Abstract
Metabotropic glutamate receptor subtype 5 (mGluR5) is a Group I mGlu subfamily of receptors coupled to the inositol trisphosphate/diacylglycerol pathway. Like other mGluR subtypes, mGluR5s contain a phylogenetically conserved, extracellular orthosteric binding site and a more variable allosteric binding site, located on the heptahelical transmembrane domain. The mGluR5 receptor has proved to be a key pharmacological target in conditions affecting the central nervous system (CNS) but its presence outside the CNS underscores its potential role in pathologies affecting peripheral organs such as the gastrointestinal (GI) tract and accessory digestive organs such as the tongue, liver and pancreas. Following identification of mGluR5s in the mouth, various studies have subsequently demonstrated its involvement in mechanical allodynia, inflammation, pain and oral cancer. mGluR5 expression has also been identified in gastroesophageal vagal pathways. Indeed, experimental and human studies have demonstrated that mGluR5 blockade reduces transient lower sphincter relaxation and reflux episodes. In the intestine, mGluR5s have been shown to be involved in the control of intestinal inflammation, visceral pain and the epithelial barrier function. In the liver, mGluR5s have a permissive role in the onset of ischemic injury in rat and mice hepatocytes. Conversely, livers from mice treated with selective negative allosteric modulators and mGluR5 knockout mice are protected against ischemic injury. Similar results have been observed in experimental models of free-radical injury and in vivo mouse models of acetaminophen intoxication. Finally, mGluR5s in the pancreas are associated with insulin secretion control. The picture is, however, far from complete as the review attempts to establish in particular as regards identifying specific targets and innovative therapeutic approaches for the treatment of GI disorders.
Keywords: Metabotropic glutamate receptor subtype 5, Esophagus, Intestine, Liver, Pancreas
Core tip: Metabotropic glutamate 5 receptors (mGluR5s) belong to Group I mGlu receptors which are coupled to the inositol trisphosphate/diacylglycerol pathway. As well as in the brain, mGluR5s and their ligands have been found in peripheral organs, including those associated with the gastrointestinal (GI) tract. We review published findings about their identification and role in the mouth, esophagus, stomach and intestine and accessory digestive organs such as the tongue, liver and pancreas. We conclude that mGluR5s are still poorly studied in the GI tract and their peripheral non-synaptic signaling, and that their therapeutic potential needs to be further investigated.
INTRODUCTION
Before 1985, quisqualate was known to be an agonist of ionotropic receptors (AMPA, kainate), but, in that year, its ability to stimulate the synthesis of inositol phosphates in striatal neurons was also demonstrated[1]. Soon after, in 1986, Nicoletti et al[2] showed that glutamate stimulates polyphosphoinositide (PI) hydrolysis in hippocampal slice brains from newborn rats. These pioneering studies opened up the way for the discovery and identification of a new family of receptors, now called metabotropic glutamate receptors (mGluRs). The term metabotropic was adopted to distinguish them from ionotropic glutamate receptors.
The mGluR family belongs to Class C G-protein coupled receptors (GPCRs) and consists of eight different subtypes (mGluR1-8) classified into three groups on the basis of sequence similarities, intracellular signaling and pharmacological properties: Group I receptors (Subtypes mGluR1 and mGluR5); Group II receptors (Subtypes mGluR2 and mGluR3) and Group III receptors (Subtypes mGluR4, mGluR6, mGluR7 and mGlu8). Group I receptors are coupled to Gq proteins and activate phospholipase C, which in turn hydrolyzes phosphatidylinositole 4,5-bisphosphate to inositol trisphosphate (IP3) and diacylglycerol (Figure 1). This pathway leads to calcium release from endoplasmic reticulum and activation of numerous kinases, such as protein kinase A, protein kinase C, extracellular signal-related kinase (ERK) and mitogen-activated protein kinase (MAPK). On the contrary, Group II and Group III receptors are coupled to Gi proteins, leading to the inhibition of cAMP synthesis[3]; these mGluRs act as regulators, providing negative feedback that inhibits glutamate release.
Figure 1.
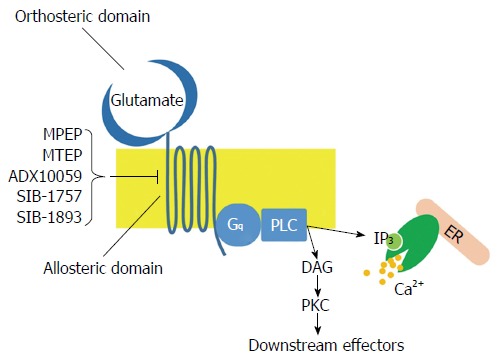
Schematic representation of metabotropic glutamate 5 receptor functions. DG: Diacylglycerol; ER: Endoplasmic reticulum; IP3: Inositol trisphosphate; PKC: Protein kinase C; PLC: Phospholipase C.
