Abstract
Mononuclear phagocytes are the most common cells in the kidney associated with immunity and inflammation. Although the presence of these cells in the kidney has been known for decades, the study of mononuclear phagocytes in the context of kidney function and dysfunction is still at an early stage. The purpose of this review is to summarize the present knowledge regarding classification of these cells in the mouse kidney and to identify relevant questions that would further advance the field and potentially lead to new opportunities for treatment of acute kidney injury and other kidney diseases.
Keywords: acute kidney injury, inflammation, ischemia-reperfusion, macrophages, renal fibrosis
the most common cells in the kidney associated with immunity are mononuclear phagocytes (MPs), which are essentially ubiquitous throughout the kidney tissue (21, 29, 51). Therefore, a priori one would conclude that they are an important component in kidney homeostasis and disease. Although the presence of MP in the kidney has been known for decades, the study of MP in the context of kidney function and dysfunction is still at an early stage. To a large extent, their overall characteristics, morphology, lineage relationships, surface markers, tissue distribution, and function are only now being determined. During the last 10 yr, a number of laboratories have engaged in the study of inflammation in the context of a variety of renal disease models using mice. Therefore, there is a significant new body of information regarding MP phenotypes and function in the mouse kidney. The purpose of this review is to provide a survey of the literature pertaining to the identification, classification, and characterization of MP in the mouse kidney and to facilitate thinking about comparison and potential classification schemes for renal MP. We will begin with the discovery of MP in the 19th century, proceed to the identification and classification of MP in the mouse kidney, and end with a brief discussion of the role of MP in murine models of acute kidney injury. For more information on the latter subject, the reader can find a number of recent excellent reviews (6, 24, 60).
General Characteristics and Functions of the Mononuclear Phagocyte System
Metchnikoff is widely credited with the discovery of phagocytic cells that, in 1883, he called “macrophages” (large eaters) and “microphages” (small eaters) after studying cells that appeared around thorns that he placed in starfish larvae (44). At that time, it was concluded that these cells were simply scavengers, but Metchnikoff theorized that the phagocytes played a role in host defense by engulfing and digesting invading microorganisms. Because it appeared that there could be several different types of cells, Metchnikoff called the aggregate population the “macrophage system.” In 1924, Aschoff refined this definition by considering the heterogeneous populations with phagocytic capabilities and calling the conglomeration of the cells the “reticuloendothelial system” because it included what were then called “reticular cells” in the spleen, lymph nodes, and peripheral tissues (1, 2). This term was used until the 1970s when van Furth and colleagues (57, 58) published a new consensus document reclassifying macrophages and associated cells as “the mononuclear phagocyte system.” At that time, it was noted that the morphology of MPs was frequently dependent on the organ or tissue in which they were found and that they appeared to be almost entirely bone marrow derived. It is now known, as we will discuss below, that there are other sources of hematopoiesis from which macrophages can be derived, and this can determine which cellular microenvironments are occupied by the cells. Under definitions presently in use, the mononuclear phagocyte system (MPS) contains cells that are commonly referred to as circulating monocytes, tissue-resident macrophages, and dendritic cells (DCs). Monocytes are large cells of the MPS with a classic unilobular nucleus and are largely agranular. They comprise ~2–10% of leukocytes in the peripheral blood, and a large proportion of the total number, possibly as much as half, are found in the spleen poised for recruitment to inflamed tissue (54). Macrophages are less uniformly shaped and are often named based on the tissue in which they are found (e.g., Kupffer cells in the liver, microglial cells in the central nervous system). DCs, which some have argued are simply specialized macrophages and not an independent lineage (28), present antigen to T lymphocytes with extremely high efficiency. It is believed that this, in fact, is their primary function.
