This work provides a direct comparison of commonly used positive inotropic medications, comparing contractility, heart rate, diastolic function, and myocardial oxygen consumption using a rodent isolated working heart model. When loading conditions were held constant, dobutamine and norepinephrine exhibited the most potent effects on systolic and diastolic function. In this model, milrinone and triiodothyronine exhibited minimal effects on contractility.
Keywords: inotropy, contractility, oxygen consumption
Abstract
Inotropic medications are routinely used to increase cardiac output and arterial blood pressure during critical illness. However, few comparative data exist between these medications, particularly independent of their effects on venous capacitance and systemic vascular resistance. We hypothesized that an isolated working heart model that maintained constant left atrial pressure and aortic blood pressure could identify load-independent differences between inotropic medications. In an isolated heart preparation, the aorta and left atrium of Sprague Dawley rats were cannulated and placed in working mode with fixed left atrial and aortic pressure. Hearts were then exposed to common doses of a catecholamine (dopamine, epinephrine, norepinephrine, or dobutamine), milrinone, or triiodothyronine (n = 10 per dose per combination). Cardiac output, contractility (dP/dtmax), diastolic performance (dP/dtmin and tau), stroke work, heart rate, and myocardial oxygen consumption were compared during each 10-min infusion to an immediately preceding baseline. Of the catecholamines, dobutamine increased cardiac output, contractility, and diastolic performance more than clinically equivalent doses of norepinephrine (second most potent), dopamine, or epinephrine (P < 0.001). The use of triiodothyronine and milrinone was not associated with significant changes in cardiac output, contractility or diastolic function, either alone or added to a baseline catecholamine infusion. Myocardial oxygen consumption was closely related to dP/dtmax (r2 = 0.72), dP/dtmin (r2 = 0.70), and stroke work (r2 = 0.53). In uninjured, isolated working rodent hearts under constant ventricular loading conditions, dobutamine increased contractility and cardiac output more than clinically equivalent doses of norepinephrine, dopamine, and epinephrine; milrinone and triiodothyronine did not have significant effects on contractility.
NEW & NOTEWORTHY
This work provides a direct comparison of commonly used positive inotropic medications, comparing contractility, heart rate, diastolic function, and myocardial oxygen consumption using a rodent isolated working heart model. When loading conditions were held constant, dobutamine and norepinephrine exhibited the most potent effects on systolic and diastolic function. In this model, milrinone and triiodothyronine exhibited minimal effects on contractility.
medications with positive inotropic effects are commonly used to augment cardiac output, reverse hypotension, or improve oxygen delivery in critically ill patients. In addition to effects on the myocardium itself, these agents may have mixed effects on vascular receptors, altering systemic vascular resistance and venous capacitance, and heart rate. These changes alter the loading conditions of the ventricle, making it difficult to understand how each medication affects the myocardium proper. Distinguishing myocardial from vascular effects is critical to understanding how each medication affects myocardial work, myocardial oxygen consumption and cardiac output in clinical use, particularly so in critically ill patients.
The choice of inotropic agent in the treatment of patients with heart failure, sepsis, and other forms of critical illness varies widely and is largely guided by expert opinion and institutional preference (1, 3, 24). Few randomized trials exist to inform decisions, and the majority of direct comparisons examine clinical outcomes, rather than measured cardiac output, and show few between-group differences (4). To understand the effects of a drug on systolic and diastolic function requires direct, invasive measurements of cardiac output and simultaneous measures of ventricular pressure and volume, measurements that are rarely performed in critically ill patients. Although literature describing the in vitro effects of many of these agents is abundant, few compare multiple currently used medications.
When reviewing our own intensive care unit practices, we identified the following medications to be most commonly used: catecholamines (dopamine, epinephrine, norepinephrine, and dobutamine), milrinone, and triiodothyronine (T3). These medications are also commonly described in the critical care literature. To enhance our understanding of these medications, we investigated the relative effects of each on contractility, diastolic function, and heart rate in an experimental preparation in which ventricular loading conditions were kept constant. We also investigated the primary determinants of myocardial oxygen consumption in hearts exposed to these medications.
MATERIALS AND METHODS
Animal preparation.