Like other mGluR subtypes, mGluR5s contain a phylogenetically conserved, extracellular orthosteric binding site and a more variable allosteric binding site, located on the heptahelical transmembrane domain. Given allosteric binding sites’ variability, subtype-selective allosteric modulators have been found and are frequently used in basic, preclinical and clinical research. A recent review, focused on mGluR5 localization, reported that this receptor is not restricted to postsynaptic membrane and extrasynaptic regions but is also found in intracellular membranes in many different brain regions such as inner nuclear, outer nuclear and endoplasmic reticulum membranes[4]. Numerous studies have also demonstrated the presence of mGluR5s in a number of peripheral non-neuronal cells. For instance, mGluR5s are found in insulin-containing vesicles purified from clonal pancreatic beta-cells[5], in rat and human testis[6] as well as in murine thymocytes and thymic stromal cells[7].
The present study reviews and discusses published data on the localization and role of mGluR5s in the gastrointestinal tract (GI) and accessory digestive organs such as the liver and pancreas. We used articles searched for in PubMed, MEDLINE, Google Scholar, and Google databases using the acronyms mGluR5 and mGlu5 in combination with mouth, esophagus, stomach, intestine, tongue, liver and pancreas to identify articles relating to mGluR5s in the GI published in the English-language literature from January 1985 to December 2016.
MGLUR5S IN THE GASTROINTESTIONAL TRACT
mGluR5s in the mouth
The analysis of Group I mGluR localization in the mouth shows that mGluR5s, but not mGluR1s, are constitutively expressed in the trigeminal ganglion[8] and the masseter muscle[9]. During mandibular disorders characterized by pain in the temporomandibular joint (TMJ), inflammation is associated with mGluR5 upregulation that differentially modulates the expression of glutamate receptors in male rats, suggesting that the use of mGluR5 antagonists may be effective in the control of inflammation pain conditions[10].
Some studies concerned with the orofacial area, highlight the role of peripheral Group I mGluRs in interleukin-1beta (IL-1beta)-induced mechanical allodynia: the results show that rat treatment with mGluR5 antagonists, Methyl-6-(phenylethynyl)pyridine (MPEP) or SIB1893, 10 min prior to injection of IL-1beta suppress IL-1beta-induced mechanical allodynia[11-13].
Using immunofluorescence, mGluR5s have also been identified in the terminal region of axon innervating human dental pulp suggesting their involvement in receptor-mediated inflammatory nociception. Odontoblasts also express mGluR5s and may contribute to the transduction of nociceptive signals[14].
mGluR1s and mGluR5s have been studied in oral cancer as the mGluR family is known to participate in tumorigenesis[15]: mGluR5 has been detected in oral squamous cell carcinoma (SCC) using both human cell cultures and tissue specimens[16]. Furthermore, in addition to cell membrane localization, mGluR5s were found in cytoplasm; by using a specific agonist (DHPG) and antagonist (MPEP) the authors of this study concluded that these receptors may be involved in tumor progression and prognosis[16].
mGluR5s in the esophagus/stomach
The expression of mGluR5 has been found in gastroesophageal vagal pathways in ferret, dog and mouse models and is also involved in the peripheral excitatory modulation of vagal afferent mechanosensitivity input[17-19]. The use of the mGluR5 antagonist, MPEP, has highlighted a decrease in reflux events through inhibition of transient lower sphincter relaxation (TLESR)[17,20]. Immunohistochemical localization of mGluR5 along the vago-vagal neural pathway has been found in human tissues indicating a potential peripheral effect of mGluR5 modulators[21]. In a trial involving a small group of patients with gastroesophageal reflux disease (GERD), use of ADX10059 a mGluR5 negative allosteric modulator, was associated with an improvement in clinical symptoms characterized by reduced acid reflux[22]. The results of randomized clinical trials on the effects of mGluR5 blockers in patients with GERD have confirmed and supported the reduction in TLESR and reflux events[23-25]. The poor tolerability (dizziness and nausea) associated with the use of ADX10059, has been reduced using a modified-release formulation or mono-therapy[23,24]. However, ADX10059 did not show to be significantly effective in refractory GERD patients[26]; furthermore, in a study on migraine, high incidence of hepatic transaminase was observed[27]. This effect appears to be related specifically to ADX10059 and not to the mGluR5-inhibiting mechanism of action[27], since the use of single doses of AZD2066, a non-competitive antagonist of mGluR5, in a small group of healthy subjects, has reduced the number of postprandial reflux episodes and appears to be better tolerated[25]. However, these preliminary results deserve further confirmation[27].
