Abstract
Percutaneous hepatic interventions are generally safe given the fact that liver closely abuts the abdominal wall and hence it is easily accessible. However, the superior portion of liver, adjacent to the diaphragm, commonly referred as the “hepatic dome”, presents unique challenges for interventionists. Percutaneous access to the hepatic dome may be restricted by anatomical factors and special considerations may be required to avoid injury to the surrounding organs. The purpose of this review article is to discuss certain specific maneuvers and techniques that can enhance the success and safety of interventions in the hepatic dome.
Keywords: Hepatic dome, Radiofrequency ablation, Hepatocellular carcinoma, Percutaneous intervention
Core tip: Percutaneous interventions for lesions in the hepatic dome can be technically challenging. This review article discusses various maneuvers and techniques to safely access and treat lesions in this region.
INTRODUCTION
Image guided hepatic interventions are integral to management of infective and neoplastic liver lesions[1-5]. A gamut of hepatic interventions including abscess drainage, thermal ablation, biopsy of focal liver lesions have significantly improved the morbidity and mortality associated with hepatic surgeries[1,2,4-6]. They offer several advantages over other invasive procedures in the liver such as laparascopy/laparotomy including absence of a laparotomy scar, shorter hospital stay, avoidance of general anesthesia and lower risk of complications, morbidity and mortality[2,4-6]. Liver lesions are generally easily accessible for percutaneous procedures, however access to certain regions may be challenging such as the hepatic dome. Certain interventional procedures in the hepatic dome, particularly thermal ablative procedures including radiofrequency ablation (RFA) can be associated with complications related to diaphragmatic and/or pleural injury[6]. Therefore, it is important to adhere to certain guiding principles of safety when performing percutaneous interventions in the hepatic dome. The purpose of this article is to review the anatomy, challenges, technical considerations and various different adjunctive maneuvers to safely access and treat lesions in the hepatic dome.
HEPATIC DOME: ANATOMIC CONSIDERATIONS AND TECHNICAL CHALLENGES
The term hepatic dome in general refers to the liver parenchyma close to the diaphragm and roughly accounts for nearly one-third of the liver volume. For most of its part, the hepatic dome is related on the anterior, lateral and posterior aspects to diaphragm, lung parenchyma with the accompanying pleura and thoracic cage (Figure 1). On the medial aspect, the hepatic dome is related to the cardia and inferior vena cava (IVC) anteriorly and the vertebral column posteriorly. Given the intricate anatomic relations, there is potential risk of severe pain during thermal ablative procedures due to diaphragmatic irritation that can limit complete treatment and can increase need for deeper sedation/anesthesia[7-12]. Percutaneous catheter drainage of hepatic dome abscesses can be difficult due to the need for transgression of the sterile pleural space and/or the lung which increases risk of pleural space contamination and the resulting pleural fluid collections and/or empyemas can often be challenging to treat[1].
Figure 1.
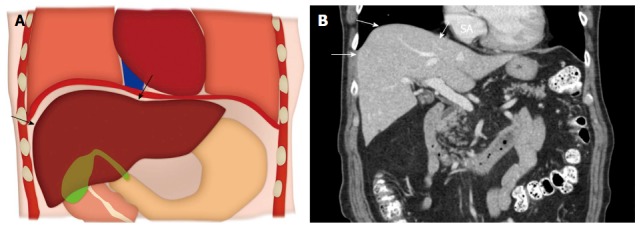
Anatomy of the hepatic dome. A: Colored schematic diagram; B: Coronal reformatted computed tomography image demonstrating the anatomy of the hepatic dome (arrows).
Other technical challenge encountered during percutaneous intervention of hepatic dome lesions is accessibility and localization. The technical difficulty is particularly amplified in patients receiving conscious sedation or general anesthesia as the liver becomes increasingly subcostal in position due to shallow respirations brought on by sedation[13].
HEPATIC DOME INTERVENTIONS: TIPS AND TRICKS
Imaging modality
Ultrasound can be useful in approaching lesions of hepatic dome as various angles can be used, given non-axial nature of ultrasound imaging. However ultrasound guidance can be challenging for deeply seated lesions. Computed tomography (CT) provides a 3-dimensional orientation of the needle/catheter and the target during navigation and allows performance of several additional maneuvers as discussed later in the review article. CT permits fluoroscopic capabilities and allows access to the hepatic dome through the transpleural/transpulmonic route. Particularly in patients undergoing ablation, the role of CT encompasses planning, positioning of needles, ablation monitoring, verification of completion and post-ablation assessment. Disadvantages of CT include inability to visualize certain lesions thereby necessitating administration of intravenous contrast and exposure to ionizing radiation. C-arm cone beam CT (CBCT) application may be useful in hepatic dome interventions. Respiratory gating application in CBCT can minimize motion mis-registration during navigation in thoracic and hepatic dome tumors[14]. Ablations of the liver with CBCT are often performed after administration of intra-arterial or intravenous contrast and obtaining an intra-arterial access might be logistically difficult at certain centers[15,16]. Real time magnetic resonance imaging (MRI) guidance for biopsy of hepatic dome lesion has been described by Lu et al[3] to be beneficial in targeting lesions best depicted on MRI. MRI guidance has its own caveats such as need for specialized equipment (including open magnet configuration), limited availability and expertise.
Lesion localization
Precise lesion localization within the hepatic dome is crucial, particularly during biopsies and ablations to maximize diagnostic yield and achieve complete tumor destruction respectively. Accurate definition and localization of lesions on CT can be accomplished by either use of anatomic landmarks, contrast administration or additional techniques[17].
