Muscle metaboreflex activation is augmented in type 2 diabetes patients, and this contributes, in part, to augmented pressor and sympathetic responses to exercise in this patient group. These findings provide important insight to the neural mechanisms that contribute to the exaggerated increases in exercise blood pressure in type 2 diabetes.
Keywords: exercise, hypertension, muscle sympathetic nerve activity, postexercise ischemia, blood pressure
Abstract
Previous studies have reported exaggerated increases in arterial blood pressure during exercise in type 2 diabetes (T2D) patients. However, little is known regarding the underlying neural mechanism(s) involved. We hypothesized that T2D patients would exhibit an augmented muscle metaboreflex activation and this contributes to greater pressor and sympathetic responses during exercise. Mean arterial pressure (MAP), heart rate (HR), and muscle sympathetic nerve activity (MSNA) were measured in 16 patients with T2D (8 normotensive and 8 hypertensive) and 10 healthy controls. Graded isolation of the muscle metaboreflex was achieved by postexercise ischemia (PEI) following static handgrip performed at 30% and 40% maximal voluntary contraction (MVC). A cold pressor test (CPT) was also performed as a generalized sympathoexcitatory stimulus. Increases in MAP and MSNA during 30 and 40% MVC handgrip were augmented in T2D patients compared with controls (P < 0.05), and these differences were maintained during PEI (MAP: 30% MVC PEI: T2D, Δ16 ± 2 mmHg vs. controls, Δ8 ± 1 mmHg; 40% MVC PEI: T2D, Δ26 ± 3 mmHg vs. controls, Δ16 ± 2 mmHg, both P < 0.05). MAP and MSNA responses to handgrip and PEI were not different between normotensive and hypertensive T2D patients (P > 0.05). Interestingly, MSNA responses were also greater in T2D patients compared with controls during the CPT (P < 0.05). Collectively, these findings indicate that muscle metaboreflex activation is augmented in T2D patients and this contributes, in part, to augmented pressor and sympathetic responses to exercise in this patient group. Greater CPT responses suggest that a heightened central sympathetic reactivity may be involved.
NEW & NOTEWORTHY
Muscle metaboreflex activation is augmented in type 2 diabetes patients, and this contributes, in part, to augmented pressor and sympathetic responses to exercise in this patient group. These findings provide important insight to the neural mechanisms that contribute to the exaggerated increases in exercise blood pressure in type 2 diabetes.
type 2 diabetes (T2D) patients exhibit exaggerated increases in arterial blood pressure (BP) during exercise (23, 25, 39, 44). Augmented BP responses have been observed even during moderate-intensity handgrip (38), a level of isometric forearm muscle contraction that is equivalent to many activities of daily living such as opening jars, or carrying groceries. This is important because repeated surges in BP throughout the day have been related to increased cardiovascular risk (11, 37). Likewise, exaggerated increases in exercise BP are related to adverse cardiovascular and cerebrovascular events both during and after physical activity (19, 27, 33). Indeed, the incidence of cardiovascular and cerebrovascular events such as myocardial infarction and stroke are significantly elevated among T2D patients (5, 26, 28, 57). An augmented pressor response to exercise is also a predictor for the development of hypertension (HTN) (10, 47), a common comorbidity among T2D patients (1, 3, 49, 50). However, despite exaggerated BP responses to exercise and the associated increase in morbidity and mortality in T2D, little is known regarding the underlying neural mechanism(s) involved.
Exercise evokes increases in BP, muscle sympathetic nerve activity (MSNA), and heart rate (HR) that are a result of an integration of central signals originating from higher brain centers (i.e., central command) (15), feedback signals from mechanically and metabolically sensitive afferents in contracting skeletal muscle (i.e., exercise pressor reflex; EPR) (2), and input from the arterial and cardiopulmonary baroreceptors (14, 21). During static handgrip exercise, central command increases heart rate and cardiac output by withdrawing parasympathetic tone (15), whereas the metabolic component of the EPR (i.e., muscle metaboreflex) is primarily responsible for the intensity-dependent increase in MSNA and peripheral vasoconstriction (30, 45). A number of studies have examined the muscle metaboreflex in subjects with risk factors for T2D (e.g., obesity) and have yielded mixed results (9, 29, 35, 40, 41, 52). Surprisingly, there is a paucity of studies examining the muscle metaboreflex in subjects with overt T2D. Furthermore, no studies have examined whether muscle metaboreflex activation in T2D leads to excessive MSNA responses that could contribute to an exaggerated EPR. A focus on the regulatory mechanisms underlying the augmented neural cardiovascular responses to exercise in T2D is important and clinically relevant.
Given the vital contribution of the muscle metaboreflex to the BP response to exercise, and the previous work demonstrating exaggerated BP responses to exercise in T2D patients, the purpose of this study was to test the hypothesis that BP and MSNA responses to muscle metaboreflex activation would be greater in T2D patients compared with healthy control subjects. Additionally, because T2D is commonly associated with HTN, and previous work has shown that the muscle metaboreflex is augmented with HTN (9, 34, 41), we also hypothesized that muscle metaboreflex activation would be further enhanced in T2D patients with HTN. To test these hypotheses, BP, MSNA, and HR were measured during graded isolation of the muscle metaboreflex using postexercise ischemia (PEI) following static handgrip performed at 30% and 40% maximal voluntary contraction (MVC). PEI was used to trap local metabolites produced during exercise and isolate activation of metabolically sensitive skeletal muscle afferent nerve endings from the mechanical component of the EPR and central command (2, 30). A cold pressor test (CPT) was also performed to quantify BP and MSNA responses to a generalized nonexercise sympathoexcitatory stimulus.
