Abstract
Glucagon like peptide-1 (GLP-1) is an incretin hormone constantly secreted from the intestine at low basal levels in the fasted state; plasma concentrations rise rapidly after nutrient ingestion. Upon release, GLP-1 exerts insulinotropic effects via a G protein-coupled receptor, stimulation of adenylyl cyclase, and cAMP generation. Although primarily involved in glucose homeostasis, GLP-1 can induce diuresis and natriuresis when administered in pharmacological doses in humans and rodents. However, whether endogenous GLP-1 plays a role in regulating renal function remains an open question. This study aimed to test the hypothesis that blockade of GLP-1 receptor (GLP-1R) signaling at baseline influences renal salt and water handling. To this end, the GLP-1R antagonist exendin-9 (100 μg·kg−1·min−1) or vehicle was administered intravenously to overnight-fasted male Wistar rats for 30 min. This treatment reduced urinary cAMP excretion and renal cortical PKA activity, demonstrating blockade of renal GLP-1R signaling. Exendin-9-infused-rats exhibited reduced glomerular filtration rate, lithium clearance, urinary volume flow, and sodium excretion compared with vehicle-infused controls. Exendin-9 infusion also reduced renal cortical Na+/H+ exchanger isotope 3 (NHE3) phosphorylation at serine 552 (NHE3pS552), a PKA consensus site that correlates with reduced transport activity. Collectively, these results provide novel evidence that GLP-1 is a physiologically relevant natriuretic factor that contributes to sodium balance, in part via tonic modulation of NHE3 activity in the proximal tubule.
Keywords: GLP-1, NHE3, proximal tubule, incretin
glucagon-like peptide-1 (GLP-1) is an incretin hormone produced through posttranslational processing of proglucagon by prohormone convertase 1/3 in the enteroendocrine L cells located predominantly in the ileum and colon (22). The basal rate of GLP-1 secretion during the fasting state is low, but plasma GLP-1 concentration rises rapidly after nutrient intake, including carbohydrates, fats, and proteins (6). Upon release, GLP-1 potentiates glucose-stimulated insulin secretion, suppresses glucagon release, and reduces gastric emptying and food intake (5). Furthermore, GLP-1 increases insulin production by activating proinsulin gene transcription, stimulating β cell proliferation, and conferring glucose sensitivity to glucose-resistant β cells (5, 13). The importance of GLP-1 in the maintenance of blood glucose provided the basis for using GLP-1 as a therapeutic target for type 2 diabetes mellitus (T2DM).
The glycemic effects of GLP-1 are primarily mediated via binding to its G protein-coupled receptor and triggering of intracellular cAMP generation (27). Activation of the GLP-1R also elicits extraglycemic effects. Continuous infusion of GLP-1 or administration of GLP-1R agonists induces diuresis and natriuresis in humans and experimental models (4, 11, 18, 21, 23). We previously showed that pharmacological doses of GLP-1 reduce Na+/H+ exchanger isotope 3 (NHE3)-mediated NaHCO3 reabsorption in the renal proximal tubule and increase intrarenal cAMP generation, PKA activation, and phosphorylation of NHE3 at the PKA consensus sites serines 552 and 605 (NHE3pS552, NHE3pS605) (4). In addition, the GLP-1/GLP-1R agonists are associated with reduced resistance of preglomerular vessels (14, 26), increased glomerular blood flow, and glomerular filtration rate (GFR) in rodents (4, 14, 18, 26).
While these studies demonstrate that pharmacological doses of GLP-1 and GLP-1R agonists induce diuresis and natriuresis, a physiological role for endogenous GLP-1 in regulating basal renal function remains an open question. Therefore, this study tested the hypothesis that physiological activation of GLP-1R under basal condition exerts a tonic effect on sodium and water handling via modulation of proximal tubule NHE3.
METHODS
Reagents and antibodies.
