Abstract
Both serology‐based and genetic studies have reported an association between pancreatic cancer risk and ABO blood groups. We have investigated this relationship in a cohort of pancreatic cancer patients from Western Norway (n = 237) and two control materials (healthy blood donors, n = 379; unselected hospitalized patients, n = 6149). When comparing patient and blood donor ABO allele frequencies, we found only the A1 allele to be associated with significantly higher risk for pancreatic ductal adenocarcinoma (PDAC) (23.8% vs. 17.9%; OR = 1.43, P = 0.018). Analyzing phenotypes, blood group A was more frequent among PDAC cases than blood donors (50.8% vs. 40.6%; OR = 1.51, P = 0.021), an enrichment fully explained by the A1 subgroup. Blood group O frequency was lower in cases than in blood donors (33.8% vs. 42.7%; OR = 0.69, P = 0.039). This lower frequency was confirmed when cases were compared to hospitalized patients (33.8% vs. 42.9%; OR = 0.68, P = 0.012). Results for blood group B varied according to which control cohort was used for comparison. When patients were classified according to surgical treatment, the enrichment of blood group A was most prominent among unresected cases (54.0%), who also had the lowest prevalence of O (28.7%). There was a statistically significant better survival (P = 0.04) for blood group O cases than non‐O cases among unresected but not among resected patients. Secretor status did not show an association with PDAC or survival. Our study demonstrates that pancreatic cancer risk is influenced by ABO status, in particular blood groups O and A1, and that this association may reflect also in tumor resectability and survival.
Keywords: ABO, blood group, FUT2, glycosyltransferase, pancreatic ductal adenocarcinoma, risk factor
Introduction
Pancreatic cancer is one of the most dreaded malignant diseases. It ranks the fourth most common cause of cancer‐related death in many Western countries and has a remarkably dismal prognosis with a 5‐year survival rate of <5% 1. The manifestation of symptoms occurs relatively late in the disease process. Most pancreatic cancer cases are therefore diagnosed at an unresectable stage and only 15–20% of the patients undergo surgical resection, which is the only potentially curative treatment.
Histologically, the large majority of pancreatic cancer cases are exocrine and classified as pancreatic ductal adenocarcinoma (PDAC), in which the characteristic morphological pattern consists of abundant duct‐like neoplastic structures embedded in a dense desmoplastic stroma 2. Accepted risk factors for PDAC include advanced age, cigarette smoking 3, and long‐standing chronic pancreatitis 4, whereas some studies also have implicated Helicobacter pylori infection 5 and diabetes mellitus 6. In addition, inherited susceptibility plays a role in the disease, both as high‐risk gene variants in the context of familial cancer syndromes 7 and as the presence of variants with modest effect, usually discovered by genome‐wide association studies (GWAS) 8.
In a landmark GWAS paper, the Pancreatic Cancer Cohort Consortium (PanScan) reported that the statistically most significant variants associating with pancreatic cancer risk belonged to the ABO locus on chromosome 9q34 9. ABO codes for a glycosyltransferase that gives rise to the histo‐blood group antigens of the ABO system. Single‐nucleotide polymorphisms (SNPs) of this gene determine the specificity of the enzyme 10, 11. Hence, by adding either N‐acetyl‐d‐galactosamine or d‐galactose to the precursor H antigen, the A and B glycosyltransferases produce A or B antigens, respectively, on cellular surfaces and secretions. A frequent ABO variant is a one‐base pair deletion that inactivates the encoded enzyme, leaving the H antigen unaltered and corresponding to the O phenotype 10. This deletion is in strong linkage disequilibrium with the T allele of SNP rs505922, which was identified as being associated with decreased susceptibility for pancreatic cancer 9. Accordingly, individuals with blood group O have a lower risk for this disease than those with other blood groups 12.
There are two major subtypes of the ABO allele determining the blood group A, namely A 1 and A 2. Data from PanScan demonstrated that, among all common ABO variants, the greatest risk of pancreatic cancer was conferred by the A 1 allele 13 which gives rise to the ABO protein with highest enzymatic activity 14. This finding suggests that it is the glycosyltransferase activity itself that is linked to cancer risk rather than actions of other nearby genes on chromosome 9q34. Somewhat surprisingly though, the association between blood group and pancreatic cancer was not influenced by the secretor phenotype, that is, a person's ability to secrete A, B, and H antigens into body fluids 13. This property is determined by FUT2, the gene encoding the glycosylating enzyme fucosyltransferase 2.
The initial reports 9, 12, 13 were followed up by genetic studies in various populations that confirmed the influence of ABO blood group alleles on pancreatic cancer risk 15, 16, 17. The finding that blood group O confers protection was also consistent with older papers that had reported an association between ABO phenotype and gastrointestinal cancers including pancreatic cancer 18, 19, 20. A meta‐analysis including over 20 studies, both genetic and serology‐based, concluded that all non‐O blood groups have elevated risks for pancreatic cancer as compared with the O phenotype 16.
In this study, we aimed at evaluating the link between pancreatic cancer and ABO histo‐blood groups in patients from Norway, a population in which this association has not yet been investigated. Our patients were carefully characterized to exclude non‐PDAC cases and also classified according to tumor resectability and survival. Two different sets of controls were included for statistical comparisons.