METHODS
Subjects.
A total of 26 subjects participated in the present study: Sixteen patients with T2D (reported duration of disease: 8 ± 2 yr) and 10 healthy controls matched to T2D patients for age, sex, and body weight. General baseline characteristics of the T2D patients and healthy control subjects are provided in Table 1. Eight of the T2D patients also had a clinical diagnosis of hypertension. These patients were all being treated for their hypertension, but we excluded any patients taking medications directly influencing MSNA (e.g., central sympathoinhibitors such as clonidine). A listing of the medications being taken by the T2D patients is provided in Table 2. Importantly, none of the T2D patients were being treated for or had symptoms of peripheral neuropathy. Table 3 provides a comparison of baseline characteristics between the T2D patients with and without hypertension. Each subject received a verbal and written explanation of the goals of the study, the experimental measurements, and risks and benefits associated with the study after which each subject provided written informed consent. All subjects also completed a medical health history questionnaire and a 12-h fasting blood chemistry screening including a lipid panel and a metabolic panel that also includes insulin, glucose, and HbA1c measurement. The experimental procedures and protocols used conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board.
Table 1.
Main subject characteristics
| Control | T2D | P Value | |
|---|---|---|---|
| Sex, men/women | 5/5 | 9/7 | |
| Age, yr | 46 ± 3 | 50 ± 2 | 0.334 |
| BMI, kg/m2 | 29 ± 2 | 31 ± 4 | 0.312 |
| Glucose, mg/dl | 95 ± 2 | 198 ± 22* | 0.001 |
| HbA1c, % | 5.3 ± 0.1 | 8.6 ± 0.5* | <0.001 |
| Insulin, μIU/ml | 7.6 ± 0.7 | 11 ± 2.3 | 0.293 |
| HOMA-IR | 1.8 ± 0.2 | 4.6 ± 0.7* | 0.013 |
| Triglycerides, mg/dl | 114 ± 20 | 184 ± 38 | 0.172 |
| Cardiovascular variables | |||
| Heart rate, beats/min | 63 ± 4 | 69 ± 3 | 0.212 |
| Systolic BP, mmHg | 122 ± 4 | 128 ± 4 | 0.313 |
| Diastolic BP, mmHg | 78 ± 2 | 81 ± 3 | 0.394 |
| Mean BP, mmHg | 91 ± 3 | 97 ± 3 | 0.258 |
| MSNA | N =10 | N =12 | |
| Burst frequency, bursts/min | 25 ± 4 | 31 ± 2 | 0.230 |
| Burst incidence, bursts/100 heartbeat | 39 ± 6 | 46 ± 4 | 0.361 |
| Total activity, AU/min | 1,241 ± 219 | 1,411 ± 139 | 0.520 |
Values are means ± SE.
T2D, Type 2 diabetes patient; BMI, body mass index; BP, blood pressure; MSNA, muscle sympathetic nerve activity.
P < 0.05 vs. control.
Table 2.
Subject medications
| Control | T2D + NTN | T2D + HTN | |
|---|---|---|---|
| Hypoglycemic medications, N | |||
| Biguanide | 0 | 5 | 7 |
| Sulfonylurea | 0 | 1 | 0 |
| DPP-4 inhibitor | 0 | 0 | 3 |
| Insulin | 0 | 2 | 4 |
| Cardiovascular medications, N | |||
| ACE inhibitor | 0 | 0 | 5 |
| ANG II receptor blocker | 0 | 0 | 1 |
| Diuretic | 0 | 0 | 4 |
| β-Blocker | 0 | 0 | 1 |
| Statin | 0 | 2 | 5 |
Values (N) are no. of subjects.
NTN, normotensive; HTN, hypertensive.
Table 3.
T2D subject characteristics
| T2D + NTN | T2D + HTN | P Value | |
|---|---|---|---|
| Sex, men/women | 4/4 | 5/3 | |
| Age, yr | 48 ± 4 | 51 ± 2 | 0.576 |
| BMI, kg/m2 | 29 ± 2 | 34 ± 1† | 0.032 |
| Glucose, mg/dl | 209 ± 34 | 187 ± 29 | 0.637 |
| HbA1c, % | 8.8 ± 0.8 | 8.5 ± 0.7 | 0.797 |
| Insulin, μIU/ml | 11.4 ± 4.9 | 10.8 ± 1.5 | 0.904 |
| HOMA-IR | 4.5 ± 1.3 | 4.8 ± 0.8 | 0.860 |
| Triglycerides, mg/dl | 175 ± 44 | 92 ± 64 | 0.833 |
| Cardiovascular variables | |||
| Heart rate, beats/min | 69 ± 3 | 68 ± 4 | 0.890 |
| Systolic BP, mmHg | 126 ± 6 | 130 ± 5 | 0.551 |
| Diastolic BP, mmHg | 80 ± 4 | 83 ± 5 | 0.604 |
| Mean BP, mmHg | 95 ± 4 | 99 ± 5 | 0.568 |
| MSNA | N = 6 | N = 6 | |
| Burst frequency, bursts/min | 31 ± 3 | 31 ± 3 | 0.980 |
| Burst incidence, bursts/100 heartbeats | 46 ± 5 | 45 ± 7 | 0.955 |
| Total activity, AU/min | 1,382 ± 209 | 1,439 ± 201 | 0.846 |
Values are means ± SE.