Extrapolation based on anatomic landmarks: Tumors seen on pre-procedural MR scans may not be well visualized on preliminary CT images or ultrasound during the procedure. In such circumstances, a comprehensive review of the imaging modality best depicting the lesion helps to identify the orientation of the lesion relative to adjacent landmarks such as blood vessels, bones, vascular or parenchymal calcification. Extrapolation of lesion relationship to hepatic veins, cardiac margin, aorta and IVC can be particularly helpful while performing interventions in hepatic dome. Sainani et al[17] reported that this strategy is highly accurate (98%) for percutaneous biopsy in liver and can obviate the need of intravenous contrast during the procedure (Figure 2).
Figure 2.
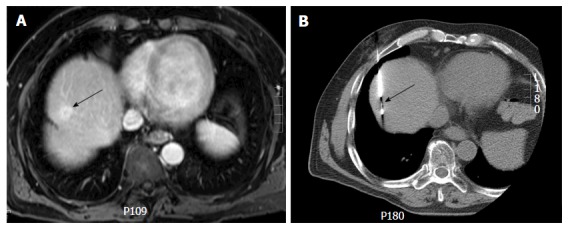
Computed tomography guided biopsy of a liver dome lesion in a 61-year-old man. A: Axial post gadolinium T1-weighted magnetic resonance image shows a 2 cm lesion (arrow) in the hepatic dome. On pre-procedural computed tomography, the tumor was not well seen and contrast could not be administered due to iodine allergy; B: Needle placement for biopsy was done based on use of anatomic landmarks (arrow) (configuration of inferior vena cava, cardiac margin and aorta) via a transpulmonary approach. Histopathology: Hepatocellular carcinoma.
Contrast administration: Administration of intravenous contrast can be used to guide exact placement of biopsy needle or RF electrode and is generally done after the guiding needle has been placed in to the presumed lesion location[11]. The relationship of the guiding needle and the lesion then facilitates the accurate placement of the biopsy needle or the RF electrode. It is important to ensure that the patient’s serum urea and creatinine are within normal limits prior to administering contrast to avoid the risk of contrast induced nephropathy[18].
Other techniques: Several techniques have been described for targeting poorly visible hepatic lesions during interventional procedures[19-22]. Image fusion techniques combining real time ultrasonography with preprocedural CT/MRI images can be used effectively to enhance detectability for focal hepatic lesions with poor sonographic visibility[19,20]. A variety of tracking methods are available for image fusion such as image-based, optical, and electromagnetic tracking (most frequently used)[19]. The image fusion techniques however have limitations related to mis-registration because while the reference images (CT, MRI) are often obtained in a static breath-holding state, real-time ultrasound is affected by tissue deformation due to patient’s respiration and movements. Most commercially available image fusion systems lack compensating mechanism for patient respiration and movement[23,24]. Hookwire and Suture localization under CT guidance followed by microwave ablation under ultrasound guidance for a sonographically invisible lesion has also been described by Kanazawa et al[21]. Such an approach however is cumbersome as it requires two different procedures for an ablation that can be entirely performed under CT guidance. Prior use of lipiodol might be helpful in the visualisation of hepatocellular carcinomas treated with lipoidal-transarterial chemoembolization (TACE)[22].
Optimizing access route
Selection of the approach and proper patient positioning are two important considerations for optimizing access to localization of lesions in the hepatic dome.
Percutaneous approach: The lesions of the hepatic dome can be accessed either by subcostal, intercostal or epipericardial fat pad approach. The choice of percutaneous approach is often based on operator experience and the access route to the regional anatomy of each individual patient is determined by reviewing prior imaging or preliminary scans. Subcostal approach is most preferred whenever feasible as this usually avoids transgression of lung and pleura (Figure 3). This approach is particularly beneficial in drainage of hepatic abscesses especially while using ultrasound guidance and can be facilitated by placing the patient in decubitus position. However this approach may not always be feasible and an intercostal route is frequently required (Figure 4) which may necessitate pleural or pulmonic transgression[1]. Pulmonary transgression can be avoided by creation of artificial pleural effusion or pneumothorax. Although pulmonary transgression may be unavoidable, for example, in patients with pleural adhesions[25]. In cases where pleural or pulmonary transgression is unavoidable it is important to limit the number of punctures to minimize the risk of pneumothorax. Furthermore, the interventionalist and patient must be prepared for the possibility of a pneumothorax and be aware of the management of such a complication. The technique of pulmonary transgression is of limited value in patients with severe emphysema or coagulopathy[25,26].
Figure 3.
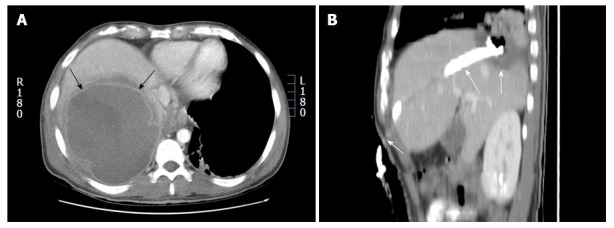
Percutaneous drainage of hepatic dome abscess in a 36-year-old man. A: Axial contrast computed tomography shows the large hepatic dome abscess (arrows). Pleural transgression carried an increased risk of pleural complications; B: Percutaneous catheter drainage using a subcostal approach (arrows) allowed successful abscess treatment while avoiding pleural transgression.
Figure 4.
