A. N., Phelan C., Servais L., et al. 2015. A high frequency of BRCA mutations in young black women with breast cancer residing in Florida. Cancer 121:4173–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Cancer Institute (NCI) . BRCA1 and BRCA2: cancer risk and genetic testing 2016. Available at: http://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet (accessed 15 February 2016).
- 12. Rebbeck, T. R. 2014. Precision prevention of cancer. Cancer Epidemiol. Biomarkers Prev. 23:2713–2715. [DOI] [PubMed] [Google Scholar]
- 13. Marcus, P. M. , Pashayan N., Church T. R., Doria‐Rose V. P., Gould M. K., Hubbard R. A., et al. 2016. Population‐based precision cancer screening: a symposium on evidence, epidemiology, and next steps. Cancer Epidemiol. Biomarkers Prev. 25:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCarthy, A. M. , and Armstrong K.. 2014. The role of testing for BRCA1 and BRCA2 mutations in cancer prevention. JAMA Intern. Med. 174:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruddy, K. J. , Risendal B. C., Garber J. E., and Partridge A. H.. 2015. Cancer survivorship care: an opportunity to revisit cancer genetics. J. Clin. Oncol. 34:539–541. https://doi.org/10.1200/JCO.2015.63.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Comprehensive Cancer Network (NCCN) . 2016. Genetic/familial high‐risk assessment: breast and ovarian. Available at: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf (accessed 14 August 2015).
- 17. Andersen, R. M. 1995. Revisiting the behavioral model and access to medical care: does it matter? J. Health Soc. Behav. 36:1–10. [PubMed] [Google Scholar]
- 18. Jahangir, E. , Irazola V., and Rubinstein A.. 2012. Need, enabling, predisposing, and behavioral determinants of access to preventative care in Argentina: analysis of the national survey of risk factors. PLoS ONE 7:e45053 https://doi.org/10.1371/journal.pone.0045053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Comprehensive Cancer Network (NCCN) . 2007. Clinical Practice Guidelines in Oncology. Genetic/Familial high risk assessment: Breast and Ovarian. Version 1. Retrieved from https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf
- 20. American Association for Public Opinion Research (AAPOR) . 2008. Response rate definition #4, standard definitions: final dispositions of case codes and outcome rates for surveys, 4th edn American Association for Public Opinion Research (AAPOR), Retrieved from http://www.aapor.org/Standards-Ethics/Standard-Definitions-(1).aspx. [Google Scholar]
- 21. The American Association for Public Opinion Research; 2011. Standard definitions: final dispositions of case codes and outcome rates for surveys, 7th edn AAPOR, Retrieved from http://www.aapor.org/Standards-Ethics/Standard-Definitions-(1).aspx. [Google Scholar]
- 22. Armstrong, K. , Micco E., Carney A., Stopfer J., and Putt M.. 2005. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA 293:1729–1736. [DOI] [PubMed] [Google Scholar]
- 23. Peters, N. , Domchek S. M., Rose A., Polis R., Stopfer J., and Armstrong K.. 2005. Knowledge, attitudes, and utilization of BRCA1/2 testing among women with early‐onset breast cancer. Genet. Test. 9:48–53. [DOI] [PubMed] [Google Scholar]
- 24. Peters, N. , Rose A., and Armstrong K.. 2004. The association between race and attitudes about predictive genetic testing. Cancer Epidemiol. Biomarkers Prev. 13:361–365. [PubMed] [Google Scholar]
- 25. Rose, A. , Peters N., Shea J. A., and Armstrong K.. 2005. The association between knowledge and attitudes about genetic testing for cancer risk in the United States. J. Health Commun. 10:309–321. [DOI] [PubMed] [Google Scholar]
- 26. Rose, A. L. , Peters N., Shea J. A., and Armstrong K.. 2005. Attitudes and misconceptions about predictive genetic testing for cancer risk. Comm. Genet. 8:145–151. [DOI] [PubMed] [Google Scholar]
