Abstract
In females, menopause, the cessation of menstrual cycling, is associated with an increase in risk for several diseases such as cardiovascular disease, osteoporosis, diabetes, the metabolic syndrome, and ovarian cancer. The majority of women enter menopause via a gradual reduction of ovarian function over several years (perimenopause) and retain residual ovarian tissue. The VCD mouse model of menopause (ovarian failure in rodents) is a follicle-deplete, ovary-intact animal that more closely approximates the natural human progression through perimenopause and into the postmenopausal stage of life. In this review, we present the physiological parameters of how to use the VCD model and explore the VCD model and its application into the study of postmenopausal disease mechanisms, focusing on recent murine studies of diabetic kidney disease, the metabolic syndrome, and hypertension.
Menopause has been associated with a variety of health risks in women. These include increases in cardiovascular disease, the metabolic syndrome, osteoporosis, Alzheimer's disease, and ovarian cancer (1, 25, 29). It is estimated that, by the year 2025, 19.5% of the population of the U.S. will be menopausal-aged women (33). Currently, with the average age of menopause at 51 and a life expectancy of ∼80, the average woman could live 30% of her life in a postmenopausal state (2). Studies have found that the risk factors for postmenopausal diseases begin to develop during perimenopause, the 5- to 10-yr period preceding the onset of menopause (7, 12). Therefore, the study of the perimenopausal period may lead to new understanding of preventive therapies for postmenopausal health risks (30). However, the lack of an adequate animal model, one that includes a progressive transition from perimenopause into menopause, has hindered studies on how hormonal changes impact disease onset and has hindered identification of the mechanisms that can be targeted to reduce disease severity after menopause. Lack of a model has also prevented studies into how the timing of hormone replacement (HT) can influence disease outcomes.
Ovarian Function, Perimenopause, and Menopause
The ovary is the primary site of female sex steroid hormone production, including estrogens and progestins. At birth, the mammalian ovary contains its full complement of oocyte-containing follicles. Oocytes cannot be generated after birth, thus the number of primordial follicles represents a finite source of germ cells for ovulation. Successful ovulation requires appropriate follicular development, during which the follicle passes through a number of distinct developmental stages (8) (FIGURE 1). The most immature stage of follicular development is termed “primordial,” and the vast majority of these follicles do not develop to ovulation but undergo cell death by atresia. Follicular atresia occurs continuously in the ovary from birth until the supply of follicles is depleted. Once the ovary is depleted of primordial follicles, ovarian failure (menopause) ensues.
FIGURE 1.
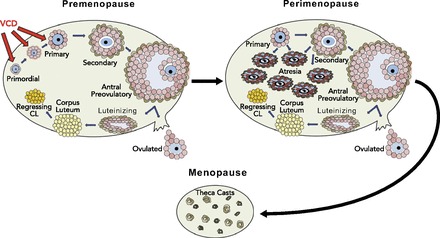
Ovarian follicular development and VCD action on primordial and primary follicular populations
The basic unit of the mammalian ovary is the ovarian follicle, containing an oocyte surrounded by somatic cells. The immature follicle is called the primordial and is surrounded by a single layer of squamous granulosa cells. The follicle goes through several stages of development: primordial to primary (now contains a layer of cuboidal granulosa cells); primary to secondary (granulosa cell layer proliferates, and the theca interna cells form); and secondary to pre-ovulatory (antrum develops). The pre-ovulatory follicle is responsible for production of 17β-estradiol. 4-Vinylcyclohexene diepoxide (VCD) is used to induce menopause since it specifically targets primordial and primary follicles, depleting the ovary of these follicle populations via accelerated atresia. The remaining secondary and antral follicles are reduced in number during progressive cycles until the ovary is follicle deplete. The remaining ovarian tissue contains tissue that continues to produce androgens.
