Abstract
The role of imaging as a tool for investigating lung physiology is growing at an accelerating pace. Looking forward, we wished to identify unresolved issues in lung physiology that might realistically be addressed by imaging methods in development or imaging approaches that could be considered. The role of imaging is framed in terms of the importance of good spatial and temporal resolution and the types of questions that could be addressed as these technical capabilities improve. Recognizing that physiology is fundamentally a quantitative science, a recurring emphasis is on the need for imaging methods that provide reliable measurements of specific physiological parameters. The topics included necessarily reflect our perspective on what are interesting questions and are not meant to be a comprehensive review. Nevertheless, we hope that this essay will be a spur to physiologists to think about how imaging could usefully be applied in their research and to physical scientists developing new imaging methods to attack challenging questions imaging could potentially answer.
Keywords: magnetic resonance imaging, computed tomography, ventilation, pulmonary perfusion, spatial and temporal resolution
two classes of measurements have principally shaped our understanding of lung function: first, measurements made at the mouth of gas flows, pressures, and concentration profiles led to the initial concepts; and second, those concepts were extended by the development of methods to acquire images of regional lung function. Although the methods utilizing measurements made at the mouth are all well established, new approaches to lung imaging have continued to evolve. Imaging is minimally invasive and makes it possible to investigate the human lung directly. Although static lung imaging is a standard clinical tool, imaging methods in development promise to address unanswered questions about both normal lung physiology and lung pathophysiology. In recognition of the continuing evolution of lung imaging, we offer a series of brief historical perspectives to provide context for the very broad question of what sorts of future imaging developments might be on our physiology “wish list.” Minding the quote that “when the only tool you have is a hammer, every problem looks like a nail,” we discuss alternatives to our current imaging “hammers” to consider the insights that novel imaging approaches might contribute to our understanding of lung physiology and lung pathophysiology. We have chosen to limit our focus to imaging approaches applicable to intact organisms, thereby excluding some innovative microscopy methods requiring a surgical preparation. The rationale for our choice was based on the belief that a wealth of physiological questions remain to be addressed at a scale of resolution between whole lung physiology and alveolar microscopy, and it is across that intermediate range of scale where new imaging developments may offer unique insights into lung function.
All imaging methods aspire to twin goals that often are in direct conflict: good spatial resolution and good temporal resolution. Although we will discuss each of these issues separately, the important subtext is that for every scientific imaging question, the investigator may have to accept some compromise between those two goals. In addition, imaging methods often suffer from what we can call the specificity problem, where the imaging signal is not a clean measurement of one specific physiological variable. For example, a “perfusion-weighted” image of a portion of lung could mean that the image is a qualitative reflection of local perfusion in the sense that increased perfusion should translate to an increase in the imaging signal, but it is not possible to measure the fractional change in perfusion quantitatively nor measure perfusion in standard physiological units of volume of blood delivered to a unit volume of tissue per minute. Hence for this example, qualitative maps of ventilation and perfusion could characterize the extent of regional ventilation-perfusion heterogeneity, but could not assign a quantitative anchor to that distribution. For physiological applications, the most valuable imaging methods will be those that provide quantitative measurements of specific physiological variables.
IMPORTANCE OF SPATIAL RESOLUTION
Spatial gradients of function within the lung.
Understanding of the regional distribution of both blood flow and ventilation in the lung advanced dramatically with the development of methods to generate radioactive gases applicable to human respiratory research. Measurements of regional lung activity during a breath hold following a single breath of O-15 labeled CO2, followed by measurements after resumption of normal ventilation, provided the first measurements of regional blood flow, from the loss of activity during the breath hold, and measurements of regional ventilation from the decrease of activity during resumed ventilation (65). The initial demonstrations of apex to base gradients of both blood flow and ventilation provided a model to explain how the newly demonstrated spatial heterogeneity of both ventilation and blood flow interacted to preserve overall VA/Q homogeneity (8, 64). The measurements introduced the concept of the upright lung as a weighted elastic structure with its regional inflation determined by the vertical gradient of pleural pressure. Hence for ventilation, a larger fraction of each inspired breath would be delivered to the less-inflated parenchyma at the lung base. For perfusion, the relatively low pulmonary arterial pressure yielded an appreciable vertical gradient of flow, in addition to the effects of pleural pressure gradients on relative lung inflation. Thus with gravitationally directed gradients in both ventilation and blood flow, a compelling combined model was formulated that explained how the spatial heterogeneity of both ventilation and blood flow combined to preserve overall VA/Q homogeneity.
The acquisition of high-resolution lung images with x-rays, beginning with the dynamic spatial reconstructor and continuing on with subsequent generations of CT scanners, documented the expected parenchymal density gradient in the supine position. However, images acquired in the prone position revealed a nearly uniform parenchymal density, contrary to the expected gravitationally directed tissue density gradient (38). Radionuclide studies of both ventilation (5) and perfusion (49) with SPECT imaging also confirmed the nearly complete loss of gravitationally oriented gradients of ventilation and blood flow in the prone position. A satisfactory explanation for this exception to the gravitational gradient hypothesis came with a recent lung model that utilized CT measurements of chest wall and diaphragm contours in both prone and supine positions (60). Utilizing an assumption of uniform tissue compliance and gravitational parenchymal stresses along with the measured chest cavity dimensions in different positions, that model successfully predicted the observed prone and supine parenchymal density gradients. That model emphasized the importance of the shape of the chest wall cavity on the pattern of regional lung inflation in different positions. Despite the success of that prediction, however, limitations of imaging lung parenchymal shifts remain. The simplest limitation relates to the design of CT and MRI scanners in that current standard instruments only permit image acquisition of the lung in horizontal postures. However, there are a few MRI systems with an open design that make it possible to image an upright human, and high resolution images of the same human lung in the prone, supine, and upright postures would be an important advance, making it possible to test whether the model described by Tawhai et al. (60) will be equally successful in accurately predicting the upright parenchymal density gradients.
Although studies of static gradients of parenchymal inflation have focused almost exclusively on the extent of inflation and gravitationally directed pleural pressure gradients, there are additional anatomic influences, as noted above, that influence regional alveolar inflation. These include the influence of shifts in diaphragm, chest wall, and mediastinum in different body positions, shifts among lung lobes with tidal breathing, and local inflation influences on parenchyma adjacent to large conducting airways or blood vessels. An unknown concerning local regional inflation is whether there are appreciable differences in regional compliance in a normal lung. Because of uncertainty about both local pleural pressures and intraparenchymal recoil pressures adjacent to large conducting airways or vessels, simple static imaging studies acquired at different levels of inflation cannot determine whether lung compliance is constant or regionally variable. However, a benefit of sophisticated lung models that utilize the assumption of uniform compliance (60) is that the tissue density gradients predicted in various postures and levels of inflation could be compared with CT measurements of in vivo tissue density as one test of whether alveolar tissue compliance is uniform throughout the lung. New MRI elastography methods utilizing MRI images acquired during external high-frequency chest wall compression provide measurements of lung parenchymal shear stiffness at different levels of inflation, providing at least one means of characterizing the uniformity of mechanical properties of lung parenchyma. (47). Figure 1 illustrates the range of regional stiffness measurements acquired over a range of inflation pressures.