All chemicals were obtained from Sigma (St. Louis, MO) unless otherwise noted. GLP-1 and the GLP-1R antagonist exendin-9 were purchased from Bachem (Philadelphia, PA). The following antibodies were used: a monoclonal antibody (mAb) to NHE3 (Biemesderfer and Aronson, Yale University, New Haven, CT), mAb anti-NHE3pS552 (Santa Cruz Biotechnology), mAb anti-actin (JLA20, Merck Millipore), a rabbit polyclonal to phosphorylated substrates of PKA (Cell Signaling Technology), and horseradish peroxidase-conjugated secondary antibodies (Life Technologies).
Experimental animals.
All experiments were performed in accordance with the ethical principles of the Brazilian College of Animal Experimentation and approved by the Institutional Animal Care and Use Committee. Male Wistar rats (8–10 wk old, State University of Campinas, São Paulo, Brazil) were housed at the Heart Institute (InCor) animal facility at a constant temperature and a 12:12-h dark-light cycle. The acute renal effects of GLP-1R blockade were evaluated after 8 h of fasting to avoid possible differences in postprandial circulating levels of endogenous GLP-1. Rats were anesthetized with thiopental (60 mg/kg ip) and placed on a heated surgical table to maintain a body temperature of 37°C. Supplemental doses of the anesthetic were administered as required. After a tracheotomy, polyethylene catheters were inserted into the jugular vein (drug infusion), urinary bladder (urine collection), and right carotid (mean blood pressure and blood sampling). After an equilibration period of 45 min, exendin-9 (100 μg·kg−1·min−1) or vehicle (4% BSA/saline) was intravenously infused at a rate of 40 μl/min for 30 min. Experiments designed to evaluate the effects of GLP-1R blockade on renal NHE3 phosphorylation levels were also performed in rats infused with 1 μg·kg−1·min−1 GLP-1 or 1 μg·kg−1·min−1 GLP-1 plus 100 μg·kg−1·min−1 exendin-9. Anesthetized rats were euthanized by decapitation, and the kidneys were immediately removed, and arterial blood collected.
Blood and urine analysis.
Blood glucose, blood and urinary sodium concentrations, and pH were measured on a Radiometer ABL5 blood-gas analyzer (Radiometer). Blood arterial samples for measurements of active GLP-1 were also collected into Vacutainer K2 EDTA tubes (BD Biosciences) containing 10 μM DPPIV inhibitor sitagliptin and centrifuged at 3,000 rpm at 4°C for 15 min. Plasma was collected and stored at −80°C. Endogenous lithium concentrations were determined by flame photometry (Micronal B262, São Paulo, Brazil). Urinary creatinine concentration was determined with a kit (Labtest, Lagoa Santa, Brazil), and plasma creatinine was measured on a Beckman Coulter Synchron CX7 Analyzer (Beckman Coulter). An ELISA was employed to determine plasma active GLP-1 (7–36), insulin (Merck Millipore), and urinary cAMP (Arbor Assays).
Stationary microperfusion.
In the experiments designed to investigate the role of GLP-1R blockade on NHE3-mediated HCO3− reabsorption (JHCO3−), proximal tubules were perfused with control solution (HCO3 saline-stained solution) in the presence of 20 nM GLP-1, 2 μM exendin-9, 20 nM GLP-1 plus 2 μM exendin-9 or vehicle, as previously described (4, 19).
Renal cortical membrane protein isolation.
Kidneys were removed, cut in half, and the cortices were isolated and homogenized as previously described (20). Protein concentration was determined by the Lowry method (17).
SDS-PAGE and immunoblotting.
Membrane proteins were solubilized in Laemmli sample buffer and resolved using 7.5% SDS-PAGE gels. Immunoblotting was performed as previously described (3). Relevant bands were digitized using an ImageScanner III (GE HealthCare) and quantified using Scion Image Software (Scion, Frederick, MD).
Statistical analysis.
Results are reported as means ± SE, with n indicating the number of rats, unless otherwise stated. Comparisons between two groups were performed using unpaired t-tests. If more than two groups were compared, statistical significance was determined by using one-way ANOVA followed by the Tukey post hoc test. P < 0.05 was considered significant.
RESULTS
Acute systemic infusion of the GLP-1R antagonist exendin-9 reduces renal GLP-1R signaling and alters renal function.
