The two major findings are as follows: 1) during unilateral dialysis of 50 mM atropine into the ventral respiratory column to block excitatory muscarinic receptor activity, a compensatory increase in other neuromodulators was state independent, but the ventilatory response appears to be state dependent; and 2) the hypothesis that absence of decreased V̇i and f during unilateral dialysis of excitatory receptor antagonists was due to compensation by the contralateral VRC was not supported by findings herein.
Keywords: breathing, neuromodulation, pre-Bötzinger complex, ventral respiratory column, sleep
Abstract
Unilateral dialysis of the broad-spectrum muscarinic receptor antagonist atropine (50 mM) into the ventral respiratory column [(VRC) including the pre-Bötzinger complex region] of awake goats increased pulmonary ventilation (V̇i) and breathing frequency (f), conceivably due to local compensatory increases in serotonin (5-HT) and substance P (SP) measured in effluent mock cerebral spinal fluid (mCSF). In contrast, unilateral dialysis of a triple cocktail of antagonists to muscarinic (atropine; 5 mM), neurokinin-1, and 5-HT receptors does not alter V̇i or f, but increases local SP. Herein, we tested hypotheses that 1) local compensatory 5-HT and SP responses to 50 mM atropine dialyzed into the VRC of goats will not differ between anesthetized and awake states; and 2) bilateral dialysis of the triple cocktail of antagonists into the VRC of awake goats will not alter V̇i or f, but will increase local excitatory neuromodulators. Through microtubules implanted into the VRC of goats, probes were inserted to dialyze mCSF alone (time control), 50 mM atropine, or the triple cocktail of antagonists. We found 1) equivalent increases in local 5-HT and SP with 50 mM atropine dialysis during wakefulness compared with isoflurane anesthesia, but V̇i and f only increased while awake; and 2) dialyses of the triple cocktail of antagonists increased V̇i, f, 5-HT, and SP (<0.05) during both day and night studies. We conclude that the mechanisms governing local neuromodulator levels are state independent, and that bilateral excitatory receptor blockade elicits an increase in breathing, presumably due to a local, (over)compensatory neuromodulator response.
NEW & NOTEWORTHY The two major findings are as follows: 1) during unilateral dialysis of 50 mM atropine into the ventral respiratory column to block excitatory muscarinic receptor activity, a compensatory increase in other neuromodulators was state independent, but the ventilatory response appears to be state dependent; and 2) the hypothesis that absence of decreased V̇i and f during unilateral dialysis of excitatory receptor antagonists was due to compensation by the contralateral VRC was not supported by findings herein.
although it is well accepted that all neuronal networks are highly modulated by excitatory and inhibitory neurochemicals, the control of neuromodulators is not well understood. Mechanisms have been postulated to prevent excesses and deficits in excitatory or inhibitory neuromodulation (17). Such mechanisms are critical for stabilizing a network against the multiple neuromodulators acting within a given neuronal network. Dysfunction of these mechanisms may underlie or contribute to diseases such as Parkinson’s disease, epilepsy, schizophrenia, and depression; thus there is a need for studies on control of neuromodulators.
Insights into control of neuromodulators have been obtained by studies on neuromodulation of respiratory control networks. For example, acetylcholine (3, 4, 6, 20), substance P (SP) (13, 22, 23), and serotonin (5-HT) (23, 25, 29) are generally excitatory to breathing and are important excitatory neuromodulators of the pre-Bötzinger complex (preBötC), which is critical for respiratory rhythm generation (1, 26, 27). In rhythmic brain stem slice preparations, Doi and Ramirez (10, 11) found that administration of an SP receptor [neurokinin-1 (NK-1); RP67580] antagonist reduced fictive respiratory frequency, but the same antagonist did not alter spontaneous breathing when injected into the preBötC in young, urethane-anesthetized mice. However, if the NK-1 receptor antagonist was injected concurrently with antagonists of α1-noradrenergic and 5-HT2A receptors, spontaneous respiratory frequency was reduced and irregularity increased. These findings support the concept of neuromodulatory interdependence, whereby changes in one neuromodulator are compensated by local changes in other neuromodulator(s) to maintain normal neuronal network function and/or breathing (10, 11).
Further support for a local neuromodulatory compensation within the neural respiratory network was provided by Muere et al. (20–23), who found that unilateral microdialysis of antagonists to muscarinic, NK-1, or 5-HT2A receptors, individually or in combination, into the ventral respiratory column (VRC; near the preBötC) of awake goats did not alter pulmonary ventilation (V̇i) and breathing frequency (f). However, the investigators also found significant changes in the levels of excitatory and/or inhibitory neuromodulators in the effluent dialysate on the ipsilateral (but not contralateral) side of antagonist dialysis, which could have compensated for the presumed decrease in local cellular activity via the reduced activity of muscarinic, NK-1, and 5-HT2A receptors (20–23). These data suggest that there are mechanisms within the network that monitor the activation of receptors and quickly compensate with an increased release of neuromodulators when receptor activity has been decreased. Such a mechanism is very specific and is different from those proposed by Marder (17) to prevent deficits and excess in neuromodulation. However, either through anatomic connections or other undetectable reflex mechanisms, the contralateral VRC could also be contributing to compensation and maintenance of V̇i and f during excitatory receptor blockade. Accordingly, one major goal of the present study was to determine if bilateral dialysis into the VRC of antagonists to excitatory neuromodulator receptors also does not alter V̇i and f. Consistent with the concept of neuromodulatory interdependence, we hypothesized that bilateral dialysis of antagonists to muscarinic, NK-1, and 5-HT2A receptors will not alter V̇i and f, but would result in compensatory changes in neuromodulators.
Simultaneous administration into the preBötC of multiple excitatory receptor antagonists decreased respiratory activity in in vitro and in vivo anesthetized rodent preparations (11), but simultaneous dialysis into this region of multiple antagonists did not decrease V̇i and f in awake and sleeping goats (23). This difference could be due to a state dependence of compensatory increases in neuromodulators with receptor blockade, whereby anesthesia may alter the fundamental mechanisms governing local neurochemical release. Accordingly, a second goal of the present study was to test the hypothesis that, during unilateral dialysis of 50 mM atropine into the VRC, the expected increases in SP and 5-HT measured in the effluent mock cerebrospinal fluid (mCSF) would not differ between anesthetized and awake states.
METHODS
Animals.
Studies were on nine nonpregnant female adult goats (48 ± 7.9 kg). The goats were housed and studied in an environmental chamber with ambient temperature and a controlled light-dark cycle (lights on 6:00 AM to 6:00 PM). With the exception of a 24-h fasting period before surgery and during study protocols, food and water were otherwise freely available. The goats were acclimated to required study instrumentation [mask, valve, electroencephalogram (EEG)/electrooculogram (EOG) cables] and to standing and/or lying in the sternal position in a stanchion before the initiation of studies. All animal care and study protocols were approved by the Medical College of Wisconsin’s Institutional Animal Care and Use Committee before studies were completed.