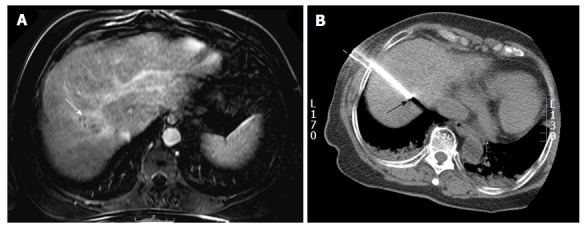
Percutaneous radiofrequency ablation of a hepatic dome hepatocellular carcinoma in a 54-year-old man. A: Axial post gadolinium T1-weighted image in the portal venous phase demonstrates a 3.4 cm hepatocellular carcinoma in the hepatic dome (arrow); B: During the radiofrequency ablation procedure, the patient was placed in the oblique position and using a lateral intercostal approach the tumor was accessed for a successful ablation (arrow).
Brennan et al[27] described a novel epipericardial fat pad approach for safe access to hepatic dome lesions. The epipericardial fat pad is a variable sized structure located in the anterior mediastinum, outside the fibrous pericardium[27]. The authors recommend that an epipericardial fat pad exceeding 1 cm in thickness, may provide a safe window for percutaneous image guided RFA using CT fluoroscopy (Figure 5)[27].
Figure 5.
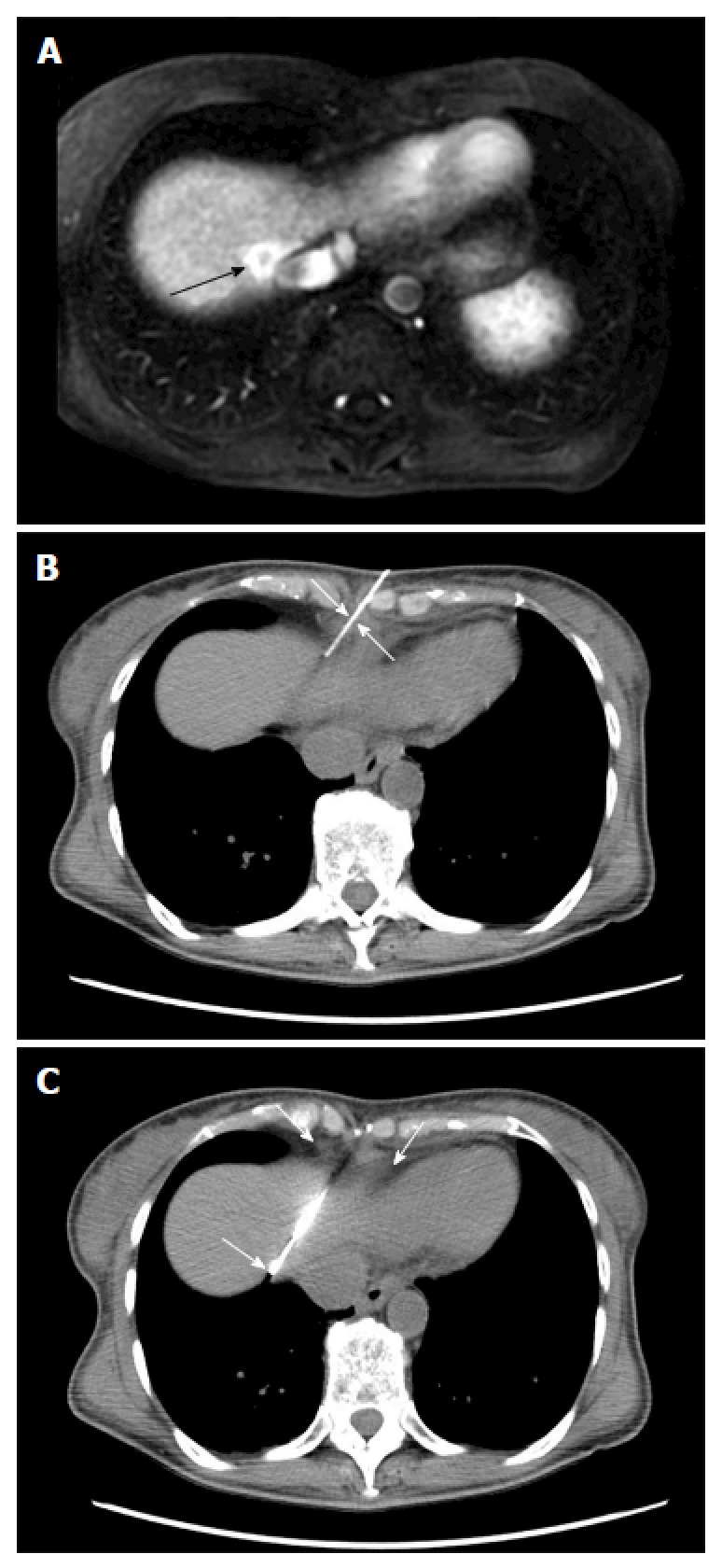
Computed tomography guided biopsy of a liver lesion adjacent to the inferior vena cava in a 56-year-old woman with breast cancer. A: Axial post gadolinium image T1 weighted magnetic resonance image demonstrates an enhancing lesion adjacent to the inferior vena cava (arrow); B and C: Intraprocedural computed tomography images demonstrate placement of the biopsy needle into the lesion through the epipericardial fat pad (arrows). Biopsy: Breast cancer metastases.
Patient positioning: Optimal patient positioning not only determines a safe percutaneous path to the lesion but also ensures patient comfort and minimizes motion. An ideal position is one which allows the least complicated access to the hepatic dome. Supine position is the most common position employed and is generally the most comfortable one. It allows the use of anterior and lateral approach to access the dome (Figure 6). Oblique patient position can also be employed to improve the safety of a percutaneous path to the hepatic dome (Figure 4). Oblique patient position is usually employed when using a lateral approach to the hepatic dome. Lateral decubitus position can also be used when accessing the hepatic dome using a lateral approach. A lateral decubitus position is beneficial in obese patients and women with large amount of breast tissue where an anterior approach is not feasible.