P < 0.05 vs. T2D + NTN.
Cardiovascular and metabolic measurements.
HR and BP were continuously monitored using a lead II surface ECG (Q710; Quinton, Bothell, WA) and a servo-controlled finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands), respectively. For Finometer measurements, return to flow calibrations were performed and physiocal turned off before each recording. The changes in BP measured using the Finometer have been shown to provide an accurate estimate of directly measured intra-arterial BP (18, 46). Also, an automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) recorded resting BP by the auscultation of the brachial artery of the right arm for absolute values of BP and to validate BP measurements from the Finometer (7, 54). Respiratory movements were monitored using a strain-gauge pneumograph placed around the abdomen (Pneumotrace, UFI, Morro Bay, CA) to avoid potential confound of large respiratory excursions on cardiovascular measurements during handgrip and PEI. Insulin was measured via an EIA assay (ALPCO, Salem, NH). Insulin resistance was assessed using the homeostatic model assessment of insulin resistance (HOMA-IR): HOMA-IR = (glucose × insulin)/22.5.
Muscle sympathetic nerve activity.
Multiunit postganglionic MSNA was recorded using standard microneurographic techniques, as previously described (13, 36, 53, 54). Briefly, a tungsten microelectrode was placed into the peroneal nerve near the left fibular head, and a reference microelectrode was inserted 2–3 cm away. Signals were amplified, filtered (bandwidth 0.7–2.0 kHz), rectified, and integrated (0.1 s time constant) to obtain mean voltage neurograms using a nerve traffic analyzer (Nerve Traffic analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA). MSNA was identified by the presence of spontaneous pulse synchronous bursts that were responsive to end-expiratory breath holds, but not to arousal or stroking of the skin. Although MSNA signals were obtained in all control subjects, 1 normotensive and 1 hypertensive T2D patient were highly sensitive to the procedure so it was stopped, and we were unable to attain quality signals in 2 others (1 normotensive and 1 hypertensive T2D patient). All neural cardiovascular data was acquired at a frequency of 1,000 Hz using Chart version 5.2 (Powerlab, ADInstruments, Bella Vista, NSW, Australia).
Isometric handgrip.
Subjects were seated in a semirecumbent position with a handgrip dynamometer held in the right hand (model 76618; Lafayette Instrument, Lafayette, IN) with the limb supported on an adjustable bedside table. Maximum voluntary contraction (MVC) was determined as the highest of three to five maximal efforts each separated by 1 min, and was used to calculate relative work rates of 30 and 40% MVC for the experimental protocol. During the experimental protocol, ratings of perceived exertion (RPE) were acquired using the Borg scale of 6 to 20 at the end of each bout of handgrip.
Experimental protocol.
All experiments were performed in a dimly-lit room at an ambient room temperature of 22–24°C with external stimuli minimized. On the experimental day, subjects arrived at the laboratory following an overnight fast, and were also requested to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol for at least 24 h. T2D patients were also instructed to refrain from all medication use the morning of the study. Before the performance of the experimental protocol, each subject was familiarized with all measurements, the equipment, and testing procedures.
After instrumentation for all experimental measurements, a 10-min baseline recording was performed to determine resting cardiovascular variables and MSNA. Subjects then performed 2 min of isometric handgrip at either 30% or 40% MVC followed by 2 min and 15 s of forearm ischemia to isolate muscle metaboreflex activation (PEI). PEI was achieved by inflation of a blood pressure cuff around the upper arm to suprasystolic pressure (>240 mmHg) 5 s before the end of handgrip exercise. The additional 15 s of PEI was included to account for the initial decrease in BP and MSNA that occurs immediately following the cessation of handgrip exercise. Visual feedback regarding the handgrip force exerted was provided via a personal computer displayed at eye level (Chart v5.2, Powerlab). In all cases except one, the 30% MVC trial was performed first due to the greater probability for muscle tension and loss of the MSNA signal during 40% MVC handgrip. The handgrip trials were separated by at least 15 min to allow BP, MSNA, and HR to return to baseline values.
Cold pressor test.
A cold pressor test was used to determine BP, MSNA, and HR responses to a generalized, nonexercise sympathoexcitatory stimulus (55). The right hand was placed in ice water for 2 min. All variables were recorded during a 2-min baseline period, during the cold pressor test, and for 2 min during recovery.
Data analysis.