Experimental design.
After arrival, goats were allowed to acclimate for at least 72 h before two surgical procedures, each separated by 2 wk. After 2 wk of recovery from the second surgery, the goats were studied in the awake, sleep, and anesthetized state for 4-6 wk thereafter. At the completion of the protocol, goats were euthanized and the brain tissue was extracted for histological confirmation of microtubule (MT) placement.
Surgical procedures.
Before beginning surgery, goats were anesthetized with ketamine [intravenous (IV)], intubated, and then mechanically ventilated with isoflurane in 100% oxygen for the duration of the surgery. During and for 24 h after surgery, rectal body temperature (TR), oxygen saturation, and heart rate (HR) were continuously monitored. Postoperative monitoring occurred at scheduled intervals for 2 wk following surgeries. For pain control, goats were given flunixin meglumine (2 mg/kg) before surgery and once daily for 2 days thereafter. To minimize the risk of infection following surgery, triple antibiotic ointment was applied to all surgical sites daily for 1 wk. Additionally, ceftriaxone (25 mg/kg iv) was administered twice daily for 3 days, starting the morning of surgery for craniotomy procedures. The antibiotics ceftiofur sodium (Naxcel, 4 mg/kg im) and gentamicin (6 mg/kg im) were administered chronically once daily for the remainder of the protocol. To minimize cerebral edema following the craniotomy procedure, dexamethasone (4 mg/ml) was administered three times a day for the first 3 days following surgery, then tapered off to twice daily for 1 wk after surgery.
In the initial surgery, EEG and EOG electrodes were implanted in the midline cranium and superior orbital ridge, respectively. The electrodes were implanted using steel screws and secured with dental acrylic and were used to record brain activity to score sleep state during night studies. Additionally, during the same procedure, a carotid artery was relocated and secured just beneath the skin. This procedure facilitated subsequent chronic catheterization of the artery for recording blood pressure (BP), HR, and sampling arterial blood for gas analysis.
After at least 2 wk of recovery, the second surgery was performed to chronically implant unilaterally (n = 2) or bilaterally (n = 7) stainless steel MTs (70.0 mm length, 1.27 mm outer diameter, 0.84 mm inner diameter) into the VRC, including the preBötC region. After the occipital craniotomy, an incision was made in the dura mater to visualize the brain stem and cerebellum. Once obex was visualized, a stereotaxic device and predetermined coordinates (2.5–3.5 mm rostral to obex, 4.0–5.0 mm lateral to midline, and 4.0–6.0 mm from the dorsal surface of the medulla) were utilized to place the MT(s) into the preBötC region of the VRC. These coordinates were based on previous studies in goats (15, 33). Occasionally, placement had to be slightly altered to avoid blood vessels. To secure the MT(s), screws were placed in the cranium, and the craniotomy was then filled with dental acrylic. The incision was closed with suture, and stainless steel stylets were inserted into the MT(s), such that they did not penetrate the tissue. Triple antibiotic ointment and bandages were applied to all surgical sites. Following surgery, the goats were monitored for at least 24 h by experienced laboratory staff.
Physiological data collection.
Following the second surgery, the goats were allowed a minimum of 2 wk to recover before the initiation of studies. During this time, the goats were accustomed to the study apparatus and protocol. To measure ventilatory data, a custom-made mask was specially fitted to each animal and secured to their muzzles during studies. A two-way valve was inserted into the end of the mask, and tubing was attached to inspiratory and expiratory sides of the valve. The inspiratory tubing was attached to a pneumotachograph connected to a Windaq data recording system and used to obtain breath-by-breath V̇i (l/min), f (breaths/min), tidal volume (Vt, l/breath), inspiratory (Ti) and expiratory (Te) time (s), and inspiratory drive (Vt/Ti). During daytime studies, the expiratory tubing was attached to a Tissot spirometer. The expired air was collected at 5-min intervals and analyzed for O2 and CO2 concentration for calculation of O2 consumption. Additionally, at least every 30 min of each protocol, arterial blood was withdrawn from a catheterized carotid artery for analysis of pH, Pco2, and Po2. The arterial catheter line was attached to a transducer to obtain BP and HR using the Windaq data analysis system. To determine and score sleep state during night studies, signals from EEG and EOG electrodes were recorded and later scored always by the same investigator. Finally, TR was recorded throughout the studies herein.
Glutamate receptor agonist injection studies.
Increased f has been observed following injection of a glutamate receptor agonist into the region of the preBötC region of the VRC (20–23). Accordingly, as in prior studies, injections of N-methyl-d-aspartic acid (NMDA) were used as a physiological indicator of the proximity of MT(s) placement to the preBötC target area. During these studies in the awake state, either 500 nl of mCSF or 500 nl of NMDA (100 mM) were injected into the MTs, separated by at least 30 min. Ventilatory data were measured as described above.
Microdialysis studies.
Microdialysis day studies were completed between 9 AM and 2 PM, and night studies were between 8 PM and 2 AM All studies were separated by a minimum of 36 h. Microdialysis probes (HSP Harvard Apparatus) were 72 mm in length, with the final 2 mm being a porous membrane (0.5-mm membrane diameter, 2-kDa cut off, 3-μl internal volume). Only the membrane portion of the probe penetrated the tissue when inserted into the MTs. Dialysis perfusate consisted of mCSF (124 mM NaCl, 2.0 mM KCl, 2.0 mM MgCl2, 1.3 mM K2PO4, 2.0 mM CaCl2, 11 mM glucose, 26 mM , and pH 7.32 in sterile water), with or without a dissolved drug. Before dialysis, the mCSF was equilibrated with 6.4% CO2, 12% O2, balance N2 and heated to 39°C utilizing a tonometer. For drug dialysis studies, either 50 mM atropine (Sigma, a0257) or a triple cocktail of 500 µM spantide (US Biological, S5370), 5 mM atropine, and 0.5 mM MDL 11939 (Tocris, 0780) were dissolved in the mCSF. To completely dissolve the triple cocktail, a final concentration of 1.25% DMSO was required. To minimize behavioral responses of the goats during awake and sleep studies, the syringe pump (Harvard Apparatus) utilized for delivery of the solutions (flow rate: 25 μl/min) was located outside the study chamber. A 150- to 180-cm length of polypropylene tubing was used to connect the syringe with the dialysis probe; thus there was a delay of ~20 min between switching the pump on and dialysate reaching the probe. Following probe insertion, there was a 30-min acclimation period, followed by a 30-min control period and then 3 continuous hours of dialysis. During the first and third hours, only mCSF was dialyzed. During the second hour, the dialyzed solution was either 1) mCSF (day awake), 2) 50 mM atropine in mCSF (day awake and day anesthetized), or 3) triple cocktail in mCSF [day awake and night awake or in non-rapid-eye-movement sleep (NREM) sleep]. Effluent dialysate was collected in separate cryovials for each hour, aliquoted, and frozen for subsequent neurochemical analysis.