mGluR5s in the intestine
The study of mGluRs in the enteric nervous system (ENS), which is unique in its ability to mediate reflex activity independently of the CNS, has demonstrated that the Group I mGluRs are present in the submucous plexus of guinea-pig ileum; mGluR5-immunoreactivity has been found to be located exclusively on submucous neurons with other types of cells devoid of staining. In general, the immunolabeling was cytoplasmic, filling the perikarya and occasionally proximal dendrites. In addition, the staining intensity of the cell bodies varied: some were heavily stained, others only lightly. Immunoreactivities for mGluR1 and mGluR5 were also found in submucous plexus neurons of the jejunum and colon[28]. Other findings have demonstrated that Group I mGluRs are present in the ENS supporting their role in enteric reflexes and suggesting that the observed internalization might be a major mechanism for regulation of mGluR activity[29].
Because enteric neurons, like their equivalents in the CNS, are intimately associated with glial cells, one study has been carried out to test for glial mGluR5 expression in various species and assess whether enteric glia express mGluR5s and, if so, whether mGluR5 expression changes during intestinal inflammation[30]. mGluR5 expression has also been found on enteric glia in the ileum and colon of mice, rats and pigs; mGluR5 stimulation increases c-Fos and pERK1/2 activation specifically in enteric glia, establishing that mGluR5s are expressed in these cells. In addition, in this study, mGluR5 expression was shown to be decreased during chronic inflammation suggesting that mGluR5 signaling may be reduced in colitis[30]; enteric glia can be considered a target of the glutamatergic neurotransmitter system in the ENS and that changes in mGluR5 expression may play a modulatory role in inflammation[30].
mGluR5s are also involved in visceral pain: because mGluR5 antagonists inhibit colorectal distension (CRD)-evoked visceromotor (VMR) in rats, it has been concluded that mGluR5s participate in mediating mechanically evoked visceral nociception in the GI tract[31].
Recent evidence has demonstrated that in a model of total parenteral nutrition (TPN), glutamate supplementation prevents intestinal mucosal atrophy and a reduced epithelial barrier function (EBF) as compared with untreated animals[32]. It has further been reported that mGluR5s are involved in the effects of glutamate on EBF as 3-[(2-Methyl-4-thiazolyl)ethynyl]pyridine (MTEP), an mGluR5 antagonist, alleviates the decreased EBF observed in TPN animals treated with glutamate[32].
MGLUR5S IN ACCESSORY DIGESTIVE ORGANS
mGluR5s in the tongue
Inflammatory tongue pain is a more recently identified area of involvement for mGluR5s; extracellular signal-regulated kinase signaling (pERK signaling) is involved in the development of mechanical and heat hypersensitivity that appears to evolve in the inflamed tongue[33].
Assessment of mGluR5 localization has also been carried out on oral tongue cancer cells: HSC3 cells exhibit mGluR5; the use of the agonist DHPG increased tumor cell migration, invasion, and adhesion; this event has been shown to be reversed by the mGluR5 antagonist MPEP[16]. Similar findings - mGluR5 functions as an oncogene in solid cancers, including oral cancer - were found using B88 cells obtained from a patient with tongue cancer[34].
mGluR5s in the liver
The presence of mGluRs in the liver was first suggested by Sureda et al[35], who showed that the competitive agonists, quisqualate and ACPD, stimulated polyphosphoinositide hydrolysis in primary rat hepatocytes. In this work, 100 μmol/L quisqualate and 100 μmol/L ACPD significantly increased the formation of [3H]InsP after 2 and 5 d of incubation, with respect to basal levels. This data suggested the activation of a Group I mGlu receptor, although at the time the subtype was not identified, since both quisqualate and ACPD were unspecific agonists. The presence of mGluR5s in rat hepatocytes was subsequently demonstrated for the first time by Storto et al[36], with mGluR5 protein and mRNA expression levels comparable to those observed in rat cerebral cortex. In this article, Storto et al[36] show that ACPD and quisqualate worsen cell injury in hepatocytes exposed to anoxic conditions; on the contrary MPEP, the selective negative allosteric modulator of mGluR5, protects hepatocytes against necrosis, suggesting that the activation of mGluR5s by endogenous glutamate is involved in the development of hypoxic injury[36]. In a subsequent study, Storto et al[36], demonstrated that the onset of hypoxic damage was significantly delayed in hepatocytes from mGlu5 receptor knockout mice, as well as in hepatocytes from wild type mice treated with 30 μmol/L MPEP. This study also observed substantial improvements in cell viability and intracellular free-radical production, with respect to wild type control hepatocytes[37].