Materials and Methods
Study population
The study was performed according to the Helsinki Declaration and all patients gave their written informed consent. The project was approved by the Regional Ethical Committee of Western Norway. The patient cohort consisted of 237 cases of pancreatic adenocarcinoma seen at Haukeland University Hospital, Bergen, Norway between the years 1998 and 2012 (Table 1). Medical records, pathology reports, and/or tissue sections were examined by two pathologists (HI and SA) for diagnosis confirmation. Final classification resulted in 195 PDAC cases and 42 other adenocarcinomas located within the pancreas. The latter group consisted of intraductal papillary mucinous neoplasm (5 cases) or mucinous cystic neoplasm with malignant component (1 case), intrapancreatic adenocarcinoma of the ampulla/papilla of Vateri (19 cases) or of ductus choledochus (9 cases), and unspecified adenocarcinoma of the pancreas (8 cases).
Table 1.
Overview of pancreatic cancer patients and controls included in the study
| Cohort | Total n | Females | Males | ||||
|---|---|---|---|---|---|---|---|
| n | % | Median age | n | % | Median age | ||
| Cases | |||||||
| All pancreatic adenocarcinoma cases | 237 | 119 | 50.2 | 69 | 118 | 49.8 | 69 |
| Pancreatic ductal adenocarcinoma (PDAC) | 195 | 97 | 49.7 | 69 | 98 | 50.3 | 69 |
| Resected | 108 | 50 | 46.3 | 70 | 58 | 53.7 | 68 |
| Not resected | 87 | 47 | 54.0 | 70 | 40 | 46.0 | 67 |
| Other adenocarcinomasa | 42 | 22 | 52.4 | 67 | 20 | 47.6 | 68 |
| Controls | |||||||
| DNA‐typed blood donors | 379 | 189 | 49.9 | 39 | 190 | 50.1 | 44 |
| Serotyped hospital patients | 6149 | 2805 | 45.6 | 66 | 3344 | 54.4 | 64 |
See Materials and Methods for description.
For the statistical comparison, two different control groups were employed (Table 1). One consisted of 379 healthy blood donors from Haukeland University Hospital (49.9% females) that were genotyped in the same way as the cases. The other control group contained 6149 patients (45.6% females) born before 1.1.1970 and admitted to the same hospital during a randomly chosen period of six consecutive months in 2007. All patients had been blood‐typed by serological means as part of their health care. No selection with regard to diagnosis was done.
DNA extraction and genotyping
EDTA‐blood, frozen tissue and formalin‐fixed paraffin‐embedded (FFPE) tissue blocks were used for DNA extraction from the 237 pancreatic adenocarcinoma cases. DNA from frozen buffy coats from EDTA‐blood (195 cases) were purified using MagAttract DNA Blood Midi M48 kit on the BioRobot M48 workstation (both from Qiagen, Hilden, Germany) or manually processed with the E.Z.N.A DNA extraction kit (Omega Bio‐Tek, Norcross, GA, USA), according to the manufacturers’ protocol. For 38 cases, only FFPE tissue samples were available; 10‐micron sections were then sliced and subjected to manual deparaffinization with xylene, followed by ethanol washes before overnight incubation at 56°C with Proteinase K (Qiagen) and processing with the E.Z.N.A. DNA extraction kit. For the final four cases, only fresh‐frozen tissue samples were available. These were incubated directly with Proteinase K and then processed as the FFPE samples. DNA from the blood donor controls was isolated from EDTA‐blood buffy coats using the same purification system as for the patient blood samples.
Genotyping was performed using TaqMan predesigned genotyping assays (Cat. No. 4351379; Applied Biosystems, Foster City, CA). Each sample was tested for three common SNPs at the ABO locus (Table S1): rs8176704 (intron 3) for the A2 allele (Assay ID: C_30336657_10), rs8176746 (exon 7) for the B allele (Assay ID: C_25610772_20), and rs505922 (intron 1) for the O allele (Assay ID: C_2253769_10). The samples were also screened for the FUT2 variant rs601338 (Assay ID: C_2405292_10), which determines secretor status of ABH antigens.
The genotyping assays were performed on the 7900 Fast Real‐time PCR System with the corresponding 7900 Fast System SDS 2.4 Software (Applied Biosystems). Positive and negative controls were included to ensure appropriate clustering. Each assay was performed using 10 ng template DNA, TaqMan Universal Master Mix buffer (Applied Biosystems), and 20x primer and probe mix as recommended by the manufacturer. Thermal cycling was performed by first activating the DNA polymerase at 95°C for 10 min and then running 40 amplification cycles, each consisting of denaturation at 92°C for 15 sec and combined annealing/extension at 60°C for 1 min.
Quality control
Misclassification of ABO genotypes was expected to be minimal, as genotyping results from the three different ABO SNPs matched the haplotype phasing of the ABO gene (Table 1 in 13) for all analyzed samples. In addition, we compared our genotyping results for 40 cases (20 extracted from blood and 20 from FFPE tissues) with serologically determined ABO status as stated in the patients’ medical records. The concordance rate was 100%. Genotyping quality for the blood donor control group was assessed by testing for Hardy–Weinberg equilibrium using the Haploview Software 21. The distribution of genotypes was as expected from the SNP frequencies.
Statistical analysis
Each SNP was tested under various genetic models (dominant, codominant, recessive, additive) using the software PLINK ( http://pngu.mgh.harvard.edu/~purcell/plink). Assessment of differences in genotype or phenotype distributions between cases and controls was carried out by the two‐tailed Fisher`s exact test or Pearson`s chi‐square test. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using two‐by‐two contingency table analysis on the SISA webpage ( http://www.quantitativeskills.com/sisa). Survival analysis was performed using the software package STATISTICA version 12 (StatSoft, Tulsa, OK). The Product–Limit (Kaplan–Meier) Analysis Module was employed for comparing survival between groups by log‐rank test of significance. Survival times versus cumulative proportion surviving, according to breakdown by blood group, were plotted. In all tests, P ≤ 0.05 was chosen for statistical significance.