For dialysis studies conducted under anesthesia, goats were anesthetized with ketaject (IV) and intubated. Anesthesia was maintained using a mechanical ventilator providing isoflurane in oxygen. In four of seven goats, the level of anesthesia was adjusted to prevent spontaneous breathing, and mechanical ventilation was adjusted accordingly to maintain arterial blood gases at control levels. In the other three goats, the anesthesia was adjusted to prevent movement, but breathing and arterial blood gases were not controlled. A custom-made apparatus was attached to the endotracheal tube to allow recording of breathing via a Windaq data recording system. Arterial blood was sampled at regular intervals. The dialysis protocol consisted of 1 h of mCSF dialysis, followed by 1 h of dialysis of 50 mM atropine, and then a third hour of mCSF alone. HR and BP were continuously monitored, and TR was recorded every 30 min. At the conclusion of the third hour of dialysis, the goats were allowed to recover from anesthesia and were supervised until fully alert and were then extubated.
Neurochemical analysis.
Effluent dialysate samples were submitted to an internal institution core laboratory facility for analysis of neuromodulator content, as cited by previous studies (20–23). To measure levels of glycine (Gly) and γ-aminobutyric acid (GABA) reverse-phase liquid chromatography was used with the following parameters: Waters Resolve C18 column (150 × 3.9) with a fluorescent detector that was excited at 229 nm and emits at 470 nm. The detector used a β-alanine standard and o-phthaldialdehyde derivatization. An identical column was used for the electrochemical detection of 5-HT; however, the potential was set at 0.6 V vs. an Ag/AgCl reference electrode using an N-methyl serotonin internal standard. To measure SP levels, a commercially available immunoassay was used (Assay Designs 900–018, range 9.76–10,000 pg/ml) with a microplate reader at 405 nm.
Data and statistical analysis.
At the conclusion of each study, inspiratory flow and BP were calibrated. Subsequent breath-by-breath analysis was performed for V̇i, f, Vt, Ti, Te, and Vt/Ti. Oxygen consumption was calculated at 5-min intervals from measured expired volume and expired O2 and CO2 concentrations. EEG and EOG signals recorded during night studies were used to determine sleep state for each breath. A single experienced investigator visually scored each breath as occurring during the awake, rapid eye movement (REM) sleep or NREM sleep state of consciousness. There was insufficient REM sleep for inclusion in statistical analyses. In all statistical analyses (detailed below), a P value < 0.05 was considered significant (Sigmaplot version 12.5 or greater).
For day studies, the breath-by-breath data were compiled into 5- and 15-min bins, which were used for statistical analyses. The coefficient of variation (CV) in breath-by-breath V̇i, f, and Vt was computed for 5- and 15-min bins. To limit statistical analyses to stable conditions, we included only data in the last 15 min of hour 1 (control dialysis), the final 45 min of hour 2 (drug dialysis), and all but the first 5 min of hour 3 (washout). For all ventilatory variables, two-way repeated-measures ANOVAs were used to compare triple cocktail and atropine dialyses to mCSF dialysis (one-factor repetition, with drug and time as factors). The Holm-Sidak post hoc test was used when appropriate. These statistics were completed both for the absolute ventilatory values and also after normalizing the second and third hour data to the last 15 min of hour 1 (%hour 1). The level of statistical significance was the same for absolute and normalized data and for 5- and 15-min bins; thus only the normalized 5-min bins are included in the results section for the day studies.
For night studies, only 15-min bins were used for statistical analyses because the intermittent sleep pattern resulted in low numbers for 5-min bins. The CV in breath-by-breath V̇i, f, and Vt was computed for 15-min bins. A two-way repeated-measures ANOVA with state and time (bin) as factors was used to determine whether there were significant differences between awake and NREM sleep states in the ventilatory responses to the triple cocktail dialysis. Additionally, a two-way repeated-measures ANOVA was used to compare the effect of bilateral triple cocktail dialysis during the day to the awake state at night (15-min bins). Finally, a one-way repeated-measures ANOVA was used to determine if there were significant ventilatory effects during the night awake or NREM sleep state during triple cocktail dialysis.
Two determine whether changes in 5-HT, SP, Gly, GABA, and glutamate during dialyses of receptor antagonists differed between anesthetized and awake states or between day and night studies, a two-way ANOVA was used. All significant P values from ANOVA analyses were followed by Holm-Sodak post hoc analysis.
Histological confirmation of MT placement.
After the completion of the study protocol, the goats were euthanized for tissue perfusion, fixation, and collection. Goats were first anesthetized with ketamine-xylazine (IV), one carotid artery was cannulated and the other ligated, and jugular veins were isolated. The goats were then euthanized (β-euthanasia, IV), the jugular veins opened, and the carotid artery was perfused with phosphate-buffered saline (PBS; volume 4–6 liters), followed by an equal volume of 4% paraformaldehyde in PBS. The brain stems were extracted, dehydrated (20% and 30% sucrose in PBS for 3–7 days each), and frozen (−80°C). The brain stems were serially sectioned at 25 µM in the transverse plane utilizing a microtome. One series of the sectioned brain stem tissue was then Nissl (cresyl violet) stained. Sections were imaged (4,000 dpi; Nikon Super Coolscan 9000) to determine the extent and location that the MTs penetrated the tissue. Metamorph image analysis software was then used to calibrate the images and measure the location of the MT(s) in millimeters.
RESULTS
Placement of MTs.
The VRC in goats extends several millimeters along the ventrolateral aspect of the medulla (Fig. 1A). Within the VRC, the preBötC in goats is ~2.5–3.5 mm rostral to obex, 4−5 mm lateral to the midline, and 4–6 mm from the dorsal surface of the medulla (16, 33). Based on postmortem histological analyses, 8 of 16 MTs (in 9 goats) were placed such that the ventral-most aspect of the MT placement (indicated by numbers in Fig. 1A), or a conservative estimated region of diffusion of the dialyzed substances (gray boxes; Fig. 1A) reached the VRC. As in past studies, we also used the f response to the glutamate receptor agonist NMDA as a functional marker of proximity to the preBötC. We found that, in 11 of 14 MT placements, injection of NMDA increased f to at least 115% of control, providing functional evidence of relative proximity to the preBötC (Fig. 1B). However, goats 2 and 3 had minimal or no response to NMDA, and the MTs were located in the most rostral region of the VRC. Because of some variation in the responses to NMDA and the corresponding histologically identified MT placement, and because it is very likely that the diffusion of administered substances with microdialysis were not restricted to the targeted regions (preBötC), we present and interpret our findings as effects within the VRC. However, the physiological responses to dialyses of 50 mM atropine and the triple antagonist cocktail were not different across all goats; thus all goats were included in group data.
Fig. 1.