Morphology and Characterization of the MPS in the Kidney
Recent information regarding patterns of gene expression in macrophages and DCs has enhanced our ability to discern lineage relationships of various MP subpopulations (16, 45). As noted above, MPs are virtually ubiquitous, and reports in the mid to late 20th century can be found in which populations of major histocompatibility complex (MHC) class II-positive cells were shown to reside in the kidney (3, 21, 29). These initial reports indicated that macrophages or DCs (the latter, at that time, indicated by very bright MHC class II staining) were distributed throughout the kidney. These cells were associated with proximal tubules, collecting ducts, glomeruli, and macula densa. Within the medulla, cells were concentrated along medullary rays (21, 29). When kidneys were digested in collagenase, and myeloid cells enriched on bovine serum albumin gradients, the cells were found to be phagocytic and capable of activating T cells (3). This is convincing evidence that the MHC class II-positive cells are, in fact, macrophages or DCs. This concept was reinforced by Hume and Gordon’s (29) observation that the cells were also positive for F4/80, a commonly used surface antigen for distinguishing tissue macrophages. From these studies and others enumerating isolated myeloid cells (33, 53), we can conclude that MPs may be integral to renal homeostasis and, by extension, to events that take place during and after renal injury. Therefore, MPs are anatomically and functionally positioned to serve as a sensor network and can initiate and maintain housekeeping, crisis management, and repair. Given this diversity of potential functional needs, we posit that many of these tasks are handled by different cellular subsets. This concept is supported by studies outlined below that demonstrate that the MPs in the kidney encompass a number of subpopulations with distinct phenotypes and functional characteristics.
Basic Markers of MP in the Kidney
The first detailed flow cytometry analyses of intrarenal MPs showed that the majority of them are CD11c+, MHCII+, and F4/80+ (33), which is consistent with the observations of renal tissue sections made two decades earlier (21, 29). In fact, the majority of renal mononuclear cells were found to be CD11c+. They are capable of activating T cells and are phagocytic, and some appear to leave for renal lymph nodes after kidney injury (11, 26), indicating that they are indeed MPs and, given their strong expression of CD11c, possibly DCs. Analyses of the cells using other antibodies specific for surface markers showed that the MPs in the kidney show differential expression of CD11b, Cd11c, and F4/80 (8, 26, 31, 33, 38). Dong et al. (11) showed similar results in the context of the ischemia/reperfusion model of AKI. CD11c+ renal MPs could stimulate histoincompatible T cells and, in fact, could present protein antigens to antigen-specific T cells. These studies indicate that the well-known histological observations of renal cellular infiltrates in injured kidneys are the result of a combination of resident MPs, such as those observed in quiescent uninjured kidneys, and MPs that arrive from elsewhere, presumably the blood. The obvious conclusion, then, is that the MPs are not bystanders or simply providers of janitorial functions associated with maintenance but are active participants in injury from some inciting event and in the subsequent recovery.
Identification of Renal MP Subpopulations
The initial questions that must be addressed are largely technical issues. What are the optimal means to isolate renal MPs, and, once isolated, how do we interpret the data that show what surface markers they express? MPs constitute a small minority of cells in the renal tissue, usually <1%. Therefore, detailed analyses require either enrichment of MPs or examination of large numbers of cells followed by selective analysis of the data. Detailed multiparameter analyses of individual cells require disaggregation of the tissue. Enrichment of the MPs in the renal tissue preparation can simplify analyses, particularly if one intends to isolate specific cellular subsets. Initial disaggregation of saline-perfused kidneys is typically performed by cutting the tissue into small pieces and digesting them in collagenase for a period of time that must be determined empirically. In unpublished methodological studies (Hull TD, Agarwal A, George JF), we have found that enrichment of MP from collagenase-digested preparations by density gradient centrifugation, even with osmolality and buffer temperature tightly controlled, can alter the proportional representation of MP subsets that are ultimately isolated, and the bias is dependent on the gradient materials used (e.g., BSA, ficoll, or nycodenz). We therefore filter disaggregated cellular preparations through nylon mesh (40 or 70 μm depending on application). Methodology used for obtaining single cell suspensions must be considered when evaluating studies of disaggregated kidney cells in the literature.
Initial classifications of renal MP subpopulations were based on investigations of the peripheral blood and secondary lymphoid organs. With those criteria, the majority of MPs in the kidney appear to be DCs because they are CD11c+ and MHC class II+. Soos and colleagues (51) imaged kidneys of transgenic mice bearing a green fluorescent protein (gfp) transgene driven by the CX3Cr1 promoter, which is active in the majority of renal MPs. They found that most of the CD11c+ cells in the kidney are stellate in appearance and closely associated with the tubules. On the basis of the information available at that time, they concluded that the gfp+ cells were DCs and that they form a network throughout the kidney tissue. However, detailed gene expression and flow cytometry analyses have shown that lineage relationships among the MPs in the kidney are far more complex (4, 7, 8, 25, 31, 32, 35, 38, 41).