The 5-10 years preceeding menopause is termed perimenopause and is characterized by irregular cycle lengths and fluctuating levels of estrogen, with extended periods of low estrogen interspersed with periods of high estrogen, until circulating levels of 17β-estradiol drop to continuously low levels at menopause (23). However, the residual ovarian tissue maintains its capacity to secrete androgens following menopause (27).
VCD Mouse Model of Menopause
The occupational chemical 4-vinylcyclohexene diepoxide (VCD) causes the loss of ovarian small follicles (primary and primordial) in mice and rats by accelerating the natural process of atresia (FIGURE 1), and can be used to mimic human menopause in rodent models of disease (26).1 The VCD mouse model of menopause is a follicle-deplete, ovary-intact animal that closely approximates the natural human progression through perimenopause and into the postmenopausal stage of life. Because the majority of women enter menopause by a gradual reduction of ovarian function and retain residual ovarian tissue, the progressive transition to a follicle-deplete, ovary-intact animal more closely approximates the natural human progression through the events leading up to (perimenopause) and into the postmenopausal stage of life than an ovariectomized (OVX) animal (the current laboratory animal most widely used for menopause-related studies).
The VCD mouse model of menopause requires repeated daily injections of VCD (160 mg·kg−1·day−1 in sesame oil, ip) to cause selective loss of primordial and primary follicles in the ovary (FIGURE 1) via the direct inhibition of autophosphorylation of the survival receptor c-kit, located on the plasma membrane of the oocyte (11). Within 15 days after the cessation of daily dosing, VCD has depleted all primordial follicles. During this time frame of impending ovarian failure, there is an increase in cycle length, estrogen levels fluctuate until they reach very low levels, and FSH levels increase as the inhibitory effects of estrogen are removed, thus mimicking perimenopause in humans.
Cyclicity studies (daily vaginal cytology) have shown that, when B6C3F1 female mice are dosed for 10 consecutive days, menopause occurs by approximately day 135 after the onset of dosing (FIGURE 2A). Varying the duration of VCD dosing allows for the length of the perimenopausal period to be manipulated. When mice are dosed with VCD for 20 consecutive days, the onset of menopause can be accelerated to day 52 (FIGURE 2B). This allows the investigator to optimize the length of perimenopause for each individual study. Once each animal has demonstrated 10 days of persistent diestrus (measured via daily vaginal cytology), ovarian failure (menopause) is termed complete. Menopause is accompanied by a concomitant expedition in the rise of FSH levels (10, 13). Histological analysis confirms that ovaries are follicle deplete at this stage (FIGURE 3) (13).
FIGURE 2.
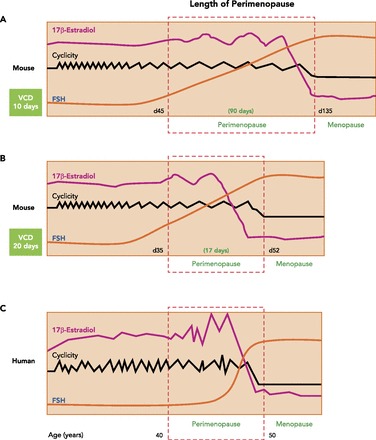
Hormone profile and length of perimenopause in the VCD mouse model of menopause
Dosing of VCD involves daily ip injections for 10, 15, or 20 days. The length of the dosing determines the extent of depletion of the primary and primordial follicles. This in turn determines the length of the perimenopause period, where cycle lengths extend, and hormone profiles fluctuate.
FIGURE 3.
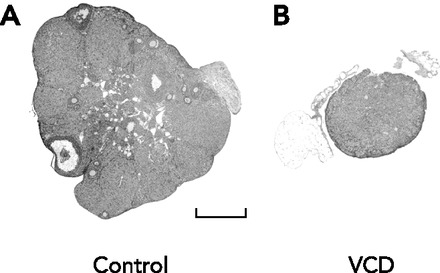
Effect of VCD treatment on ovarian morphology
Micrograph of sections of representative ovaries stained with hematoxylin and eosin collected from vehicle control (A) and VCD-treated (B) animals on day 120. Bar in A = 500 μm. Figure is from Ref. 13 and used with permission from Biology of Reproduction.