Resting values for BP, MSNA, and HR were calculated as mean values over a 10-min steady-state period. MSNA was analyzed using a custom LabVIEW program (12, 13). MSNA was quantified as burst frequency (bursts/min), burst incidence (bursts/100 cardiac cycles) and total activity (burst frequency multiplied by mean burst amplitude; AU/min). To account for variation in burst amplitude, MSNA burst amplitudes were expressed as a percentage of the average of the three largest bursts during baseline (assigned a mean value of 100 arbitrary units; AU). Thirty- second averages of handgrip exercise (30–60 s and 90–120 s) and the final 60-s averages of PEI were used for group comparisons. The first 60 s and second 60 s of the cold pressor test were averaged and used for group comparisons.
To examine the interaction between the muscle metaboreflex and the arterial baroreflex, spontaneous baroreflex control of MSNA was calculated during PEI and compared with resting measures. Briefly, MSNA was averaged over 3-mmHg diastolic BP ranges (bins), and a weighted linear regression analysis between the spontaneous changes in MSNA and diastolic BP was performed. MSNA within each pressure bin was calculated as total MSNA (total area of all MSNA bursts relative to the number of cardiac cycles) and expressed as AU/beat. Burst incidence within each pressure bin was also calculated. Diastolic BP was used for this analysis because changes in MSNA correlate closely with changes in diastolic BP but not systolic BP (51).
Statistical analysis.
All data are reported as means ± SE. Statistical comparisons of resting physiological variables between groups were made using one-way analysis of variance (ANOVA) or t-tests when appropriate. Statistical comparisons of changes in BP, MSNA, and HR between groups during handgrip and PEI, and during the CPT, were made using two-way repeated-measures ANOVA. Bonferonni post hoc testing was applied where significant main effects were found. Pearson product-moment correlation coefficients were performed between metabolic parameters and BP and MSNA responses to handgrip, PEI, and the CPT. Data were analyzed using SigmaPlot 13 (Systat Software).
RESULTS
Subject characteristics.
Age and BMI were not different between controls and T2D patients (Table 1). As expected, T2D patients had significantly elevated plasma glucose, HbA1c, and HOMA-IR compared with control subjects (Table 1). No significant differences in resting systolic pressure, diastolic pressure, or MAP were found between controls and T2D patients (Table 1). In this regard, all hypertensive T2D patients were currently on an active treatment regimen (≥ 1 antihypertensive medications) (Table 2). Resting MSNA burst frequency and burst incidence were also not different between controls and T2D patients (Table 1). A comparison of normotensive and hypertensive T2D patients demonstrated no significant differences in resting metabolic, cardiovascular, or MSNA variables (Table 3). The only significant difference found was a greater body mass index (BMI) in hypertensive T2D patients. MVC was not different between groups (control: 40 ± 3 kg; T2D + NTN: 38 ± 3 kg; T2D + HTN: 42 ± 3 kg; P = 0.908).
Isometric handgrip and PEI.
Original recordings of BP and MSNA at baseline, during 30% MVC handgrip, and during PEI in 3 T2D patients and 3 control subjects are displayed in Fig. 1. The increase in MAP was significantly greater during 30% and 40% MVC handgrip in T2D patients compared with control subjects, and these augmented pressor responses in T2D patients were maintained during PEI (Fig. 2). Similar results were found with systolic BP (30% MVC, P = 0.001 vs. control; 40% MVC, P = 0.011 vs. control) and diastolic BP (30% MVC, P < 0.001 vs. control; 40% MVC, P = 0.021 vs. control) (data not shown). The increase in HR during handgrip was also significantly greater in T2D patients, but only during 30% MVC handgrip (P < 0.001 vs. control), and returned toward baseline values during PEI following both 30% and 40% handgrip in both T2D patients and controls (Fig. 2). RPE values obtained at the end of handgrip were not different between groups (30% MVC: T2D 13.5 ± 0.6 vs. control 11.8 ± 0.6, P = 0.057; 40% MVC: T2D 15.7 ± 0.5 vs. control 14.3 ± 0.8, P = 0.161).
Fig. 1.
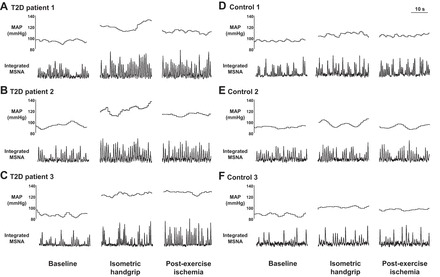
Original recordings of muscle sympathetic nerve activity (MSNA) and mean arterial pressure (MAP) in 3 type 2 diabetes patients (T2D; A–C) and 3 control subjects (D–F) at baseline, during 30% maximal voluntary contraction (MVC) isometric handgrip, and during postexercise ischemia (PEI).
Fig. 2.
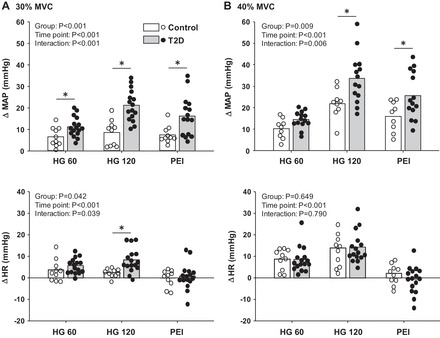
Mean and individual data showing the change in mean arterial pressure (MAP) and heart rate (HR) at 60 (HG 60) and 120 s (HG 120) of 30% MVC (A) and 40% MVC (B) handgrip followed by subsequent periods of PEI in T2D and control subjects. *P < 0.05 vs. control.