Although all published kidney MP classification strategies depend on similar markers, three are notable (Fig. 1). Most groups have based their classification schemes on expression of F4/80, MHCII, CD11b, CD11c, Ly6C, Ly6G, and CX3Cr1. At the time of this writing, the most comprehensive studies, in terms of the number of surface antigens investigated and the number of subpopulations defined, have been described by Li et al. (38), Kawakami et al. (31), Cao et al. (7, 8), and Hull et al. (26). These studies, as well as those listed in Table 1, generally used flow cytometric analyses of the expression of CD11b, CD11c, and F4/80 to delineate major renal subpopulations defined by the presence, absence, or relative surface density of a given antigen. They used similar initial gating schemes in which intrarenal leukocytes are discerned by forward and side scatter characteristics (including doublet exclusion), viable cells were identified using a vital dye, such as 7-actinomycin D, and CD45, a protein tyrosine kinase, allowed identification of intrarenal leukocytes. Following these initial steps, groups have used two analysis methods. The first, initially published by Li et al. (38) and subsequently modified by Hull et al. (26) relies on exclusion of neutrophils and separation of renal MPs into two populations based on expression of F4/80 and CD11b: F4/80HiCD11bInt and F4/80IntCD11bHi, termed hereafter as POP1 and POP2, respectively (Fig. 1). Li et al. (38) and Hull et al. (26) showed that these two subpopulations were phenotypically distinct using a variety of surface antigens and that they possessed different functional characteristics. The F4/80IntCD11bHi (POP2) cells are CX3Cr1Low, Ly6CHi, and CD62L+, and the F4/80HiCD11bInt (POP1) cells are Cx3Cr1Hi, Ly6C-, CD11c+, and MHCIIHi. The expression of Ly6C has been previously associated with blood monocyte-derived tissue macrophages (23, 47, 48, 55). A similar role in the kidney is supported by data in which, after ischemic injury, there is an influx of Ly6C+ F4/80IntCD11bHi (POP2) cells into the kidney, roughly concomitant with, or just following an influx of, Gr-1Hi neutrophils (26, 37, 39). This classification system is particularly well suited for studies involving fate mapping and the embryonic origins of specific subpopulations. Furthermore, independent from the above-described tissue macrophages and monocytes, populations of DCs have been observed and can be classified based on their CD103 expression (7).
Fig. 1.
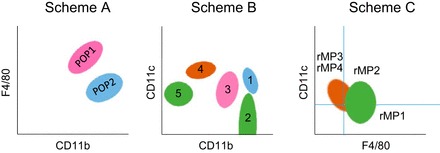
A schematic comparison of 3 schemes used to differentiate subpopulations of renal mononuclear phagocytes. Scheme A has been reproduced by a number of laboratories and shows populations consistent with embryological and fate-mapping studies, indicating that at least a portion of POP1 cells are derived from the embryonic yolk sac. Scheme B provides differentiation similar to that shown in Scheme A (pink and blue populations) and also indicates subpopulations containing CD103+ dendritic cells (DCs) (brown population). Because of a greater reliance on variations in fluorescence intensity, it is, however, critically dependent on the choice of fluorochromes, antibody clones, and cytometer settings. Therefore, it is more difficult to reproduce. Both Scheme A and Scheme B use similar preliminary gating based on forward and side scatter and selection-based CD45 and viability staining. Scheme C is additionally gated on MHCII+ cells. It provides excellent definition of DCs and allows differentiation of CD103+ DC with additional gating of CD11c+F4/80- cells (rMP3 and rMP4) based on CD103 and CD11b.
Table 1.
Surface markers on renal myeloid cells detected by flow cytometry
| Marker | POP1 | POP2 | Reference |
|---|---|---|---|
| CCR2 | + | ± | 27, 31 |
| CD103 | − | − | 6, 7, 23, 27 |
| CD11b | Lo/Int | Hi | 6, 7, 23, 27, 28, 31 |
| CD11c | + | ± | 6, 7, 23, 27, 28, 31 |
| CD14 | Hi | Int | 27 |
| CD16 | Hi | Hi | 27 |
| CD62L | − | + | 27, 31 |
| CD64 | Hi/Int | Int/Lo | 23, 27 |
| CD86 | + | − | 6, 7, 27, 31 |
| CSF1R | + | + | 27, 55 |
| CX3Cr1 | Lo/Int | Hi | 23, 27, 21, 44 |
| F4/80 | Hi | Lo/Int | 18, 23, 26, 27, 28, 31 |
| Gr-1 | − | Int | 23, 27, 31 |
| LFA-1 | Lo | Hi | 23, 31 |
| Ly6C | − | ± | 27, 31 |
| MD1 | Lo | Lo | 31 |
| MHCII | Hi | Hi | 6, 7, 18, 23, 26, 27, 28, 31 |
| TLR2 | Lo | Lo | 31 |
| VLA-4 | Lo | Hi | 31 |
This is not a comprehensive list of all possible markers. CD11b designates a mixed population. POP1, population 1 (F4/80HiCD11bInt); POP2, population 2 (F4/80IntCD11bHi). See text for a discussion of these subpopulations.