To test the hypothesis that endogenous activation of the GLP-1R influences renal function at baseline, we infused the GLP-1R antagonist exendin-9 in overnight-fasted anesthetized Wistar rats for 30 min at a dose of 100 μg·kg−1·min−1. This dose was chosen based on binding studies performed in Chinese hamster ovary cells expressing rat GLP-1R which showed that exendin-9 at a concentration 100 times higher than that of GLP-1 completely blocks GLP-1R activation (7). The exendin-9 dose chosen corresponds to 100 times the pharmacological dose of GLP-1 shown to induce natriuresis and diuresis in rodents (4). The levels of fasting blood glucose, insulin, and GLP-1 were similar between exendin-9- and vehicle-infused rats (Table 1). As seen in Fig. 1A, rats infused with exendin-9 excreted lower levels of urinary cAMP compared with vehicle-infused rats (8.8 ± 1.7 vs. 14.5 ± 1.4 pmol/min, P = 0.02). In addition, phosphorylation of PKA substrates in the renal cortex was lower in exendin-9-infused vs. vehicle-infused rats (73 ± 3 vs. 100 ± 2%, P < 0.001) (Fig. 1B).
Table 1.
Effects of acute GLP-1R blockade by exendin-9 on glycemia and renal function of overnight-fasted rats
| CTRL | Exendin-9 | |
|---|---|---|
| Body wt, g | 254 ± 5 (18) | 253 ± 9 (16) |
| Glucose, mg/dl | 106 ± 8 (14) | 113 ± 12 (12) |
| Insulin, ng/ml | 0.41 ± 0.04 (14) | 0.44 ± 0.07 (12) |
| GLP-1, pg/ml | 11.5 ± 0.6 (14) | 11.3 ± 0.9 (12) |
| MAP, mmHg | 105 ± 5 (14) | 116 ± 4 (12) |
| Urinary flow, μl·min−1·kg−1 | 130 ± 11 (14) | 47 ± 3*** (12) |
| Urinary Na+, μeq ·min−1·kg−1 | 2.62 ± 0.34 (14) | 1.22 ± 0.26* (12) |
| GFR, ml·min−1·kg−1 | 8.7 ± 0.5 (14) | 7.0 ± 0.6* (12) |
| FE Na+, % | 1.06 ± 0.20 (14) | 0.55 ± 0.09* (12) |
| Urinary pH | 6.36 ± 0.15 (14) | 5.90 ± 0.15* (12) |
| Li+ clearance, ml·min−1·kg−1 | 2.08 ± 0.12 (14) | 1.56 ± 0.10* (12) |
| FE Li+, % | 29 ± 2 (14) | 21 ± 1*** (12) |
Values are means ± SE. Nos. in parentheses are no. of rats in a group. Wistar rats were infused with vehicle (CTRL) or the glucagon-like-1 receptor (GLP-1R) antagonist exendin-9 for 30 min. Glomerular filtration rate (GFR) was estimated with creatinine clearance.
MAP, mean arterial pressure; FE Na+, fractional excretion of sodium.
P < 0.05 and
P < 0.001 vs. CTRL.
Fig. 1.
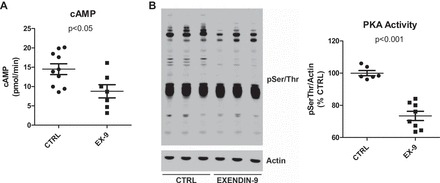
Acute systemic infusion of the glucagon-like peptide-1 receptor (GLP-1R) antagonist exendin-9 reduces urinary cAMP and renal cortical PKA activity. A: urinary cAMP collected from rats infused with 100 μg·kg−1·min−1 exendin-9 (n = 7) or vehicle (n = 10) for 30 min was determined by ELISA and adjusted to urinary flow. B: renal cortical PKA activity was indirectly determined by immunoblotting using an antibody that specifically recognizes phosphorylated PKA substrates (anti-pSer/Thr). The sum total of all phospho-PKA proteins per lane was estimated by densitometry and normalized to actin. Values are means ± SE; n = 6 rats/group.