The following protocol was approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital. Sprague Dawley rats (n = 24, 343-452 g) were housed in the Animal Resources at Children's Hospital (ARCH) with a 12:12-h day-night cycle and with free access to food and water until the date of experimentation. Animals were anesthetized with inhalational isofluorane (1.5–2.5%) and anticoagulated (heparin 100 U/100 g ip). The heart and lungs were explanted en bloc by a single researcher (J. N. Kheir) and placed immediately in ice-cold modified Krebs Henseleit buffer (KHB). The aorta was then cannulated and retrograde perfused using a perfusion pressure of 90 mmHg (Harvard Apparatus IH51-B; Fig. 1). The pulmonary veins were then isolated and ligated bilaterally. The left atrium was cannulated for the provision of preload KHB, as was the pulmonary artery for the collection of coronary effluent. A pressure-volume catheter (6.0-mm spacing; Millar Instruments) was inserted into the left ventricle retrograde through the aortic valve, or when this was technically challenging, via transapical puncture. There were no differences noted between these two approaches in baseline or postdrug measurements of dP/dtmax or peak systolic pressure. The heart was then transitioned into working heart mode with a fixed left atrial pressure of 10 mmHg, and mean aortic pressure was fixed at 90 mmHg. All perfusates passed through a 0.22-μm filter prior to entering the heart.
Fig. 1.
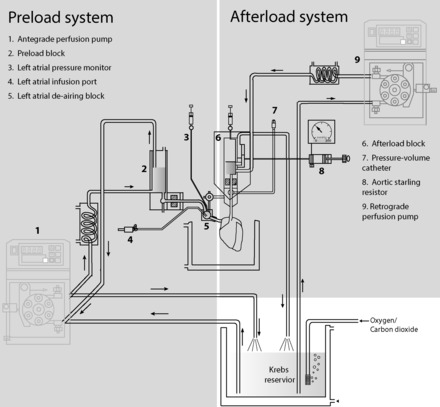
Depiction of experimental preparation, including the preload and afterload system.
Following a 10-min period of baseline observation, a medication was infused into the left atrial block. At the initiation of each inotropic infusion, a bolus of 0.40 ml was delivered via the pump to prime the tubing. An acute change in hemodynamics was noted for ∼30 s following each bolus, which returned to a new baseline rapidly thereafter. Thus hemodynamic data were excluded for a 2-min equilibration period, and hemodynamic measurements were collected for the subsequent 10-min infusion. Only one drug or drug combination was tested in each preparation.
Treatment groups.
Isolated hearts were treated with one of the following medications and doses: dopamine (3, 5, 10, or 15 μg·kg−1·min−1), dobutamine (3, 5, 10, or 15 μg·kg−1·min−1), epinephrine (0.05, 0.1, or 0.2 μg·kg−1·min−1), norepinephrine (0.05, 0.1, or 0.2 μg·kg−1·min−1), milrinone (0.25, 0.5, or 1 μg·kg−1·min−1), and triiodothyronone (0.05 or 0.1 μg·kg−1·min−1). Because the volume of distribution prior to coronary perfusion was limited to the left atrium and ventricle, no milrinone load was given. For the purposes of comparison of drugs with different potencies, the lowest tested doses of each drug were considered to be “clinically equivalent,” as were the highest doses. To further examine whether milrinone has a synergistic inotropic effect when combined with catecholamines, the same end points were measured when milrinone (0.25, 0.5, or 1 μg·kg−1·min−1) was added to a baseline of either dopamine 5 μg·kg−1·min−1 or epinephrine 0.1 μg·kg−1·min−1. All dosages were based on measured whole body weight.
Measurements.
We calculated maximum rate of pressure rise (dP/dtmax, mmHg/s) and stroke work (ml·mmHg) as measures of systolic function. The maximum rate of pressure fall (dP/dtmin, mmHg/s) and isovolumic relaxation constant (tau, ms, determined by curve fit of the isovolemic pressure-time curve) were measured as diastolic variables. Heart rate (HR, beats/min) and cardiac output (CO, μl/min) were determined from conductance data. Signals were sampled by a MPVS Ultra (Millar Instruments) and recorded and analyzed in LabChart 7 Pro (PowerLab 16/35; ADInstruments). Raw data were recorded at a frequency of 1,000 samples per second. The median value for each minute was calculated for each end point using the DataPad function within the software. Median values over the baseline period prior to each dose of each medication were calculated and compared between drugs (Table 1). The effect of treatment on each end point was represented as a percentage of change in this baseline value.
Table 1.
Characteristics of hearts in each group as measured at baseline
| dP/dtmax, mmHg/s | LVSP, mmHg | Tau, s | HR, beats/min | CO, ml/min | |
|---|---|---|---|---|---|
| Dobutamine | 3627 [3386–4128]* | 106 [101–112] | 12.9 [11.7–16.7] | 396 [376–407]† | 23.1 [21.6–31.2] |
| Norepinephrine | 3570 [3498–4470] | 86 [88–105] | 13.8 [13.8–16.3]* | 322 [314–325] | 17.5 [16.5–20.1] |
| Dopamine | 4539 [4154–4816] | 107 [99–107] | 12.5 [12.0–13.2] | 331 [323–349] | 22.9 [18.9–25.2] |
| Epinephrine | 4627 [3633–4821] | 112 [95–112] | 16.8 [14.4–24.4]† | 328 [318–335] | 14.1 [16.2–29.9] |
| Milrinone | 3654 [3536–4899] | 95 [90–101] | 12.8 [12.1–17.4] | 331 [323–343] | 25.5 [20.5–26.3] |
| T3 | 3705 [3532–4252] | 109 [106–110] | 12.8 [12.3–14.0] | 356 [352–363]* | 17.5 [16.4–20.1] |
Data are medians, 95% confidence interval shown in brackets.