Additionally, two classification strategies based on CD11b, CD11c, and F4/80 expression have been published (Fig. 1). Kawakami et al. (31) discerned five subpopulations as determined by fluorescence intensity of CD11b and CD11c (Fig. 1). They classified the F4/80HiCD11bInt (POP1) as CD11bIntCD11cHi, which they called MPC3, and the F4/80IntCD11bHi (POP2) cells as CD11bHiCD11cHi (called MPC1). In addition to these major subpopulations, they also found small subpopulations that were CD11bHiCD11cLo (MPC2), CD11bLoCD11cHi (MPC4), and CD11b-CD11cInt (MPC5). Characterization of MP-related gene expression and other surface antigens generated significant clues regarding the lineage relationships of the identified subpopulations. The CD11bLoCD11cHi MPC4 subpopulation was found to be lacking expression of F4/80 but positive for expression of CD103 and strongly positive for Zbtb46, Batf3, IRF8, and CCR7, all indicative of the DC lineage or associated with DC function. Batf3 and Irf8 are characteristic of CD8+ DCs found in the secondary lymphoid organs and CD103+ DCs found in nonlymphoid organs that are known to actively cross present antigens and are one of the two known subpopulations of conventional (or classical) DCs. They appear to constitute <5% of the CD45+ cells in the kidney. Second, Cao and colleagues (7) identified a CD103+ DC-like subpopulation in a model of adriamycin-induced nephropathy. Importantly, these studies unequivocally identified a subpopulation of renal DCs based on both phenotype and function in the context of a renal disease process. Renal CD103+ DCs are present primarily in the cortex, can prime CD8+ T cells, and appear to be involved in the pathogenesis of adriamycin-induced nephropathy (7, 8). Furthermore, Evers et al. (14) recently published a study indicating protective effects of CD103+ DCs in murine crescentic glomerulonephritis.
Because macrophages and DCs have overlapping function and surface antigen expression, their distinction has not always been clear. This has caused some uncertainty regarding their identification, especially in nonlymphoid organs (18, 27, 28). To some extent, this problem has been ameliorated by comparison of gene expression profiles and regulatory pathways in various macrophage and DC populations in several tissues by the Immunological Genome Consortium (16, 45), which has identified a gene expression profile they termed a core macrophage signature. This core signature includes an array of 39 genes, among which are canonical genes such as CD64 (Fc gamma receptor 1) and MerTK (an efferocytosis receptor). High-quality antibodies are available for the protein products of these two genes; therefore, Gautier and colleagues (16) proposed that they serve as a starting point for identification of macrophages in tissues, because DCs do not coexpress MerTK and CD64. We (26) and Kawakami et al. (31) have noted that the F4/80HiCD11bInt (POP1) population in the kidney strongly expresses both MerTK and CD64, which suggests that these cells are macrophages, not DCs as previously thought. This conclusion is supported by functional studies (8). Therefore, these data best support the conclusion that the vast majority of renal MPs are, in fact, macrophages, and DCs constitute a minority of MPs isolated from renal tissue.
Subsetting Kidney MPs Based on Ontogeny
A substantial literature exists in which MPs have been studied in the context of hematopoiesis and lineage relationships (13, 19, 22, 42, 50, 61). In the kidney, F4/80HiCD11bInt (POP1) and F4/80IntCD11bHi (POP2) populations are present in mice by embryonic day 12.5 (E12.5). The F4/80HiCD11bInt (POP1) population appears at E9.5 to E10.5 and arises primarily from the fetal yolk sac. Liver hematopoiesis becomes active at approximately E12.5, coincident with the appearance of the F4/80IntCD11bHi (POP2) population. These subpopulations are readily detectable in the kidney by E14.5 and persist for the life of the animal. It appears that at least part of the F4/80HiCD11bInt (POP1) population seen during embryonic life originates from the fetal yolk sac, and the F4/80IntCD11bHi (POP2) population is a product of fetal liver/bone marrow hematopoiesis.