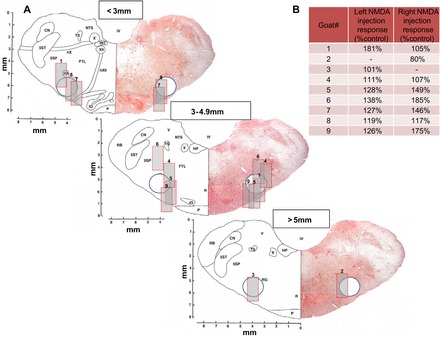
A: sections of the goat brain stem at three caudal to rostral distances from obex, depicting the microtubule placement in nine goats. Each goat is numbered (1–9), and the nos. for the left and right microtubule are located in the images, where the distal end of the microtubule was identified by postmortem histological analysis. The 0.5-mm-wide porous membrane of the dialysis probe extended 2 mm beyond the distal end of the microtubule; thus we inserted a 2-mm long and 1-mm wide gray rectangle for each number to represent a conservative estimate of the region of diffusion of the dialyzed substances. The circle in each section is the approximate VRC in goats rostral to obex. B: quantitation of the breathing frequency response to NMDA injections displayed as percent increase from control levels before injection. Note goats 2 and 3 had unilaterally placed microtubules rostral to the preBötC, and these goats had the lowest response to NMDA injections. Note also that the left-side microtubule placement in goat 6 was the only case in which the rectangle did not extend to the estimated location of the VRC, which suggests widespread diffusion of NMDA in particular, but also dialyzed substances. CN, cuneate nucleus; FTL, lateral tegmental field; HP, nucleus praepositus hypoglossi; INT, nucleus intercalatus; IO, inferior olivary complex; IV, trochlear nucleus; NA, nucleus ambiguus; NTS, nucleus tractus solitarii; nX, vagus nerve; nXII, hypoglossal nerve; P, pyramidal tract; R, raphe nucleus; RB, restiform body; RG, nucleus gigantocellularis reticularis; 5SP, spinal trigeminal nucleus; 5ST, spinal trigeminal tract; TS, tractus solitaries; V, vestibular nucleus; X, dorsal motor nucleus of the vagus; XII, hypoglossal nucleus.
Bilateral microdialysis of antagonists to multiple excitatory receptors.
Our laboratory has shown previously that unilateral dialysis of the triple cocktail of antagonists to muscarinic, NK-1, and 5-HT2A receptors (5 mM atropine, 500 µM spantide, and 0.5 mM MDL in 1.25% DMSO; referred to as “triple cocktail” hereafter) during the day has no effect on V̇i and f, but significantly alters local neurochemicals (23). Here, bilateral dialysis of the triple cocktail increased V̇i (Fig. 2A, P < 0.001) and f (Fig. 2B, P < 0.001), but did not alter Vt (Fig. 2C, P = 0.567) compared with dialysis of mCSF alone (n = 7), which was the case for all raw data and the hour 1-normalized data. The increased f with bilateral triple cocktail was due to decreased Ti (P = 0.001, Fig. 3A) and Te (P < 0.001, Fig. 3B), and there also was an increased drive to breath (as measured by Vt/Ti; P < 0.001, Fig. 3C). Bilateral triple cocktail dialysis did not significantly (P ≥ 0.422) alter variability of V̇i, f, or Vt during the day, which was measured by CV in either 5-min or 30-min intervals (data not shown). Oxygen consumption (P = 0.022) and body temperature (P = 0.02, Fig. 4) were significantly increased during dialysis of the triple cocktail of receptor antagonists. BP, HR, and blood arterial Pco2 (), and arterial Po2 were not significantly (P ≥ 0.095) affected by bilateral daytime triple cocktail dialysis (data not shown).
Fig. 2.
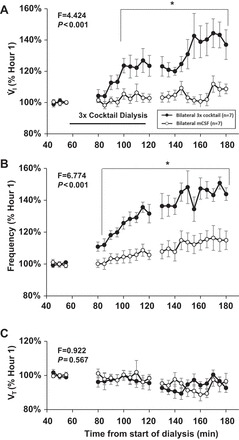
During 180 min of bilateral dialysis during the day, the triple cocktail of antagonists to muscarinic, NK-1, and 5-HT2A receptors (5.0 mM atropine, 500 µM spantide, 0.5 mM MDL) (●; n = 7) dialyzed between 60 and 120 min (indicated by horizontal solid line) significantly (P < 0.001) increased ventilation (V̇i; A) and breathing frequency (f; B), but did not alter (P = 0.567) tidal volume (Vt; C) compared with bilateral dialysis of mCSF alone (○, n = 7). Values are means ± SE. The F and P values shown are the interaction terms obtained from two-way repeated-measures ANOVA using time and dose as factors. *Values during and after triple cocktail dialysis that differed from hour 1 mCSF dialyses, as indicated by Holm-Sidak post hoc analysis.
Fig. 3.
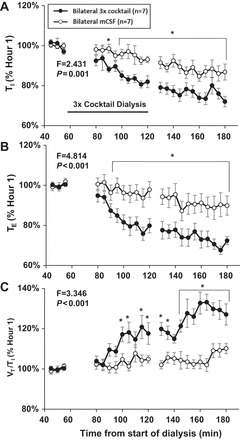
During 180 min of bilateral dialysis during the day, the triple cocktail of antagonists to muscarinic, NK-1, and 5-HT2A receptors (5.0 mM atropine, 500 µM spantide, 0.5 mM MDL) (●; n = 7) dialyzed between 60 and 120 min (indicated by horizontal solid line) significantly (P < 0.001) decreased inspiratory time (Ti; A) and expiratory time (Te; B) and increased respiratory drive (Vt/ Ti; C) during hours 2 and 3 compared with bilateral dialysis of mCSF alone (○, n = 7). Values are means ± SE. A two-way repeated-measures ANOVA using time and dose as factors was used to obtain the F and P values. *Values during and after triple cocktail dialysis that differed from hour 1 mCSF dialyses, as indicated by Holm-Sidak post hoc analysis.
Fig. 4.
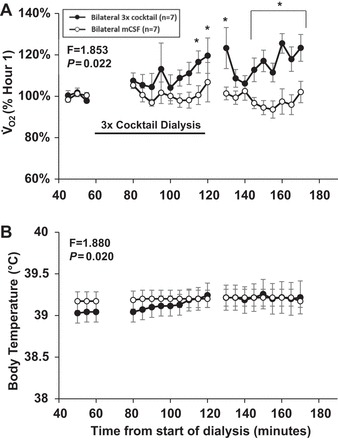
Daytime bilateral dialysis of a triple cocktail of antagonists to muscarinic, NK1, and 5-HT2A receptors (5.0 mM atropine, 500 µM spantide, 0.5 mM MDL) (●; n = 7) dialyzed between 60 and 120 min (indicated by horizontal solid line) significantly increased oxygen consumption (V̇o2, P = 0.022; A) and body temperature (P = 0.02; B) compared with bilateral dialysis of mCSF alone (○, n = 7). Values are means ± SE. A two-way repeated-measures ANOVA (time and dose as factors) was used to determine if significant interactions occurred between treatments. *Values during and after triple cocktail dialysis that differed from hour 1 mCSF dialyses, as indicated by Holm-Sidak post hoc analysis.