Figure 6.
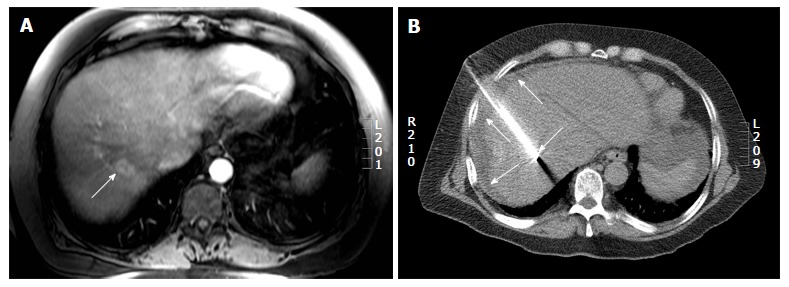
Computed tomography guided radiofrequency ablation in a 56-year-old lady with colorectal liver metastases. A: Axial post gadolinium T1 weighted magnetic resonance image shows a 2.7 cm (arrow) hepatic dome metastases; B: The radiofrequency ablation was performed with the patient in supine position and needle placement through the anterolateral intercostal approach. Hydrodissection was performed in this patient (arrows).
Adjunctive techniques
Non-target organ injury is the most feared complication during ablative procedures of hepatic dome. Several maneuvers could be performed in order to minimize collateral damage such as CT gantry angulation, creation of artificial ascites/pleural effusions and artificial pneumothorax.
Gantry angulation: Angulation of the CT gantry is a useful approach when the presence of overlying structures precludes a safe path to the hepatic dome (Figure 7). Angling the gantry allows optimum needle track visualization and permits a lower site of entry relative to lesion location, helping avoid transgression of pleura and lungs and thus reducing the risk of pneumothorax and pleural contamination. In this technique, the CT gantry is tilted towards the patient feet to achieve a caudo-cephalad beam direction. After preliminary scanning and identification of a safe path to the lesion, the needle shaft and hub are aligned to the CT gantry with the help of the localizing light. The needle is then advanced by constantly monitoring the needle tip position at frequent intervals while maintaining the angle of the needle. Gantry angulation technique is frequently performed for hepatic dome lesions and allows a subcostal approach to lesions high in the subphrenic location[28].
Figure 7.
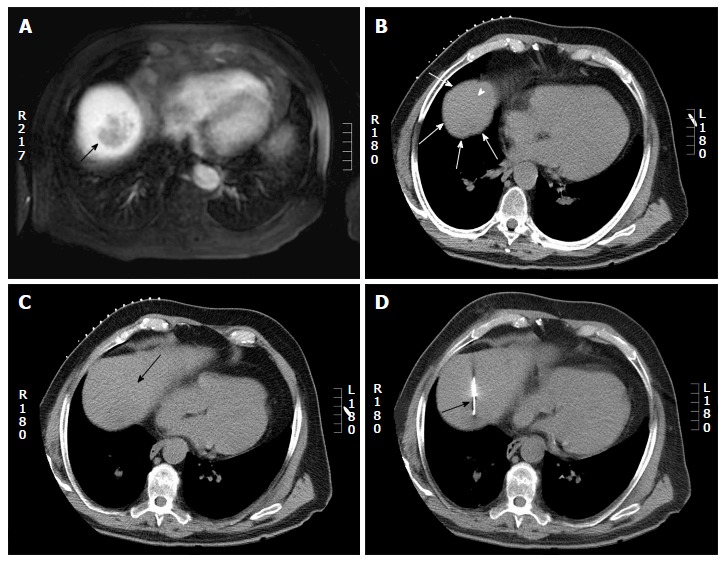
Computed tomography guided biopsy in a 65-year-old man. A: Axial post gadolinium magnetic resonance imaging shows a 4 cm hepatic dome lesion; B: On preprocedural CT, the lesion in the high dome is surrounded by lung (arrow head) on all sides. Pulmonic transgression was not possible as the patient had severe emphysema; C: The CT gantry was angulated in the craniocaudal direction (20 degrees) which created a safe path to the tumor from the anterior aspect (arrow); D: Axial intraprocedural CT image shows biopsy needle within the lesion (arrow). Biopsy: Hepatocellular carcinoma. CT: Computed tomography.
Hydro dissection/artificial ascites: Creation of artificial ascites or hydrodissection is an effective techniques for safe percutaneous ablation of hepatic dome lesions[7,8,29,30]. Hydrodissection involves injection of fluid into the peritoneal space around the liver to create separation of hepatic dome from the diaphragm, thereby preventing damage to the diaphragm and pleura during thermal ablation. Additionally, this technique diminishes post procedure pain resulting from diaphragmatic irritation, and reduces the need for general anesthesia for pain control allowing use of conscious sedation[30]. Hydrodissection can be performed using ultrasound or CT guidance and we most commonly use a 14-20 G Chiba needle or a 5 French vascular catheter/sheath for instilling fluid (Figure 8)[7,8,29]. For hepatic dome interventions, the puncture sites for creation of artificial ascites are typically at the level of left subphrenic space. Prolonged procedures such as ablations necessitate continued hydrodissection throughout the primary procedure to maintain sustained separation of the lesion from the diaphragm. Five percent dextrose water (D5W) is preferred over normal saline for hydrodissection since it provides significantly better electrical isolation, reduces unwanted heat dissipation to the adjacent organs and is least likely to cause volume shifts due to its iso-osmolar nature[7,8,29,31]. While no definitive amount of separation has been universally agreed upon, at least 5 mm separation between the diaphragmatic margin and liver capsule is recommended to minimize organ damage. The instilled fluid usually resorbs spontaneously within a week and does not decrease the therapeutic efficacy of RFA[29]. Despite its benefits, occasionally the fluid dissipates away from the intended site and hydrodissection is not effective in the presence of peritoneal adhesions due to prior treatments such as surgical resection, TACE or thermal ablation. Additionally, lesions located in the bare area of liver cannot be separated by hydrodissection as this area is surrounded by peritoneal ligaments. Omentum interposed during hepatic surgeries can also impede successful induction of artificial ascites[7].