Fig. 1.
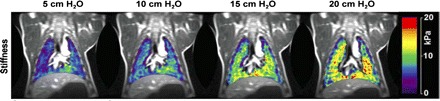
Lung mechanical properties measured with imaging. Effective lung stiffness maps measured in a porcine model at four levels of opening airway pressure with an MRI elastography method. Stiffness is calculated by detecting shear waves produced by a noninvasive mechanical driver placed on the chest wall. With synchronized motion-sensitizing magnetic field gradients, small displacements of a lung element (<10 μm) are detectable as phase changes of the MR signal. [Adapted with permission from Mariappan et al. (48)].
Over the past decade a progression of sophisticated lung perfusion models have been developed based on detailed anatomic data derived from static CT lung images. Making the assumption that the pulmonary airway tree dimensions and bifurcation angles reasonably reflect the dimensions of the pulmonary arterial tree, Burrowes et al. (16, 17) compiled an 8-generation model of the human pulmonary arterial tree (Fig. 2A) and applied equations of fluid dynamics to predict the distribution of blood flow in different postures and different gravitational stresses. Those initial models demonstrated striking concurrence with measurements reported in experimental animals of shifts in regional blood flow both in different postures and with different gravitational stresses (31). One of the advantages of an established and validated model of perfusion distribution is that in silico experiments can be performed with the model. Figure 2B from Burrowes et al. (14) shows changes from baseline flow in their pulmonary vascular model after model administration of enough 7-mm pulmonary emboli to occlude 40% of the vascular cross section. This figure also illustrates the interactive role comprehensive lung models can now play in physiological imaging, in that models with specified characteristics can be compared with in vivo imaging measurements to test the assumptions of the models. The validity of such models will progress with the availability of higher resolution images of both airway and vascular trees. In addition, high-resolution measurements of regional airway diameters at different points in the respiratory cycle could add the capacity to model important phenomena such as air trapping for disease models of the lung.
Fig. 2.
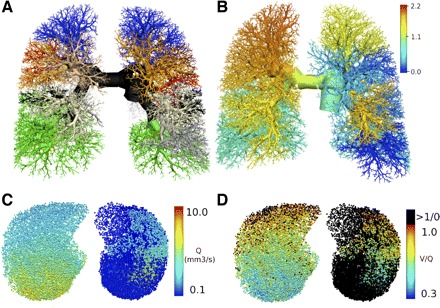
Detailed modeling of lung structure and function: predictions of perfusion changes following embolus occlusion. A: a detailed anatomically based model of the pulmonary arterial tree with different colors identifying 10 lobar or segmental branches. B: redistribution of blood flow following an embolus occlusion of the model, where the colors now represent the occluded flow divided by the baseline flow. From another study, a transverse section through the model, showing the effect of sufficient 7-mm (model) emboli to occlude 40% of the acinar units on pulmonary perfusion (Q; C) and ventilation/perfusion matching (V/Q; D). [Images courtesy of K. S. Burrowes; A and B adapted with permission from (14), C and D adapted with permission from (15)].
Scale-dependent heterogeneity of regional blood flow.
The first high-resolution maps of regional blood flow were constructed from flow measurements on multiple small samples cut from lungs administered intravenous injections of radioactive 15-μm microspheres (10, 35, 39). Those initial perfusion mapping studies demonstrated an unexpected extent of blood flow heterogeneity relative to the earlier in vivo radionuclide measurements of blood flow. Furthermore, when microsphere lung blood flow measurements were examined with successively smaller samples of lung tissue, the measured flow heterogeneity was noted to increase as the volume of the tissue sampled decreased (34). That finding of scale-dependent flow heterogeneity had been described earlier in myocardium by King et al. (45), and based on that finding, Bassingthwaighte et al. (9) described how that scale-dependent heterogeneity was consistent with a fractal model of blood flow distribution. When that fractal model was applied to blood flow measurements using a range of different sample volumes in the lung, the same scale-dependent characteristics were observed (34). The relatively low fractal dimension calculated applying Bassinthwaighte's model to the lung data sets also demonstrated that there was local structure to that flow heterogeneity. That is, high flow areas were adjacent to other high flow regions, and low flow regions likewise were adjacent to other low flow regions. This local similarity characteristic of flow heterogeneity could be modeled by considering a model of a progressively branching vascular tree that included a constant small difference between flows at each successive vascular bifurcation (33). This model, with successive minimally asymmetrical flow allocations at each branching generation, yields both progressive overall scale-dependent flow heterogeneity and local flow similarity. Imaging evidence supporting such a fractal distribution of pulmonary blood flow was confirmed in MRI studies of blood flow in the human lung (7), with a fractal dimension in the same range as that found in the animal studies using tissue maps of flow.
Although scale-dependent blood flow heterogeneity cannot continue past an alveolar level, a microsphere study in rat lungs demonstrated fractal-characteristic increases in perfusion heterogeneity at least to an acinar level of scale (30). However, an important limitation to the interpretation of all of the current measurements of scale-dependent blood flow heterogeneity is that those measurements use rectilinearly cut or rectilinearly imaged samples of lung. Given the implicit assumption that the fractal model represents the successive branching of the pulmonary vasculature, sampling of the flow measurements along rectilinear boundaries does not necessarily conform to the anatomic distribution of flow. Identification of the true heterogeneity of lung perfusion at an acinar level of scale would require a means of subdividing progressively smaller volumes defined according to the pulmonary vascular anatomy. Until image-processing algorithms can be developed to provide anatomically defined subdivisions of lung volumes down to an acinar level, an appropriate estimate of pulmonary perfusion distribution will remain inadequately defined. Hence future blood flow imaging studies to determine the heterogeneity of perfusion among acinar units would need to include both very high resolution measurements of flow and the image processing methods to divide sections of lung parenchyma into anatomically appropriate vascular outflow beds.
Imaging measurement of regional ventilation.
As an example of the difficulty of measuring a specific physiological quantity, consider the difference between the measurement of total ventilation to a unit lung volume compared with an estimate of the delivery of fresh gas to the same lung volume, the alveolar ventilation. One approach to measuring total regional ventilation has been to assess the deformation of the lung or changes in local density with tidal breathing. Studies of lung with implanted markers were the first to demonstrate substantial scale-dependent heterogeneity of lung parenchymal movement (41), subsequently confirmed at higher resolution with analysis of computed tomography images (55). However, those measurements of tidal parenchymal volume changes do not account for the fraction of each volume change that includes reinspired dead space. The imaging techniques utilizing measurements of the wash-in or wash-out of labeled gases represent the best current measurements of regional ventilation. Multiple images acquired during washout of either radioactive gases [for PET (62)], radio-dense gases [for CT (19)], or hyperpolarized gases [for MR (24)] yield estimates of regional ventilation. Those estimates of ventilation heterogeneity account for the influence of reinspired dead space on regional alveolar ventilation. However, those marker gas methods require either sustained image acquisition or multiple images over time and cannot separate the conducting airways from alveolar structures. Hence if images are acquired in exhalation, conducting airways register as alveolar spaces, and if images are acquired in end inspiration the conducting airways register as very well ventilated units. Hence both of those approaches include a conducting airways artifact that requires correction. Finally, ventilation images produced by alveolar deposition of very small aerosols that do not deposit on airways can represent delivery of ventilation down to acinar units, but at the acinar level of scale, gas diffusion becomes the primary transport mode, and even the smallest aerosols fail to represent alveolar gas delivery. In addition, deposited aerosol could come from reinspired dead space and hence fail to accurately represent alveolar ventilation. In summary, all of these imaging ventilation methods fail to exactly represent the theoretical concept of alveolar ventilation as representing the delivery of fresh gas to the gas-exchanging units.