LVSP, left ventricular systolic pressure.
P < 0.05;
P < 0.01 different from dopamine baselines.
Myocardial oxygen consumption was calculated using the following formula as previously described (16): [arterial − venous oxygen tension (mmHg)] × [solubility of oxygen at 37°C (ml oxygen/ml saline)] × [coronary flow rate (ml/min)], where “arterial” and “venous” oxygen tension were measured in left atrial perfusate and coronary sinus effluent, respectively. Coronary vascular resistance was calculated as follows: (diastolic aortic root pressure − mean right atrial pressure)/coronary flow rate. Coronary flow rate was quantified each 5 min using timed collections in a graduated cylinder.
In this model, we chose to infuse set doses (i.e., μg·kg−1·min−1) of medication from a syringe into the KHB perfusing the left atrial block (whose flow rate was equivalent to the cardiac output); in many other models, the left atrial block is perfused with KHB containing a set concentration of a given agent. We chose to deliver the infusions in this manner to make the analyses more applicable to clinical practice. To permit comparisons to literature in which fixed concentrations are infused, we calculated drug concentration for each 10-min administration as follows: {[administered dose (μg·kg−1·min−1)] × animal body wt × 10 min}/total cardiac output over the same 10-min period.
Statistical methods.
For each condition (i.e., drug and dose combination), the median value of each end point was determined for the immediately preceding 10-min baseline period. The percent change from that baseline was calculated for minutes 2–12 following initiation of the medication infusion. The change from baseline was then calculated for each experimental replicate. Because dopamine is the most commonly utilized inotrope in our intensive care unit, we compared end points at the maximum tested doses of each inotrope to that of dopamine using a Kruskall-Wallis test with Dunn's multiple comparisons posttest (Prism version 6.0; GraphPad, LaJolla, CA). Dose-response relationships were compared within and between treatments by linear regression analysis. The relationship between myocardial V̇o2, dP/dtmax, and treatment group was assessed by multiple regression analysis (SPSS Statistics for Windows, version 22.0; IBM, Armonk, NY). A P value of <0.05 was considered statistically significant.
RESULTS
Baseline function.
As shown in Table 1, there were minimal differences in baseline myocardial function between treatment groups. Compared with dopamine, hearts treated with dobutamine exhibited lower baseline dP/dt and higher heart rate, those treated with norepinephrine and epinephrine exhibited a higher baseline tau, and those treated with T3 exhibited a higher baseline heart rate. Cardiac output was similar between all baseline periods in all groups (P = 0.19, ANOVA).
Inotrope concentrations.
The calculated concentrations of each dose of each drug are listed in Table 2. As expected, each drug concentration increased with the dose administered. However, medications that caused more significant increases in cardiac output were present in relatively lower concentrations as each set drug rate was diluted by the increased cardiac output.
Table 2.
Calculated effective drug concentrations in coronary perfusate for each drug combination
| “Low Dose” | “Low-Moderate Dose” | “Moderate-High Dose” | “High Dose” | |
|---|---|---|---|---|
| Dopamine | ||||
| Dose, μg·kg−1·min−1 | 3 | 5 | 10 | 15 |
| Concentration, ng/ml | 60.7 ± 43.3 | 96.8 ± 79.3 | 189.3 ± 176.8 | 263.7 ± 221.3 |
| Dobutamine | ||||
| Dose, μg·kg−1·min−1 | 3 | 5 | 10 | 15 |
| Concentration, ng/ml | 30.8 ± 11.9 | 47.6 ± 17.6 | 95.4 ± 37.8 | 144.4 ± 61.6 |
| Epinephrine | ||||
| Dose, μg·kg−1·min−1 | 0.05 | 0.1 | 0.2 | |
| Concentration, ng/ml | 0.69 ± 0.49 | 1.46 ± 0.94 | 2.57 ± 1.59 | |
| Norepinephrine | ||||
| Dose, μg·kg−1·min−1 | 0.05 | 0.1 | 0.2 | |
| Concentration, ng/ml | 1.02 ± 0.54 | 1.86 ± 0.58 | 3.38 ± 2.09 | |
| Milrinone | ||||
| Dose, μg·kg−1·min−1 | 0.25 | 0.5 | 1 | |
| Concentration, ng/ml | 5.5 ± 2.9 | 10.8 ± 3.7 | 24.4 ± 8.2 | |
| Triiodothyronine | ||||
| Dose, μg·kg−1·min−1 | 0.05 | 0.1 | ||
| Concentration, ng/ml | 1.24 ± 0.27 | 2.51 ± 0.42 |
Data are means ± SD. Doses shown each minute were distributed within the cardiac output for that minute; thus, for medications with small effects on cardiac output (e.g., triiodothyronine), the relationship between drug dose and drug concentration was linear, but was discrepant in drugs with more pronounced effects on cardiac output. Doses in the “low dose” column were considered “clinically equivalent” low doses, and those in the “high dose” column were considered “clinically equivalent” high doses.