Physiological Benefits of Using the VCD Model of Menopause
There are several physiological advantages to using the VCD model for the study of sex differences. The first of these is the retention of residual ovarian tissue. Despite the loss of estrogen-producing follicles, the remaining theca cells in the ovary of a VCD-treated mouse continue to secrete androgens, demonstrating that the androgenic capacity of the ovary in the VCD model is retained, similar to ovary-intact women (FIGURE 4, A AND B) (18). During the perimenopause transition, ovarian hormone production is gradually decreased, and FSH and LH levels rise accordingly and remain elevated after ovarian failure (Table 1) (10, 13). This is in contrast to the abrupt depletion of estrogen in an OVX animal. An additional benefit of the VCD model is the presence of the aforementioned perimenopause phase, allowing investigators to evaluate the impact of impending menopause on disease mechanisms.
FIGURE 4.
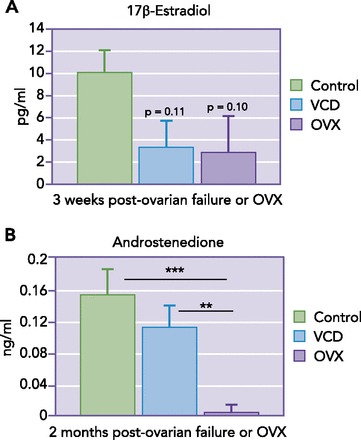
Comparison of estrogen and androstenedione levels in OVX vs. VCD mouse models of menopause
Table 1.
Plasma values in control vs. VCD-treated mice
| Control | VCD-Treated | |
|---|---|---|
| LH, ng/ml | 1.50 ± 0.21 | 3.11 ± 0.23* |
| FSH, ng/ml | 2.17 ± 0.06 | 27.57 ± 2.17* |
| Progesterone, ng/ml | 4.54 ± 0.07 | 3.39 ± 0.06* |
| Androstenedione, ng/ml | 0.44 ± 0.02 | 0.29 ± 0.02* |
| Estradiol, pg/ml | 2.21 ± 0.05 | Nondetectable |
Female B6C3F1 mice were treated daily with VCD (160 mg · kg · day, 15 days, ip) or vehicle control. Plasma samples were collected on day 127 after the onset of VCD treatment, and hormones were measured as described. Values are means ± SE (n = 6).
P < 0.05.
The VCD model can be used in animals with normal or altered genotypes and can be induced at any age, allowing the separation of aging mechanisms from those associated with ovarian failure. This latter point is an important consideration when discussing the use of aged rodents to study human menopause. Humans can spend nearly 40% of their life span in peri- and postmenopausal states. Rodents, however, often do not become acyclic or have low estrogen (menopause) until 18–24 mo of age (variable between mouse strains), at which time they are termed “old” and do not mimic the physiology of a 50- to 60-yr-old woman undergoing menopause.
Specificity of VCD for Ovarian Follicles: Lack of Tissue Toxicity
The best evidence that daily dosing of mice and rats with VCD does not produce generalized toxicity or effects in other tissues comes from studies conducted by the National Toxicology Program (NTP, 1989). In an extensive 2-yr-long study investigating potential toxic and carcinogenic effects of VCD, B6C3F1 mice were exposed dermally to VCD for a period of 103 wk, and adverse effects were limited to the reproductive tract. These effects related to the development of ovarian neoplasms at high doses. In generating the menopause model, the lowest total animal exposure necessary to induce ovarian failure is considerably less (by 980-fold) than that observed to induce any toxic or carcinogenic effects in the NTP study. Extensive histopathological evaluation was performed on of a number of tissues 6 mo after daily dosing (15 days, 160 mg·kg−1·day−1 VCD), and no pathological effects were seen in uteri, kidneys, adrenals, spleen, liver, lung, heart, brain, intestine, or pituitary tissues (31).