Results
Patient characteristics
From our biobank of patients with pancreatic tumors, we initially selected the patients diagnosed with adenocarcinoma of the exocrine gland. This cohort consisted of 237 cases (50.2% females), with a median age at diagnosis of 69 years in both sexes (Table 1). We reviewed all cases to identify those that were consistent with a diagnosis of PDAC (n = 195, 82.3%). The cases were also classified according to whether or not the tumor had been judged resectable at the time of diagnosis (Table 1).
Association between ABO blood group and pancreatic cancer risk
The genotype frequencies for all adenocarcinoma cases and blood donor controls are given in Table S2. In both groups, the most common ABO genotypes were A1O and OO, and the least frequent were BB and A2A2. We first compared allele frequencies of A1, A2, B, and O in the whole adenocarcinoma cohort with frequencies observed in the blood donors (Table 2, ‘All cases’). The A1 allele frequency was higher among the patients (22.4% vs. 17.9%) but the difference did not quite reach statistical significance (P = 0.057). When the analysis was limited to PDAC cases only, there was a significant difference in A1 frequency (23.8% vs. 17.9%; OR = 1.43, CI = 1.06–1.93; P = 0.018). Interestingly, the A2 frequency appeared almost identical between the groups compared (7.6–7.8%). The B and O allele frequencies varied, but were not statistically different.
Table 2.
ABO and FUT2 allele frequencies of blood donor controls compared with pancreatic cancer cases
| Allele | Controls (n = 758) | All cases (n = 474) | PDAC cases only (n = 390) | ||||
|---|---|---|---|---|---|---|---|
| % | % | P | OR (95% CI) | % | P | OR (95% CI) | |
| ABO | |||||||
| A1 | 17.9 | 22.4 | 0.057 | 1.32 (0.99–1.75) | 23.8 | 0.018 | 1.43 (1.06–1.93) |
| A2 | 7.8 | 7.6 | 1.000 | 0.97 (0.63–1.50) | 7.7 | 1.000 | 0.99 (0.63–1.56) |
| B | 8.7 | 7.6 | 0.491 | 0.86 (0.56–1.32) | 7.7 | 0.556 | 0.87 (0.56–1.37) |
| O | 65.6 | 62.4 | 0.266 | 0.87 (0.69–1.11) | 60.8 | 0.109 | 0.81 (0.63–1.05) |
| FUT2 | |||||||
| Se | 51.5 | 51.5 | 1.000 | 1.00 (0.79–1.26) | 52.1 | 0.847 | 1.02 (0.80–1.31) |
| Se0 | 48.5 | 48.5 | 1.000 | 1.00 (0.79–1.26) | 47.9 | 0.847 | 0.98 (0.77–1.25) |
n, number of genotyped alleles; PDAC, pancreatic ductal adenocarcinoma; P, P ‐value from chi‐square test (df = 1); OR (95% CI), odds ratio (95% confidence interval). Significant P‐value is shown in bold face.
For the further analyses, we restricted our analysis to the PDAC cases only. We deduced ABO phenotypes from the genotype data of the cases and the blood donors. The blood group distributions are shown in Table 3. The blood group A prevalence was clearly different (50.8% vs. 40.6%; OR = 1.51, CI = 1.06–2.13; P = 0.021,) and, in keeping with the data of Table 2, the subgroup A1 frequencies fully explained the observed difference (42.6% vs. 29.3%; OR = 1.79, CI = 1.25–2.56; P = 0.001). Moreover, the prevalence of blood group O was lower in cases than in controls (33.8% vs. 42.7%; OR = 0.69, CI = 0.48–0.98; P = 0.039). Blood group B did not show a statistically significant difference in distribution. Neither did blood group AB, although in this case, the number of subjects was too small for meaningful comparisons to be made.
Table 3.
ABO blood group and secretor phenotype frequencies of blood donor controls compared with PDAC cases
| Phenotype | Controls (n = 379)% | PDAC cases (n = 195) | PDAC cases according to resection status | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resected (n = 108) | Unresected (n = 87) | |||||||||
| % | P | OR (95% CI) | % | P | OR (95% CI) | % | P | OR (95% CI) | ||
| ABO | ||||||||||
| A | 40.6 | 50.8 | 0.021 | 1.51 (1.06–2.13) | 48.1 | 0.163 | 1.36 (0.88–2.09) | 54.0 | 0.023 | 1.72 (1.07–2.74) |
| A1 | 29.3 | 42.6 | 0.001 | 1.79 (1.25–2.56) | 40.7 | 0.024 | 1.66 (1.07–2.59) | 44.8 | 0.005 | 1.96 (1.22–3.16) |
| A2 | 11.3 | 8.2 | 0.241 | 0.70 (0.38–1.27) | 7.4 | 0.238 | 0.63 (0.29–1.37) | 9.2 | 0.562 | 0.79 (0.36–1.75) |
| B | 12.1 | 12.8 | 0.814 | 1.06 (0.63–1.79) | 11.1 | 0.771 | 0.91 (0.46–1.78) | 14.9 | 0.478 | 1.27 (0.65–2.47) |
| ABa | 4.5 | 2.6 | 0.359 | 0.56 (0.20–1.54) | 2.8 | 0.586 | 0.61 (0.18–2.12) | 2.3 | 0.548 | 0.50 (0.11–2.21) |
| O | 42.7 | 33.8 | 0.039 | 0.69 (0.48–0.98) | 38.0 | 0.374 | 0.82 (0.53–1.27) | 28.7 | 0.016 | 0.54 (0.33–0.90) |
| FUT2 | ||||||||||
| Secretor | 77.6 | 76.4 | 0.753 | 0.94 (0.62–1.41) | 72.2 | 0.248 | 0.74 (0.46–1.22) | 81.6 | 0.410 | 1.28 (0.71–2.32) |
| Non‐secretor | 22.4 | 23.6 | 0.753 | 1.07 (0.71–1.61) | 27.8 | 0.248 | 1.33 (0.82–2.16) | 18.4 | 0.410 | 0.78 (0.43–1.41) |
PDAC, pancreatic ductal adenocarcinoma; P, P ‐value from chi‐square test (df=1); OR (95% CI), odds ratio (95% confidence interval). Significant P ‐values are shown in bold face.