Systolic function.
Across the range of doses tested, dobutamine resulted in the steepest dose-response curve for dP/dtmax and stroke work, followed by norepinephrine, then dopamine, then epinephrine (Fig. 2A). The slope of the contractility dose-response curve was not different from 0 for milrinone and T3. Maximal doses of dobutamine resulted in greater increases in contractility than maximal doses of dopamine (P < 0.05), which was superior to maximal doses of milrinone (P < 0.01). Relative to dopamine, only maximum doses of dobutamine increased stroke work more than maximum doses of dopamine (P < 0.01). Changes in stroke work were not different between maximum doses of dopamine, epinephrine, norepinephrine, milrinone, or T3 (Fig. 2B).
Fig. 2.
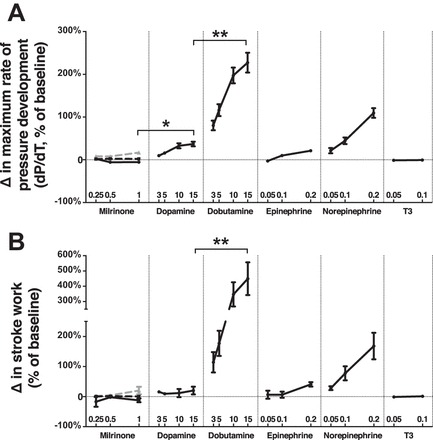
Effects on systolic function. Percentage of change in dP/dtmax (A) and stroke work (B) from an immediately preceding baseline for each dose of inotrope measured. Within milrinone group, end points were measured with milrinone alone (solid black lines) and in addition to a baseline of dopamine 5 μg·kg−1·min−1 (dashed black lines) and epinephrine 0.1 μg·kg−1·min−1 (dashed gray lines). *P < 0.05, **P < 0.01, n = 10 replicates per group. Error = SE.
Diastolic function.
Relative to maximum doses of dopamine, the maximum rate of pressure decrease (dP/dtmin) compared with baseline was greater in dobutamine and norepinephrine-treated hearts (P < 0.05), suggesting enhanced diastolic function with these agents. Changes in dP/dtmin across dosing ranges tested were not significant for epinephrine-, milrinone-, or T3-treated hearts (P > 0.05, Fig. 3A). Across the doses tested, dobutamine and norepinephrine resulted in greater decreases in tau (left ventricular time constant) relative to baseline than did dopamine, milrinone, or T3 (P < 0.001, Fig. 3B). Relative to dopamine, left ventricular end diastolic volume was similar during treatment with maximum doses of dobutamine and norepinephrine, but was higher compared with baseline following treatment with maximal doses of milrinone (P < 0.01), epinephrine (P < 0.01), and T3 (P < 0.05) (Fig. 3C).
Fig. 3.
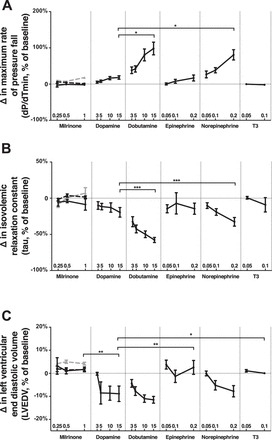
Effects on diastolic function. Percentage of change from baseline in maximum rate of pressure fall (dP/dtmin, A), isovolemic relaxation constant (tau, B), and left ventricular end diastolic volume (C). Within milrinone group, end points were measured with milrinone alone (solid black lines) and in addition to a baseline of dopamine 5 μg·kg−1·min−1 (dashed black lines) and epinephrine 0.1 μg·kg−1·min−1 (dashed gray lines). *P < 0.05, **P < 0.01, ***P < 0.001, n = 10 replicates per group. Error = SE.
Heart rate, stroke volume, and cardiac output.