The lower renal GLP-1R/cAMP/PKA activation induced by exendin-9 was associated with a 50% reduction in urine output and urinary sodium excretion (Table 1). The antidiuretic and antinatriuretic actions of GLP-1R blockade were accompanied by a reduction in GFR. In addition, fractional excretion of sodium, urinary pH, and lithium clearance (an index of volume flow out of the proximal tubule) were significantly reduced in exendin-9- vs. vehicle-treated rats. A trend toward increased blood pressure in 30-min exendin-9-infused rats was evident but not significant (P = 0.10) (Table 1). Taken together, these results suggest that acute blockade of the renal GLP-1R/cAMP/PKA pathway exerts strong antinatriuretic and antidiuretic effects that can be attributed, at least in part, to lower GFR and higher renal proximal tubule sodium reabsorption.
Effects of acute GLP-1R blockade by exendin-9 on activity and phosphorylation of proximal tubule NHE3.
We previously reported that luminal perfusion of proximal tubules with GLP-1 inhibits NHE3 transport activity (4). Therefore, we hypothesized that GLP-1R antagonism would stimulate NHE3-mediated Na+/H+ exchange. As shown in Fig. 2A, NHE3-mediated JHCO3−, in proximal tubules perfused with 2 μM exendin-9, measured by stationary in situ microperfusion, was very similar to those tubules perfused with control solution (2.18 ± 0.07 vs. 2.00 ± 0.08 nmol·cm−2·s−1). At first glance, this finding appears to refute the hypothesis that endogenous GLP-1 is a tonic inhibitor of NHE3. However, during stationary in situ microperfusion, the tubular flow is interrupted and the proximal tubule cell is solely exposed to the perfusion solution. Since the proximal tubule does not synthesize or secrete GLP-1, the blockade of GLP-1R in the absence of its ligand is not expected to affect proximal tubular function. Indeed, proximal tubular perfusion with exendin-9 in the presence of its ligand completely blocked the inhibitory effect of GLP-1 on JHCO3− (1.19 ± 0.11 vs. 2.00 ± 0.08 nmol·cm−2·s−1, P < 0.001).
Fig. 2.
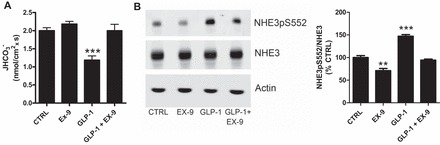
Effects of acute GLP-1R blockade on activity and phosphorylation of proximal tubule Na+/H+ exchanger isoform 3 (NHE3). A: NHE3-mediated bicarbonate reabsorption (JHCO3−) was determined by in situ stationary microperfusion and continuous measurement of luminal pH in proximal tubules from rats perfused with control solution (n = 14), 20 nM GLP-1 (n = 11), 2 μM exendin-9 (n = 18), or 20 nM GLP-1+2 μM exendin-9 (n = 8); n = number of perfused tubules from 4–5 rats/group. B: levels of phosphorylated (NHE3pS552) and total NHE3 rats in the renal cortex of rats infused with 100 μg·kg−1·min−1 exendin-9 or vehicle for 30 min were determined by immunoblotting; n = 6 rats/group. Values are means ± SE. **P < 0.01 and ***P < 0.001 vs. CTRL.
To evaluate whether GLP-1R blockade could alter NHE3 phosphorylation levels at the PKA consensus site serine 552 (PS552), rats were infused with exendin-9 (100 μg·kg−1·min−1), GLP-1 (1 μg·kg−1·min−1), or GLP-1 (1 μg·kg−1·min−1) plus exendin-9 (100 μg·kg−1·min−1) for 30 min, and renal cortical membrane proteins were isolated from these rats and subjected to SDS-PAGE and immunoblotting. As illustrated in Fig. 2B, acute exendin-9 infusion reduced the levels of NHE3pS552 in the renal cortex compared with vehicle-infused rats (71 ± 5 vs. 100 ± 4%, P < 0.01). Consistent with our previous findings, GLP-1 increased renal cortical NHE3pS552 (147 ± 4%). In line with our data from the stationary in situ microperfusion, exendin-9 was capable of preventing the effects of GLP-1 on NHE3 (94 ± 2 vs. 147 ± 4%, P < 0.001). Collectively, these results suggest that acute systemic administration of exendin-9 reduces NHE3pS552, which is consistent with higher NHE3 activity and the lower estimated with endogenous lithium clearance.