MSNA responses to handgrip and PEI were also significantly greater in T2D patients compared with controls (Fig. 3). In this regard, during handgrip exercise (HG) at 30% MVC, the change in MSNA burst frequency and percent change in total activity were augmented in T2D patients compared with controls, and this augmented MSNA response was sustained during PEI (Fig. 3A). Likewise, MSNA burst incidence was greater in T2D patients during 30% MVC handgrip and PEI (HG 120: T2D Δ20.1 ± 3.8 vs. control Δ6.5 ± 1.7 burst/100 heartbeats, P < 0.001; PEI: T2D Δ23.8 ± 6.5 vs. control Δ9.9 ± 2.5 burst/100 heartbeats, P < 0.001). During handgrip and PEI at 40% MVC, the percent change in MSNA total activity was also augmented in T2D patients, whereas the change in MSNA burst frequency did not reach statistical significance, although there was a tendency for a greater response in T2D patients (Fig. 3B, top panel). The latter may be due to maintaining MSNA recordings in only 8 T2D patients during 40% MVC handgrip. This was primarily due to muscle tension and loss of the MSNA signal with this higher intensity of handgrip. In contrast, quality MSNA recordings were maintained during 30% MVC handgrip and PEI in 12 T2D patients. Nevertheless, MSNA burst incidence was greater in the T2D patients during 40% MVC handgrip and PEI (HG 120: T2D Δ27.4 ± 6.1 vs. control Δ13.0 ± 5.6 burst/100 heartbeats; PEI: T2D Δ31.7 ± 4.9 vs. control Δ16.7 ± 3.6 burst/100 heartbeats, P = 0.04).
Fig. 3.
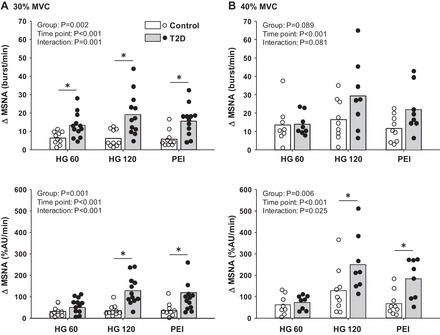
Mean and individual data showing the change in MSNA at 60 and 120 s of 30% MVC (A) and 40% MVC (B) handgrip followed by subsequent periods of PEI in T2D and control subjects. *P < 0.05 vs. control.
Among the T2D patients, BP and MSNA responses to isometric handgrip at 30% MVC were similar between those with and without hypertension. Likewise, BP responses to 40% MVC handgrip were not different between normotensive and hypertensive T2D patients, whereas the MSNA response to 40% MVC handgrip was greater in hypertensive T2D patients. Nevertheless, BP and MSNA responses during PEI following both 30% and 40% MVC were not different between normotensive and hypertensive T2D patients (Fig. 4). We also tested for potential sex differences in cardiovascular responses to handgrip and PEI, since our groups were composed of both men and women. We found no effect of sex on any of the variables of interest both during handgrip and PEI. For example, in the control group (N = 5 men and 5 women), the increase in MSNA during PEI following 30% MVC handgrip was Δ5.2 ± 1.5 bursts/min in the men and Δ6.4 ± 2.6 bursts/min in the women (P = 0.702) and in the T2D patients (N = 6 men and 6 women), the increase in MSNA during PEI was Δ17 ± 5.4 bursts/min in the men and Δ14.5 ± 3.1 bursts/min in the women (P = 0.615).
Fig. 4.
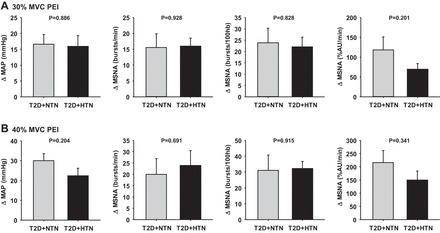
Mean summary data showing the changes in MAP and MSNA during isolation of the muscle metaboreflex with PEI following 30% MVC handgrip (A) and 40% MVC handgrip (B) in normotensive (T2D + NTN) and hypertensive type 2 diabetes patients (T2D + HTN).
The increases in MSNA total activity during PEI following both 30% MVC and 40% MVC handgrip were significantly correlated with fasting glucose, HbA1c, and HOMA-IR (Fig. 5). In contrast, weaker relationships between fasting insulin and MSNA responses during PEI were found (30% MVC PEI: R = 0.2, P = 0.417; 40% MVC PEI: R = 0.4, P = 0.153).
Fig. 5.
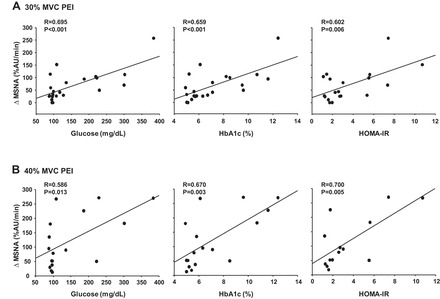
Correlations between the change in MSNA during PEI following 30% MVC handgrip (A) and 40% MVC handgrip (B) and fasting glucose, glycated hemoglobin (HbA1c), and homeostatic model assessment of insulin resistance (HOMA-IR) in all subjects.