Furthermore, several studies have shown convincing evidence that VCD directly targets the primordial and primary follicles of the ovary, leading to follicular atresia. At the ultrastructural level, VCD-induced follicle loss is identical to natural atresia, with no evidence of necrotic changes (14). The results of these studies demonstrate the lack of toxicity to non-ovarian tissue and point to the utility of using this model to study the mechanisms underlying postmenopausal disease pathology.
Use of the VCD Mouse Model to Study Postmenopausal Disease
Postmenopausal Diabetic Kidney Disease and the Metabolic Syndrome
A woman's risk of insulin resistance and metabolic syndrome increases after menopause, correlating with an increase in circulating triglycerides and low-density lipoproteins, and a decrease in high-density lipoproteins. HT has been associated with beneficial effects on glycemic control in women with Type 2 diabetes (5). The incidence of kidney disease in women is lower than in men in the same age group, and the loss of ovarian hormones is thought to accelerate kidney damage. The VCD model of menopause has been used to examine the impact of menopause on the onset and progression of diabetic kidney disease and the metabolic syndrome (4, 9). First, to examine the impact of menopause on the progression of diabetic kidney disease, we used streptozotocin (STZ) (model of Type 1 diabetes, hyperglycemia) to induce diabetes in VCD-treated animals during perimenopause or 2 wk after the onset of menopause. Induction of diabetes during menopause resulted in higher blood glucose levels relative to cycling diabetic and perimenopausal diabetic mice (9). Diabetic kidney disease was evaluated, and renal proliferation and hypertrophy were significantly increased in menopausal diabetic females compared with cycling diabetic females. Gene array studies were used to identify genes associated with the accelerated progression of diabetic kidney disease after menopause, and the expression of genes associated with inflammation and proliferation were significantly increased in the kidneys of menopausal diabetic mice compared with cycling diabetic mice (4). These studies provided evidence that the VCD model of menopause could be utilized to evaluate the impact of hormone levels on hyperglycemia and the onset of diabetic kidney disease.
In male mice, the metabolic syndrome can be induced by feeding a high-fat diet for 1 mo; however, studies in our laboratory have suggested that cycling female mice are resistant to high-fat diet-induced diabetes. To examine the impact of hormone loss on the metabolic syndrome, we fed cycling and VCD-treated menopausal mice a high-fat diet for 12–16 wk (23). Cycling female mice on a high-fat diet (58% fat) for 12 wk did not develop insulin resistance or high fasting blood glucose. In contrast, menopausal mice on the high-fat diet demonstrated a more rapid weight gain, higher fasting insulin levels, and greater insulin resistance (FIGURE 5, A AND B). Interestingly, after 16 wk, even menopausal mice fed a standard chow diet exhibited elevated free fatty acid levels. In a separate cohort of mice also fed a standard chow, insulin resistance and increased fasting blood glucose were apparent after 26 wk of menopause and could be prevented by estrogen replacement (19).
FIGURE 5.