Across the doses tested, the magnitude of change above baseline heart rate (i.e., chronotropic effect) was greatest in dopamine-treated hearts, followed by dobutamine-treated hearts and then norepinephrine- and epinephrine-treated hearts. Milrinone and T3 did not exert a significant chronotropic effect (Fig. 4A). There was no difference in heart rate change between maximal doses of dopamine and dobutamine or norepinephrine, and dopamine exerted a significantly greater chronotropic effect than epinephrine (P < 0.01) and milrinone and T3 (P < 0.001).
Fig. 4.
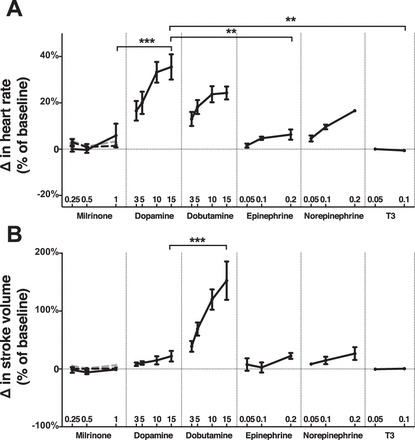
Effects on heart rate and stroke volume. Percentage of change in heart rate (A) and stroke volume (B). Within milrinone group, end points were measured with milrinone alone (solid black lines) and in addition to a baseline of dopamine 5 μg·kg−1·min−1 (dashed black lines) and epinephrine 0.1 μg·kg−1·min−1 (dashed gray lines). **P < 0.01, ***P < 0.001, n = 10 replicates per group. Error = SE.
Compared with dopamine, only dobutamine exhibited a significantly greater increase in stroke volume above baseline (P < 0.001, Fig. 4B). None of the other agents tested, including dopamine, exhibited a dose-dependent increase in stroke volume above baseline (P > 0.05), though dobutamine did (P < 0.001).
Compared with dopamine 3 μg·kg−1·min−1, only dobutamine (all doses) resulted in a significantly greater increase in cardiac output, which afforded up to a 200% increase in cardiac output at the highest doses tested (Fig. 5). The slope of the dose-response relationship was greater for dobutamine than for dopamine and epinephrine (P < 0.01). Milrinone and T3 did not change cardiac output across the doses tested (P > 0.05).
Fig. 5.
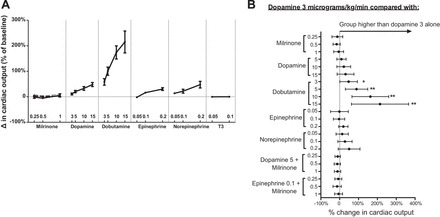
Effects on cardiac output. Percentage of change from baseline in cardiac output as categorized by inotrope and dose (A). Compared with an infusion of dopamine 3 μg·kg−1·min−1, only dobutamine resulted in a significantly higher cardiac output (B). A: Error = SE. Within milrinone group, end points were measured with milrinone alone (solid black line) and in addition to a baseline of dopamine 5 μg·kg−1·min−1 (dashed black line) and epinephrine 0.1 μg·kg−1·min−1 (dashed gray line); n = 10 replicates per group. B: Error = 95% confidence interval. *P < 0.05, **P < 0.01.
Myocardial oxygen consumption.
Increases in myocardial oxygen consumption correlated most closely with increases in markers of systolic function, including dP/dtmax (r2 = 0.72) and stroke work (r2 = 0.48), as well as diastolic function (dP/dtmin, r2 = −0.71) (Fig. 6). When controlling for differences in dP/dtmax, myocardial oxygen consumption was not significantly different between the inotropes tested.
Fig. 6.
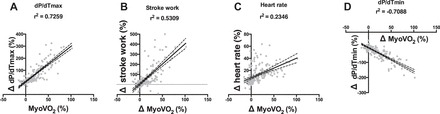
Relationship between changes in myocardial oxygen consumption and contractility (dP/dtmax, A), stroke work (B), heart rate (C), and maximum rate of pressure fall (dP/dtmin, D) for all groups. Data are individual points (n = 190); lines are linear regression lines. Error = 95% confidence interval of linear regression lines. MyoVO2, myocardial oxygen consumption.
DISCUSSION
The key findings of this work are as follows. 1) Of the catecholamines tested, dobutamine affords the greatest increase in contractility and cardiac output and the most pronounced dose-response effect. 2) Within the dosing range and time course examined here, neither milrinone nor triiodothyronine significantly alters myocardial contractility or lusitropy. 3) In an isolated heart model, changes in myocardial oxygen consumption are primarily related to changes in contractility rather than class of drug.
Relative potency of catecholamines.