DISCUSSION
The diuretic and natriuretic effects of GLP-1R activation have been consistently reported by numerous studies (4, 11, 18, 21). However, these studies did not address whether endogenous GLP-1 plays a physiological role in the basal regulation of sodium balance. Herein, we provide novel evidence that baseline levels of GLP-1R signaling tonically influence renal sodium reabsorption. Thus GLP-1R activation is now added to the list of factors that, working in concert, match sodium and volume output to intake.
The physiological role of endogenous GLP-1 in the regulation of renal function was established by acutely blocking the GLP-1R with its peptide antagonist exendin-9, which lowered cAMP urinary levels and PKA activity in the renal cortex, consistent with a blockade of renal GLP-1R signaling. Renal GLP-1R blockade exerted antidiuretic and antinatriuretic effects in normoglycemic rats with an accompanying stimulation of proximal tubule sodium reabsorption, possibly due to reduced PKA-mediated inhibition of NHE3 transport function. These data imply that, under physiological conditions, an endogenous GLP-1R ligand, most likely GLP-1 itself, is filtered by the glomerulus, binds to GLP-1R in the proximal tubule, and activates the cAMP/PKA signaling cascade, phosphorylates NHE3 at serine 552, and tonically reduces NHE3 transport activity. Noteworthy, our ongoing unpublished studies suggest that proximal tubule NHE3 transport activity declines postprandially compared with fasting values. The role of GLP-1 in this phenomenon is a focus of further investigation.
Kim and colleagues (15) reported that the natriuretic and blood pressure-lowering effects of the GLP-1R agonist liraglutide are mediated by stimulation of atrial natriuretic peptide (ANP) release. Specifically, they found that liraglutide did not stimulate natriuresis nor lower blood pressure in ANP knockout mice at baseline nor during ANG II infusion hypertension (15). This GLP-1-ANP axis has not been detected in humans (16, 24). Our current and previous studies (1, 4) provide evidence for a direct effect of GLP-1R stimulation on kidney sodium transport; in particular, NHE3 activity is inhibited in situ when GLP-1 is added to the luminal tubular proximal tubule of rats, and when the GLP-1R agonist exendin-4 is added to the incubation medium of cultured porcine renal proximal tubular cells. These results, in line with the studies conducted in humans, indicate that the GLP-1 natriuretic effect is unlikely to be caused exclusively via ANP secretion. One interesting possibility is that the GLP-1R may undergo compensatory activation in ANP knockout mice such that further activation is not evident with ligand addition.
Baseline levels of GLP-1R signaling may also modulate renal function by regulating renal hemodynamics, since the antidiuretic and antinatriuretic effects of renal GLP-1R blockade reduce GFR. This hemodynamic effect does not seem to be secondary to tubular effects of GLP-1R blockade, since tubuloglomerular feedback (TGF) predicts GFR would increase in response to increased proximal tubular fluid reabsorption. Accordingly, Thomson and colleagues (26) have found that the GLP-1R agonist exenatide is capable of increasing single-nephron GFR, despite its inhibition of proximal tubular fluid reabsorption, at both extremes of TGF activation. These authors conclude that renal GLP-1R activation causes a direct effect on the renal vasculature, most likely by decreasing preglomerular resistance. Accordingly, Jensen and colleagues (14) recently detected GLP-1R expression on vascular smooth cells of the afferent arterioles and demonstrated that receptor activation causes vasodilation and increases renal blood flow and GFR.
There is ample evidence in the literature that hormones and compounds associated with the regulation of glucose metabolism, including insulin (8), ATP (2), and glucose itself (19, 28), regulate the activity of NHE3 in both the kidneys and intestine. Moreover, experimental diabetes (12) as well as several classes of antidiabetic agents, namely, DPPIV inhibitors (9, 10), GLP-1R agonists (1, 21, 26), thiazolidinediones (25), and SGLT inhibitors (19), modulate NHE3. Furthermore, it has been recently shown that the sodium-glucose cotransporter SGLT-2 colocalizes with NHE3 in the proximal tubule, where inhibition of SGLT inhibits NHE3 (19), indicating that these transporters functionally interact. Our current finding that endogenous GLP-1R activation exerts a tonic effect on renal sodium handling, at least in part through inhibition of NHE3, further illustrates the importance of the relationship between glucose and salt homeostasis.