It has been suggested that peripheral mGluRs could be activated by non-synaptic glutamate[38], probably synthesized from α-Ketoglutaric acid, a by-product of the Krebs cycle. During anoxia, blockade of the Krebs cycle causes accumulation of this intermediate, favoring its conversion into glutamate by various enzymes, such as glutamate dehydrogenase or aspartate transaminase[39]. Furthermore, the release of toxic glutamate concentrations in the CNS, in hypoxic conditions, has been variously attributed to increased activity in the mitochondrial enzyme glutaminase[40] or reduced activity of the ATP-dependent enzyme glutamine synthetase[41]. Similarly, Storto et al[36], had previously demonstrated an increase in glutamate release in hypoxic conditions in isolated rat hepatocytes. Subsequently, Storto et al[42] showed that the noncompetitive mGluR5 antagonists MPEP, SIB-1757 and SIB-1893 reduce ROS-induced toxicity in both in vitro and in vivo models. This study shows that selective blockade of mGluR5s reduce ROS production, malondialdehyde formation and thiol group oxidation, and improve hepatocyte viability in isolated hepatocytes treated with 0.5 mmol/L tert-butylhydroperoxide. Furthermore, in mice treated with acetaminophen (300 mg/kg), MPEP protects against toxicity, reducing the formation of ROS, due to acetaminophen-induced GSH depletion. The mechanism responsible for the protection against acetaminophen has not been clearly elucidated. However, in liver homogenates, Western Blot analysis has shown a significant acetaminophen-induced increase in inducible nitric oxide synthase (iNOS) expression, markedly reduced in mice co-injected with MPEP (20 mg/kg). Storto et al[42] also rules out the possibility of mGluR5 negative allosteric modulators (NAMs) providing protection through free-radical scavenging activity; somewhat curiously, MPEP maintains its beneficial effects even though it significantly depletes glutathione (GSH) in hepatocytes, whereas SIB-1893, another mGluR5 selective NAM, structurally different from MPEP, shows similar effects without reducing GSH stores. Based on these observations, Storto et al[42] have posited an indirect, GSH-independent effect of MPEP against acetaminophen toxicity. Indeed, the formation of GSH conjugates with mGluR5 ligands containing an acetylene group, has recently been demonstrated[43]. Since MPEP, but not SIB-1893, contains an acetylene bond, MPEP probably forms GSH-conjugates, which, in retrospect, explains the MPEP-induced GSH depletion. This observation further supports the hypothesis that MPEP protection may well occur without any interference with the GSH-mediated tert-buthylhydroperoxide metabolism, and cannot be entirely ascribed to reduced ROS formation[42]. Furthermore, Jesse et al[44] had found a decrease in liver injury and mortality in a hepatitis model induced by lipopolysaccharide and D-galactosamine using the same dose of MPEP reported by Storto et al[42]. Of note, this new study has reported a reduction in malondialdehyde formation and no changes in GSH-S-transferase after the administration of MPEP in agreement with the previous study by Storto et al[42].
Recently, hepatic mitochondrial dysfunctions have been observed in a rodent model of Parkinson disease. Rats with nigrostriatal degeneration induced by 6-hydroxydopamine intrastriatal administration have lower mitochondrial membrane potential and higher ROS production, with respect to sham operated animals[45]. In the same model, the administration of MPEP reduces ROS and improves ATP production with respect to Parkinsonian rats not treated with MPEP. This supports the hypothesis that MPEP may reduce ROS indirectly by improving mitochondrial efficiency rather than through direct ROS scavenging action[46].
The effects of inhibiting mGluR5 activity in hepatocellular carcinoma (HCC) have been analyzed using hepatocarcinoma cell lines and a xenograft model. Inactivation of mGluR5s with MPEP has been shown to cause inhibition of cell growth, migration, and invasion of HepG2 and Bel-7402 cells. Moreover, inhibition of tumor growth and potential metastasis of HCC has also been found in nude mice. Furthermore, mGluR5-mediated extracellular signal-regulated kinase (ERK) phosphorylation has been shown to be partially involved in cell growth and migration, as found by stimulation of (S)-3,5-Dihydroxyphenylglycine (DHPG), an agonist of the mGluR5 and blockade of MPEP and U0126, an inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (MEK)[47].
mGluR5s in the pancreas
Using RT-PCR and immunoblotting analysis, one study has detected mGluR5s in rat and human islets of Langerhans whereas mGluR1s were not found[48]. The treatment of pancreatic beta cells, MIN6, with a specific Group I agonist DHPG, stimulates insulin release at low glucose concentrations (3-10 mmol/L). This, however, is absent in the presence of high concentrations of glucose (25 mmol/L). A subsequent study has demonstrated that anti-mGluR5 antibodies do not immunostain any islet cells, but do immunostain synaptic terminals and blood vessels in the pancreas[49].