Figure 8.
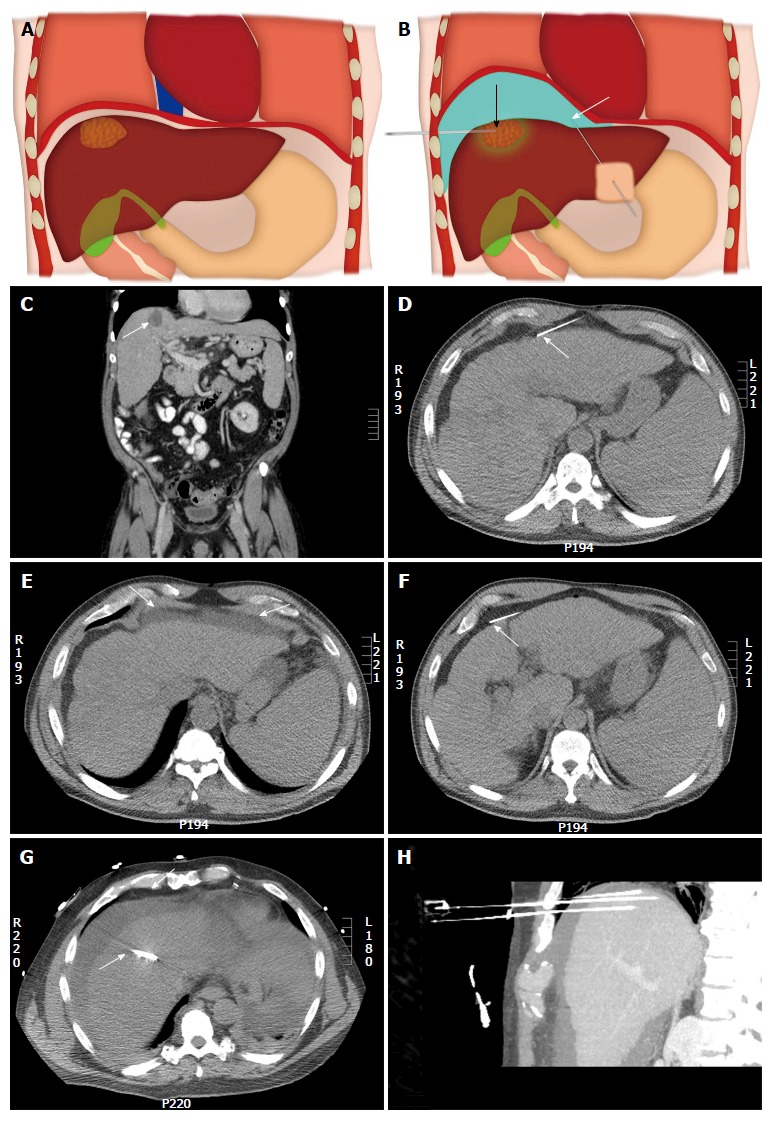
Illustration of the upper abdomen demonstrating the hydro-dissection technique. A: Coronal colored image shows a hepatic dome lesion very close to the diaphragm; B: Coronal colored image after hydro-dissection (shown in blue color) shows the separation of the dome of liver from the diaphragm which improves percutaneous access to the lesion and limits diaphragmatic injury. White arrow shows the needle for hydodissection and black arrow shows the needle into the lesion. An example of a hepatic dome lesion (C) (arrow) where hydrodissection was attempted by needle placed anteriorly (D) (arrow); E: Axial computed tomography showed accumulation of fluid within the properitoneal fat (arrows); F: The needle was repositioned with the tip of the needle into the peritoneal cavity (arrow); G: Successful hydrodissection achieved using instillation of 500 cc of D5W through the needle (arrow); H: The fluid was used to create a safe path for radiofrequency ablation of the hepatic dome hepatocellular carcinoma. Sagittal Maximum intensity projection image demonstrating the artificial ascites and electrodes in position in the hepatic dome lesion.
Artificial pleural effusion: Bare area of liver (not lined by peritoneum) is in direct contact with the diaphragm. The fluid form an artificial ascites cannot dissect this region from the diaphragm. Similar situation arises when intra-peritoneal adhesions limit separation of the diaphragm from the liver. Artificial pleural effusion using saline is a valuable adjunctive technique in such situations. It also creates a safe percutaneous path or good sonographic window when using ultrasound for image guidance[32-36].
Iatrogenic pneumothorax: An iatrogenic pneumothorax can be created when other approaches fail[37,38]. In this technique, an 18 gauge epidural needle is appropriately positioned in the pleural space and around 50 mL of air, obtained through a micro-porous filter, is injected into the pleural space. Subsequently, serial boluses of 200, 400, 600 and 800 mL are injected to separate the lung from the pleura (Figure 9). Following completion of the interventional procedure, the intrapleural air is aspirated through the catheter into the syringe and expelled through the stopcock[37]. The patient is usually admitted overnight for observation and serial radiographs are obtained to monitor the resolution of the pneumothorax. Pneumothorax tends to accumulate in non-dependent locations and hence patient positioning is of critical importance. For example, if anterior approach is adopted, the patient should be placed in supine position to direct the pneumothorax anteriorly[37].