These subpopulations are dependent on unique transcription factors. F4/80HiCD11bInt (POP1) cells are dependent on PU.1, whereas the F4/80IntCD11bHi (POP2) cells are dependent on the c-myb transcription factor (50). It is not clear whether the yolk sac and hematopoietic stem cell (HSC, bone marrow/fetal liver)-derived cells remain well delineated in adult kidneys. Preliminary experiments using inducible c-myb knockout mice reconstituted with CD45.1 congenic bone marrow show that the proportion of renal F4/80HiCD11bInt (POP1) cells that are derived from the yolk sac or HSC is variable, possibly depending on age (50). It is notable that the F4/80HiCD11bInt (POP1) population is the first to colonize the kidney tissue and appears to colocate with collagen IV+ tubules (50). Soos et al. (51) noted that CX3Cr1+ stellate cells in the kidney are closely associated with the tubules, which suggests that the tubule-associated MPs are yolk sac derived and that, if so, they are likely important for maintaining homeostasis within the kidney because they are the earliest population to appear in the embryonic organ.
How the makeup of the kidney MPS changes with age, in the normal adult kidney or in the context of kidney disease, remains to be studied. Candidate master transcription factor regulators (42) and gene enhancers (19, 34) provide a potential means of manipulation of MP subpopulations. MPs of distinct origin are likely to have different functions in the kidney, making these immune cells an interesting target for treatment of kidney diseases (13, 52).
Renal MPs in AKI and Other Forms of Kidney Inflammation
MP populations change substantially following AKI. The kinetics of these changes varies as a function of the type [e.g., ischemia-reperfusion (IR), unilateral ureteral obstruction (UUO), or rhabdomyolysis] and the severity of injury. The latter variable in IR models changes depending on the ischemic time and species. The models that are most informative are those in which kidney function recovers after injury because such models are more clinically relevant. Kinetic studies of cell types in the kidney show that neutrophils infiltrate injured kidneys within hours (4, 38, 62). The details of neutrophil kinetics after injury are not clear because, in mouse models, detailed studies employed the RB6-8C5 monoclonal antibody, which, not only binds Ly6G (Gr-1), but also binds Ly6C, which is found on activated blood-derived monocytes. The 1A8 antibody, which is also useful for depletion studies, is specific only for Ly6G (9). The 7/4 antibody, an allotypic marker of neutrophils, is also useful. Results of kinetic studies using the latter antibody clone have not been published.
Depletion studies have shown that MPs are clearly involved in the loss of kidney function and cytoarchitectural disruption that follows an injurious event. The bisphosphonate, clodronate, can be incorporated into liposomes, which induce apoptosis when they accumulate in macrophages that phagocytose them. When injected into mice, clodronate depletes ~75% of MPs in the kidney and reduces the severity of AKI after IR injury or rhabdomyolysis (10, 12, 15, 63). In the first demonstration of this effect, Day et al. (10) showed that the increase in plasma creatinine induced by 32 min of ischemia was effectively abrogated by liposomal clodronate treatment and further showed that injecting treated animals with RAW 264.7 cells restored the rise in creatinine. These data suggest that renal MPs promote damage and the associated decline in renal function after injury. In contrast, when MPs are more effectively depleted (i.e., >90%) by diphtheria toxin treatment of mice transgenic for the diphtheria toxin receptor under control of the CD11b promoter, mice are not protected from IR injury (15). This result supports the idea that clodronate-resistant CD11b+ cells may be required for recovery from AKI. Zhang et al. (63) noted that clodronate treatment spared some or all of an F4/80Hi population. One can speculate that the clodronate-resistant MPs, which likely have low phagocytic activity, are kidney-resident macrophages, perhaps yolk sac derived, which may be a key component in maintenance or restoration of homeostasis.
Given the data from investigations of MP ontogeny and from detailed studies of MP populations in the kidney after AKI, it is possible to theorize the role of MPs in injury and restoration of homeostasis. The F4/80HiCD11bInt/Lo (POP1) population is closely associated with the kidney tubules beginning in embryonic life. This anatomical distribution is consistent with the notion that these cells are important in tubular homeostasis. Indeed, these F4/80Hi cells demonstrate monitoring of immune complexes transported in a transendothelial fashion into the kidney (52). Furthermore, this MP population would be the first point of contact for potential infectious threats originating from inside the lumen of the tubules, such as in pyelonephritis. When Escherichia coli are instilled into the urethra of normal mice, a decline in the numbers of these resident cells occurs in the kidney, concomitant with an increase in the number of neutrophils. This is shortly followed by an increase in the numbers of F4/80IntCD11bHi(POP2) macrophages, which appear to be directly involved in clearing the infection (56). Notably, CD11c+ cells were required for recruitment of neutrophils. The same apparatus is involved in IRI, with the important difference that AKI is a “sterile” injury. Nonetheless, after IRI, there is a rapid increase in the number of CD45+ cells in the kidney, with an initial influx of neutrophils (4, 38, 40, 62, 63) that peaks at ~24 h (38). This influx of monocyte-derived macrophages and neutrophils is dependent on the chemokine CCR2, which is necessary for egress of precursors from the bone marrow (38). A lack of CX3Cr1 also prevents infiltration of injured kidneys by monocyte-derived macrophages (38). The bulk of the evidence shows that blood monocyte-derived macrophages are Ly6C+, and these, in turn, appear to differentiate into other subpopulations upon infiltration into the tissues (38, 40).