A recent MRI method called specific ventilation imaging (SVI) overcomes the confounding effect of dead space by measuring the time dependence of the MR signal after a subject begins breathing 100% oxygen (58). The method is based on the idea that the specific ventilation of a lung unit, defined as the volume of fresh gas delivered with one breath divided by the gas volume of the unit at FRC, defines the time required for the alveolar O2 concentration to equilibrate with the new level of inspired O2. The method exploits the phenomenon that O2 dissolved in tissue alters the longitudinal relaxation time and so alters the MR signal. Importantly, the method does not depend on how much the signal changes (which depends on several factors), but instead just on how many breaths it takes for the MR signal to equilibrate at a new value. Modeling showed that this equilibration is exponential with a time constant that depends just on specific ventilation. In essence, this experiment is analogous to a nitrogen washout experiment, with the alveolar nitrogen being replaced by oxygen. Figure 3D shows a sagittal lung map of ventilation portrayed by this technique. This approach largely avoids the conducting airway problem in that the delivered “agent” (O2) is not directly detected, but instead is only detected after it reaches the tissue where it can affect the MR signal.
Fig. 3.
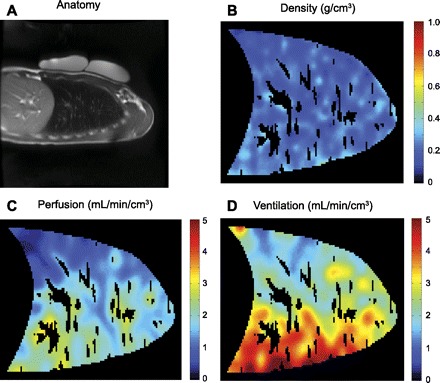
Quantitative information available with noninvasive proton MRI. A: anatomy: a sagittal slice through the right lung, with phantoms for density calibration resting on the subject's chest. B: density: acquired with a multiple gradient echo method to account for the short T2* decay of the signal (61). C: perfusion: acquired with an arterial spin labeling (ASL) method (40). D: ventilation: acquired with a specific ventilation imaging (SVI) technique, based on the time to equilibration of an inhaled hyperoxic gas (58) and then combined with the density data to calculate ventilation. Masked areas within the lung correspond to large conduit vessels that were identified and removed from the high-resolution perfusion image based on an intensity threshold (13), and the images were subsequently smoothed to ∼1 cm3 spatial resolution. (Example images courtesy of A. C. Henderson.)
To date there have been few direct comparisons of the different methods for assessing regional ventilation. For the healthy lung, where dead space issues are probably relatively minor, the differences among methods in measurement of regional ventilation may be quite small, but those differences may be magnified in situations of disease. A combination of techniques may in fact provide a way to probe the dead space issues. For example, differences in ventilation measured by density changes during tidal breathing compared with SVI could provide a measure of regional dead space.
Scale-dependent heterogeneity of ventilation.
Exhaled nitrogen profiles following a single breath of 100% oxygen in 3-mm airways of experimental animals provided the first evidence for a substantial extent of ventilation heterogeneity within isogravitational segments of normal lung (25). Subsequent human PET studies of regional ventilation utilizing positron-emitting gases (12) and animal CT studies of washout of inhaled xenon (46) had adequate resolution to demonstrate that, like perfusion, substantial ventilation heterogeneity is also present within iso-gravitational regions of the lung. Utilizing deposition of a 1.0-μm fluorescent aerosol to produce ventilation maps in juvenile pigs the overall heterogeneity of ventilation was comparable in scale to the heterogeneity of perfusion measured by intravascular injection of 15-μm fluorescent microspheres, and application of the same fractal model used for perfusion distribution to the ventilation distribution yielded a comparable fractal dimension (2). Very high-resolution synchrotron CT measurements of xenon washout in rabbits at a 200-mm3 level of resolution described an overall coefficient of variation of ventilation of 25% (52). Maps of regional ventilation in rats utilizing deposition of 1-μm fluorescent aerosol reported a coefficient of variation of ventilation of 35% at a sample size of ∼120 mm3 (52). However, as noted above, the aerosol ventilation estimates below the ∼120 mm3 volume may no longer appropriately represent gas distribution somewhere below that scale of measurement. In addition, both the aerosol and CT ventilation studies employ arbitrary partitions of lung to calculate ventilation heterogeneity, once again underlining the need for image processing methods that can divide the lung into volumes defined by airway anatomy.
How big is the functional unit of gas exchange?
In light of the extreme extent of scale-dependent ventilation and perfusion heterogeneity attained proximal to the acinar lung units, the nearly ideal gas exchange performance of normal lungs mandates close matching of those two parameters, especially for large animals where inspired gases and blood flow must pass through multiple generations of branches before the acinar units are reached. Studies reporting simultaneous measurement of both ventilation and blood flow in large animals utilizing tissue maps of deposited aerosol and vascular fluorescent microspheres at a 1-cm3 level of scale showed roughly equal heterogeneity of ventilation and perfusion, with strong correlation between the two parameters (3, 54). Similar measurements in rats at a ∼120-mm3 scale level showed less heterogeneity than that observed in larger animals, with weaker correlation between ventilation and blood flow, possibly a consequence of fewer branching generations in a small animal prior to reaching respiratory bronchioles (53). Unfortunately those aerosol measurements cannot appropriately represent ventilation at any smaller level of scale and no measurements of ventilation and blood flow at an acinar level of resolution have been reported.
Although perfusion heterogeneity appears likely to increase progressively to the level of the alveolar wall, alveolar gas composition is homogenized by diffusion, and gas composition could be homogenized at some larger level of scale within the acinus. Uniform gas composition within units larger than an alveolus would define both a functional unit of ventilation and a functional unit of gas exchange, regardless of perfusion heterogeneity that continued to smaller levels of scale. Both gas exchange studies following graded bead embolization (67) and gas exchange models (50) have suggested that the functional unit of ventilation and gas exchange exists at some subdivision of volume within the acinus. However, the challenge to answering the question of the size of a functional unit of gas exchange turns back to the requirement to develop very high-resolution ventilation images. Ventilation images based on calculations from synchrotron CT images of xenon washout in rabbits represent the highest in vivo spatial resolution measurements of ventilation reported to date (52), but as noted earlier, that ventilation technique with a 200-mm3 resolution [roughly the volume of 50 rabbit acini (56)] must have the measurement units apportioned according to anatomical boundaries to appropriately identify the size of a ventilation and gas exchange unit of function.