Figure 9.
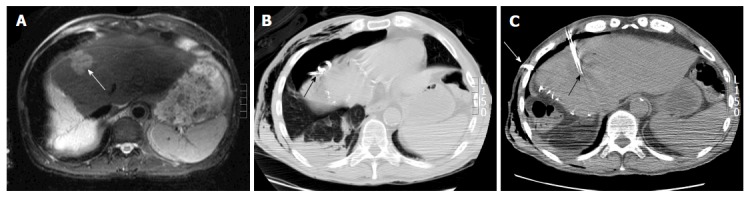
Artificial pneumothorax for radiofrequency ablation of hepatic dome hepatocellular carcinoma in a 69-year-old man. A: Axial T2WI magnetic resonance imaging shows A 4 cm lesion (arrow) in the hepatic dome; B: Artificial pneumothorax was created after instillation of intrapleural air. A chest tube was placed for drainage (arrow); C: Intra-procedural computed tomography shows radiofrequency electrode within the lesion for a successful ablation (black arrow).
Other techniques: Investigators have tried using different barriers for diaphragmatic protection such as intraabdominal carbon dioxide insufflations or angiographic balloon interposition, although the experience with their use is limited[10,39]. Raman et al[10] studied the use of intraperitoneal carbon dioxide insufflations for diaphragmatic protection during hepatic RFA ablations in porcine model and proved its efficacy in limiting diaphragmatic injury during superficial hepatic RFA. Knuttinen et al[39] interposed an angiographic balloon catheter during RFA ablation of the hepatic dome in a porcine model and demonstrated that balloon interposition is an effective technique for diaphragmatic protection. Balloon interposition has been reported to be superior to hydro-dissection or carbon dioxide insufflation, as the balloon remains stable during the procedure, while fluid and gas have a tendency to dissipate, however evidence in this regard is limited[39]. Electrode “retraction/torquing” technique is another maneuver with the use of expandable RFA probes in kidney, liver and lung tumors[40]. In this technique, the expandable electrode is retracted or torqued to displace the organ after the electrode is in position and fully expanded. This technique may be ineffective in isolation as only a few millimetres of displacement is achieved but could be used as an adjunct to other techniques.
COMPLICATIONS
Most dreaded complications during hepatic dome interventions include diaphragmatic and lung injury, pleural effusion, pneumothorax and empyema. Specific maneuvers like CT-guided transpulmonary needle insertion for liver tumors may lead to pneumothorax, lung hemorrhage and hemothorax, pleural effusion, diaphragmatic injury, tumor seeding in the pleura and/or lung parenchyma, lung abscess and systemic air embolism[26]. Serious complications such as massive pulmonary hemorrhage and systemic air embolism may result from transpulmonary RF needle insertion[41-43]. Diaphargmatic injury can lead to severe pain due to irritation, diaphragmatic palsy and/or perforation[7-10,30,31,39,44]. Diaphragmatic injury can also lead to fistulization of hepatic dome processes into the thorax (Figure 10). Injury to the lung and pleura can result in pneumothorax, pleural effusion and empyema which often need chest tube drainage[9,45]. The reported incidence of major diaphragmatic complications is low and has been reported to be more frequent with deployable radiofrequency electrodes and multiple treatments[29,46-49]. Post-ablation local tumor progression may be slightly higher for peri-diaphragmatic tumors as compared to central tumors as these tumors are ablated more cautiously because of concern for collateral damage[50].
Figure 10.
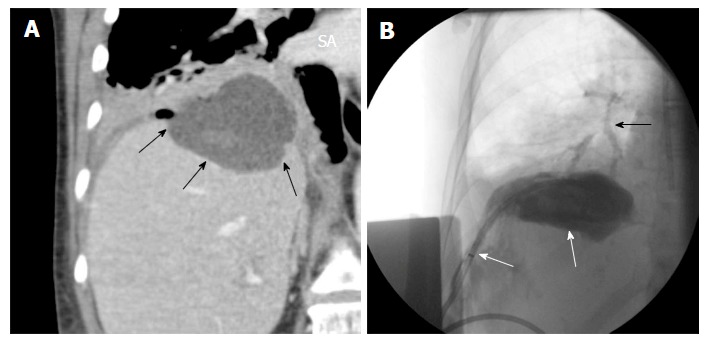
Hepatic abscess complicating a hepatic dome metastases ablation. A: Coronal reformatted image shows a abscess in the dome of liver (arrows); B: Percutaneous drainage was performed and drain injection shows communication with the bronchi (black arrow).
CONCLUSION
Image guided interventions in the hepatic dome often pose unique challenges to interventional radiologists. Interventionists should use their anatomic expertise along with the wide range of available imaging and interventional techniques to safely access and successfully manage hepatic dome lesions.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Nothing to disclose.