In summary, our findings show that GLP-1R signaling at baseline decreases proximal sodium reabsorption via reduction of NHE3 transport activity and possibly by increasing GFR. Therefore, endogenous GLP-1R signaling exerts a tonic natriuretic action that contributes to regulation of sodium balance and may prevent volume expansion and maintain blood pressure in the normal range.
GRANTS
This work was supported by Grants 2012/10146-0 and 2013/10619-8 from the São Paulo Research Foundation (FAPESP).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.X.F., V.V., T.D.P., and G.M. performed experiments; L.X.F., V.V., T.D.P., and G.M. analyzed data; L.X.F., V.V., T.D.P., G.M., A.A.M., and A.C.C.G. interpreted results of experiments; L.X.F., V.V., T.D.P., G.M., A.A.M., and A.C.C.G. approved final version of manuscript; A.A.M. and A.C.C.G. edited and revised manuscript; A.C.C.G. provided conception and design of research; A.C.C.G. prepared figures; A.C.C.G. drafted manuscript.
REFERENCES
- 1.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297: F1647–F1655, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Cassel D, Katz M, Rotman M. Depletion of cellular ATP inhibits Na+/H+ antiport in cultured human cells. Modulation of the regulatory effect of intracellular protons on the antiporter activity. J Biol Chem 261: 5460–5466, 1986. [PubMed] [Google Scholar]
- 3.Crajoinas RO, Lessa LM, Carraro-Lacroix LR, Davel AP, Pacheco BP, Rossoni LV, Malnic G, Girardi AC. Posttranslational mechanisms associated with reduced NHE3 activity in adult vs. young prehypertensive SHR. Am J Physiol Renal Physiol 299: F872–F881, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab 1: 22–31, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1(7–36) amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute postprandial and 24-h secretion patterns. J Endocrinol 138: 159–166, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Fehmann HC, Jiang J, Schweinfurth J, Wheeler MB, Boyd AE 3rd, Goke B. Stable expression of the rat GLP-I receptor in CHO cells: activation and binding characteristics utilizing GLP-I(7–36)-amide, oxyntomodulin, exendin-4, and exendin (9–39). Peptides 15: 453–456, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Fuster DG, Bobulescu IA, Zhang J, Wade J, Moe OW. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol 292: F577–F585, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+- H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol 294: F414–F422, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Harris RC, Brenner BM, Seifter JL. Sodium-hydrogen exchange and glucose transport in renal microvillus membrane vesicles from rats with diabetes mellitus. J Clin Invest 77: 724–733, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holz GGt Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37). Nature 361: 362–365, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen EP, Poulsen SS, Kissow H, Holstein-Rathlou NH, Deacon CF, Jensen BL, Holst JJ, Sorensen CM. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol 308: F867–F877, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 19: 567–575, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 38: 132–139, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 18.Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol 434: 163–167, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol 25: 2028–2039, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR, Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol 308: F848–F856, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouille Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J Biol Chem 270: 26488–26496, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Skov J, Dejgaard A, Frokiaer J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 98: E664–E671, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Skov J, Holst JJ, Gotze JP, Frokiaer J, Christiansen JS. Glucagon-like peptide-1: effect on pro-atrial natriuretic peptide in healthy males. Endocr Connect 3: 11–16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Knepper MA, Hu X, Verbalis JG, Ecelbarger CA. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J Pharmacol Exp Ther 308: 426–433, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Thomson SC, Kashkouli A, Singh P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am J Physiol Renal Physiol 304: F137–F144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89: 8641–8645, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na+-glucose cotransport in intestinal epithelia. Am J Physiol Cell Physiol 281: C1533–C1541, 2001. [DOI] [PubMed] [Google Scholar]