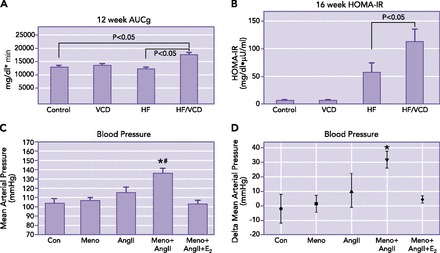
Effect of ovarian status on glucose tolerance and ANG II-induced hypertension
A: ovarian failure causes impaired glucose tolerance after 12 wk on a high-fat diet. Intraperitoneal glucose tolerance tests (IPGTT) were performed in all groups after 12 wk on a high-fat (58%) or standard diet (6%). Glucose tolerance curves were plotted, and areas under the glucose clearance (AUCg) were calculated (n = 3 in each group). Results are expressed as means ± SE. *P < 0.05 vs. control cycling mice on a standard diet or cycling mice on a high-fat diet. B: effect of ovarian status on diet-induced insulin resistance. Fasting insulin and glucose values were determined at 16 wk to calculate HOMA-IR values. Results are expressed as means ± SE. Figure is from Ref. 19 and used with permission. C and D: hemodynamic responses in menopausal mice after ANG II infusion. A: mean arterial pressure (MAP) was elevated in menopausal mice (Meno/ANGII) compared with cycling mice (Con) after 14 days of ANG II infusion. The ANG II-induced increase in MAP was prevented in menopausal mice by 17β-estradiol infusion (Meno/ANGII/E2). B: changes in MAP are presented as Δ change from day 0 to day 14 after onset of ANG II infusion. Results are expressed as means ± SE; n = 7–11 mice/group. *Significant difference in MAP vs. control mice. #Significant difference vs. ANG II cycling mice (P ≤ 0.05, ANOVA, Tukey). Figure is from Ref. 17 and is used with permission.
Exercise is known to be protective against the development of diabetes and the metabolic syndrome. Therefore, the VCD model has also been used to assess the ability of menopause to influence physical activity and the response to exercise in female mice (3, 6, 16). In young female mice, wheel-running distance was similar between control animals and VCD-treated animals with or without 17-β estradiol treatment (6).
However, using the VCD model, several beneficial estrogenic effects on muscle function were identified. Soleus muscle concentric, isometric, and eccentric in vitro forces were greater in menopausal mice that received 17β-estradiol treatment. In a separate study, voluntary and forced treadmill running capacity was assessed in female B6C3F1 and C57BL/6 mice after VCD induction of menopause. All groups demonstrated similar voluntary and forced treadmill running capacity, and menopause did not affect exercise-induced cardiac hypertrophy. However, changes were identified in cellular cardiac adaptation to exercise, specifically to downstream AMPK signaling, in VCD-treated menopausal mice (16).
These data confirmed that the VCD model of menopause can be utilized to model the postmenopausal changes in diabetic kidney damage that occur in humans and can be used to further improve our understanding of how menopause impacts the onset of the metabolic syndrome. Future studies could utilize the VCD model to determine the extent to which pharmacological and/or lifestyle interventions during the perimenopause period protect females from diabetic complications.
Postmenopausal Hypertension and Cardiovascular Disease
In addition to diabetic kidney disease and the metabolic syndrome, premenopausal women are also protected from hypertension and stroke. However, after menopause, the incidence and severity of heart disease increases substantially (15). Early observational studies of the effects of hormone therapy (HT) to reduce disease risk in postmenopausal women supported protective effects of estrogen on coronary heart disease (CHD) (21). As a result, HT usage in women increased during the last half of the 20th Century. In 1991, a randomized controlled study was designed by the NIH, the Women's Health Initiative (WHI), and enrolled ∼100,000 women, ages 50–79 yr, making it the largest study of postmenopausal women in the U.S. In 2002, results from this study were published and reported an overall increase in total cardiovascular disease, with an odds ratio relative risk (RR) of 1.22 in women taking combined HT (conjugated equine estrogen and progestin), relative to placebo controls (20). These risks were assessed on the basis of increased coronary heart disease, stroke, and pulmonary embolism. However, other end points, such as the incidence of breast cancer, were also evaluated. Although the WHI was designed to evaluate cardiovascular risks, this study was halted 5.2 years early due to an increased incidence of breast cancer (RR 1.26) in women taking HT. Subsequent analyses of the data from this study sorted subjects into age groups and found that, relative to controls, women in the HT group who had recently gone through menopause (age 50–59, RR 0.93) trended toward a reduced risk for CHD, whereas older women who had been in menopause for a longer period of time (age 70–79) were at greater risk (RR 1.26) (22). These observations have led to the generation of the “timing hypothesis,” which proposes that estrogen replacement instituted close to menopause is protective from CHD, while that instituted at a later time poses substantial risk for CHD (21).