Consistent with previous findings of changes in local neurochemicals (23), daytime bilateral triple cocktail led to local increases in SP and 5-HT (P ≤ 0.033) measured in the effluent mCSF, but Gly, GABA, and glutamate were not affected (P ≥ 0.379, Fig. 5). With one exception, there was no difference (P ≥ 0.109) in the levels of all measured neurochemicals in the effluent collected from the left or right MT, but there were small but significant differences in Gly levels (P = 0.005) between sides (data not shown).
Fig. 5.
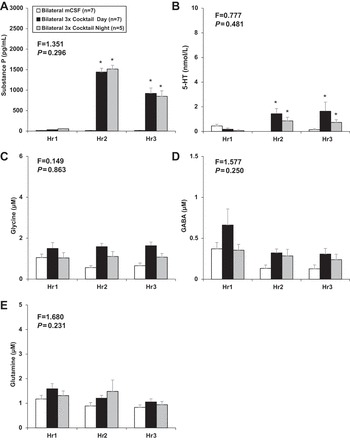
There were no significant differences between day and night studies in the measured neurochemical responses to the triple cocktail dialysis, as indicated by a two-way repeated-measures ANOVA using time and dose as factors (F and P values presented at the top left of each panel). Daytime (solid bars) and nighttime (shaded bars) bilateral dialysis of a triple cocktail of antagonists to muscarinic, NK-1, and 5-HT2A receptors (5.0 mM atropine, 500 µM spantide, 0.5 mM MDL) during hour 2 of dialysis significantly (P ≤ 0.033) increased levels of substance P (A) and 5-HT (B) in effluent mCSF during hour 2 and hour 3 of dialysis compared with dialysis of mCSF alone (open bars) (n = 7). C: glycine was significantly (P = 0.011) lower throughout the 3 h of mCSF dialysis compared with the 3 h when the triple cocktail was dialyzed in hour 2. GABA (D) and glutamine (E) were not significantly (P ≥ 0.379) altered by triple cocktail dialysis during the day or at night. Values are means ± SE. *Values during and after triple cocktail dialysis that differed from hour 1 mCSF dialyses, as indicated by Holm-Sidak post hoc analysis. Hr1–Hr3, hours 1–3.
During bilateral triple cocktail dialysis at night, there was no significant difference in V̇i (P = 0.129), f (P = 0.218), and Vt (P = 0.761) between awake and NREM sleep states, as indicated by the interaction term of two-way ANOVA (Fig. 6). Analysis of these data using one-way repeated-measures ANOVA (with time used as the factor) indicated there was an increase in f during both the awake (P = 0.002) and NREM states (P < 0.001), and an increase in V̇i (P = 0.007) during NREM sleep but not during the awake state. Vt was not significantly altered in either state at night.
Fig. 6.
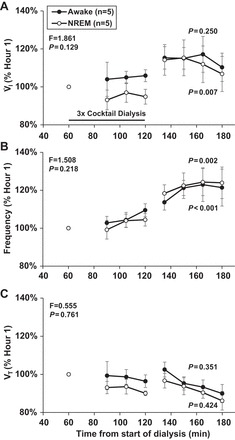
A two-way repeated-measures ANOVA analysis of the effects of bilateral triple cocktail dialysis indicates that the ventilation (V̇i; A), breathing frequency (f; B), and tidal volume (Vt; C) responses did not differ between awake (●) and NREM sleep (○) (F and P values, top left of each panel). B: bilateral dialysis of a triple cocktail of antagonists to muscarinic, NK1, and 5-HT2A receptors (5.0 mM atropine, 500 µM spantide, 0.5 mM MDL) (n = 5) (indicated by solid horizontal line) significantly (P ≤ 0.002) increased f at night during awake and NREM sleep states (one-way ANOVA). A: V̇i was significantly increased during NREM sleep (P = 0.007), but not in the night awake state. C: Vt was not significantly affected in either state. Values are means ± SE.
During the night studies, the CV for f (P = 0.038) and Vt (P = 0.04) within 15-min periods were greater for the awake state than for NREM sleep, as indicated by the interaction term of two-way ANOVA (Fig. 7). A one-way ANOVA indicated that, in the awake state at night, the triple cocktail significantly increased the CV for V̇i (P = 0.042), f (P = 0.032), and Vt (P = 0.049), whereas, for NREM sleep, the triple cocktail only significantly increased the CVs for V̇i (P = 0.039). The CVs for V̇i, f, and Vt for night awake and for night NREM sleep were significantly (P < 0.05) greater than for day awake (comparisons not shown).
Fig. 7.
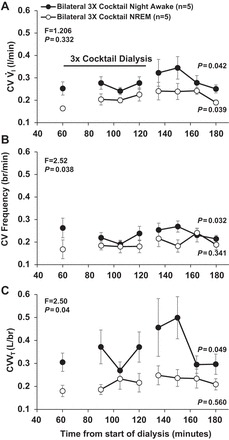
During the night studies, the coefficient of variation (CV) for f (P = 0.038; B) and Vt (P = 0.04; C) within 15-min periods was greater for the awake state (●) than for NREM slee (○), indicated by the interaction term of two-way ANOVA (F and P at top left). A: the CV of V̇i did not differ between awake and NREM sleep. A one-way ANOVA indicated that, in the awake state at night, the triple cocktail significantly increased the CV for V̇i, f, and Vt (P < 0.05), whereas for NREM sleep, the triple cocktail only significantly increased the CV for V̇i (P = 0.039) (P, right side). The horizontal solid line in A denotes that the triple cocktail was dialyzed between 60 and 120 min. Values are means ± SE.
For all neurochemicals, there was no significant difference between day and night studies in the responses to the triple cocktail, as indicated by F and P values (see top left of figure panels) from two-way ANOVA. Similar to daytime studies, bilateral dialysis of the triple cocktail of antagonists at night resulted in a significant (P ≤ 0.021) increase in SP and 5-HT (Fig. 5). During hour 1 of dialysis when mCSF was dialyzed, there were no significant differences between day and night studies.
Effects of unilateral dialysis of 50 mM atropine in awake and anesthetized goats.
As in prior studies, unilateral dialysis of 50 mM atropine into the VRC of awake goats significantly (P < 0.001) increased V̇i, and f, but did not affect Vt (P = 0.578) compared with dialysis of mCSF alone (n = 8) (Fig. 8). In awake goats, dialysis of 50 mM atropine significantly (P < 0.001) increased SP and 5-HT, but glutamine, Gly, and GABA were unchanged (P ≥ 0.052) compared with dialysis of mCSF alone (Fig. 9). Unilateral dialysis of 50 mM atropine in anesthetized animals (n = 7) also significantly (P < 0.001) increased 5-HT and SP in effluent mCSF during hours 2 and 3 of studies compared with dialysis of mCSF dialysis during the first hour. In contrast, GABA was decreased significantly (P = 0.011) during hour 2, and glutamine and Gly were unaffected (P ≥ 0.052, Fig. 9). During 50 mM atropine dialyses, there were no significant (P ≥ 0.123) differences in effluent mCSF 5-HT, SP, Gly, or GABA levels between awake or anesthetized state, but glutamine levels was significantly (P = 0.034) greater during anesthesia (Fig. 9).