Measuring structure on a scale smaller than the image resolution.
Sometimes it is possible to measure microscopic aspects of anatomic structures without being able to resolve those structures in an image. MRI measurement of diffusion is an example in which the MR signal is made sensitive to random motions due to diffusion that are on a spatial scale much smaller than the resolution of the images. Essentially, the displacement of a molecule between application of two lobes of a magnetic field gradient pulse creates a phase offset of the MR signal arising from that molecule, and a random spread of such phase offsets reduces the net MR signal to a degree that depends on the extent of the local displacements due to diffusion. When diffusing molecules are restricted in their motion by membranes or other boundaries, the apparent diffusion coefficient is reduced. With the development of hyperpolarized gas MRI it is possible to measure signals from inhaled 3He or 129Xe, and in the lung the diffusion of these gases is restricted by the diameter of the alveoli (11). These methods have led to new ways to estimate aspects of lung anatomy such as alveolar size and density (66) and are showing promising results in COPD patients (44). Xenon-129 offers additional possibilities to measure signals sensitive to aspects of exchange from the gas phase to dissolved gas in tissue, such as thickness of alveolar septa, because these two phases are distinguishable in the MR signal (22). As these methods mature, they could potentially provide measurements that are not possible with other techniques.
Imaging measurement of ventilation-perfusion heterogeneity.
Combined measurements of regional ventilation and regional blood flow have been used to produce high-resolution images of the distribution of the ventilation-perfusion ratio in the lung. This was initially described with PET scans utilizing two different positron emitting markers (12) and later with a PET technique utilizing a single injection of N-13 during a breath hold, followed by images acquired during gas washout (62). A CT method utilizing sequential images following injection of intravascular contrast followed by sequential images of the ventilatory washout of radiodense xenon also provided high-resolution images of ventilation perfusion distribution (18, 19). Finally animal studies utilizing simultaneous injection of intravascularly injected fluorescent microspheres with inhalation of fluorescent aerosol microspheres provided detailed post mortem tissue maps of ventilation perfusion heterogeneity that successfully predicted both respiratory and multiple inert gas exchange in those same animals (3). However, a subsequent study of a pulmonary embolus model utilizing that same FMS technique significantly underestimated the measured gas exchange abnormality, suggesting that a higher level of resolution was required to characterize the gas exchange properties of abnormal lungs (4). One potential solution to the requirement for a single technique with better resolution to characterize ventilation perfusion heterogeneity has been with MRI images of inhaled hyperpolarized 3He. Because of the sensitivity of the longitudinal relaxation rate to oxygen, hyperpolarized 3He measurements offer the possibility of measuring local alveolar PO2 (68). An accurate measurement of alveolar PO2 with an appropriate O2-CO2 curve could yield a map of VA/Q distribution. Current MRI 3He estimates of PaO2 are promising and are now capable of assessing the whole human lung (36), although the method does yield some physiologically impossible results in some lung regions, and the artifact responsible for those results remains undefined. If the technique could be corrected, an accurate regional measurement of alveolar PO2 would provide a noninvasive high-resolution map of VA/Q heterogeneity, and that distribution, combined with an MRI measurement of regional ventilation utilizing measurements of 3He washout (21) could yield quantitative maps of both regional ventilation and regional blood flow, a valuable tool for investigation of a broad range of lung diseases.
Spatial patterns of acute and chronic lung diseases.
High-resolution CT images are an essential diagnostic tool for the practice of chest medicine, and the spatial distribution of parenchymal abnormalities can be very suggestive of specific diagnoses. Two of the best large-scale examples are the basal-peripheral pattern of scarring characteristic of usual interstitial pneumonia (UIP) and the basal distribution pattern of emphysema seen in patients with alpha-1 antitrypsin deficiency. At the resolution level of the secondary lobule, centrilobular emphysema is associated with loss of airway parenchyma surrounding the central respiratory bronchiole, whereas panacinar emphysema, as its name suggests, is associated with a uniform loss of alveoli within the entire secondary lobule. Those examples suggest that regional differences in parenchymal stress or environment within the lung influence the local cellular expression of the disease and that identification of differences in mechanical stress or local metabolic environment would provide valuable insights into the pathogenesis of disease processes. A high-resolution method to detect differences in regional strain and/or compliance, such as the illustrated above in Fig. 1 (47), could provide valuable insights into lung pathology.
High-resolution lung imaging has revealed consistent patterns of acute edema formation. The initial animal studies of acute cardiogenic pulmonary edema revealed regions of frank alveolar flooding adjacent to lung regions that appeared perfectly “dry.” That same heart failure pattern of flooded secondary lobules adjacent to dry secondary lobules is now confirmed on a daily basis in human CT images of acute cardiogenic pulmonary edema. CT images of acute capillary leak pulmonary edema (ARDS) revealed a pattern of larger patches of pulmonary edema, and high-altitude pulmonary edema (HAPE) revealed yet a third pattern of edema formation. Although different pathophysiology is involved in all three of these edema conditions, the relatively consistent patterns of each of their manifestations in lung tissue suggest differences in local parenchymal characteristics that govern the presence or absence of edema fluid. The ability to acquire repeated, anatomically segmented high-resolution measurements of regional blood flow in any of the conditions leading to acute pulmonary edema would provide insight into the role local vascular reactivity might play in edema development.
Both PET and MRI ventilation images of patients with asthma have focused attention on the patchy air trapping. Figure 4 illustrates that patchy air trapping in a PET study of a patient with asthma. Although current CT imaging has demonstrated that the large conducting airways in patients with asthma are indeed abnormal, oscillometry studies have suggested that air trapping develops within the 2-mm airways, rather than the larger conducting airway serving an entire collapsed segment (43). Recent studies have brought into focus the importance of the interaction between airway mucosal edema, bronchoconstriction, and variable local parenchymal forces on those small airways, leading to combinations of airway collapse and airway dilation (20). These findings were anticipated in models of small airway responses to bronchoconstriction that predicted the coexistence of collapsed and dilated small airways (6), but current imaging resolution for those airways in that critical 2-mm range remains a challenge. In addition to the spatial heterogeneity of the airway abnormalities in asthma, the temporal variability represents another component that is clinically relevant. What is needed to confirm those hypotheses of variable spatial and temporal small airway patency are methods to obtain multiple measurements of very small airway diameter and means to divide lung ventilation images according to their bronchovascular anatomy. In addition to studies of asthma, the interaction between small airways inflammation and local parenchymal forces in COPD also determines the extent of regional air trapping, and the same imaging needs described above for asthma would also be relevant, particularly for investigation of the early stages of COPD.
Fig. 4.