Peer-review started: March 5, 2017
First decision: March 28, 2017
Article in press: April 24, 2017
P- Reviewer: Chen YJ, Ji FP, Sirin G S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Johnson RD, Mueller PR, Ferrucci JT, Dawson SL, Butch RJ, Papanicolaou N, vanSonnenberg E, Simeone JF, Wittenberg J. Percutaneous drainage of pyogenic liver abscesses. AJR Am J Roentgenol. 1985;144:463–467. doi: 10.2214/ajr.144.3.463. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesan AM, Gervais DA, Mueller PR. Percutaneous radiofrequency thermal ablation of primary and metastatic hepatic tumors: current concepts and review of the literature. Semin Intervent Radiol. 2006;23:73–84. doi: 10.1055/s-2006-939843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu DS, Lee H, Farahani K, Sinha S, Lufkin R. Biopsy of hepatic dome lesions: semi-real-time coronal MR guidance technique. AJR Am J Roentgenol. 1997;168:737–739. doi: 10.2214/ajr.168.3.9057526. [DOI] [PubMed] [Google Scholar]
- 4.Rhim H, Goldberg SN, Dodd GD, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21 Spec No:S17–S35; discussion S36-S39. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 6.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009;34:371–380. doi: 10.1007/s00261-008-9408-4. [DOI] [PubMed] [Google Scholar]
- 8.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190:91–98. doi: 10.2214/AJR.07.2384. [DOI] [PubMed] [Google Scholar]
- 9.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–418. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 10.Raman SS, Aziz D, Chang X, Sayre J, Lassman C, Lu D. Minimizing diaphragmatic injury during radiofrequency ablation: efficacy of intraabdominal carbon dioxide insufflation. AJR Am J Roentgenol. 2004;183:197–200. doi: 10.2214/ajr.183.1.1830197. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Kim CS, Lee JM, Chung GH, Chon SB. Efficacy and safety of radiofrequency ablation of hepatocellular carcinoma in the hepatic dome with the CT-guided extrathoracic transhepatic approach. Eur J Radiol. 2006;60:100–107. doi: 10.1016/j.ejrad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Chun JY, Ho CS. Management of Pericardial Effusion following Cardiac Perforation during Radiofrequency Ablation of Hepatocellular Carcinoma. Semin Intervent Radiol. 2014;31:101–103. doi: 10.1055/s-0033-1363850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond GB, Allan PL, Logan MR. Changes in diaphragmatic position in association with the induction of anaesthesia. Br J Anaesth. 1986;58:1246–1251. doi: 10.1093/bja/58.11.1246. [DOI] [PubMed] [Google Scholar]
- 14.Floridi C, Radaelli A, Abi-Jaoudeh N, Grass M, Lin M, Chiaradia M, Geschwind JF, Kobeiter H, Squillaci E, Maleux G, et al. C-arm cone-beam computed tomography in interventional oncology: technical aspects and clinical applications. Radiol Med. 2014;119:521–532. doi: 10.1007/s11547-014-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto M, Numata K, Kondo M, Nozaki A, Hamaguchi S, Takebayashi S, Tanaka K. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentgenol. 2010;194:W452–W454. doi: 10.2214/AJR.09.3514. [DOI] [PubMed] [Google Scholar]
- 16.Iwazawa J, Ohue S, Hashimoto N, Mitani T. Ablation margin assessment of liver tumors with intravenous contrast-enhanced C-arm computed tomography. World J Radiol. 2012;4:109–114. doi: 10.4329/wjr.v4.i3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sainani NI, Schlett CL, Hahn PF, Gervais DA, Mueller PR, Arellano RS. Computed tomography-guided percutaneous biopsy of isoattenuating focal liver lesions. Abdom Imaging. 2014;39:633–644. doi: 10.1007/s00261-014-0089-x. [DOI] [PubMed] [Google Scholar]
- 18.McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, Williamson EE, Kallmes DF. Intravenous Contrast Material-induced Nephropathy: Causal or Coincident Phenomenon? Radiology. 2016;278:306. doi: 10.1148/radiol.2015154044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227–239. doi: 10.14366/usg.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HJ, Lee MW, Lee MH, Hwang J, Kang TW, Lim S, Rhim H, Lim HK. Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med. 2013;32:1557–1564. doi: 10.7863/ultra.32.9.1557. [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa S, Sadamori H, Mimura H, Yoshimura K, Inagaki M, Yagi T, Tanaka N, Hiraki Y. Localization of hepatocellular carcinoma in the hepatic dome before tumor ablation: using a system that includes a hookwire and suture. AJR Am J Roentgenol. 2000;175:1259–1261. doi: 10.2214/ajr.175.5.1751259. [DOI] [PubMed] [Google Scholar]
- 22.Basile A, Calcara G, Montineri A, Brisolese V, Lupattelli T, Patti MT. Application of a new combined guiding technique in RF ablation of subphrenic liver tumors. Eur J Radiol. 2008;66:321–324. doi: 10.1016/j.ejrad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Hakime A, Deschamps F, De Carvalho EG, Teriitehau C, Auperin A, De Baere T. Clinical evaluation of spatial accuracy of a fusion imaging technique combining previously acquired computed tomography and real-time ultrasound for imaging of liver metastases. Cardiovasc Intervent Radiol. 2011;34:338–344. doi: 10.1007/s00270-010-9979-7. [DOI] [PubMed] [Google Scholar]