Fig. 8.
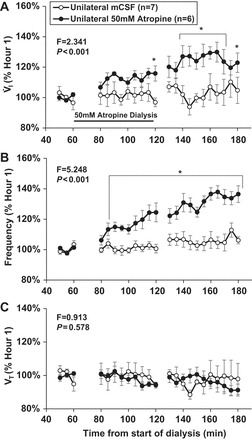
Unilateral dialysis of 50 mM atropine during the day (n = 6; ●) (indicated by horizontal solid line) significantly (P < 0.001) increased ventilation (V̇i; A) and breathing frequency (f; B), but did not significantly (P = 0.578) alter tidal volume (Vt; C) compared with mCSF time control studies (○). The F and P values are the interaction terms from two-way repeated-measures ANOVA using dose and time as factors. Values are means ± SE. *Values during and after atropine dialysis that differed from hour 1 mCSF dialyses, as indicated by Holm-Sidak post hoc analysis.
Fig. 9.
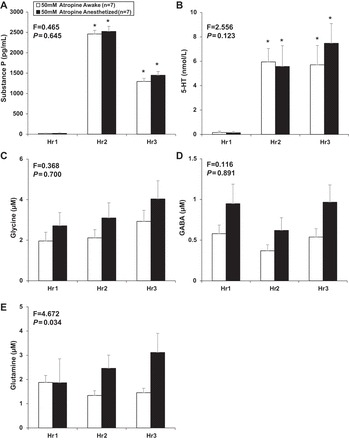
There were no statistically significant differences between the awake (open bars) and anesthetized (solid bars) state in substance P (A), 5-HT (B), glycine (C), or GABA (D) levels, as indicated by the F and P values from the interaction term (top left in each panel) of the two-way repeated-measures ANOVA. E: glutamine was significantly (P = 0.034) greater during anesthesia studies. Values are means ± SE. *For awake and anesthesia, unilateral dialysis of 50 mM atropine significantly (P < 0.001) increased substance P (A) and 5-HT (B) in effluent dialysate during both hours 2 and 3 (obtained from Holm-Sidak post hoc analysis).
In four of seven goats studied under anesthesia, the ventilator was set to maintain a constant within the range of control levels (Fig. 10). However, in three of the goats, we maintained a level of anesthesia in which they were immobilized but still able to breathe spontaneously. In two of these, we measured , which increased during dialysis (Fig. 10). Near the end of the atropine dialysis, both goats stopped breathing spontaneously. Therefore, mechanical ventilation was initiated; however, remained elevated.
Fig. 10.
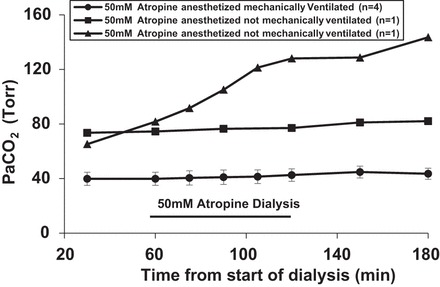
did not change during unilateral dialysis of 50 mM atropine in anesthetized goats that were mechanically ventilated (●). However, increased in goats under anesthesia that were not mechanically ventilated (▲ and ■). Values are means ± SE.
DISCUSSION
One aim of the present study was to test the hypothesis that combined bilateral dialysis of antagonists to muscarinic, NK-1, and 5-HT2A receptors into the VRC would not decrease V̇i and f, but would result in compensatory changes in neuromodulators in the effluent mCSF. This hypothesis was validated as bilateral dialysis of these receptor antagonists paradoxically increased V̇i and f with concomitant increases in local excitatory neuromodulators in the effluent mCSF. A second aim of the present study was to test the hypothesis that, during unilateral dialysis of 50 mM atropine, increases in SP and 5-HT in the effluent mCSF would not differ between anesthetized and awake states. This hypothesis was also validated as the increases in SP and 5-HT in the effluent mCSF did not differ between the awake and anesthetized states.
Neuromodulator compensation in awake and sleeping states.
Previous studies demonstrated that dialysis of atropine at one VRC site and simultaneously dialyzing mCSF at the contralateral VRC site, changes in neuromodulators measured in the effluent dialysate, only occurred at the site of atropine dialysis (20). These data suggested that the compensatory changes in neurochemicals in response to antagonist dialyses in the VRC were mediated by a local mechanism. However, it was unclear whether this local compensatory response was truly sufficient to overcome the presumptive local, unilateral decrease in cellular activity in the VRC in response to the antagonist. For example, the compensatory response to unilateral blockade of NK-1 receptors was a local increase in SP, which should have been an ineffective means for compensation if indeed NK-1 receptors are the primary/exclusive mediator of SP stimulation in this region (22, 23). Other potential contributing mechanisms to maintain V̇i and f could be through anatomical connections with the contralateral preBötC/VRC, or through reflex compensation independent of neuromodulator changes on the contralateral side, which is a possibility supported by previous findings. Wenninger et al. found that abrupt bilateral destruction of the preBötC in awake goats caused terminal apnea (33), whereas abrupt unilateral destruction of this region induced several hours of hypoventilation without apnea (32). Also St-Jacque and St-John (28) found in decerebrate, vagotomized rats that attenuation of preBötC activity with unilateral injections of muscimol (inhibitory GABA agonists) resulted in several minutes of intermittent apneas but no terminal apnea. These examples demonstrate that respiratory rhythm continues with unilateral silencing of the preBötC, presumably due to compensation by the contralateral preBötC and/or redundancy within the control network. Accordingly, if indeed contralateral changes prevented V̇i and f from decreasing during unilateral dialysis of receptor antagonists in our previous studies, then herein there should have been a decrease in V̇i and f during bilateral dialysis of the antagonists. However, if local neuromodulator compensation is a robust mechanism as we postulated, a decrease in V̇i and f during bilateral dialysis of multiple excitatory receptor antagonists should be prevented.