P ‐values from two‐tailed Fisher`s exact test.
Healthy blood donors may not always serve as an optimal control group in case–control studies 22. We therefore collected information on ABO blood group distribution from a large control cohort of unselected hospitalized patients (see Materials) from the same geographical region as our pancreatic cancer patients. The subgroup A1/A2 distribution was not known for the hospitalized patient cohort. Notably, the hospitalized patients had a significantly higher blood group A prevalence than the blood donors (45.8% vs. 40.6%; OR = 1.24, CI = 1.00–1.53; P = 0.049). Similarly, blood group B was significantly less frequent (7.7% vs. 12.1%; OR = 0.60, CI = 0.44–0.83; P = 0.002). Blood group O had very similar prevalence in the two control groups (42.7% and 42.9%).
When the PDAC blood group distribution was compared with that of the hospitalized patients, the enrichment of blood group A among the PDAC cases no longer reached statistical significance (50.8% vs. 45.8%; OR = 1.22, CI = 0.92–1.62; P = 0.173) (Table 4). On the other hand, the blood group B difference was now significant (12.8% vs. 7.7%; OR = 1.76, CI = 1.15–2.71; P = 0.009). The lower frequency of blood group O in the PDAC cohort remained significant with almost identical odds ratio (33.8% vs. 42.9%; OR = 0.68, CI = 0.50–0.92; P = 0.012).
Table 4.
ABO phenotypic frequencies of hospital patient controls compared with PDAC cases
| Blood types | Controls (n = 6149) % | PDAC cases (n = 195) | PDAC cases according to resection status | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resected (n = 108) | Unresected (n = 87) | |||||||||
| % | P | OR (95% CI) | % | P | OR (95% CI) | % | P | OR (95% CI) | ||
| A | 45.8 | 50.8 | 0.173 | 1.22 (0.92–1.62) | 48.1 | 0.632 | 1.10 (0.75–1.61) | 54.0 | 0.128 | 1.39 (0.91–2.12) |
| B | 7.7 | 12.8 | 0.009 | 1.76 (1.15–2.71) | 11.1 | 0.188 | 1.50 (0.82–2.75) | 14.9 | 0.012 | 2.11 (1.16–3.83) |
| ABa | 3.5 | 2.6 | 0.690 | 0.72 (0.29–1.76) | 2.8 | 1.000 | 0.78 (0.25–2.47) | 2.3 | 0.771 | 0.64 (0.16–2.62) |
| O | 42.9 | 33.8 | 0.012 | 0.68 (0.50–0.92) | 38.0 | 0.301 | 0.81 (0.55–1.20) | 28.7 | 0.008 | 0.54 (0.34–0.86) |
PDAC, pancreatic ductal adenocarcinoma; P, P‐value from chi‐square test (df=1); OR (95% CI), odds ratio (95% confidence interval). Significant P ‐values are shown in bold face.
P‐values from two‐tailed Fisher`s exact test
Tumor resectability
To further explore the association between blood group frequencies and PDAC, the patients were stratified into two subgroups: those who had their pancreatic tumor resected and those who were considered surgically unresectable at the time of cancer diagnosis (Table 1). The latter group consisted of patients with locally advanced tumors with encasement of adjacent large blood vessels (Clinical stage III) or with metastatic disease at the time of diagnosis (Clinical stage IV) 23. We observed that the blood group A and subgroup A1 prevalences were highest among the unresected cases (54.0% and 44.8%, respectively) and both were significantly different from the frequencies found in blood donors (A: OR = 1.72, CI = 1.07–2.74, P = 0.023; A1: OR = 1.96, CI = 1.22–3.16, P = 0.005) (Table 3). Moreover, the unresected cases had the lowest frequency of blood group O (28.7% vs. 42.7%; OR = 0.54, CI = 0.33–0.90; P = 0.016). A comparison with the cohort of hospitalized patients (Table 4) revealed significant differences for the unresected patients, both with regard to blood group B (14.9% vs. 7.7%; OR = 2.11, CI = 1.16–3.83; P = 0.012) and blood group O (28.7% vs. 42.9%; OR = 0.54, CI = 0.34–0.86; P = 0.008).