- 27. Armstrong, K. , Putt M., Halbert C. H., et al. 2013. Prior experiences of racial discrimination and racial differences in health care system distrust. Med. Care 51:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong, K. , Rose A., Peters N., Long J. A., McMurphy S., and Shea J. A.. 2006. Distrust of the health care system and self‐reported health in the United States. J. Gen. Intern. Med. 21:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LaVeist, T. A. , Isaac L. A., and Williams K. P.. 2009. Mistrust of health care organizations is associated with underutilization of health services. Health Serv. Res. 44:2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rose, A. , Peters N., Shea J. A., and Armstrong K.. 2004. Development and testing of the health care system distrust scale. J. Gen. Intern. Med. 19:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyons, J. S. , Perrotta P., and Hancher‐Kvam S.. 1988. Perceived social support from family and friends: measurement across disparate samples. J. Pers. Assess. 52:42–47. [DOI] [PubMed] [Google Scholar]
- 32. Procidano, M. E. , and Heller K.. 1983. Measures of perceived social support from friends and from family: three validation studies. Am. J. Community Psychol. 11:1–24. [DOI] [PubMed] [Google Scholar]
- 33. Anderson, L. A. , and Dedrick R. F.. 1990. Development of the Trust in Physician scale: a measure to assess interpersonal trust in patient‐physician relationships. Psychol. Rep. 67(3 Pt 2):1091–1100. [DOI] [PubMed] [Google Scholar]
- 34. Becker, E. R. , and Roblin D. W.. 2008. Translating primary care practice climate into patient activation: the role of patient trust in physician. Med. Care 46:795–805. [DOI] [PubMed] [Google Scholar]
- 35. Kowalski, C. , Nitzsche A., Scheibler F., Steffen P., Albert U. S., and Pfaff H.. 2009. Breast cancer patients' trust in physicians: the impact of patients' perception of physicians' communication behaviors and hospital organizational climate. Patient Educ. Couns. 77:344–348. [DOI] [PubMed] [Google Scholar]
- 36. Thom, D. H. , Hall M. A., and Pawlson L. G.. 2004. Measuring patients' trust in physicians when assessing quality of care. Health Aff. (Millwood) 23:124–132. [DOI] [PubMed] [Google Scholar]
- 37. Singletary, S. E. , Allred C., Ashley P., Bassett L. W., Berry D., Bland K. I., et al. 2003. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg. Clin. North Am. 83:803–819. [DOI] [PubMed] [Google Scholar]
- 38. Ricks‐Santi, L. , McDonald J. T., Gold B., Dean M., Thompson N., Abbas M., et al. 2017. Next generation sequencing reveals high prevalence of BRCA1 and BRCA2 variants of unknown significance in early‐onset breast cancer in African American Women. Ethn. Dis. 27:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glenn, B. A. , Chawla N., and Bastani R.. 2012. Barriers to genetic testing for breast cancer risk among ethnic minority women: an exploratory study. Ethn. Dis. 22:267–273. [PubMed] [Google Scholar]
- 40. Ramirez, A. G. , Chalela P., Gallion K. J., Muñoz E., Holden A. E., Burhansstipanov L., et al. 2015. Attitudes toward breast cancer genetic testing in five special population groups. J. Health Dispar. Res. Pract. 8:124–135. [PMC free article] [PubMed] [Google Scholar]
- 41. Jagsi, R. , Griffith K. A., Kurian A. W., Morrow M., Hamilton A. S., Graff J. J., et al. 2015. Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J. Clin. Oncol. 33:1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oberguggenberger, A. , Sztankay M., Morscher R. J., Sperner‐Unterweger B., Weber I., Hubalek M., et al. 2016. Psychosocial outcomes and counselee satisfaction following genetic counseling for hereditary breast and ovarian cancer: a patient‐reported outcome study. J. Psychosom. Res. 89:39–45. [DOI] [PubMed] [Google Scholar]