Studies of the quiescent kidney show that there is a small subpopulation of Ly6C+ cells that are predominantly CD11c-CD11b+ (31, 40). Upon injury, these cells greatly increase in number in the renal tissue. Experiments utilizing bone marrow chimeras show that the Ly6C+ cells are bone marrow derived and arrive in the kidney via the peripheral blood (40). These findings are consistent with studies of monocyte-derived populations in cardiac tissue, which show that tissue injury is followed by an influx of Ly6CHi cells that decline in numbers over time such that Ly6Int and Ly6CLo cells become predominant. While events in the kidney are similar, the ultimate fate of these cells is not clear. The numbers and proportion of Ly6CHi cells wane after the initial influx following injury. The most facile explanation is that they either undergo apoptosis or downregulate Ly6C. In either scenario, which are not mutually exclusive, the available evidence supports a model in which Ly6C+ cells arrive via the blood supply and infiltrate the kidney shortly after injury and are therefore a key component of the inflammatory phase after AKI.
To better understand the dynamics of MP subpopulations in the kidney after injury, some investigators have invoked the M1/M2 classification scheme, which describes macrophage activation states that mirror Th1 and Th2 polarization of T cells. The M1 phenotype is characterized by production of predominantly proinflammatory cytokines and chemokines, generation of reactive oxygen/nitrogen species, and antimicrobial activity. This phenotype can be generated in vitro by stimulation of TLR receptors in the presence of IFN-γ (41, 46). M2 macrophages, which can be generated in vitro in the presence of IL-4 and IL-13, appear to participate in tissue remodeling, wound healing, immunoregulation, and parasite containment (17, 18, 46). This dichotomous classification can be readily studied in vitro, but in vivo it is more usual to find a spectrum of macrophage phenotypes and considerable plasticity in which macrophage populations are able to shift phenotypes. Nonetheless, the M1 and M2 classifications can be useful in determinations of the proinflammatory or anti-inflammatory/wound-healing characteristics of microenvironments at specific times relative to some event. Analysis of macrophage functional phenotypes as a function of time after renal injury found that F4/80+ renal MPs isolated 24 h after injury exhibited markedly increased levels of inducible nitric oxide synthase and IL-12, whereas arginase-1 expression characteristic of the M2 phenotype was minimal, indicating a predominance of the M1 phenotype. However, at 7 days after injury, renal MPs expressed increased levels of the mannose receptor and arginase-1 (35). Therefore, the functional characteristics of the renal MPs had substantially changed, acquiring an M2-like character.
Human Kidney MPs
Finally, of relevance to translation of these findings to human health, some laboratories have begun to delineate leukocyte populations in the human kidney (30, 36, 49). Although these studies are preliminary in comparison to the body of data generated from mice, they have shown that leukocyte populations in human kidneys share a number of similarities with those found in mouse kidneys. There is a substantial number of intrarenal subpopulations with similar markers to those found in the peripheral lymphoid organs but expressed on cell types with different functional characteristics. Gulliams et al. (20) published a study using unsupervised high-dimensional analyses of flow cytometry data to map conventional DC populations across mice and humans. They showed that cDC1s can be identified as CADM1HiCD172aloCD11cint-HiCD26HiIRF8HiIRF4Lo and cDC2s as CADM1loCD172aHiCD1cHiCD11cHiIRF4HiIRF8lo. This is a powerful approach that will likely yield additional information about MP function in both humans and mice as well as species-specific differences that will inform our interpretation of studies of renal disease models in rodents. Systematic studies of MP subpopulations from individuals with acute and chronic kidney disease will also provide useful insights into the course of injury and repair as well as possible targets for prognosis and treatment.