Survival
Data on survival after time of diagnosis was available for all PDAC cases. As expected, survival was significantly better among resected than among unresected cases (median survival 18.1 months vs. 5.6 months, respectively; P < 0.001) (Fig. 1A). Given that blood group is a risk factor for PDAC and that it also may influence resection status, we also analyzed survival according to ABO phenotype. When all 195 patients were classified as O or non‐O cases, survival of the two groups was not significantly different (median 10.4 vs. 9.3 months, respectively; P = 0.23) (Fig. 1B). We then looked at resected and unresected cases separately. Among the resected cases, survival did not differ between patients with O and non‐O blood group (P = 0.93) (Fig. 1C). However, in the group of unresected cases, patients with blood group O survived longer than non‐O patients (median 6.7 vs. 5.5 months, respectively; P = 0.04) (Fig. 1D). When the non‐O cases were split into blood group A or B and compared to the O cases, the difference in survival reached significance for blood group B (median 2.7 months; P = 0.03), but not for A (median 5.6 months; P = 0.14) (Fig. 1E–F). Although it should be noted that the number of A2 cases is relatively small (16/195 cases), we also examined whether there was a survival difference between patients of blood group A1 and A2. Neither among all cases nor when cases were classified according to resection status was any statistically significant difference observed (data not shown).
Figure 1.
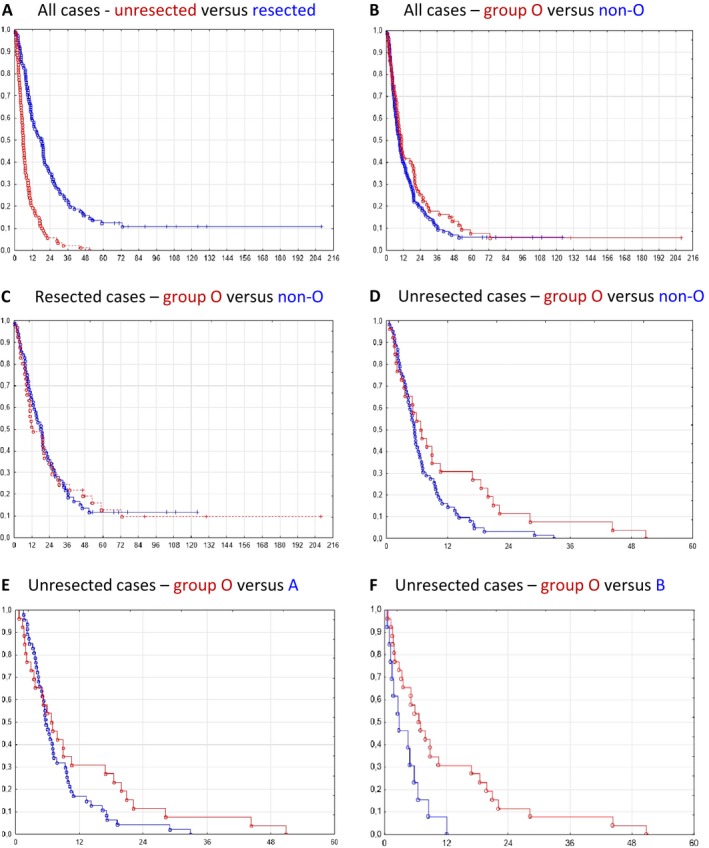
Cumulative proportion survival (Kaplan–Meier) plot for the 195 pancreatic ductal adenocarcinomas according to breakdown by resection status and blood group phenotype. (A)‐(F) Pairwise comparison of subgroups as specified in the heading of each panel. The observed survival times (months along the X‐axis) are indicated by circles (complete) or crosses (censored observations). In panel A, survival curves for resected and unresected cases are shown by a blue and a red line, respectively. In all other panels, survival curves for blood group O and the comparison group are shown by a red and blue line, respectively.
Secretor status
Because the secretion of soluble H antigen is associated with susceptibility to multiple pathogens through adherence to the gastrointestinal mucosa we looked at the secretor phenotype determined by the rs601338 FUT2 polymorphism (Tables S1, S2). Consistent with another study 13, there were no significant differences in prevalence of alleles or phenotypes when compared with the blood donor controls (Tables 2, 3). We further checked whether FUT2 could have an effect on resection status by comparing the groups of resected and unresected PDAC cases in Table 3 directly against each other. The difference in secretor status was not significant (72.2% vs. 81.6%; OR=0.59, CI=0.30–1.16; P = 0.124). Finally, we examined if secretor status might associate with survival in our patient cohort. No such association was seen (Fig. S1).
Conflict of Interest
None declared.
Supporting information
Figure S1. Cumulative proportion survival (Kaplan–Meier) plot for the 195 pancreatic ductal adenocarcinomas according to breakdown by FUT2 secretor phenotype and resection status.
Table S1. SNPs used for ABO and FUT2 allele genotyping.
Table S2. ABO and FUT2 genotype frequencies of blood donor controls and pancreatic cancer cases.
Discussion
A variety of biological traits have been linked to ABO histo‐blood group phenotypes. Reported associations include, among others, plasma lipid levels 24, susceptibility to H. pylori–associated disorders 25, cardiovascular disease 26, and cancer 27. Here, we have investigated ABO genetic variants and phenotypes in the context of pancreatic cancer. A strength of our study is that a re‐evaluation of the recorded pancreatic adenocarcinomas was performed to select only those cases consistent with a diagnosis of PDAC. Thus, from our initial cohort of adenocarcinomas with an anatomical location in the pancreas, 42 (17.7%) cases were excluded. Materials of pancreatic head cancers may contain a significant number of adenocarcinomas originating from the distal bile duct, ampulla, or duodenum 28, and proper classification of cases collectively regarded as pancreatic cancer should always be attempted.
One limitation of our study is the relatively low number of cases and blood donor controls. Another relates to the selection of control groups, which may pose a challenge for ABO phenotype and other traits with a distribution that varies by geography and ethnicity. The optimal control group for our study would have been randomly drawn individuals from the population of Western Norway, matched to cases by age, sex and county of residence. However, such a cohort could not be obtained within the restrictions of the present study.