In the WHI study, 37.8% of women included in the study were hypertensive. Of those with hypertension, 64% percent reported using anti-hypertensive medications. However, only a small percentage of these women had their blood pressure under control (36.1%, overall average), with responsiveness worsening with increasing age (28). Hypertensive women who were taking monotherapies of a β-blocker, an ACE inhibitor, or a calcium-channel blocker were less likely to have their blood pressure under control than women who took a diuretic alone. Yet, the reasons for these discrepancies in disease pathology between pre- and postmenopausal women, as well as the reduced effectiveness of the major anti-hypertensive therapeutics, remain unclear.
Experimentally, significant sex differences are known to exist in a number of animal models of hypertension, including angiotensin II (ANG II)-induced hypertension. Several studies have shown that, compared with males, female mice are resistant to ANG II-induced increases in blood pressure and that this protection is lost in OVX females (reviewed in Ref. 32). To determine whether the VCD model of menopause is useful for the study of how hypertension progresses across the peri- to postmenopausal transition, we infused ANG II into VCD-treated mice during either perimenopause or menopause. Similar to control mice (cycling), ANG II did not elicit an increase in mean arterial pressure when infused during perimenopause (FIGURE 5C). However, this protection was lost after ovarian failure, as blood pressure increased significantly in VCD-treated menopausal mice. 17β-Estradiol treatment prevented this increase in blood pressure (FIGURE 5D) (17). These results confirm the utility of the VCD model of menopause to study the mechanisms of hypertension in menopausal females and further demonstrate the vital role that female sex hormones play in regulating disease pathogenesis.
These results become increasingly important when considering the notion that the previously described WHI findings have resulted in a precipitous decline in women using HT for health benefits after menopause. As a result, a recent report estimated that, between 2002 and 2011, since the dramatic decline in HT use, there have been 18,601–90,610 premature deaths in women of ages 50–59 with hysterectomy (who have presumably not used HT) (24). The authors concluded that a failure to adequately interpret findings of the HT studies during this time interval may have “cost thousands of lives.” Consequently, there remains an ongoing controversy about the advisability of menopausal women taking estrogen. Further research is needed into the delivery method of HT (oral vs. subcutaneous), and into the source and purity of the estrogen used for HT in women. The WHI study administered conjugated equine estrogen, which contains estrogens that are not endogenous to the human body. Additional studies utilizing the VCD model of menopause could determine the time points and underlying mechanisms driving the increased disease pathogenesis in women after menopause.
Summary
Collectively, the studies outlined here demonstrate that VCD-induced ovarian failure can provide a valuable approach to assess a variety of physiological end points related to human menopause. We suggest that the gradual progression into menopause and the presence of follicle-deplete residual ovarian tissue contributes to a hormonal milieu more closely resembling natural menopause in women. In summary, the VCD-treated animal has proven useful for modeling physiological processes involved in the increased health risks associated with peri- and postmenopause, and highlights the benefit of using the VCD animal model for menopause-related studies.
Footnotes
This work was funded by National Institutes of Health Grants DK-073611 (H. L. Brooks), AG-021948 (P. B. Hoyer), and T32 HL-007249 (D. P. Pollow); American Heart Association Predoctoral Fellowship (D. P. Pollow); University of Arizona Sarver Heart Center Heart Disease in Women Research Grant (H. L. Brooks and D. P. Pollow); and the Stevie and Karl Eller Achievement Rewards for College Scientists Award (D. P. Pollow).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: H.L.B., D.P.P.J., and P.B.H. prepared figures; H.L.B., D.P.P.J., and P.B.H. drafted manuscript; H.L.B., D.P.P.J., and P.B.H. edited and revised manuscript; H.L.B., D.P.P.J., and P.B.H. approved final version of manuscript.