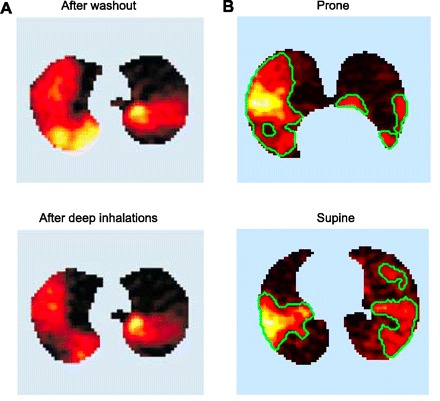
PET images of ventilation defects identified by lack of clearance of 13N labeled N2. An intravenously injected bolus of labeled N2 is cleared from the lung by ventilation, revealing ventilation defects as high levels of retained label. A: asthmatic lung, showing ventilation defects after 2 min of tidal breathing and then after 3 subsequent deep breaths [tracer concentration increasing from black (complete clearance of label) through red, yellow, and white (no clearance); adapted with permission from Venegas et al. (63)]. B: alteration of ventilation defects in the prone compared with the supine posture in a subject with mild asthma following bronchoconstriction induced by methacholine [adapted with permission from Harris et al. (37)].
IMPORTANCE OF TEMPORAL RESOLUTION
Need for specific and quantitative temporal imaging methods.
Physiology is a quantitative science, and for imaging to be most useful it should provide quantitative values of specific physiological variables. This is a primary challenge for future imaging developments, particularly because technical aspects of the methodology can enter into the interpretation of the image signals in subtle ways. For example, coil heterogeneity in an MRI experiment can directly affect the measured distribution of perfusion as milliliters blood per milliliters tissue per minute, but the density-normalized perfusion, calculated by dividing the perfusion image by a separately acquired density image provides measurements of perfusion in units of milliliters blood per gram tissue per minute that are insensitive to coil inhomogeneity. Identifying and clarifying the quantitative limitations of new imaging methods is vital for widespread acceptance of the results and adoption of the techniques.
Both CT and PET estimates of regional ventilation require multiple images and radiation exposure. For MRI there is no radiation dose, so repeating the imaging many times is not a problem, but there remains the basic conflict between temporal and spatial resolution for each image. Higher spatial resolution means that more bits of information need to be encoded in the measured signal, and this translates to a longer time to acquire sufficient information for each image. A traditional approach to dealing with this problem in cardiovascular imaging is to gate the data acquisition to the heart cycle and acquire only a part of the information needed for the full image after each heart beat. However, this approach depends on the reproducibility of each cycle, which is likely a poor assumption for the lungs of a spontaneously breathing subject. The rate at which spatial information can be encoded in MRI is often limited by the gradient switching rates. Interestingly, these limitations are not due to the hardware, but rather the need to keep these switching rates below levels that produce nerve stimulation in the subjects. However, two approaches are being actively pursued to overcome this problem. The first is the design of array coils, essentially consisting of many independent coils each with a unique spatial sensitivity. By using the intrinsic spatial sensitivity of the coils, less information on the spatial distribution needs to be encoded into the signal itself with the imaging gradients. The second approach, compressed sensing, attempts to reconstruct an image without the fully encoded spatial information using prior information about basic features of the images (1). The combination of these methods could provide substantial improvements in being able to quickly acquire lung images with good spatial resolution.
Are there continuous, coordinated VA and Q shifts in a normal lung?
The ability to measure fluctuations of blood flow and ventilation repeatedly over time would make it possible to look at the lung as a dynamic system in which the magnitude of the fluctuations may be as interesting as the mean values. The observation that normal ventilation perfusion heterogeneity is both minimal and stable over time mandates that the observed extent of small-scale spatial blood flow and perfusion heterogeneity in the lung must show a very strong correlation between those two parameters. The temporal fluctuation of spatial heterogeneity of blood flow is small, ranging around 5 to 7% of the total variability over several hours of measurement, leading to the conclusion that the normal lung has stable and relatively fixed regional allocations of ventilation and blood flow (32). However, a study making repeated simultaneous measurements of ventilation and blood flow in small units of lung showed an infrequent but progressive accumulation of bidirectional coordinated shifts of ventilation and blood flow within small units of lung (54). The sample piece size for this study was ∼2 cm3, and those few pieces experiencing large coordinated shifts remained stable at the new values during subsequent measurements. Although those findings await validation by other measurement techniques, they suggest that there is active coordinated control of both ventilation and blood flow in the lung taking place at a sporadic temporal pace and at a relatively small level of scale. This form of small-scale coordinated temporal heterogeneity of ventilation and blood flow will remain undetected by most experimental approaches, as the VA/Q ratio of those shifting units is changed only minimally. However, the capacity of small regions within the lung to spontaneously readjust their allocation of ventilation and blood flow might represent an important mechanism to shape and defend the long-term maintenance of overall VA/Q homogeneity. Confirmation of these coordinated temporal shifts would require a noninvasive imaging method to make multiple high-resolution simultaneous measurements of both ventilation and blood flow.
Challenge of dynamic within-breath imaging.
The lung is fundamentally a dynamic organ. At the most basic level, dynamic imaging could provide insights on the regional motions of the lung, such as the timing of expansion and contraction for different regions and the relative motion of lobes as they slide past one another. The ability to measure these motions in different body positions would add an interesting dynamic axis to the detailed mechanical modeling. These mechanical tidal motions, by stretching and compressing both blood vessels and airways, in turn may have significant effects on local blood flow and local ventilation. Finally, for any sophisticated modeling of particulate deposition in the lung, the normal tidal excursions of the upper airways and larynx need to be characterized with dynamic imaging. Regardless of the technique selected, all of these imaging goals once again will require choices to be made between spatial and temporal resolution. The basic requirement for temporal imaging is that the time to acquire an image must be short compared with the dynamics of the system being imaged, and it must be possible to repeat the imaging many times. For CT or radionuclide imaging this translates to a dose problem, with multiple measurements over time requiring multiple administrations of tracers. In addition, for studies utilizing an injected or inhaled marker, the implicit assumption is that the underlying physiology during the time of data acquisition is in a steady state, with constant rates of blood flow or ventilation delivering or clearing the agent. This effectively limits the temporal resolution of these methods.
The ability to characterize the tidal dynamics of pulmonary parenchymal motion would add a new level of sophistication to our understanding of lung function. Even for normal lungs at normal respiratory rates, it seems possible that the interaction between the lung and tidal thoracic cavity movement might contribute some additional temporal component to regional ventilation. Although the lungs of most mammals are separated into lobes, the functional significance of the tidal movement of lung lobes relative to each other is undetermined, but might reduce parenchymal strain on the normal lung. However, imaging methods to fully characterize tidal chest cavity shape, lung volume changes, and tidal lobar movements are still in development. A CT image acquisition method for ventilated rodents (42) acquired gated images of the lung at different points in the respiratory cycle, revealing the very appreciable tidal variation in airway diameter. A technologically complex system utilizing a synchrotron radiation source to produce phase contrast computed tomography tidal images of spontaneously breathing mice has been described by Fouras et al. (28). A subsequent study with this methodology applied to both normal and abnormal mouse lungs applied the technique of velocitrometry to illustrate tidal regional lung movement (27). Figure 5A from that study revealed appreciable regional differences in lung tissue velocity during normal tidal breathing and showed yet larger regional differences when subsequent images were acquired of rats with injured lungs. Figure 5B shows another application with that imaging system to measure regional ventilation. The potential to characterize regional shifts, both within parenchyma and among lobes throughout a tidal cycle would be interesting in normal subjects but would be especially relevant to the investigation of regional ventilation characteristics of both obstructive and restrictive lung diseases.