- 43. Jones, T. , Lockhart J. S., Mendelsohn‐Victor K. E., et al. 2016. Use of cancer genetics services in African‐American young breast cancer survivors. Am. J. Prev. Med. 51:427–436. https://doi.org/10.1016/j.amepre.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 44. Armstrong, J. , Toscano M., Kotchko N., et al. 2015. Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: the ABOUT study. JAMA Oncol. 1:1251–1260. [DOI] [PubMed] [Google Scholar]
- 45. Cragun, D. , Bonner D., Kim J., et al. 2015. Factors associated with genetic counseling and BRCA testing in a population‐based sample of young Black women with breast cancer. Breast Cancer Res. Treat. 151:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Underhill, M. L. , Jones T., and Habin K.. 2016. Disparities in cancer genetic risk assessment and testing. Oncol. Nurs. Forum 43:519–523. [DOI] [PubMed] [Google Scholar]
- 47. National Comprehensive Cancer Network (NCCN) . Clinical Practice Guidelines in Oncology. Genetic/Familial High Risk Assessment: Breast and Ovarian. 2016. Version 2. Retrieved from https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf [Google Scholar]
- 48. Armstrong, K. , McMurphy S., Dean L. T., Micco E., Putt M., Halbert C. H., et al. 2008. Differences in the patterns of health care system distrust between blacks and whites. J. Gen. Intern. Med. 23:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kennedy, B. R. , Mathis C. C., and Woods A. K.. 2007. African Americans and their distrust of the health care system: healthcare for diverse populations. J. Cult. Divers 14:56–60. [PubMed] [Google Scholar]
- 50. Armstrong, K. , Putt M., Halbert C. H., Grande D., Schwartz J. S., Liao K., et al. 2012. The influence of health care policies and health care system distrust on willingness to undergo genetic testing. Med. Care 50:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sheppard, V. B. , Mays D., LaVeist T., and Tercyak K. P.. 2013. Medical mistrust influences black women's level of engagement in BRCA 1/2 genetic counseling and testing. J. Natl Med. Assoc. 105:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tung, N. , Lin N. U., Kidd J., et al. 2016. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J. Clin. Oncol. 34:1460–1468. https://doi.org/10.1200/JCO.2015.65.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kensler, T. W. , Spira A., Garber J. E., Szabo E., Lee J. J., Dong Z., et al. 2016. Transforming cancer prevention through precision medicine and immune‐oncology. Cancer Prev. Res. (Phila.) 9:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Subbiah, V. , and Kurzrock R.. 2016. Universal genomic testing needed to win the war against cancer: genomics is the diagnosis. JAMA Oncol. 2:719–720. https://doi.org/10.1001/jamaoncol.2016.0078. [DOI] [PubMed] [Google Scholar]
- 55. Eccles, B. K. , Copson E., Maishman T., Abraham J. E., and Eccles D. M.. 2015. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer 15:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Panel CMBR . 2016. Cancer moonshot blue ribbon panel report 2016. Available at: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf (accessed 9 October 2016).
- 57. Mays, D. , Sharff M., DeMarco T., Williams B., Beck B., Sheppard V. B., et al. 2012. Outcomes of a systems‐level intervention offering breast cancer risk assessments to low‐income underserved women. Fam. Cancer 11:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Appel, S. J. , and Cleiment R. J.. 2015. Identifying women at risk for hereditary breast and ovarian cancer syndrome utilizing breast care nurse navigation at mammography and imaging centers. J. Natl Black Nurses Assoc. 26:17–26. [PubMed] [Google Scholar]
- 59. Joseph, G. , Kaplan C., Luce J., Lee R., Stewart S., C. Guerra , et al. 2012. Efficient identification and referral of low‐income women at high risk for hereditary breast cancer: a practice‐based approach. Public Health Genomics 15:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]