Spontaneous baroreflex control of MSNA at rest was not different between T2D patients and controls for either burst incidence or total MSNA (burst incidence: T2D, −4.7 ± 0.4 vs. control, −4.3 ± 0.5 bursts·100 heartbeats−1·mmHg−1, P = 0.534; total MSNA: T2D, −2.5 ± 0.2 vs. control, −2.5 ± 0.3 AU·beats−1·mmHg−1, P = 0.936). Likewise, the increase in total MSNA gain during PEI was not different between groups (30% MVC PEI: T2D, −3.4 ± 0.6 vs. control, −4.4 ± 0.3 AU·beats−1·mmHg−1, P = 0.323).
Cold pressor test.
Although the increase in MAP during the cold pressor test appeared to be greater in T2D patients (n = 9) compared with control subjects (n = 9), this did not reach statistical significance (Fig. 6). However, the change in MSNA burst frequency (Fig. 6B) and MSNA total activity (CPT minute 2: T2D 151 ± 20 vs. control 55 ± 21 %AU/min, P = 0.005) were significantly greater in T2D patients (n = 7) compared with control subjects (n = 9). HR responses to the cold pressor test were not different between groups (CPT minute 2: T2D Δ6 ± 3 vs. control Δ3 ± 2 beats/min, P = 0.330).
Fig. 6.
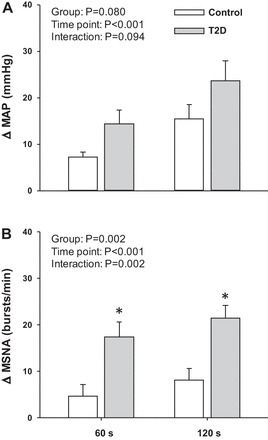
Mean summary data showing the change in MAP (A) and MSNA (B) at 60 and 120 s of a cold pressor test in T2D and control subjects. *P < 0.05 vs. control.
For the CPT, significant correlations were noted between increases in MSNA and fasting glucose (R = 0.55, P = 0.027) and HOMA-IR (R = 0.79, P = 0.001), but not HbA1c (R = 0.39, P = 0.134) or fasting insulin (R = 0.35, P = 0.218). Interestingly, no significant correlations were observed between the increases in MSNA during the CPT and during PEI following 30% MVC (R = 0.368, P = 0.161) or 40% MVC (R = 0.472, P = 0.103).
DISCUSSION
The major and novel finding of the present study is that T2D patients exhibit a heightened activation of the metabolic component of the EPR. Indeed, augmented pressor and MSNA responses during handgrip were maintained during isolation of the muscle metaboreflex with PEI. Thus greater MSNA and BP responses remained in T2D patients when input from central command and the muscle mechanoreflex were removed. Notably, MSNA responses were also greater in T2D patients compared with controls during the CPT. Collectively, these findings indicate, for the first time, that the metabolic component of the EPR is augmented with T2D and this contributes, in part, to augmented pressor and sympathetic responses to exercise in T2D patients. Greater MSNA responses to a generalized nonexercise sympathoexcitatory stimulus such as the CPT suggest that a heightened central sympathetic reactivity may be involved.
Given the fairly well-documented augmentation in exercise BP in T2D patients (23, 25, 38, 39, 43, 44), it was surprising that few studies have attempted to examine potential alterations in the underlying neural cardiovascular mechanisms in this patient group. Furthermore, to our knowledge, no studies have measured MSNA responses during exercise in T2D patients. Given the significance of the muscle metaboreflex to the pressor response to exercise, we chose to begin with the muscle metaboreflex. To this end, graded PEI was used to trap local metabolites produced by active skeletal muscle and preserve activation of metabosensitive afferent nerve endings, and therefore isolate the metabolic component of the EPR (2, 30). We now demonstrate that PEI following 30 and 40% MVC handgrip resulted in augmented pressor responses, and that these augmented pressor responses were accompanied by enhanced increases in MSNA. Interestingly, the increase in MSNA during PEI was significantly correlated with glucose control and insulin resistance markers (i.e., fasting glucose, HbA1c, and HOMA-IR), implying that the effectiveness of T2D control may play a role (see Fig. 5). Indeed, the higher the fasting glucose and HbA1c and the greater level of insulin resistance, the greater augmentation in muscle metaboreflex activation. Taken together, our results suggest that the effects of T2D on the regulatory mechanisms underlying the neural cardiovascular responses to exercise involve at least a heightened metabolic component of the EPR that appears to be related to the severity of T2D.
T2D is commonly associated with hypertension (1, 3, 49, 50), and indeed, a significant number of T2D patients recruited for the present study had hypertension. Given that both human and animal studies have suggested an exaggerated activation of the muscle metaboreflex in hypertension (9, 34, 40, 41), we compared responses between normotensive and hypertensive T2D patients to test if the coexistence of hypertension and T2D would further augment the pressor and MSNA responses to PEI. However, we found that the heightened metaboreflex activation observed in the T2D patients was unaffected by hypertensive status (see Fig. 4). Both the BP and MSNA responses to handgrip and PEI were similar between normotensive and hypertensive T2D patients. Nonetheless, it is important to note that our data are only reflective of hypertensive T2D patients that have well controlled BP and it is possible that uncontrolled hypertensive T2D patients or never-treated hypertensive T2D patients might have different responses. Additional studies are warranted in this regard to further understand the influence of uncontrolled hypertension among T2D patients on muscle metaboreflex activation.