- 24.Ewertsen C, Săftoiu A, Gruionu LG, Karstrup S, Nielsen MB. Real-time image fusion involving diagnostic ultrasound. AJR Am J Roentgenol. 2013;200:W249–W255. doi: 10.2214/AJR.12.8904. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, Takagi H, Mori M, Nakajima T. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006;12:608–611. doi: 10.3748/wjg.v12.i4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iguchi T, Inoue D, Yabushita K, Sakaguchi K, Tatsukawa M, Sasaki H, Kanazawa S. Effect of CT fluoroscopy-guided transpulmonary radiofrequency ablation of liver tumours on the lung. Br J Radiol. 2012;85:e373–e377. doi: 10.1259/bjr/34646739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan DD, Ganguli S, Brecher CW, Goldberg SN. Thinking outside the abdominal box: safe use of the epipericardial fat pad window for percutaneous radiofrequency ablation of hepatic dome tumors. J Vasc Interv Radiol. 2008;19:133–136. doi: 10.1016/j.jvir.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Maher MM, Gervais DA, Kalra MK, Lucey B, Sahani DV, Arellano R, Hahn PF, Mueller PR. The inaccessible or undrainable abscess: how to drain it. Radiographics. 2004;24:717–735. doi: 10.1148/rg.243035100. [DOI] [PubMed] [Google Scholar]
- 29.Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196:907–913. doi: 10.2214/AJR.10.4584. [DOI] [PubMed] [Google Scholar]
- 30.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 31.Laeseke PF, Sampson LA, Brace CL, Winter TC, Fine JP, Lee FT. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol. 2006;186:S249–S254. doi: 10.2214/AJR.04.1240. [DOI] [PubMed] [Google Scholar]
- 32.Kondo Y, Yoshida H, Tateishi R, Shiina S, Kawabe T, Omata M. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95:996–1004. doi: 10.1002/bjs.6058. [DOI] [PubMed] [Google Scholar]
- 33.Shibata T, Iimuro Y, Ikai I, Hatano E, Yamaoka Y, Konishi J. Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol. 2002;13:313–315. doi: 10.1016/s1051-0443(07)61725-4. [DOI] [PubMed] [Google Scholar]
- 34.Iwai S, Sakaguchi H, Fujii H, Kobayashi S, Morikawa H, Enomoto M, Tamori A, Kawada N. Benefits of artificially induced pleural effusion and/or ascites for percutaneous radiofrequency ablation of hepatocellular carcinoma located on the liver surface and in the hepatic dome. Hepatogastroenterology. 2012;59:546–550. doi: 10.5754/hge11988. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Liang P, Yu X, Cheng Z, Han Z, Yu J, Liu F. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia. 2013;29:663–670. doi: 10.3109/02656736.2013.833347. [DOI] [PubMed] [Google Scholar]
- 36.Han Y, Yu L, Hao YZ, Yang M, Liu S, Deng YB, He LF, Cai JQ, Chen MH. [Percutaneous radiofrequency ablation with artificial hydrothorax for liver cancer in the hepatic dome] Zhonghua Zhong Liu Za Zhi. 2012;34:846–849. doi: 10.3760/cma.j.issn.0253-3766.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 37.de Baère T, Dromain C, Lapeyre M, Briggs P, Duret JS, Hakime A, Boige V, Ducreux M. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology. 2005;236:666–670. doi: 10.1148/radiol.2362040992. [DOI] [PubMed] [Google Scholar]
- 38.Golse N, Ducerf C, Rode A, Gouillat C, Baulieux J, Mabrut JY. Transthoracic approach for liver tumors. J Visc Surg. 2012;149:e11–e22. doi: 10.1016/j.jviscsurg.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Knuttinen MG, Van Ha TG, Reilly C, Montag A, Straus C. Unintended thermal injuries from radiofrequency ablation: organ protection with an angioplasty balloon catheter in an animal model. J Clin Imaging Sci. 2014;4:1. doi: 10.4103/2156-7514.126018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsoumakidou G, Buy X, Garnon J, Enescu J, Gangi A. Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol. 2011;14:170–176. doi: 10.1053/j.tvir.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, Teratani T, Shiina S, Ohtomo K. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25 Suppl 1:S57–S68. doi: 10.1148/rg.25si055505. [DOI] [PubMed] [Google Scholar]
- 42.Herrera LJ, Fernando HC, Perry Y, Gooding WE, Buenaventura PO, Christie NA, Luketich JD. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–937. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 43.Okuma T, Matsuoka T, Tutumi S, Nakmura K, Inoue Y. Air embolism during needle placement for CT-guided radiofrequency ablation of an unresectable metastatic lung lesion. J Vasc Interv Radiol. 2007;18:1592–1594. doi: 10.1016/j.jvir.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 44.Yamagami T, Yoshimatsu R, Matsushima S, Tanaka O, Miura H, Nishimura T. Diaphragmatic hernia after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2011;34 Suppl 2:S175–S177. doi: 10.1007/s00270-010-9832-z. [DOI] [PubMed] [Google Scholar]
- 45.Miura H, Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Nishimura T. Pneumothorax induced by radiofrequency ablation for hepatocellular carcinoma beneath the diaphragm under real-time computed tomography-fluoroscopic guidance. Acta Radiol. 2010;51:613–618. doi: 10.3109/02841851003786001. [DOI] [PubMed] [Google Scholar]
- 46.Head HW, Dodd GD, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243:877–884. doi: 10.1148/radiol.2433060157. [DOI] [PubMed] [Google Scholar]
- 47.Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, Lim HK. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34–42. doi: 10.3348/kjr.2009.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koda M, Ueki M, Maeda N, Murawaki Y. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;180:1561–1562. doi: 10.2214/ajr.180.6.1801561. [DOI] [PubMed] [Google Scholar]
- 49.Shibuya A, Nakazawa T, Saigenji K, Furuta K, Matsunaga K. Diaphragmatic hernia after radiofrequency ablation therapy for hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186:S241–S243. doi: 10.2214/AJR.04.0931. [DOI] [PubMed] [Google Scholar]
- 50.Smolock AR, Lubner MG, Ziemlewicz TJ, Hinshaw JL, Kitchin DR, Brace CL, Lee FT. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. AJR Am J Roentgenol. 2015;204:197–203. doi: 10.2214/AJR.14.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]