The relative potency of several catecholamines has been well studied both in vitro and in vivo, though few studies have compared multiple medications head-to-head. We found that dobutamine increased markers of contractility, including dP/dtmax and stroke work, more so than any other catecholamine at clinically used doses. This is consistent with prior reports that dobutamine (a derivative of isoproterenol designed to mitigate its chronotropic effect) increases contractility more than norepinephrine and with a lesser chronotropic effect in dogs (21). In clinical studies, dobutamine caused a greater increase in systemic oxygen delivery and cardiac index than did equivalent doses of dopamine in adult patients with critical illnesses (20). We found that the increase in heart rate across dobutamine's dose-response curve was of lesser magnitude than equivalent doses of dopamine. This suggests that increases in dP/dtmax were not solely due to increases in heart rate, which is known to positively affect contractility (the Treppe effect) (5).
We also found that norepinephrine, dopamine, and epinephrine were similar to each other in their inotropic effects, each exhibiting a dose-dependent increase in contractility and cardiac output that was less than that of dobutamine but that was significantly different from baseline. It has been previously described that the inotropic effects of dopamine and norepinephrine are similar in adult ex vivo canine hearts (19) and that myocardial α-adrenergic receptors may independently mediate contractility (11). Further, no difference in cardiac index or survival was noted in a large clinical trial comparing dopamine and norepinephrine as primary therapies for septic shock (4).
In these acute experiments, we also found that the catecholamines had favorable effects on diastolic function, as indicated by a decreased tau and an increased rate of pressure fall (i.e., more negative dP/dtmin). Tau, the time constant of exponential ventricular pressure fall during isovolumic relaxation, has been regarded as a load-independent marker of ventricular relaxation (18). In this acute experiment, we found that tau was inversely correlated with dP/dtmax for all of the catecholamines, suggesting that the myocardium relaxes more rapidly (resulting in a shorter tau) as contractility increases (as indicated by an increased dP/dtmax) with catecholamine administration, a finding that has been described in other settings (18).
Direct cardiac effects of milrinone.
Milrinone, an inhibitor of cardiac adenosine 3′-5′-adenosine monophosphate (cAMP) phosphodiesterase, has a well-established role in the treatment of heart failure. It is clear that milrinone causes a decrease in systemic vascular resistance and an increase in cardiac output when used clinically. For example, milrinone has been shown to cause a reduction in cardiac filling pressures and peripheral vascular resistance and an increase in cardiac output in patients following cardiac surgery (1, 9). However, it remains unclear whether the increase in cardiac output is due to an intrinsic inotropic effect, a reduction in afterload, or both (23).
In another study, directed intracoronary infusion of milrinone in patients with heart failure (to primarily deliver the drug to the myocardium and a lower dose to the vasculature) resulted in a significant decrease in atrial pressures and increase in dP/dtmax. These effects were significantly potentiated when higher doses were administered systemically, causing a measureable reduction in afterload (14). The authors concluded not only that milrinone's effects were related primarily to its vasodilatory effects but also that a direct inotropic effect was possible. Because our experimental construct resulted in a fixed preload and afterload, any vasodilatory effect of milrinone infusion was obviated. In that setting, we did not find a significant inotropic or lusitropic effect related to milrinone administration, nor did we find that milrinone potentiated the inotropic effect of catecholamine infusions. It is possible that milrinone's inotropic effects would take place over a longer time course or if administered at higher concentrations, though hemodynamic changes have been well demonstrated during short-term infusions (i.e., <15 min) in in vivo experiments (14). It may also be that, in some settings, milrinone has no direct effects on the myocardium; when compared head-to-head in patients with dilated cardiomyopathy and heart failure, amrinone and sodium nitroprusside caused equal improvements in cardiac index and left ventricular end diastolic pressure in patients (22, 23). Thus our data support the notion that milrinone's effects may be primarily or even exclusively that of arterial and venodilation, thus lowering myocardial afterload and enhancing performance.
Direct cardiac effects of triiodothyronine.
Thyroid hormone is known to have important effects on the cardiovascular system, including an increase in myocardial contractility and a decrease in systemic vascular resistance when used in vivo (17). Because triiodothyronine (T3) acts via a mechanism that is distinct from other classes of inotropes and has a benign adverse effect profile and because native thyroid function is often altered in patients with heart failure (8), administration of T3 has received considerable attention in the care of critically ill patients (6). However, the acute inotropic effects of T3 independent of afterload have not been established. T3 infusions have not consistently demonstrated improvements in cardiac output in adults with heart failure (7) or in neonates following cardiac surgery (15). In the setting of this acute experiment, in which afterload was held constant, T3-treated hearts did not exhibit any significant changes in systolic or diastolic performance or in myocardial V̇o2. However, it may be that T3's effects would be subacute because of its mechanisms of action, an area that merits future study.
Effects of inotropes on myocardial oxygen consumption.