Moving from these findings, Storto et al[5], have confirmed that beta-cells express mGluR5s and have reported that these receptors are found not just in the cell surface but also in insulin-secreting granules. The endogenous activation of mGluR5s appears to be permissive to glucose-stimulate Ca2+ release and insulin secretion. The study showed that the use of mGluR5 agonists (L-glutamate, quisqualate or DHPG) act extracellularly involving no changes in Ca2+ concentration or insulin secretion in pancreatic beta-cells. On the contrary, treatment with MPEP, a highly lipophilic drug and selective antagonist of mGluR5, or the use of mice lacking mGluR5 showed a blunted insulin response to glucose[5]. Thus, drugs such as mGluR5 modulators, under development for neurologic and psychiatric disorders, may also affect glucose homeostasis.
CONCLUSION
The wide distribution of mGluRs in plants and animals, from invertebrates to primates, suggests that mGluRs represent a primitive signalling system. Some published reviews have summarized current knowledge on GluRs, including both ionotropic and metabotropic receptors, in peripheral tissues[50]. However, only a few articles have focused on metabotropic receptors in the peripheral organs[51]. The presence of mGluRs in peripheral organs supports efforts to redirect pharmacological studies originally directed to the CNS toward new targets in peripheral districts such as the GI tract. Furthermore, the identification of mGluRs in non-neuronal tissues may well be crucial for predicting potential adverse effects during the use of CNS-targeted mGluR therapies.
Due to the role of mGluR5s as critical mediators and potential therapeutic targets for the treatment of numerous CNS disorders, this review has underscored possible adverse drug reactions in the GI tract using mGluR5 modulators, attempting to draw attention to the distribution and potential role of mGluR5s in the GI tract and in accessory digestive organs, in particular, the liver and pancreas (Table 1). Hopefully, this will encourage researchers to provide additional information that identifies specific targets and innovative therapeutic approaches in the treatment of GI diseases.
Table 1.
Literature assessing metabotropic glutamate 5 receptor localization in the gastrointestinal tract and accessory digestive organs
| Organs | Role/disorder | Animal/human study | Measurement/observation | Ref. |
| Mouth | Mechanical allodynia | Rats | Antagonists block allodynia | [11-13] |
| Mouth | Inflammation/pain | Rats | Upregulation of receptor expression | [10] |
| Mouth | Inflammation | Human pulp | Receptor-mediated inflammatory nociception. | [14] |
| Mouth | Oral cancer | Human tissue | Tumor progression, cell migration | [16] |
| Esophagus/stomach | TLESR (transient lower sphincter relaxation) | Ferrets | Reduction in reflux | [17,20] |
| Esophagus/stomach | TLESR | Humans | Reduction in reflux | [22-24] |
| Esophagus/stomach | TLESR | Humans | Reduction in reflux | [25] |
| Intestine | Intestinal inflammation | Pigs and mice | Decreased mGluR5 expression | [30] |
| Intestine | Visceral pain | Rats and mice | mGluR5 antagonists inhibit colorectal distension-evoked visceromotor (VMR) | [31] |
| Intestine | Epithelial barrier function | Mice | mGluR5 antagonists improve the epithelial barrier function | [32] |
| Tongue | Inflammation | Rats | Mechanical and heat hypersensitivity | [33] |
| Tongue | Cancer | Human cell lines | Antagonists block tumor cell migration and invasion | [16] |
| Tongue | Cancer | Mice | Antagonists block tumor cell migration | [34] |
| Liver | Hypoxia | Rats and mice | Necrosis, reactive oxygen species (ROS) | [36,37] |
| Liver | Acetaminophen damage | Mice | ROS iNOS | [42] |
| Liver | Cancer | Mice | Tumor reduction | [47] |
| Liver | Fulminant hepatitis | Mice | Mortality, Lipid peroxidation | [44] |
| Liver | Mitochondrial damage | Rats | Mitochondrial membrane potential | [46] |
| ROS production | ||||
| Pancreas | Insulin release | Rats | Change in glucose concentration | [48] |
| Pancreas | Insulin secretion | Rats and mice | Glucose-stimulated intracellular Ca2+ | [5] |
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Ferrigno A, Berardo C, Di Pasqua LG, Siciliano V, Richelmi P and Vairetti M declare no conflict of interest related to this publication.