Fig. 5.
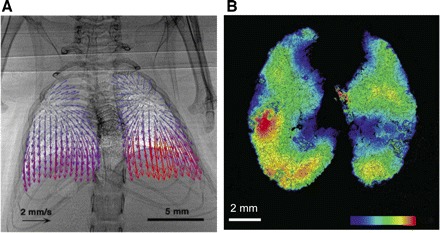
Lung motion measured with imaging. A: vector field representation of instantaneous velocity of each lung element ∼140 ms after the beginning of inspiration in a spontaneously breathing mouse, measured with synchrotron-based phase-contrast x-ray imaging [adapted from Fouras et al. (27)]. B: local ventilation measured in a mouse model with the same computed tomography methodology [adapted from Dubsky et al. (23)].
Regional allocation of dead space in tidal breathing.
Currently we remain ignorant of two of the most basic properties of dead space in pulmonary ventilation. First, it is not known if the dead space fraction of reinspired gas is constant throughout different regions of the lung. That represents a potentially important issue for lungs in large animals that must include a substantial range of different conducting airways path lengths prior to entry into the acini. Second, the composition of reinspired dead space gas may approximate the composition of the specific units that exhaled it, in which case the influence on overall VA/Q heterogeneity would be minimal, the so-called “personal” dead space option (26). Alternatively, the exhaled dead space could be well mixed within the conducting airways, the so-called common dead space option, in which case overall VA/Q heterogeneity would be minimized (51, 57).
The composition and allocation of reinspired dead space gas will be governed by the timing of exhalation in different areas of the lung. Even during a tidal breath in a normal lung, it is not known if every region receives a constant proportion of that reinspired dead space gas. MRI images of a single inhalation of hyperpolarized gas could describe the sequence of regional inspired ventilation distribution, but interpretation of the allocation of subsequent reinspired dead space would be challenging because of the sensitivity of the longitudinal relaxation rate of hyperpolarized gases to the partial pressure of oxygen. Although it is likely that the irregular distribution of parenchymal abnormalities in any lung disease will alter the within-breath regional allocation of tidal ventilation, the extent and significance of that mismatch on gas exchange efficiency in diseased lungs remains unknown. High temporal resolution images of tidal ventilation such as those described by Fouras et al. (27) will provide some insight into those challenging questions.
Complex dynamics.
It was noted earlier that the spatial distribution of blood flow in the lung has fractal characteristics. A number of physiological systems also exhibit fractal structure in their temporal dynamics, with fluctuations occurring on many time scales. Often the loss of this “irregularity” is a sign of pathophysiology. For the lung, the temporal dynamics of blood flow are just beginning to be explored with new imaging tools with sufficient temporal resolution. The evidence cited above for coordinated shifts in ventilation and perfusion on a time scale of 5 min suggests the interesting dynamics that remain to be explored as new techniques become available. Arterial spin labeling (ASL) MRI methods are a promising approach for measuring the temporal dynamics in the human lung (40). ASL approach works by manipulating the magnetization of arterial blood outside the slice to be imaged, waiting for that blood to be delivered during one cardiac cycle, and imaging the distribution of labeled blood in the imaged slice. In current implementations two images are required, one in which the magnetization of arterial blood was inverted prior to delivery and one in which it remained fully relaxed so that the difference image cancels the signal from static tissue and leaves a signal proportional to the volume of arterial blood delivered to each voxel. Each image is collected with a single-shot acquisition, so imaging is quick, but the need for two images makes the temporal resolution on the order of 10 s. Figure 3C illustrates an ASL image of human lung acquired by this technique. If this 10-s time limitation for perfusion measurement can be overcome, it is tempting to imagine a free-running image acquisition that captures the blood flow pattern at different stages of the breathing cycle. In addition to the technical MRI requirements, dynamic imaging requires efficient algorithms for deformably registering lung images, particularly when the contrast characteristics of the lung are significantly different in the images. In short, current methods look promising to be able to provide new insights on the temporal dynamics of blood flow in the human lung.
Comparably refined methods for the measurement of regional and temporal dynamics of ventilation are not yet available, but the temporal complexity of ventilation in both health and disease is already an active topic of investigation (29). If high-resolution temporal ventilation methods could be developed, they would open the door to detailed combined studies of the dynamics of blood flow, ventilation, and VA/Q matching. In particular such data would make it possible to investigate the lung as a dynamical system and test whether ideas from studies of other complex systems are applicable to the lung. For example, Venegas et al. (63) showed that a simple dynamical model for the interaction of different lung ventilation units, based on the bistable model of Anafi and Wilson (6), predicts a transition to a spatial patchiness of reduced ventilation similar to what is observed in asthma (illustrated in Fig. 3). In a number of systems, the approach to an abrupt switch to a new mode (a critical transition) has been found to be associated with characteristic features of increased temporal variance and a slowed recovery from a challenge (59). Reliable methods for measuring temporal fluctuations in blood flow and ventilation will allow us to explore these possibilities of complex dynamics in the lung.
CONCLUSIONS
Submitting a paper on respiratory phenomena that have not yet been discovered based on techniques that have not yet been developed might well be considered appropriate only for the long-deceased Journal of Irreproducible Results. Our rationale in accepting that challenge, however, was that we believe the rapidly developing technology of imaging merits the close attention of all physiologists. We hope our readers will forgive the clearly personal choices we made for the scientific questions and developing methods that we have included (or excluded). Regardless of the merits of our selections, our primary goal was to encourage physiologists to think forward to questions that might be investigated utilizing the imaging methods on the current developmental horizon and to encourage those developing new imaging methods to consider our wish list as a challenge.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.T.R. and R.B.B. conception and design of research; H.T.R. and R.B.B. interpreted results of experiments; H.T.R. and R.B.B. drafted manuscript; H.T.R. and R.B.B. edited and revised manuscript; H.T.R. and R.B.B. approved final version of manuscript.