In addition to their effects on the myocardium and vasculature, some researchers have implicated differential effects of catecholamines on oxygen consumption. In the late 1990s, Shoemaker et al. described increases in V̇o2 during administration of dopamine or dobutamine to critically ill patients (20). Similarly, Li et al. demonstrated that termination of dopamine in infants following congenital heart surgery resulted in a nearly immediate 20% decrease in oxygen consumption (13). Whether such changes in V̇o2 are related to myocardial oxygen consumption, brown fat metabolism, or other effects remains unclear, though it is intuitive that as the stroke work and heart rate increase the myocardium must consume more oxygen. Concordantly, we found that changes in myocardial oxygen consumption were most closely correlated with changes in contractility (as indicated by dP/dtmax), diastolic function (dP/dtmin), and stroke work and more loosely associated with heart rate. When controlled for these markers of myocardial work, we did not find significant between-group differences in myocardial oxygen consumption. Said another way, we did not find that any of the inotropes increased myocardial oxygen consumption disproportionate to the increase in myocardial work. Rather, between-group differences in myocardial oxygen consumption were related to the differential potency noted between agents. Thus changes in whole body oxygen consumption noted with infusions of inotropic agents are likely partially related to increased myocardial work (and the associated energy costs) and increased cardiac output and systemic oxygen delivery, which may increase total body oxygen consumption in some circumstances (20). Whether the use of these agents in vivo disproportionately increases whole body oxygen consumption (e.g., through brown fat metabolism and thermogenesis) remains a question that merits further investigation.
Limitations.
There are several limitations to this work that merit discussion. First, while ex vivo study of the myocardium allowed us to study the relative acute, load-independent effects of these medications, each of them has important vascular effects in vivo. Loading conditions of the heart are intimately related to function, and therefore this artificial construct is only useful to enhance our understanding of these effects independent of changes in ventricular loading conditions, and not to predict a patient's response to a medication per se. For example, medications that cause arteriolar vasoconstriction increase afterload and may diminish cardiac performance under some circumstances. Further, it is known that the potency of catecholamines is significantly diminished when used ex vivo compared with in vivo (12); thus our results can only be used to understand the effects of these agents on the myocardium proper in relation to one another. Second, our experimental construct differed from common preparations in the way medications were administered. Most groups have administered KHB mixed with a defined concentration of drug, rather than infusing a defined mass of drug per unit time. We chose this construct to emulate the clinical use of these medications, permitting comparisons between commonly used doses and drugs. The disadvantage to this construct is that the intracoronary concentration of medications may have differed from those measured in vivo, given differences in volume of distribution and cardiac output between species and also between in vivo and ex vivo scenarios. For example, the calculated concentrations of milrinone in our preparation are at the low end of the broad range described in patients during intravenous (10) or intracoronary (14) administration of milrinone (12–200 ng/ml), raising the possibility that the potency of some drugs in this construct may be underrepresented. However, because all drugs were dosed and infused in the same way between groups, the relative comparisons presented here should be valid. Second, this acute study avoided several factors that may affect contractility during long-term infusions of inotropes. For example, prolonged exposure to catecholamines may result in downregulation of the beta receptor, diminishing the dose responsiveness to this class of drugs over time (2). Alternatively, prolonged treatment with inotropes may cause ventricular hypertrophy or may alter calcium handling with negative effects on diastolic function in patients with heart failure. Third, our experiment took place in nonischemic, uninjured, and nondiseased hearts. The response of the myocardium to catecholamines in these settings may differ significantly. For example, while we found that healthy hearts exhibit a linear increase in cardiac output at high doses of dopamine, in vivo studies have demonstrated that cardiac index may in some cases fall in critically ill patients treated with high doses of dopamine; this may be explained by the vasoconstrictive effects of the drug which may impair myocardial contractility in myopathic patients.
Conclusions.
In uninjured, isolated working rodent hearts, dobutamine exhibits superior potency to norepinephrine, dopamine, and epinephrine. Under these circumstances, milrinone and T3 did not have significant effects on contractility. Once controlling for differences in inotropic effects, changes in myocardial oxygen consumption were similar between the drugs tested.
GRANTS
This work was funded by the Boston Children's Hospital Strategic Investment Fund, an American Heart Association Innovative Research Grant, and the Haseotes Family Philanthropic Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.S.D., K.J.B., J.A.D., F.X.M., and J.N.K. conception and design of research; E.S.D., K.J.B., and J.N.K. performed experiments; E.S.D., K.J.B., R.T., J.A.D., S.D.C., F.X.M., and J.N.K. analyzed data; E.S.D., R.T., J.A.D., S.D.C., F.X.M., and J.N.K. interpreted results of experiments; E.S.D., K.J.B., R.T., J.A.D., S.D.C., F.X.M., and J.N.K. edited and revised manuscript; E.S.D., K.J.B., R.T., J.A.D., S.D.C., F.X.M., and J.N.K. approved final version of manuscript; K.J.B., S.D.C., and J.N.K. prepared figures; R.T. and J.N.K. drafted manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Huame He for technical advice related to this preparation.