Peer-review started: February 5, 2017
First decision: April 26, 2017
Article in press: June 19, 2017
P- Reviewer: Kobayashi T S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
References
- 1.Sladeczek F, Pin JP, Récasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317:717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- 2.Nicoletti F, Iadarola MJ, Wroblewski JT, Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci USA. 1986;83:1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jong YJ, Sergin I, Purgert CA, O’Malley KL. Location-dependent signaling of the group 1 metabotropic glutamate receptor mGlu5. Mol Pharmacol. 2014;86:774–785. doi: 10.1124/mol.114.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storto M, Capobianco L, Battaglia G, Molinaro G, Gradini R, Riozzi B, Di Mambro A, Mitchell KJ, Bruno V, Vairetti MP, et al. Insulin secretion is controlled by mGlu5 metabotropic glutamate receptors. Mol Pharmacol. 2006;69:1234–1241. doi: 10.1124/mol.105.018390. [DOI] [PubMed] [Google Scholar]
- 6.Storto M, Sallese M, Salvatore L, Poulet R, Condorelli DF, Dell’Albani P, Marcello MF, Romeo R, Piomboni P, Barone N, et al. Expression of metabotropic glutamate receptors in the rat and human testis. J Endocrinol. 2001;170:71–78. doi: 10.1677/joe.0.1700071. [DOI] [PubMed] [Google Scholar]
- 7.Storto M, de Grazia U, Battaglia G, Felli MP, Maroder M, Gulino A, Ragona G, Nicoletti F, Screpanti I, Frati L, et al. Expression of metabotropic glutamate receptors in murine thymocytes and thymic stromal cells. J Neuroimmunol. 2000;109:112–120. doi: 10.1016/s0165-5728(00)00269-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Ro JY. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci Lett. 2007;421:91–95. doi: 10.1016/j.neulet.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HJ, Choi HS, Ju JS, Bae YC, Kim SK, Yoon YW, Ahn DK. Peripheral mGluR5 antagonist attenuated craniofacial muscle pain and inflammation but not mGluR1 antagonist in lightly anesthetized rats. Brain Res Bull. 2006;70:378–385. doi: 10.1016/j.brainresbull.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Lu L, Tan X, Zhong M, Guo Y, Yi X. Peripheral metabotropic glutamate receptor subtype 5 contributes to inflammation-induced hypersensitivity of the rat temporomandibular joint. J Mol Neurosci. 2013;51:710–718. doi: 10.1007/s12031-013-0052-2. [DOI] [PubMed] [Google Scholar]
- 11.Ahn DK, Kim KH, Jung CY, Choi HS, Lim EJ, Youn DH, Bae YC. Role of peripheral group I and II metabotropic glutamate receptors in IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Pain. 2005;118:53–60. doi: 10.1016/j.pain.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Jung CY, Choi HS, Ju JS, Park HS, Kwon TG, Bae YC, Ahn DK. Central metabotropic glutamate receptors differentially participate in interleukin-1beta-induced mechanical allodynia in the orofacial area of conscious rats. J Pain. 2006;7:747–756. doi: 10.1016/j.jpain.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Jung CY, Lee SY, Choi HS, Lim EJ, Lee MK, Yang GY, Han SR, Youn DH, Ahn DK. Participation of peripheral group I and II metabotropic glutamate receptors in the development or maintenance of IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Neurosci Lett. 2006;409:173–178. doi: 10.1016/j.neulet.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Kim YJ, Paik SK, Cho YS, Kwon TG, Ahn DK, Kim SK, Yoshida A, Bae YC. Expression of metabotropic glutamate receptor mGluR5 in human dental pulp. J Endod. 2009;35:690–694. doi: 10.1016/j.joen.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 16.Park SY, Lee SA, Han IH, Yoo BC, Lee SH, Park JY, Cha IH, Kim J, Choi SW. Clinical significance of metabotropic glutamate receptor 5 expression in oral squamous cell carcinoma. Oncol Rep. 2007;17:81–87. [PubMed] [Google Scholar]
- 17.Frisby CL, Mattsson JP, Jensen JM, Lehmann A, Dent J, Blackshaw LA. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterology. 2005;129:995–1004. doi: 10.1053/j.gastro.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 18.Jensen J, Lehmann A, Uvebrant A, Carlsson A, Jerndal G, Nilsson K, Frisby C, Blackshaw LA, Mattsson JP. Transient lower esophageal sphincter relaxations in dogs are inhibited by a metabotropic glutamate receptor 5 antagonist. Eur J Pharmacol. 2005;519:154–157. doi: 10.1016/j.ejphar.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Slattery JA, Page AJ, Dorian CL, Brierley SM, Blackshaw LA. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J Physiol. 2006;577:295–306. doi: 10.1113/jphysiol.2006.117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young RL, Page AJ, O’Donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol. 2007;292:G501–G511. doi: 10.1152/ajpgi.00353.2006. [DOI] [PubMed] [Google Scholar]