We confirmed the association between the blood group O phenotype and reduced susceptibility to pancreatic exocrine cancer (frequency of 33.8% in cases versus 42.7% and 42.9% in the two control groups). When comparing with blood donors, blood group A was significantly overrepresented among cases. We made the interesting observation that the association with blood group A was explained by the A1 allele/blood group only, a finding previously reported by a few papers 13, 29. Serology‐based analyses and many genetic studies of ABO blood groups in pancreatic cancer do not distinguish between A1 and A2 subgroups. However, this distinction might be of biological importance as A1 and A2 glycosyltransferases display different catalytic activities, the A2 isoform having a higher Km and an estimated enzyme activity 30–50 times lower than A1 14. The finding that A1, but not A2, associates with pancreatic cancer could therefore suggest a role of blood group A glycosyltransferase activity in carcinogenesis.
Studies of associations between ABO blood groups and human disease are challenging for at least two reasons. Firstly, across the globe there are important variations in ABO frequencies, reflecting human migration movements and possibly also selection pressures from the environment 30, 31. Within Norway, the ABO distribution differs according to geographical localization with the highest blood group O frequency observed in the Western part of the country and the highest B frequency in the northernmost region 32. Secondly, healthy blood donors might not always serve as appropriate controls for epidemiological studies in general and, studies of ABO in particular. There could, for example, be a bias in ABO distribution among donors because of the association of blood groups with a variety of diseases. Another potential selective effect could arise in that certain blood groups might be preferred as donors.
In the literature, it is well established that blood group O individuals have a lower risk of pancreatic cancer 33. For the other blood types, the data are not always consistent. Some authors report an association with blood group A, but not with B 29, 34 whereas others describe an association with B, but not with A 20 or even find AB to be protective 35. One reason for this discrepancy might therefore be the way that control groups have been selected. We employed two different sets of controls: a limited number of blood donors and a large number of random patients from the same geographical area as our patients. The control groups exhibited very similar blood group O frequencies (close to 43%). Thus, we found a very consistent protective effect of blood group O on PDAC risk. On the other hand, the frequencies of blood groups A and B varied considerably among the two control groups, resulting in a significant association of PDAC risk with blood group A when blood donors were used and with blood group B when employing hospital patients. Interestingly, in a recent large study where 1.6 million blood donors from Denmark and Sweden were followed over an average of 17 years, there was increased risk for pancreatic cancer in subjects with blood group A when compared with O subjects 36. Blood group B did not associate with increased risk, that is, a result similar to what we observed when using blood donors as controls.
We also analyzed ABO blood group distribution in the context of resectability at the time of diagnosis (Tables 3 and 4). In this respect, it should be noted that our cases were recruited at a surgical department to which patients usually are referred when they are to be evaluated for operation. Thus, those PDAC patients that at the time of diagnosis obviously are unresectable will be clearly underrepresented. This explains the relatively high fraction of operated patients in our material (55.4%) compared with the general low resection rate for pancreatic adenocarcinomas, which is around 20%.
We divided our PDAC patients into those who underwent surgery to remove the primary tumor (Clinical stages I and II) and those who presented with an unresectable tumor (Stages III and IV). In the latter group, the O phenotype frequency dropped to 28.7% and the A1 and B frequencies increased to 44.8% and 14.9%, respectively (Table 3). Thus, the ABO frequency differences that we observed for the whole PDAC group versus the control groups appeared more prominent when analyzing unresected cases only. This suggests an association between ABO phenotype and clinical stage which again could indicate that blood group O subjects diagnosed with pancreatic cancer have the best prognosis. Previous analyses in this regard are discrepant. In a study involving Han Chinese patients, blood group O subjects had better outcome that those with non‐O blood group 37. However, in a German material, although the incidence of blood group O was significantly lower among the investigated cohort of pancreatic cancer patients, no association was seen between ABO status and survival 38. Intriguingly, when we analyzed unresected cases only, there was a significant association between having blood group O and better survival (Fig. 1D–F). This effect was not seen when the group of resected cases were analyzed according to blood group O status (Fig. 1C). Overall, the data from our material indicate that individuals with blood group O may have somewhat less aggressive PDACs than patients having other blood groups.
The implication of ABO glycosyltransferases in pancreatic cancer is still enigmatic. There is, however, strong evidence that the expression of ABH antigens in the gastrointestinal tract affects the anchoring efficiency of certain pathogenic strains to the mucosa and influences bacterial and/or viral colonization and infectivity 39. In this context, H. pylori infection as risk factor in pancreatic pathologies has been extendedly researched 40, and it was shown that the increased risk of non‐O individuals for PDAC became even greater if these subjects were positive for the CagA‐negative H. pylori strain 41. FUT2 secretor status seems to affect the composition of the intestinal microbiota 42 and the susceptibility to certain gastrointestinal infections 43. Nevertheless, we did not observe a difference in FUT2 allele distribution or phenotype among pancreatic cancer cases and controls, similar to what a previous report showed 13. Similarly, there was no association between secretor phenotype and survival (Fig. S1).
Naturally occurring alloantibodies (i.e., isoagglutinins) could also play a role in the association between ABO blood groups and pancreatic cancer. These antibodies provide immunity against pathogens expressing blood group‐like antigens on their surface. Anti‐A and anti‐B isoagglutinins, present in the plasma of blood group O individuals, have been shown to react toward the Tn and T pancarcinoma antigens 44. Tn and T may be structurally related to A and B blood group antigens, respectively 45 and could possibly render cancer cells immunologically less recognized in blood group A and B individuals.