The VCD model of menopause has recently been termed the accelerated ovarian failure (AOF) model. These are the same animal model.
References
- 1.Appt SE, Ethun KF. Reproductive aging and risk for chronic disease: insights from studies of nonhuman primates. Maturitas 67: 7–14, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canderelli R, Leccesse LA, Miller NL, Unruh Davidson J. Benefits of hormone replacement therapy in postmenopausal women. J Am Acad Nurse Practitioners 19: 635–641, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Perez JN, Constantopoulos E, McKee L, Regan J, Hoyer PB, Brooks HL, Konhilas J. A method to study the impact of chemically-induced ovarian failure on exercise capacity and cardiac adaptation in mice. J Vis Exp 86: e51083, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond-Stanic MK, Romero-Aleshire MJ, Hoyer PB, Greer K, Hoying JB, Brooks HL. Midkine, a heparin-binding protein, is increased in the diabetic mouse kidney postmenopause. Am J Physiol Renal Physiol 300: F139–F146, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara A, Karter AJ, Ackerson LM, Liu JY, Selby JV, Northern California Kaiser Permanente Diabetes Registry . Hormone replacement therapy is associated with better glycemic control in women with Type 2 diabetes: The Northern California Kaiser Permanente Diabetes Registry. Diabetes Care 24: 1144–1150, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol 46: 685–693, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Kappeler CJ, Hoyer PB. 4-Vinylcyclohexene diepoxide: a model chemical for ovotoxicity. Sys Biol Reprod Med 58: 57–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL. Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol 293: F193–F199, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Lohff JC, Christian PJ, Marion SL, Hoyer PB. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause 13: 482–488, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Mark-Kappeler CJ, Sen N, Lukefahr A, McKee L, Sipes IG, Konhilas J, Hoyer PB. Inhibition of ovarian KIT phosphorylation by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol Reprod 85: 755–762, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Santoro N, Lasley B, Chang Y, Crawford S, Pasternak RC, Sutton-Tyrrell K, Sowers M. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab 91: 1789–1795, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod 71: 130–138, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol 16: 775–781, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Members WG, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation 125: e2–e220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez JN, Chen H, Regan JA, Emert A, Constantopoulos E, Lynn M, Konhilas JP. Effects of chemically induced ovarian failure on voluntary wheel-running exercise and cardiac adaptation in mice. Comp Med 63: 233–243, 2013. [PMC free article] [PubMed] [Google Scholar]
- 17.Pollow DP Jr, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol 309: R1546–R1552, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera Z, Christian PJ, Marion SL, Brooks HL, Hoyer PB. Steroidogenic capacity of residual ovarian tissue in 4-vinylcyclohexene diepoxide-treated mice. Biol Reprod 80: 328–336, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 297: R587–R592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J,. Writing Group for the Women's Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women's Health Initiative trials of menopausal hormone therapy. Obstet Gynecol 121: 172–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297: 1465–1477, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 81: 1495–1501, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health 103: 1583–1588, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ER, Wang Y, Xu XX. Development of a mouse model of menopausal ovarian cancer. Front Oncol 4: 36, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 139: 394–401, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Ushiroyama T, Sugimoto O. Endocrine function of the peri- and postmenopausal ovary. Horm Res 44: 64–68, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension 36: 780–789, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Webber KM, Casadesus G, Marlatt MW, Perry G, Hamlin CR, Atwood CS, Bowen RL, Smith MA. Estrogen bows to a new master: the role of gonadotropins in Alzheimer pathogenesis. Ann NY Acad Sci 1052: 201–209, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Williams JK. A mouse model of the perimenopausal transition: importance for cardiovascular research. Arterioscler Thromb Vasc Biol 25: 1765–1766, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res 23: 1296–1303, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II-induced hypertension. Braz J Med Biol Res 40: 727–734, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Yax L. National Population Projections. Washington, DC: Population Division U.S, Census Bureau, 2000. [Google Scholar]