- 21.Rohof WO, Aronica E, Beaumont H, Troost D, Boeckxstaens GE. Localization of mGluR5, GABAB, GABAA, and cannabinoid receptors on the vago-vagal reflex pathway responsible for transient lower esophageal sphincter relaxation in humans: an immunohistochemical study. Neurogastroenterol Motil. 2012;24:383–e173. doi: 10.1111/j.1365-2982.2011.01868.x. [DOI] [PubMed] [Google Scholar]
- 22.Keywood C, Wakefield M, Tack J. A proof-of-concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastro-oesophageal reflux disease. Gut. 2009;58:1192–1199. doi: 10.1136/gut.2008.162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerbib F, Keywood C, Strabach G. Efficacy, tolerability and pharmacokinetics of a modified release formulation of ADX10059, a negative allosteric modulator of metabotropic glutamate receptor 5: an esophageal pH-impedance study in healthy subjects. Neurogastroenterol Motil. 2010;22:859–865, e231. doi: 10.1111/j.1365-2982.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- 24.Zerbib F, Bruley des Varannes S, Roman S, Tutuian R, Galmiche JP, Mion F, Tack J, Malfertheiner P, Keywood C. Randomised clinical trial: effects of monotherapy with ADX10059, a mGluR5 inhibitor, on symptoms and reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;33:911–921. doi: 10.1111/j.1365-2036.2011.04596.x. [DOI] [PubMed] [Google Scholar]
- 25.Rohof WO, Lei A, Hirsch DP, Ny L, Astrand M, Hansen MB, Boeckxstaens GE. The effects of a novel metabotropic glutamate receptor 5 antagonist (AZD2066) on transient lower oesophageal sphincter relaxations and reflux episodes in healthy volunteers. Aliment Pharmacol Ther. 2012;35:1231–1242. doi: 10.1111/j.1365-2036.2012.05081.x. [DOI] [PubMed] [Google Scholar]
- 26.Castell DO, Zerbib F, Varannes SB des, Galmiche JP, Vaezi MF, Keywood C. Efficacy and Tolerability of ADX10059, a mGluR5 Negative Allosteric Modulator, as Add on Therapy to Proton Pump Inhibitors (PPIs) in Patients With Gastroesophageal Reflux Disease (GERD) Gastroenterology. 2011;140:S–577. [Google Scholar]
- 27.Zerbib F, Simon M. Novel therapeutics for gastro-esophageal reflux symptoms. Expert Rev Clin Pharmacol. 2012;5:533–541. doi: 10.1586/ecp.12.38. [DOI] [PubMed] [Google Scholar]
- 28.Hu HZ, Ren J, Liu S, Gao C, Xia Y, Wood JD. Functional group I metabotropic glutamate receptors in submucous plexus of guinea-pig ileum. Br J Pharmacol. 1999;128:1631–1635. doi: 10.1038/sj.bjp.0702980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Kirchgessner AL. Agonist- and reflex-evoked internalization of metabotropic glutamate receptor 5 in enteric neurons. J Neurosci. 2000;20:3200–3205. doi: 10.1523/JNEUROSCI.20-09-03200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasser Y, Keenan CM, Ma AC, McCafferty DM, Sharkey KA. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia. 2007;55:859–872. doi: 10.1002/glia.20507. [DOI] [PubMed] [Google Scholar]
- 31.Lindström E, Brusberg M, Hughes PA, Martin CM, Brierley SM, Phillis BD, Martinsson R, Abrahamsson C, Larsson H, Martinez V, et al. Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain. 2008;137:295–305. doi: 10.1016/j.pain.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Xiao W, Feng Y, Holst JJ, Hartmann B, Yang H, Teitelbaum DH. Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. FASEB J. 2014;28:2073–2087. doi: 10.1096/fj.13-238311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu MG, Matsuura S, Shinoda M, Honda K, Suzuki I, Shibuta K, Tamagawa T, Katagiri A, Kiyomoto M, Ohara K, et al. Metabotropic glutamate receptor 5 contributes to inflammatory tongue pain via extracellular signal-regulated kinase signaling in the trigeminal spinal subnucleus caudalis and upper cervical spinal cord. J Neuroinflammation. 2012;9:258. doi: 10.1186/1742-2094-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuribayashi N, Uchida D, Kinouchi M, Takamaru N, Tamatani T, Nagai H, Miyamoto Y. The role of metabotropic glutamate receptor 5 on the stromal cell-derived factor-1/CXCR4 system in oral cancer. PLoS One. 2013;8:e80773. doi: 10.1371/journal.pone.0080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sureda F, Copani A, Bruno V, Knöpfel T, Meltzger G, Nicoletti F. Metabotropic glutamate receptor agonists stimulate polyphosphoinositide hydrolysis in primary cultures of rat hepatocytes. Eur J Pharmacol. 1997;338:R1–R2. doi: 10.1016/s0014-2999(97)81950-4. [DOI] [PubMed] [Google Scholar]