In conclusion, although the choice of control material involves some particular challenges when studying ABO blood groups as a disease risk factor, our results confirm both a genetic and phenotypic association with pancreatic ductal adenocarcinoma. We found that the increased susceptibility connected with blood group A is likely to be caused by the subtype A1 only, and we suggest that also clinical stage at the time of diagnosis and survival could be influenced by blood group status. However, the biology behind the fascinating ABO‐pancreatic cancer link is poorly understood, and studies are warranted on how ABO glycosyltransferase activity may influence biological aggressiveness of pancreatic neoplastic cells.
Acknowledgements
We are very grateful to all patients who took part in this study, to Jorunn Vadheim for helping with extraction of blood type data from the hospitalized patients and to Paal Henning Borge for assistance with the blood donor samples. This project was funded by a PhD fellowship (grant no. 911831) to K.E.J. from the Western Norway Regional Health Authority (Helse Vest).
Cancer Medicine 2017; 6(7):1531–1540
References
- 1.Available at: http://www.cancerresearchuk.org/about-cancer/type/pancreatic-cancer/ (accessed 12 April 2017).
- 2. Esposito, I. , Konukiewitz B., Schlitter A. M., and Kloppel G.. 2014. Pathology of pancreatic ductal adenocarcinoma: facts, challenges and future developments. World J. Gastroenterol. 20:13833–13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosetti, C. , Lucenteforte E., Silverman D. T., Petersen G., Bracci P. M., Ji B. T., et al. 2012. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case‐Control Consortium (Panc4). Annals Oncol. 23:1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raimondi, S. , Lowenfels A. B., Morselli‐Labate A. M., Maisonneuve P., and Pezzilli R.. 2010. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Practice Res. Clin. Gastroenterol. 24:349–358. [DOI] [PubMed] [Google Scholar]
- 5. Schulte, A. , Pandeya N., Fawcett J., Fritschi L., Risch H. A., Webb P. M., et al. 2015. Association between Helicobacter pylori and pancreatic cancer risk: a meta‐analysis. Cancer Causes Control 26:1027–1035. [DOI] [PubMed] [Google Scholar]
- 6. Xu, C. X. , Zhu H. H., and Zhu Y. M.. 2014. Diabetes and cancer: associations, mechanisms, and implications for medical practice. World J. Diabetes. 5:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts, N. J. , Norris A. L., Petersen G. M., Bondy M. L., Brand R., Gallinger S., et al. 2016. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 6:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Childs, E. J. , Mocci E., Campa D., Bracci P. M., Gallinger S., Goggins M., et al. 2015. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 47:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amundadottir, L. , Kraft P., Stolzenberg‐Solomon R. Z., Fuchs C. S., Petersen G. M., Arslan A. A., et al. 2009. Genome‐wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat. Genet. 41:986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamoto, F. , Clausen H., White T., Marken J., and Hakomori S.. 1990. Molecular genetic basis of the histo‐blood group ABO system. Nature 345(6272):229–233. [DOI] [PubMed] [Google Scholar]
- 11. Clausen, H. , Bennett E. P., and Grunnet N.. 1994. Molecular genetics of ABO histo‐blood groups. Transfus. Clin. Biol. 1:79–89. [DOI] [PubMed] [Google Scholar]
- 12. Wolpin, B. M. , Kraft P., Gross M., Helzlsouer K., Bueno‐de‐Mesquita H. B., Steplowski E., et al. 2010. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 70:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolpin, B. M. , Kraft P., Xu M., Steplowski E., Olsson M. L., Arslan A. A., et al. 2010. Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: results from the pancreatic cancer cohort consortium. Cancer Epidemiol. Biomarkers Preven. 19:3140–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamamoto, F. , McNeill P. D., and Hakomori S.. 1992. Human histo‐blood group A2 transferase coded by A2 allele, one of the A subtypes, is characterized by a single base deletion in the coding sequence, which results in an additional domain at the carboxyl terminal. Biochem. Biophys. Res. Commun. 187(1):366–374. [DOI] [PubMed] [Google Scholar]
- 15. Nakao, M. , Matsuo K., Hosono S., Ogata S., Ito H., Watanabe M., et al. 2011. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 102:1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Risch, H. A. , Lu L., Wang J., Zhang W., Ni Q., Gao Y. T., et al. 2013. ABO blood group and risk of pancreatic cancer: a study in Shanghai and meta‐analysis. Am. J. Epidemiol. 177:1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu, H. L. , Cheng J. R., Zhang W., Wang J., Yu H., Ni Q. X., et al. 2014. Re‐evaluation of ABO gene polymorphisms detected in a genome‐wide association study and risk of pancreatic ductal adenocarcinoma in a Chinese population. Chin. J. Cancer. 33:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aird, I. , Lee D. R., and Roberts J. A.. 1960. ABO blood groups and cancer of oesophagus, cancer of pancreas, and pituitary adenoma. Br. Med. J. 1:1163–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newell, G. R. , Gordon J. E., Monlezun A. P., and Horwitz J. S.. 1974. ABO blood groups and cancer. J. Natl Cancer Inst. 52:1425–1430. [DOI] [PubMed] [Google Scholar]
- 20. Annese, V. , Minervini M., Gabbrielli A., Gambassi G., and Manna R.. 1990. ABO blood groups and cancer of the pancreas. Int. J. Pancreatol. 6:81–88. [DOI] [PubMed] [Google Scholar]