Conclusions
The data support the concept that, over time, the MP-mediated response to injury shifts from a proinflammatory milieu to a fibrotic or healing phase. It also appears that the process involves communication between renal MPs and kidney parenchymal cells (24, 25, 35). This likely involves a number of soluble factors and surface receptors, in addition to CSF-1, that drive phenotypic changes of MP within the local microenvironment, facilitate recruitment of MPs from the peripheral blood, and drive entry of certain MPs into the cell cycle (5, 43, 59, 63). Understanding the dynamics of individual MP subpopulations and their roles in homeostasis and recovery from injury is an important step toward devising a means of targeting the mechanisms of kidney injury and recovery by manipulation of MP populations.
DISCLOSURES
The authors acknowledge grant support from the University of Alabama at Birmingham-University of California San Diego O'Brien Center (P30 DK079337) and R01 DK059600 (to A. Agarwal and J. F. George), NIGMS MSTP T32GM008361 to Dr. Robin Lorenz for J. M. Lever; AHA 17PRE33370121 to J. M. Lever; and 16GRNT31180023 to J. F. George.
AUTHOR CONTRIBUTIONS
J.G. and A.A. prepared figures; J.G., J.M.L., and A.A. drafted manuscript; J.G., J.M.L., and A.A. edited and revised manuscript; J.G., J.M.L., and A.A. approved final version of manuscript.
REFERENCES
- 1.Aschoff L. Das reticulo-endotheliale system. Ergebnisse der Inneren Medizin und Kinderheilkunde (Berlin) 26: 1–118, 1924. [Google Scholar]
- 2.Aschoff L. Das retikulo-endotheliale system und seine Beziehungen zur Gallenfarbstoffbildung. Munch Med Wochenschr 69: 1352–1356, 1922. [Google Scholar]
- 3.Austyn JM, Hankins DF, Larsen CP, Morris PJ, Rao AS, Roake JA. Isolation and characterization of dendritic cells from mouse heart and kidney. J Immunol 152: 2401–2410, 1994. [PubMed] [Google Scholar]
- 4.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK, Colonna M, Kelley VR. IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest 125: 3198–3214, 2015. doi: 10.1172/JCI81166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30: 183–194, 2015. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q, Lu J, Li Q, Wang C, Wang XM, Lee VW, Wang C, Nguyen H, Zheng G, Zhao Y, Alexander SI, Wang Y, Harris DC. CD103+ dendritic cells elicit CD8+ T cell responses to accelerate kidney injury in adriamycin nephropathy. J Am Soc Nephrol 27: 1344–1360, 2016. doi: 10.1681/ASN.2015030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Q, Wang Y, Wang XM, Lu J, Lee VW, Ye Q, Nguyen H, Zheng G, Zhao Y, Alexander SI, Harris DC. Renal F4/80+ CD11c+ mononuclear phagocytes display phenotypic and functional characteristics of macrophages in health and in adriamycin nephropathy. J Am Soc Nephrol 26: 349–363, 2015. doi: 10.1681/ASN.2013121336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83: 64–70, 2008. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 10.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 13.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evers BD, Engel DR, Böhner AM, Tittel AP, Krause TA, Heuser C, Garbi N, Kastenmüller W, Mack M, Tiegs G, Panzer U, Boor P, Ludwig-Portugall I, Kurts C. CD103+ kidney dendritic cells protect against crescentic GN by maintaining IL-10-producing regulatory T cells. J Am Soc Nephrol 27: 3368–3382, 2016. doi: 10.1681/ASN.2015080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 82: 928–933, 2012. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 16.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 19.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159: 1327–1340, 2014. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GH, Chung TJ, Chan DK, Tan KK, Hon TL, Fossum E, Bogen B, Choolani M, Chan JK, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, Ginhoux F. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45: 669–684, 2016. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart DN, Fabre JW. Major histocompatibility complex antigens in rat kidney, ureter, and bladder. Localization with monoclonal antibodies and demonstration of Ia-positive dendritic cells. Transplantation 31: 318–325, 1981. doi: 10.1097/00007890-198105010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115: 284–295, 2014. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huen SC, Cantley LG. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol 30: 199–209, 2015. doi: 10.1007/s00467-013-2726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol 26:1334–1345, 2015. doi: 10.1681/ASN.2014060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hull TD, Kamal AI, Boddu R, Bolisetty S, Guo L, Tisher CC, Rangarajan S, Chen B, Curtis LM, George JF, Agarwal A. Heme oxygenase-1 regulates myeloid cell trafficking in AKI. J Am Soc Nephrol 26: 2139–2151, 2015. doi: 10.1681/ASN.2014080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hume DA. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol 1: 432–441, 2008. doi: 10.1038/mi.2008.36. [DOI] [PubMed] [Google Scholar]