- 36.Storto M, de Grazia U, Knöpfel T, Canonico PL, Copani A, Richelmi P, Nicoletti F, Vairetti M. Selective blockade of mGlu5 metabotropic glutamate receptors protects rat hepatocytes against hypoxic damage. Hepatology. 2000;31:649–655. doi: 10.1002/hep.510310315. [DOI] [PubMed] [Google Scholar]
- 37.Storto M, Battaglia G, Gradini R, Bruno V, Nicoletti F, Vairetti M. Mouse hepatocytes lacking mGlu5 metabotropic glutamate receptors are less sensitive to hypoxic damage. Eur J Pharmacol. 2004;497:25–27. doi: 10.1016/j.ejphar.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Nicoletti F, Battaglia G, Storto M, Ngomba RT, Iacovelli L, Arcella A, Gradini R, Sale P, Rampello L, De Vita T, et al. Metabotropic glutamate receptors: beyond the regulation of synaptic transmission. Psychoneuroendocrinology. 2007;32 Suppl 1:S40–S45. doi: 10.1016/j.psyneuen.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Ellinger JJ, Lewis IA, Markley JL. Role of aminotransferases in glutamate metabolism of human erythrocytes. J Biomol NMR. 2011;49:221–229. doi: 10.1007/s10858-011-9481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newcomb R, Sun X, Taylor L, Curthoys N, Giffard RG. Increased production of extracellular glutamate by the mitochondrial glutaminase following neuronal death. J Biol Chem. 1997;272:11276–11282. doi: 10.1074/jbc.272.17.11276. [DOI] [PubMed] [Google Scholar]
- 41.Krajnc D, Neff NH, Hadjiconstantinou M. Glutamate, glutamine and glutamine synthetase in the neonatal rat brain following hypoxia. Brain Res. 1996;707:134–137. doi: 10.1016/0006-8993(95)01372-5. [DOI] [PubMed] [Google Scholar]
- 42.Storto M, Ngomba RT, Battaglia G, Freitas I, Griffini P, Richelmi P, Nicoletti F, Vairetti M. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against acetaminophen hepatotoxicity in mice. J Hepatol. 2003;38:179–187. doi: 10.1016/s0168-8278(02)00384-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhuo X, Huang XS, Degnan AP, Snyder LB, Yang F, Huang H, Shu YZ, Johnson BM. Identification of glutathione conjugates of acetylene-containing positive allosteric modulators of metabotropic glutamate receptor subtype 5. Drug Metab Dispos. 2015;43:578–589. doi: 10.1124/dmd.114.061879. [DOI] [PubMed] [Google Scholar]
- 44.Jesse CR, Wilhelm EA, Bortolatto CF, Savegnago L, Nogueira CW. Selective blockade of mGlu5 metabotropic glutamate receptors is hepatoprotective against fulminant hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. J Appl Toxicol. 2009;29:323–329. doi: 10.1002/jat.1413. [DOI] [PubMed] [Google Scholar]
- 45.Vairetti M, Ferrigno A, Rizzo V, Ambrosi G, Bianchi A, Richelmi P, Blandini F, Armentero MT. Impaired hepatic function and central dopaminergic denervation in a rodent model of Parkinson’s disease: a self-perpetuating crosstalk? Biochim Biophys Acta. 2012;1822:176–184. doi: 10.1016/j.bbadis.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Ferrigno A, Vairetti M, Ambrosi G, Rizzo V, Richelmi P, Blandini F, Fuzzati-Armentero MT. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against hepatic mitochondrial dysfunction in 6-OHDA lesioned Parkinsonian rats. Clin Exp Pharmacol Physiol. 2015;42:695–703. doi: 10.1111/1440-1681.12410. [DOI] [PubMed] [Google Scholar]
- 47.Wu YL, Wang NN, Gu L, Yang HM, Xia N, Zhang H. The suppressive effect of metabotropic glutamate receptor 5 (mGlu5) inhibition on hepatocarcinogenesis. Biochimie. 2012;94:2366–2375. doi: 10.1016/j.biochi.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Brice NL, Varadi A, Ashcroft SJH, Molnar E. Metabotropic glutamate and GABAB receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2002;45:242–252. doi: 10.1007/s00125-001-0750-0. [DOI] [PubMed] [Google Scholar]
- 49.Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S, Moriyama Y. Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes. 2004;53:998–1006. doi: 10.2337/diabetes.53.4.998. [DOI] [PubMed] [Google Scholar]
- 50.Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol. 2001;29:208–223. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- 51.Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011;63:35–58. doi: 10.1124/pr.110.004036. [DOI] [PubMed] [Google Scholar]