REFERENCES
- 1. Ajraoui S, Lee KJ, Deppe MH, Parnell SR, Parra-Robles J, Wild JM. Compressed sensing in hyperpolarized 3He lung MRI. Magn Reson Med 63: 1059–1069, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Altemeier WA, McKinney S, Glenny RW. Fractal nature of regional ventilation distribution. J Appl Physiol 88: 1551–1557, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Altemeier WA, Robertson HT, Glenny RW. Pulmonary gas-exchange analysis by using simultaneous deposition of aerosolized and injected microspheres. J Appl Physiol 85: 2344–2351, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Altemeier WA, Robertson HT, McKinney S, Glenny RW. Pulmonary embolization causes hypoxemia by redistributing regional blood flow without changing ventilation. J Appl Physiol 85: 2337–2343, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Amis TC, Jones HA, Hughes JM. Effect of posture on inter-regional distribution of pulmonary ventilation in man. Respir Physiol 56: 145–167, 1984. [DOI] [PubMed] [Google Scholar]
- 6. Anafi RC, Wilson TA. Airway stability and heterogeneity in the constricted lung. J Appl Physiol 91: 1185–1192, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Arai TJ, Henderson AC, Dubowitz DJ, Levin DL, Friedman PJ, Buxton RB, Prisk GK, Hopkins SR. Hypoxic pulmonary vasoconstriction does not contribute to pulmonary blood flow heterogeneity in normoxia in normal supine humans. J Appl Physiol 106: 1057–1064, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ball WC, Jr, Stewart PB, Newsham LG, Bates DV. Regional pulmonary function studied with xenon 133. J Clin Invest 41: 519–531, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res 65: 578–590, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beck KC, Rehder K. Differences in regional vascular conductances in isolated dog lungs. J Appl Physiol 61: 530–538, 1986. [DOI] [PubMed] [Google Scholar]
- 11. Boudreau M, Xu X, Santyr GE. Measurement of (129) Xe gas apparent diffusion coefficient anisotropy in an elastase-instilled rat model of emphysema. Magn Reson Med. EPUB 03/12/2012. [DOI] [PubMed] [Google Scholar]
- 12. Brudin LH, Rhodes CG, Valind SO, Jones T, Jonson B, Hughes JM. Relationships between regional ventilation and vascular and extravascular volume in supine humans. J Appl Physiol 76: 1195–1204, 1994. [DOI] [PubMed] [Google Scholar]
- 13. Burrowes KS, Buxton RB, Prisk GK. Assessing potential errors of MRI-based measurements of pulmonary blood flow using a detailed network flow model. J Appl Physiol; doi:10.1152/japplphysiol.00894.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burrowes KS, Clark AR, Marcinkowski A, Wilsher ML, Milne DG, Tawhai MH. Pulmonary embolism: predicting disease severity. Philos Transact A Math Phys Eng Sci 369: 4255–4277, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ 1: 365–376, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burrowes KS, Hunter PJ, Tawhai MH. Investigation of the relative effects of vascular branching structure and gravity on pulmonary arterial blood flow heterogeneity via an image-based computational model. Acad Radiol 12: 1464–1474, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Burrowes KS, Tawhai MH. Computational predictions of pulmonary blood flow gradients: Gravity versus structure. Respir Physiol Neurobiol 154: 515–523, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Chon D, Beck KC, Larsen RL, Shikata H, Hoffman EA. Regional pulmonary blood flow in dogs by 4D-X-ray CT. J Appl Physiol 101: 1451–1465, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Chon D, Simon BA, Beck KC, Shikata H, Saba OI, Won C, Hoffman EA. Differences in regional wash-in and wash-out time constants for xenon-CT ventilation studies. Respir Physiol Neurobiol 148: 65–83, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Contoli M, Bousquet J, Fabbri LM, Magnussen H, Rabe KF, Siafakas NM, Hamid Q, Kraft M. The small airways and distal lung compartment in asthma and COPD: a time for reappraisal. Allergy 65: 141–151, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Deppe MH, Parra-Robles J, Ajraoui S, Wild JM. Combined measurement of pulmonary inert gas washout and regional ventilation heterogeneity by MR of a single dose of hyperpolarized 3He. Magn Reson Med 65: 1075–1083, 2011. [DOI] [PubMed] [Google Scholar]
- 22. Dregely I, Mugler JP, 3rd, Ruset IC, Altes TA, Mata JF, Miller GW, Ketel J, Ketel S, Distelbrink J, Hersman FW, Ruppert K. Hyperpolarized Xenon-129 gas-exchange imaging of lung microstructure: first case studies in subjects with obstructive lung disease. J Magn Reson Imaging 33: 1052–1062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubsky S, Hooper SB, Siu KK, Fouras A. Synchrotron-based dynamic computed tomography of tissue motion for regional lung function measurement. J R Soc Interface. EPUB 04/12/2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Emami K, Xu Y, Hamedani H, Xin Y, Profka H, Rajaei J, Kadlecek S, Ishii M, Rizi RR. Multislice fractional ventilation imaging in large animals with hyperpolarized gas MRI. NMR Biomed. EPUB 02/01/2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Engel LA, Utz G, Wood LD, Macklem PT. Ventilation distribution in anatomical lung units. J Appl Physiol 37: 194–200, 1974. [DOI] [PubMed] [Google Scholar]
- 26. Fortune JB, Wagner PD. Effects of common dead space on inert gas exchange in mathematical models of the lung. J Appl Physiol 47: 896–906, 1979. [DOI] [PubMed] [Google Scholar]
- 27. Fouras A, Allison BJ, Kitchen MJ, Dubsky S, Nguyen J, Hourigan K, Siu KK, Lewis RA, Wallace MJ, Hooper SB. Altered lung motion is a sensitive indicator of regional lung disease. Ann Biomed Eng 40: 1160–1169, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Fouras A, Dusting J, Sheridan J, Kawahashi M, Hirahara H, Hourigan K. Engineering imaging: using particle image velocimetry to see physiology in a new light. Clin Exp Pharmacol Physiol 36: 238–247, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Frey U, Maksym G, Suki B. Temporal complexity in clinical manifestations of lung disease. J Appl Physiol 110: 1723–1731, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Glenny RW, Bernard SL, Robertson HT. Pulmonary blood flow remains fractal down to the level of gas exchange. J Appl Physiol 89: 742–748, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Glenny RW, Lamm WJ, Bernard SL, An D, Chornuk M, Pool SL, Wagner WW, Jr, Hlastala MP, Robertson HT. Selected contribution: redistribution of pulmonary perfusion during weightlessness and increased gravity. J Appl Physiol 89: 1239–1248, 2000. [DOI] [PubMed] [Google Scholar]
- 32. Glenny RW, McKinney S, Robertson HT. Spatial pattern of pulmonary blood flow distribution is stable over days. J Appl Physiol 82: 902–907, 1997. [DOI] [PubMed] [Google Scholar]
- 33. Glenny RW, Robertson HT. Fractal modeling of pulmonary blood flow heterogeneity. J Appl Physiol 70: 1024–1030, 1991. [DOI] [PubMed] [Google Scholar]
- 34. Glenny RW, Robertson HT. Fractal properties of pulmonary blood flow: characterization of spatial heterogeneity. J Appl Physiol 69: 532–545, 1990. [DOI] [PubMed] [Google Scholar]