The above hypothesis was validated, as bilateral dialysis of multiple excitatory receptor antagonists did not decrease V̇i and f, but rather led to a paradoxical increase in breathing. The cause(s) of the increases in V̇i and f during bilateral triple cocktail dialysis of excitatory receptor antagonists into the VRC is not known. There may be one or more explanations, including, but not limited to, changes in metabolic rate and body temperature, alterations of neuromodulators not yet measured in the local milieu, and/or compensation at the level of second-messenger pathways downstream of G protein-coupled receptors. The contribution of the increases in metabolic rate and body temperature is unclear, as the hyperpnea during dialysis of 50 mM atropine (Fig. 8) was not associated with increases in metabolic rate and body temperature, suggesting that these changes in triple cocktail dialysis might not be significant factors. Other potential contributors are the compensatory increases in SP and 5-HT, which could act through excitatory receptors other than those blocked herein (NK-1 and 5-HT2A receptors), or alternatively there may have been an increase in other neuromodulators, which we have yet to measure in the local neurochemical milieu. An example may be neuropeptide gamma (derived from the same promolecule as SP), which activates NK-2 receptors and may have also been increased with the antagonist dialysis. In addition, the receptors targeted by our antagonist cocktail all couple to G proteins of the Gq/11 subtype, which are linked to other receptors known to modulate neuronal activity in the VRC, such as α1-adrenergic (34) and P2Y purinergic (12, 31) receptors. Thus another possibility that could account for the increase in V̇i and f with the triple antagonist cocktail is that compensation could occur in second-messenger systems downstream of common or additional G protein-coupled receptors, but we have no evidence to support this possibility. All three of the receptors we currently blocked converge on the same second-messenger system, the phospholipase C pathway; thus activity within this pathway may increase even though overall receptor activity decreased. Regardless of the cause, it is clear that bilateral blockade of three excitatory receptors resulted in what we consider an overcompensation, which also occurs with a high concentration of a single receptor antagonist (i.e., 50 mM atropine; Fig. 9).
Marder and colleagues (17, 18) have previously identified several potential mechanisms that could protect neural networks against overmodulation, which include the following: 1) a convergence of many modulators onto the same voltage-dependent current or on the same G protein-coupled receptor and/or G protein; 2) modulators coordinately acting on opposing cellular processes, 3) saturation of postsynaptic actions, and 4) compensation downstream of receptors by second-messenger signal transduction pathways. Despite such potentially protective mechanisms, our findings indicate that overmodulation can occur under physiological conditions, suggesting that there are limits in the mechanisms of compensatory neuromodulation and/or regulation of neuromodulators.
Increases in metabolic rate and body temperature suggest there was a general excitatory physiological response to the dialysis of the triple cocktail of receptor antagonists herein. These findings are consistent with findings during triple cocktail dialysis during night studies when there was unstable breathing (Fig. 7), large variation in breathing between goats, absence of a difference in breathing between awake and NREM states (Fig. 6), and a relative low amount of NREM sleep in some of the goats. Indeed, two of seven goats (not included in the group data) remained awake throughout the 3-h dialysis protocol, and they had a large drug-induced increase in breathing. These findings are similar to effects observed in newborn piglets when Brown et al. (7) dialyzed 8-OH-DPAT into the medullary raphe, which resulted in fragmented sleep with increases in the number and a decrease in the duration of bouts of NREM sleep and a marked decrease in amount of REM sleep. It seems conceivable that an overcompensation of neuromodulators may have prevented the normal change in neuromodulators that occurs with changes in state, such as sleep (4, 14). Indeed, unstable breathing occurs in transitions from wakefulness to NREM sleep (9) during which there are major changes in excitatory neuromodulators. Dialysis of the triple cocktail of receptor antagonists may have created a type of “sleep disordered breathing,” or at least a lack of state transition and the subsequent associated changes in breathing. It seems clear that findings herein show that perturbations of neuromodulators at night during the normal sleep period have a major disruptive effect on sleep and on breathing during sleep.
Effects of anesthesia on compensatory neuromodulation.
It is well known that perturbations of the respiratory control network in anesthetized and/or reduced preparations have greater effects on breathing than perturbations in the awake state (10, 11). Doi and Rameriz (11) found that simultaneously blocking two excitatory receptors in anesthetized rodents decreased respiratory activity. In contrast, we found that simultaneously blocking three excitatory receptors did not decrease V̇i and f (23). One possible explanation for this difference among studies is that neuromodulatory compensation does not occur in the anesthetized state. This possibility led us to compare the neuromodulatory response to dialysis of 50 mM atropine in the anesthetized and awake states in the same goats. The major compensation in neurochemicals (5-HT and SP) in response to 50 mM atropine did not differ between awake and anesthetized states, suggesting that the mechanism of local neurochemical compensation is the same in anesthetized and awake goats. Previously, our laboratory (20) showed that the neurochemical compensation to 50 mM atropine during the day does not differ from compensation at night, even though there is a mixture of awake and NREM sleep states at night. Thus it appears that the fundamental mechanism(s) that facilitates neuromodulatory compensation occurs even when there are major, presumptive changes in the modulatory state between wakefulness and NREM sleep, and between wakefulness and anesthesia. Brain disorders, such as schizophrenia (5, 8), depression, and epilepsy (2, 24), and other disorders like Rett and Prader-Willi syndromes (30) that are marked or defined by altered neuromodulators are likely associated with some degree of dysfunction in modulatory control systems. Thus a question that needs to be addressed is: does neuromodulator compensation occur normally in these disease states, or are these mechanisms aberrantly contributing to the dysfunction? Would neurochemical compensation occur normally after other experimental forms of respiratory-related dysfunction, such as carotid body denervation, which results in a major downregulation of excitatory neuromodulators, such as 5-HT (19)? These questions emphasize the need for future studies on mechanisms/determinants of the modulatory state of the brain, which our in vivo goat model is well suited to address.
In contrast to the local neuromodulatory response, the V̇i and f response to 50 mM atropine appears to be state dependent (20). Under anesthesia, the goats were unable to maintain spontaneous breathing, even when hypoventilation caused a severe hypercapnia (Fig. 10). This state-dependent effect likely reflects the fact that the compensatory increases in 5-HT and SP were local and could not counter the anesthetic depression of the entire respiratory network. Finally, there is a state-dependent effect of dialysis of 50 mM atropine on V̇i and f, which led to an overcompensation significantly greater in the awake state than during NREM sleep (20). This difference could be due to the state-dependent attenuation of excitatory drives for V̇i and f at multiple components of the respiratory control network.
Caveats and limitations.
The limitations of our laboratory's goat model have been previously published (20–23) and are summarized here. First, although the target for MT implantation was the preBötC, the exact boundaries of this nucleus are not definitively known (16, 33), and, therefore, we cannot conclusively state that MT placement was only in the preBötC. Based on histological data, we can conclude that all MTs were placed at locations whereby the microdialysis probes were within or directly adjacent to the medullary VRC and in the region of the preBötC. It also remains difficult to determine the exact radius of diffusion of the drugs dialyzed, leaving the possibility that the concentration of drug that reached the preBötC/VRC may have varied between studies and doses of the drugs. In addition, we cannot eliminate the possibility that the compensatory responses to drug dialysis occurred at sites other than the preBötC/VRC.