- 28.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835, 2008. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 29.Hume DA, Gordon S. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med 157: 1704–1709, 1983. doi: 10.1084/jem.157.5.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassianos AJ, Wang X, Sampangi S, Afrin S, Wilkinson R, Healy H. Fractalkine-CX3CR1-dependent recruitment and retention of human CD1c+ myeloid dendritic cells by in vitro-activated proximal tubular epithelial cells. Kidney Int 87: 1153–1163, 2015. doi: 10.1038/ki.2014.407. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami T, Lichtnekert J, Thompson LJ, Karna P, Bouabe H, Hohl TM, Heinecke JW, Ziegler SF, Nelson PJ, Duffield JS. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J Immunol 191: 3358–3372, 2013. doi: 10.4049/jimmunol.1300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krüger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Müller-Newen G, Floege J, Kurts C. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004. doi: 10.1097/01.ASN.0000114553.36258.91. [DOI] [PubMed] [Google Scholar]
- 34.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326, 2014. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone DA, Kozakowski N, Kornauth C, Waidacher T, Neudert B, Loeffler AG, Haitel A, Rees AJ, Kain R. The phenotypic characterization of the human renal mononuclear phagocytes reveal a co-ordinated response to injury. PLoS One 11: e0151674, 2016. doi: 10.1371/journal.pone.0151674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SL, Castaño AP, Nowlin BT, Lupher ML Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X, Wang Y, Lee VW, Zheng G, Tan TK, Wang X, Alexander SI, Harris DC, Wang Y. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int 84: 745–755, 2013. doi: 10.1038/ki.2013.135. [DOI] [PubMed] [Google Scholar]
- 42.Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, Jacome-Galarza CE, Händler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C, Geissmann F. Specification of tissue-resident macrophages during organogenesis. Science 353: 353, 2016. doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metchnikoff E. Untersuchungen uber die intracellulare Verdauung bei Wirbellosen Thieren. Arbeit Zoologischen Instituten. Universitat Wien 5: 141–168, 1883. [Google Scholar]
- 45.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M; Immunological Genome Consortium . Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol 13: 888–899, 2012. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills CD. M1 and M2 macrophages: Oracles of health and disease. Crit Rev Immunol 32: 463–488, 2012. doi: 10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 47.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 112: 1624–1633, 2013. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer MB, Vichot AA, Cantley LG, Moeckel GW. Quantification and localization of M2 macrophages in human kidneys with acute tubular injury. Int J Nephrol Renovasc Dis 7: 415–419, 2014. doi: 10.2147/IJNRD.S66936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 51.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 52.Stamatiades EG, Tremblay ME, Bohm M, Crozet L, Bisht K, Kao D, Coelho C, Fan X, Yewdell WT, Davidson A, Heeger PS, Diebold S, Nimmerjahn F, Geissmann F. Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166: 991–1003, 2016. doi: 10.1016/j.cell.2016.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steptoe RJ, Patel RK, Subbotin VM, Thomson AW. Comparative analysis of dendritic cell density and total number in commonly transplanted organs: Morphometric estimation in normal mice. Transpl Immunol 8: 49–56, 2000. doi: 10.1016/S0966-3274(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 54.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swirski FK, Wildgruber M, Ueno T, Figueiredo J-L, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Nahrendorf M. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest 120: 2627–2634, 2010. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tittel AP, Heuser C, Ohliger C, Knolle PA, Engel DR, Kurts C. Kidney dendritic cells induce innate immunity against bacterial pyelonephritis. J Am Soc Nephrol 22: 1435–1441, 2011. doi: 10.1681/ASN.2010101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 46: 845–852, 1972. [PMC free article] [PubMed] [Google Scholar]
- 58.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. [Mononuclear phagocytic system: New classification of macrophages, monocytes and of their cell line]. Bull World Health Organ 47: 651–658, 1972. [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, Abboud Werner SL, Harris RC, Zhang MZ. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int 88: 1274–1282, 2015. doi: 10.1038/ki.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weisheit CK, Engel DR, Kurts C. Dendritic cells and macrophages: sentinels in the kidney. Clin J Am Soc Nephrol 10: 1841–1851, 2015. doi: 10.2215/CJN.07100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant 15: 1562–1574, 2000. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 63.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]