The mechanisms responsible for the exaggerated muscle metaboreflex activation in T2D are not entirely clear. Although elevations in BP and MSNA during muscle metaboreflex activation are primarily driven by the muscle metaboreflex, there is an interaction between the metaboreflex and the arterial baroreflex that can modify such responses. Indeed, studies have shown exaggerated neural cardiovascular responses when input from the arterial baroreflex is removed (48, 56). Likewise, in healthy humans, an increase in the baroreflex control of MSNA has been observed during isolation of the muscle metaboreflex with PEI (8, 20, 22). Since previous work suggests that the sensitivity of the arterial baroreflex may be impaired in conditions associated with T2D such as obesity (6, 16) and hypertension (17, 31), we examined the interaction between the muscle metaboreflex and the arterial baroreflex. Our findings suggest that an impaired baroreflex control of MSNA does not appear to contribute to the augmented MSNA and BP responses observed during PEI since both groups exhibited an increase in MSNA baroreflex sensitivity with PEI, similar to previous studies (8, 20, 22). However, since only spontaneous baroreflex measures were used, additional studies are needed to more fully characterize arterial baroreflex function. It also remains possible that the group IV afferent fibers in the skeletal muscle interstitium that are responsive to changes in metabolic concentrations have greater sensitivity in T2D. Alternatively, although handgrip strength and perceived exertion were not different between T2D patients and control subjects, it remains possible that T2D patients experience greater metabolite build-up within the muscle interstitium during handgrip. In this regard, previous work suggests altered skeletal muscle metabolism in T2D patients (4, 42), which may lead to greater production of substances during muscle contraction that stimulate skeletal muscle afferents and contribute to greater metaboreflex activation in T2D patients. Identification of the particular substances responsible for stimulating muscle afferents remains an ongoing area of research (24), and future studies would be needed to characterize the responsiveness of skeletal muscle afferents to various substances in T2D.
In the present study, a CPT was used as a generalized sympathoexcitatory stimulus to assess whether a heightened central sympathetic activation may be augmented in T2D patients. Interestingly, the MSNA responses to the CPT were greater in T2D patients, and there was also a trend for a greater pressor response but this did not reach statistical significance. These results suggest that heightened sympathetic responsiveness in T2D may be global and not specific to metaboreflex activation. However, when MSNA responses to the CPT were compared to MSNA responses to isolated metaboreflex activation, no significant correlations were found. Also, correlations between metabolic measures (fasting glucose, HbA1c, and HOMA-IR) and the MSNA responses to the cold pressor test were noticeably weaker than was seen with isolated metaboreflex activation. Although not determining causality or lack thereof, these data suggest that greater central sympathetic activation may not completely account for the augmented metaboreflex activation of MSNA observed in T2D patients. Nevertheless, further studies are warranted to investigate the mechanism(s) responsible for the heightened BP and MSNA mediated metaboreflex responses in T2D as well as hypertension and will likely require animal investigations to tease apart the afferent, central, and efferent pathways.
Perspectives.
Exaggerated increases in exercise BP are related to adverse cardiovascular and cerebrovascular events both during and following exercise (19, 27, 33). Although it is known that T2D patients exhibit augmented BP responses to exercise, limited studies have focused on the sympathetic and cardiovascular responses to isometric exercise in this patient group. This is important because isometric contractions are a component of many daily activities and are capable of inducing marked increases in BP and afterload on the heart even when performed with a small muscle mass (32). This highlights the significance of the augmented increases in BP and MSNA that were observed in T2D patients and attributable in part to a heightened muscle metaboreflex activation. These findings are of vital importance given the incidence of myocardial infarction and stroke among T2D patients (5, 26, 28, 57), and the number of common daily activities that involve an isometric muscle contraction component. Given our findings of a significant contribution of the muscle metaboreflex to greater pressor and sympathetic responses to isometric contractions in patients with T2D, future studies to identify the mechanism(s) responsible to target and reduce such hyperresponses are needed. In the meantime, if prescribed to a T2D patient for better health and fitness, resistance exercise training should be prescribed at a low intensity and duration for this patient population.
In summary, we report for the first time that greater pressor responses in T2D during isometric handgrip are attributable, in part, to heightened muscle metaboreflex activation. Augmented pressor responses to handgrip and PEI in T2D patients are paralleled by exaggerated increases in MSNA. These findings demonstrate that the metabolic component of the EPR is augmented in T2D, and provide important insight to the neural mechanisms that contribute to the exaggerated increases in exercise BP in T2D.