- 21. Barrett, J. C. , Fry B., Maller J., and Daly M. J.. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. [DOI] [PubMed] [Google Scholar]
- 22. Golding, J. , Northstone K., Miller L. L., Davey Smith G., Pembrey M.. 2013. Differences between blood donors and a population sample: implications for case‐control studies. Int. J. Epidemiol. 42:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bosman F. T., Carneiro F., Hruban R. H., and Theise N. D., eds. 2010. P. 280 WHO Classification of Tumors of the Digestive system, 4th ed. International Agency for Research on Cancer, Lyon. [Google Scholar]
- 24. Chen, Y. , Chen C., Ke X., Xiong L., Shi Y., Li J., et al. 2014. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ. Cardiovasc. Genet. 7:43–48. [DOI] [PubMed] [Google Scholar]
- 25. Jaff, M. S. 2011. Relation between ABO blood groups and Helicobacter pylori infection in symptomatic patients. Clin. Exper. Gastroenterol. 4:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang, H. , Mooney C. J., and Reilly M. P.. 2012. ABO blood groups and cardiovascular diseases. Int. J. Vasc. Med. 2012:641917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rummel, S. K. , and Ellsworth R. E.. 2016. The role of the histoblood ABO group in cancer. Future Sci. OA. 2:FSO107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pomianowska, E. , Grzyb K., Westgaard A., Clausen O. P., and Gladhaug I. P.. 2012. Reclassification of tumour origin in resected periampullary adenocarcinomas reveals underestimation of distal bile duct cancer. Eur. J. Surg. Oncol. 38:1043–1050. [DOI] [PubMed] [Google Scholar]
- 29. Rizzato, C. , Campa D., Pezzilli R., Soucek P., Greenhalf W., Capurso G., et al. 2013. ABO blood groups and pancreatic cancer risk and survival: results from the PANcreatic Disease ReseArch (PANDoRA) consortium. Oncol. Rep. 29:1637–1644. [DOI] [PubMed] [Google Scholar]
- 30. Seymour, R. M. , Allan M. J., Pomiankowski A., and Gustafsson K.. 2004. Evolution of the human ABO polymorphism by two complementary selective pressures. Proceed. Royal Soc. B: Biol. Sci. 271:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cserti, C. M. , and Dzik W. H.. 2007. The ABO blood group system and Plasmodium falciparum malaria. Blood 110:2250–2258. [DOI] [PubMed] [Google Scholar]
- 32. Kornstad, L. 1997. Frequency of the blood group antigen K and the A1A2BO groups in the Norwegian counties. Gene Geog. 11:37–46. [PubMed] [Google Scholar]
- 33. Liumbruno, G. M. , and Franchini M.. 2014. Hemostasis, cancer, and ABO blood group: the most recent evidence of association. J. Thromb. Thrombolysis 38:160–166. [DOI] [PubMed] [Google Scholar]
- 34. Pelzer, U. , Klein F., Bahra M., Sinn M., Dorken B., Neuhaus P., et al. 2013. Blood group determinates incidence for pancreatic cancer in Germany. Front. Physiol. 4:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Engin, H. , Bilir C., Ustun H., and Gokmen A.. 2012. ABO blood group and risk of pancreatic cancer in a Turkish population in Western Black Sea region. Asian Pac. J. Cancer Prev. 13:131–133. [PubMed] [Google Scholar]
- 36. Vasan, S. K. , Hwang J., Rostgaard K., Nyren O., Ullum H., Pedersen O. B., et al. 2016. ABO blood group and risk of cancer: a register‐based cohort study of 1.6 million blood donors. Cancer Epidemiol. 44:40–43. [DOI] [PubMed] [Google Scholar]
- 37. Ben, Q. , Wang K., Yuan Y., and Li Z.. 2011. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case‐control study. Int. J. Cancer 128:1179–1186. [DOI] [PubMed] [Google Scholar]
- 38. Rahbari, N. N. , Bork U., Hinz U., Leo A., Kirchberg J., Koch M., et al. 2012. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer 12:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liumbruno, G. M. , and Franchini M.. 2013. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus. 11(4):491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao, M. , Wang Y., and Gao Y.. 2013. Association between Helicobacter pylori infection and pancreatic cancer development: a meta‐analysis. PLoS ONE 8:e75559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Risch, H. A. , Yu H., Lu L., and Kidd M. S.. 2010. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case‐control study. J. Natl Cancer Inst. 102:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wacklin, P. , Makivuokko H., Alakulppi N., Nikkila J., Tenkanen H., Rabina J., et al. 2011. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS ONE 6:e20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlsson, B. , Kindberg E., Buesa J., Rydell G. E., Lidon M. F., Montava R., et al. 2009. The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 Norovirus infection. PLoS ONE 4:e5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hofmann, B. T. , Stehr A., Dohrmann T., Gungor C., Herich L., Hiller J., et al. 2014. ABO blood group IgM isoagglutinins interact with tumor‐associated O‐glycan structures in pancreatic cancer. Clin. Cancer Res. 20:6117–6126. [DOI] [PubMed] [Google Scholar]
- 45. Ju, T. , Otto V. I., and Cummings R. D.. 2011. The Tn antigen‐structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 50:1770–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cumulative proportion survival (Kaplan–Meier) plot for the 195 pancreatic ductal adenocarcinomas according to breakdown by FUT2 secretor phenotype and resection status.
Table S1. SNPs used for ABO and FUT2 allele genotyping.
Table S2. ABO and FUT2 genotype frequencies of blood donor controls and pancreatic cancer cases.