REFERENCES
- 1.Allen LA, Fonarow GC, Grau-Sepulveda MV, Hernandez AF, Peterson PN, Partovian C, Li SX, Heidenreich PA, Heidenrich PA, Bhatt DL, Peterson ED, Krumholz HM; American Heart Association's Get With the Guidelines Heart Failure Investigators. Hospital variation in intravenous inotrope use for patients hospitalized with heart failure: insights from Get With the Guidelines. Circ Heart Fail 7: 251–260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allwood MJ, Cobbold AF, Ginsburg J. Peripheral vascular effects of noradrenaline, isopropylnoradrenaline and dopamine. Br Med Bull 19: 132–136, 1963. [DOI] [PubMed] [Google Scholar]
- 3.ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SAR, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371: 1496–1506, 2014. [DOI] [PubMed] [Google Scholar]
- 4.De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL, SOAP II Investigators . Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 362: 779–789, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Furnival CM, Linden RJ, Snow HM. The inotropic and chronotropic effects of catecholamines on the dog heart. J Physiol 214: 15–28, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation 122: 385–393, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, Chopra IJ, Moriguchi JD, Hage A. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol 81: 443–447, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol 16: 91–95, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 107: 996–1002, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Jaski BE, Fifer MA, Wright RF, Braunwald E, Colucci WS. Positive inotropic and vasodilator actions of milrinone in patients with severe congestive heart failure: dose-response relationships and comparison to nitroprusside. J Clin Invest 75: 643–649, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landzberg JS, Parker JD, Gauthier DF, Colucci WS. Effects of myocardial alpha 1-adrenergic receptor stimulation and blockade on contractility in humans. Circulation 84: 1608–1614, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Latini R, Zuanetti G, Conforti L, Schwartz PJ, Lazzara R. Demonstration of a different sensitivity to epinephrine in isolated and in vivo hearts. Eur J Pharmacol 156: 87–94, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhang G, Holtby H, Humpl T, Caldarone CA, Van Arsdell GS, Redington AN. Adverse effects of dopamine on systemic hemodynamic status and oxygen transport in neonates after the Norwood procedure. J Am Coll Cardiol 48: 1859–1864, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Ludmer PL, Wright RF, Arnold JM, Ganz P, Braunwald E, Colucci WS. Separation of the direct myocardial and vasodilator actions of milrinone administered by an intracoronary infusion technique. Circulation 73: 130–137, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Mackie AS, Booth KL, Newburger JW, Gauvreau K, Huang SA, Laussen PC, DiNardo JA, del Nido PJ, Mayer JE Jr, Jonas RA, McGrath E, Elder J, Roth SJ. A randomized, double-blind, placebo-controlled pilot trial of triiodothyronine in neonatal heart surgery. J Thorac Cardiovasc Surg 130: 810–816, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Neely Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212: 804–814, 1967. [DOI] [PubMed] [Google Scholar]
- 17.Novitzky D, Human PA, Cooper DK. Inotropic effect of triiodothyronine following myocardial ischemia and cardiopulmonary bypass: an experimental study in pigs. Ann Thorac Surg 45: 50–55, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Raff GL, Glantz SA. Volume loading slows left ventricular isovolumic relaxation rate: Evidence of load-dependent relaxation in the intact dog heart. Circ Res 48: 813–824, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt HD, Hoppe H, Heidenreich L. Direct effects of dopamine, orciprenaline and norepinephrine on the right and left ventricle of isolated canine hearts. Cardiology 64: 133–148, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Shoemaker WC, Appel PL, Kram HB. Oxygen transport measurements to evaluate tissue perfusion and titrate therapy: dobutamine and dopamine effects. Crit Care Med 19: 672–688, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Tuttle RR, Mills J. Dobutamine: development of a new catecholamine to selectively increase cardiac contractility. Circ Res 36: 185–196, 1975. [DOI] [PubMed] [Google Scholar]
- 22.Wilmshurst PT, Thompson DS, Jenkins BS, Coltart DJ, Webb-Peploe MM. Haemodynamic effects of intravenous amrinone in patients with impaired left ventricular function. Br Heart J 49: 77–82, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilmshurst PT, Thompson DS, Juul SM, Jenkins BS, Coltart DJ, Webb-Peploe MM. Comparison of the effects of amrinone and sodium nitroprusside on haemodynamics, contractility, and myocardial metabolism in patients with cardiac failure due to coronary artery disease and dilated cardiomyopathy. Br Heart J 52: 38–48, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, 1810–1852, 2013. [DOI] [PubMed] [Google Scholar]