GRANTS
This research was supported by an American Heart Association Grant in Aid (20160072 to P. J. Fadel), by a Mizzou Advantage Grant (to P. J. Fadel), and by an American College of Sports Medicine (ACSM) Foundation Research Grant (S. W. Holwerda). The results of the study do not constitute endorsement by ACSM.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W.H., J.P.F., and P.J.F. conception and design of research; S.W.H., R.M.R., J.P.F., and P.J.F. performed experiments; S.W.H. analyzed data; S.W.H., J.P.F., and P.J.F. interpreted results of experiments; S.W.H. prepared figures; S.W.H. drafted manuscript; S.W.H., C.M.A., J.P.F., and P.J.F. edited and revised manuscript; S.W.H., R.M.R., C.M.A., G.L., J.P.F., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank C. Jay, R.N., for assistance with screening blood draws and patient recruitment. We also thank J. W. LeMaster, M.D., and C. N. Young, PhD, for assistance during the initiation of these studies. This research was submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy for S. W. Holwerda.
REFERENCES
- 1.Anonymous. National High Blood Pressure Education Program Working Group report on hypertension in diabetes. Hypertension 23: 145–158; Discussion 159–160, 1994. [PubMed] [Google Scholar]
- 2.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arauz-Pacheco C, Parrott MA, Raskin P. Treatment of hypertension in adults with diabetes. Diabetes Care 26, Suppl 1: S80–S82, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Avogaro A, Toffolo G, Miola M, Valerio A, Tiengo A, Cobelli C, Del Prato S. Intracellular lactate- and pyruvate-interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J Clin Invest 98: 108–115, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 265: 627–631, 1991. [PubMed] [Google Scholar]
- 6.Beske SD, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol 282: H630–H635, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol 306: H1417–H1425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol (1985) 91: 1679–1686, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 106: 316–320, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens 25: 962–968, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC 2nd, Wray DW, Davis MJ, Fadel PJ. The role of alpha-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 36: 538–542, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res 29: 424–431, 1971. [DOI] [PubMed] [Google Scholar]
- 18.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci 97: 291–301, 1999. [PubMed] [Google Scholar]
- 19.Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kübler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol 65: 583–589, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex control of muscle sympathetic nerve activity by muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol 286: H701–H707, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kamiya A, Michikami D, Fu Q, Niimi Y, Iwase S, Mano T, Suzumura A. Static handgrip exercise modifies arterial baroreflex control of vascular sympathetic outflow in humans. Am J Physiol Regul Integr Comp Physiol 281: R1134–R1139, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Karavelioglu Y, Karapinar H, Gul I, Kucukdurmaz Z, Yilmaz A, Akpek M, Kaya MG. Blood pressure response to exercise is exaggerated in normotensive diabetic patients. Blood Press 22: 21–26, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 28: 355–359, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehabil 22: 178–183, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Koskinen P, Manttari M, Manninen V, Huttunen JK, Heinonen OP, Frick MH. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care 15: 820–825, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Limberg J, Morgan B, Schrage W. Mechanical and metabolic reflex activation of the sympathetic nervous system in younger adults with metabolic syndrome. Auton Neurosci 183: 100–105, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Matsukawa T, Gotoh E, Hasegawa O, Shionoiri H, Tochikubo O, Ishii M. Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J Hypertens 9: 537–542, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JH, Wildenthal K. Static (isometric) exercise and the heart: physiological and clinical considerations. Annu Rev Med 25: 369–381, 1974. [DOI] [PubMed] [Google Scholar]
- 33.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clinics 14: 263–270, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negrao CE, Trombetta IC, Batalha LT, Ribeiro MM, Rondon MU, Tinucci T, Forjaz CL, Barretto AC, Halpern A, Villares SM. Muscle metaboreflex control is diminished in normotensive obese women. Am J Physiol Heart Circ Physiol 281: H469–H475, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep 14: 421–431, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Petrofsky JS, Stewart B, Patterson C, Cole M, Al Malty A, Lee S. Cardiovascular responses and endurance during isometric exercise in patients with Type 2 diabetes compared to control subjects. Med Sci Monitor 11: CR470–CR477, 2005. [PubMed] [Google Scholar]
- 39.Regensteiner JG, Bauer TA, Reusch JE, Quaife RA, Chen MY, Smith SC, Miller TM, Groves BM, Wolfel EE. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc 41: 977–984, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrão CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens 19: 951–957, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105: 351–356, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Schultz MG, Hordern MD, Leano R, Coombes JS, Marwick TH, Sharman JE. Lifestyle change diminishes a hypertensive response to exercise in type 2 diabetes. Med Sci Sports Exerc 43: 764–769, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Scott JA, Coombes JS, Prins JB, Leano RL, Marwick TH, Sharman JE. Patients with type 2 diabetes have exaggerated brachial and central exercise blood pressure: relation to left ventricular relative wall thickness. Am J Hypertens 21: 715–721, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol (1985) 66: 2472–2478, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Shi X, Gallagher KM, SASM, Bryant KH, Raven PB. Diminished forearm vasomotor response to central hypervolemic loading in aerobically fit individuals. Med Sci Sports Exerc 28: 1388–1395, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation 99: 1831–1836, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension 26: 869–879, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 37: 1053–1059, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrão CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol 285: H974–H982, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 54.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987. [DOI] [PubMed] [Google Scholar]
- 56.Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular responses to static muscular contraction. Am J Physiol Heart Circ Physiol 249: H710–H714, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. Sex differences in the risk of stroke and HbA(1c) among diabetic patients. Diabetologia 57: 918–926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