Another caveat of our dialysis protocol is that, in order to minimize disturbance of the goats during studies, we keep the dialysis pump outside of the animal chamber, which necessitates having dead space in a length of tubing (~150–180 cm) and a delay of ~15–20 min from the start of drug dialysis to when the drug reaches the probe tip. To correct for this delay, the first 15 min of hour 2 of dialysis were omitted from statistical analysis. Moreover, during the initial 15–20 min of hour 3, drug delivery to the tissue would continue as in hour 2. In addition, thereafter, washout of the drug from the tissue would be time dependent. As a result, the sustained hyperpnea in hour 3 (shown in Figs. 2, 6, and 7) likely reflects these drug delivery and washout characteristics. Finally, due to the nature of our effluent mCSF collection protocol, we were not able to separate neuromodulator content between sleep states, but rather have one single neurochemical value for each hour for both day and night studies.
Conclusions and significance.
The present findings do not support the hypothesis that absence of decreased breathing during unilateral dialysis of excitatory receptor antagonists is due to compensation by the contralateral preBötC/VRC. In addition, findings herein show that, during unilateral dialysis of 50 mM atropine, into the preBötC/VRC, to block excitatory muscarinic receptor activity, a compensatory increase in other neuromodulators is state independent, but the V̇i and f response appear to be state dependent. These data emphasize the need/value for testing hypotheses, formulated on the basis of data obtained during anesthetized/reduced preparations, under physiological wakefulness and sleep states. It seems clear that findings herein show that perturbations of neuromodulators at night during the normal sleep period have a major disruptive effect on sleep and on breathing during sleep. Finally, we emphasize that insights gained herein regarding control of neuromodulators affecting the respiratory control network have general application to neuromodulation of all neuronal networks and thus have relevance to multiple diseases such as depression, epilepsy, and schizophrenia.
GRANTS
This project was funded by National Heart, Lung, and Blood Institute Grants HL-25739, HL-112996, and HL-007852 and by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.M.L., M.R.H., L.G.P., and H.V.F. conceived and designed research; T.M.L., S.E.N., E.C., N.J.B., S.T., M.R.H., L.G.P., and H.V.F. performed experiments; T.M.L., S.E.N., E.C., N.J.B., S.T., M.R.H., and H.V.F. analyzed data; T.M.L., S.E.N., S.T., M.R.H., L.G.P., and H.V.F. interpreted results of experiments; T.M.L., S.E.N., and M.R.H. prepared figures; T.M.L. and H.V.F. drafted manuscript; T.M.L., S.E.N., M.R.H., and H.V.F. edited and revised manuscript; T.M.L., S.E.N., M.R.H., and H.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lisa Henderson, Jenifer Phillips, and Camille Torres for help with neurochemical quantifications.
REFERENCES
- 1.Achard P, Zanella S, Rodriguez R, Hilaire G. Perinatal maturation of the respiratory rhythm generator in mammals: from experimental results to computational simulation. Respir Physiol Neurobiol 149: 17–27, 2005. doi: 10.1016/j.resp.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Al-Otaibi FA, Hamani C, Lozano AM. Neuromodulation in epilepsy. Neurosurgery 69: 957–979, 2011. doi: 10.1227/NEU.0b013e31822b30cd. [DOI] [PubMed] [Google Scholar]
- 3.Bellingham MC, Funk GD. Cholinergic modulation of respiratory brain-stem neurons and its function in sleep-wake state determination. Clin Exp Pharmacol Physiol 27: 132–137, 2000. doi: 10.1046/j.1440-1681.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 4.Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 131: 135–144, 2002. doi: 10.1016/S1569-9048(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 5.Bencherif M, Stachowiak MK, Kucinski AJ, Lippiello PM. Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med Hypotheses 78: 594–600, 2012. doi: 10.1016/j.mehy.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol (1985) 109: 159–170, 2010. doi: 10.1152/japplphysiol.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphé disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol 294: R884–R894, 2008. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- 8.Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry 165: 1594–1603, 2008. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey JA, Harms CA, Morgan BJ, Badr MS, Skatrud JB. Sleep effects on breathing and breathing stability, in The Lung: Scientific Foundations. Philadelphia: Lippincott-Raven, 1997, Chapt. 156, p. 2063–2073. [Google Scholar]
- 10.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal 1: 789–803, 2012. doi: 10.1002/wmts.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189: 55–58, 1975. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 15.Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol (1985) 106: 605–619, 2009. doi: 10.1152/japplphysiol.90966.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. Micro-opioid receptor agonist injections into the presumed pre-Botzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol (1985) 107: 1591–1599, 2009. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7: 563–574, 2006. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 19.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Hodges MR, Forster HV. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J Appl Physiol (1985) 115: 1088–1098, 2013. doi: 10.1152/japplphysiol.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Botzinger Complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol (1985) 114: 694–704, 2013. doi: 10.1152/japplphysiol.00634.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Evidence for respiratory neuromodulator interdependence after cholinergic disruption in the ventral respiratory column. Respir Physiol Neurobiol 205: 7–15, 2015. doi: 10.1016/j.resp.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muere C, Neumueller S, Olesiak S, Miller J, Hodges MR, Pan L, Forster HV. Blockade of neurokinin-1 receptors in the ventral respiratory column does not affect breathing but alters neurochemical release. J Appl Physiol (1985) 118: 732–741, 2015. doi: 10.1152/japplphysiol.00884.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muere C, Neumueller S, Olesiak S, Miller J, Langer T, Hodges MR, Pan L, Forster HV. Combined unilateral blockade of cholinergic, peptidergic, and serotonergic receptors in the ventral respiratory column does not affect breathing in awake or sleeping goats. J Appl Physiol (1985) 119: 308–320, 2015. doi: 10.1152/japplphysiol.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nune G, DeGiorgio C, Heck C. Neuromodulation in the Treatment of Epilepsy. Curr Treat Options Neurol 17: 375, 2015. doi: 10.1007/s11940-015-0375-0. [DOI] [PubMed] [Google Scholar]
- 25.Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Jacques R, St-John WM. Transient, reversible apnoea following ablation of the pre-Bötzinger complex in rats. J Physiol 520: 303–314, 1999. doi: 10.1111/j.1469-7793.1999.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tryba AK, Peña F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viemari JC, Maussion G, Bévengut M, Burnet H, Pequignot JM, Népote V, Pachnis V, Simonneau M, Hilaire G. Ret deficiency in mice impairs the development of A5 and A6 neurons and the functional maturation of the respiratory rhythm. Eur J Neurosci 22: 2403–2412, 2005. doi: 10.1111/j.1460-9568.2005.04441.x. [DOI] [PubMed] [Google Scholar]
- 31.Weisman GA, Woods LT, Erb L, Seye CI. P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets 11: 722–738, 2012. doi: 10.2174/187152712803581047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Bötzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol (1985) 97: 1620–1628, 2004. doi: 10.1152/japplphysiol.00952.2003. [DOI] [PubMed] [Google Scholar]
- 33.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol (1985) 97: 1629–1636, 2004. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- 34.Zimnik NC, Treadway T, Smith RS, Araneda RC. α(1A)-Adrenergic regulation of inhibition in the olfactory bulb. J Physiol 591: 1631–1643, 2013. doi: 10.1113/jphysiol.2012.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]