- 35. Greenleaf JF, Ritman EL, Sass DJ, Wood EH. Spatial distribution of pulmonary blood flow in dogs in left decubitus position. Am J Physiol 227: 230–244, 1974. [DOI] [PubMed] [Google Scholar]
- 36. Hamedani H, Kadlecek SJ, Emami K, Kuzma NN, Xu Y, Xin Y, Mongkolwisetwara P, Rajaei J, Barulic A, Wilson Miller G, Rossman M, Ishii M, Rizi RR. A multislice single breath-hold scheme for imaging alveolar oxygen tension in humans. Magn Reson Med 67: 1332–1345, 2012. [DOI] [PubMed] [Google Scholar]
- 37. Harris RS, Winkler T, Musch G, Vidal Melo MF, Schroeder T, Tgavalekos N, Venegas JG. The prone position results in smaller ventilation defects during bronchoconstriction in asthma. J Appl Physiol 107: 266–274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffman EA. Effect of body orientation on regional lung expansion: a computed tomographic approach. J Appl Physiol 59: 468–480, 1985. [DOI] [PubMed] [Google Scholar]
- 39. Hogg JC, Holst P, Corry P, Ruff F, Housley E, Morris E. Effect of regional lung expansion and body position on pulmonary perfusion in dogs. J Appl Physiol 31: 97–101, 1971. [DOI] [PubMed] [Google Scholar]
- 40. Hopkins SR, Prisk GK. Lung perfusion measured using magnetic resonance imaging: new tools for physiological insights into the pulmonary circulation. J Magn Reson Imaging 32: 1287–1301, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hubmayr RD, Rodarte JR, Walters BJ, Tonelli FM. Regional ventilation during spontaneous breathing and mechanical ventilation in dogs. J Appl Physiol 63: 2467–2475, 1987. [DOI] [PubMed] [Google Scholar]
- 42. Jacob RE, Lamm WJ. Stable small animal ventilation for dynamic lung imaging to support computational fluid dynamics models. PLos One 6: e27577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaczka DW, Lutchen KR, Hantos Z. Emergent behavior of regional heterogeneity in the lung and its effects on respiratory impedance. J Appl Physiol 110: 1473–1481, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaushik SS, Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kraft M, Auffermann W, Wolber J, McAdams HP, Driehuys B. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65: 1154–1165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. King RB, Bassingthwaighte JB, Hales JR, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ Res 57: 285–295, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marcucci C, Nyhan D, Simon BA. Distribution of pulmonary ventilation using Xe-enhanced computed tomography in prone and supine dogs. J Appl Physiol 90: 421–430, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Mariappan YK, Glaser KJ, Hubmayr RD, Manduca A, Ehman RL, McGee KP. MR elastography of human lung parenchyma: technical development, theoretical modeling and in vivo validation. J Magn Reson Imaging 33: 1351–1361, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mariappan YK, Kolipaka A, Manduca A, Hubmayr RD, Ehman RL, Araoz P, McGee KP. Magnetic resonance elastography of the lung parenchyma in an in situ porcine model with a noninvasive mechanical driver: correlation of shear stiffness with trans-respiratory system pressures. Magn Reson Med 67: 210–217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nyren S, Mure M, Jacobsson H, Larsson SA, Lindahl SG. Pulmonary perfusion is more uniform in the prone than in the supine position: scintigraphy in healthy humans. J Appl Physiol 86: 1135–1141, 1999. [DOI] [PubMed] [Google Scholar]
- 50. Paiva M, Engel LA. Model analysis of intra-acinar gas exchange. Respir Physiol 62: 257–272, 1985. [DOI] [PubMed] [Google Scholar]
- 51. Petrini MF, Robertson HT, Hlastala MP. Interaction of series and parallel dead space in the lung. Respir Physiol 54: 121–136, 1983. [DOI] [PubMed] [Google Scholar]
- 52. Porra L, Monfraix S, Berruyer G, Le Duc G, Nemoz C, Thomlinson W, Suortti P, Sovijarvi AR, Bayat S. Effect of tidal volume on distribution of ventilation assessed by synchrotron radiation CT in rabbit. J Appl Physiol 96: 1899–1908, 2004. [DOI] [PubMed] [Google Scholar]
- 53. Robertson HT, Krueger MA, Lamm WJ, Glenny RW. High-resolution spatial measurements of ventilation-perfusion heterogeneity in rats. J Appl Physiol 108: 1395–1401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robertson HT, Neradilek B, Polissar NL, Glenny RW. Sporadic coordinated shifts of regional ventilation and perfusion in juvenile pigs with normal gas exchange. J Physiol 583: 743–752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodarte JR, Chaniotakis M, Wilson TA. Variability of parenchymal expansion measured by computed tomography. J Appl Physiol 67: 226–231, 1989. [DOI] [PubMed] [Google Scholar]
- 56. Rodriguez M, Bur S, Favre A, Weibel ER. Pulmonary acinus: geometry and morphometry of the peripheral airway system in rat and rabbit. Am J Anat 180: 143–155, 1987. [DOI] [PubMed] [Google Scholar]
- 57. Ross BB, Farhi LE. Dead-space ventilation as a determinant in the ventilation-perfusion concept. J Appl Physiol 15: 363–371, 1960. [DOI] [PubMed] [Google Scholar]
- 58. Sa RC, Cronin MV, Henderson AC, Holverda S, Theilmann RJ, Arai TJ, Dubowitz DJ, Hopkins SR, Buxton RB, Prisk GK. Vertical distribution of specific ventilation in normal supine humans measured by oxygen-enhanced proton MRI. J Appl Physiol 109: 1950–1959, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes EH, Rietkerk M, Sugihara G. Early-warning signals for critical transitions. Nature 461: 53–59, 2009. [DOI] [PubMed] [Google Scholar]
- 60. Tawhai MH, Nash MP, Lin CL, Hoffman EA. Supine and prone differences in regional lung density and pleural pressure gradients in the human lung with constant shape. J Appl Physiol 107: 912–920, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Theilmann RJ, Arai TJ, Samiee A, Dubowitz DJ, Hopkins SR, Buxton RB, Prisk GK. Quantitative MRI measurement of lung density must account for the change in T(2) (*) with lung inflation. J Magn Reson Imaging 30: 527–534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Treppo S, Mijailovich SM, Venegas JG. Contributions of pulmonary perfusion and ventilation to heterogeneity in V(A)/Q measured by PET. J Appl Physiol 82: 1163–1176, 1997. [DOI] [PubMed] [Google Scholar]
- 63. Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005. [DOI] [PubMed] [Google Scholar]
- 64. West JB. Regional differences in gas exchange in the lung of erect man. J Appl Physiol 17: 893–898, 1962. [DOI] [PubMed] [Google Scholar]
- 65. West JB, Dollery CT. Distribution of blood flow and ventilation-perfusion ratio in the lung, measured with radioactive carbon dioxide. J Appl Physiol 15: 405–410, 1960. [DOI] [PubMed] [Google Scholar]
- 66. Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol 107: 1258–1265, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Young I, Mazzone RW, Wagner PD. Identification of functional lung unit in the dog by graded vascular embolization. J Appl Physiol 49: 132–141, 1980. [DOI] [PubMed] [Google Scholar]
- 68. Yu J, Law M, Kadlecek S, Emami K, Ishii M, Stephen M, Woodburn JM, Vahdat V, Rizi RR. Simultaneous measurement of pulmonary partial pressure of oxygen and apparent diffusion coefficient by hyperpolarized 3He MRI. Magn Reson Med 61: 1015–1021, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


