Abstract
Exercise is beneficial in pulmonary arterial hypertension (PAH), although studies to date indicate little effect on the elevated pulmonary pressures or maladaptive right ventricle (RV) hypertrophy associated with the disease. For chronic left ventricle failure, high-intensity interval training (HIIT) promotes greater endothelial stimulation and superior benefit than customary continuous exercise training (CExT); however, HIIT has not been tested for PAH. Therefore, here we investigated acute and chronic responses to HIIT vs. CExT in a rat model of monocrotaline (MCT)-induced mild PAH. Six weeks of treadmill training (5 times/wk) were performed, as either 30 min HIIT or 60 min low-intensity CExT. To characterize acute hemodynamic responses to the two approaches, novel recordings of simultaneous pulmonary and systemic pressures during running were obtained at pre- and 2, 4, 6, and 8 wk post-MCT using long-term implantable telemetry. MCT-induced decrement in maximal aerobic capacity was ameliorated by both HIIT and CExT, with less pronounced pulmonary vascular remodeling and no increase in RV inflammation or apoptosis observed. Most importantly, only HIIT lowered RV systolic pressure, RV hypertrophy, and total pulmonary resistance, and prompted higher cardiac index that was complemented by a RV increase in the positive inotrope apelin and reduced fibrosis. HIIT prompted a markedly pulsatile pulmonary pressure during running and was associated with greater lung endothelial nitric oxide synthase after 6 wk. We conclude that HIIT may be superior to CExT for improving hemodynamics and maladaptive RV hypertrophy in PAH. HIIT’s superior outcomes may be explained by more favorable pulmonary vascular endothelial adaptation to the pulsatile HIIT stimulus.
Keywords: apelin, endothelial nitric oxide synthase
pulmonary arterial hypertension (PAH) causes progressive remodeling of small to midsize pulmonary arteries that is dominated by medial hypertrophy and vasoconstriction and that leads to right ventricular (RV) failure and death (2). Despite the development of several new drug therapies for PAH that have improved overall survival rate (31), most patients continue to exhibit significantly reduced exercise tolerance (3, 53) and quality of life. Until recently, practitioners generally discouraged aerobic exercise for patients with PAH. Over the past few years, this opinion has shifted toward a more liberal recommendation in favor of exercise, including a grade 1A recommendation at the Fifth World Health Organization World Symposium on Pulmonary Hypertension (19). However, this recommendation has been made in the absence of clinical or preclinical evidence about the duration or intensity of exercise. Moreover, there is lack of evidence of salutary effects of exercise training on cardiopulmonary hemodynamics or RV function (13, 17, 23, 40, 43, 48, 62) that would suggest that there are adaptations to the afterload increase in PAH.
Individualized exercise prescription is essential, where exercise parameters are set based on objective evaluation of patient exercise response (42), but optimal mode of delivery for a prescribed exercise workload in PAH has not been investigated. The exercise prescription with the highest therapeutic index for PAH may require an approach alternative to the customary continuous exercise training (CExT) protocol used in most cardiopulmonary rehabilitation settings and patient-directed independent programs. Multiple studies indicate greater favorable cardiovascular adaptations after exercise performed at higher intensity than at low or moderate levels for healthy humans (26, 45), and for animal models and humans with coronary artery disease (50), myocardial infarction and left ventricular (LV) dysfunction (14, 25), and chronic obstructive pulmonary disease (15, 47). High-intensity interval training (HIIT), alternating intervals of high-intensity and low-intensity exercise, is superior to CExT in chronic LV failure (1), with evidence even for reversal of LV remodeling in patients following an intervention of HIIT but not of CExT (61). However, the opposite was recently reported in a pre-heart-failure hypertension rat model where 4 wk of HIIT promoted significant pathological LV adaptation not observed for CExT-trained and untrained animals (30). Such contradictory findings highlight the importance of studying disease-specific responses to HIIT, or any exercise approach, before establishing as clinical practice. Therefore, the purpose of this study was to compare outcomes and training adaptations of the lungs, RV, and soleus muscle in a rat model of PAH following a 6-wk protocol of HIIT vs. CExT. Moreover, to ascertain dynamic changes in hemodynamic responses during the two types of exercise, a rat was instrumented with implantable telemetric probes. We hypothesized that, because higher exercise pressures prompt greater flow-mediated endothelial shear stress (6), HIIT would provide greater pulmonary vascular endothelial stimulation and more robust training-induced nitric oxide (NO) pathway enhancement for improved hemodynamics, attenuated RV maladaptive remodeling, and better exercise capacity.
METHODS
PAH Induction and Experimental Groups
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Indiana University, which is in compliance with National Institutes of Health (NIH) guidelines. All animals received care in compliance with the Guide for the Care and Use of Laboratory Animals. PAH was induced in male Sprague-Dawley rats (~300 g; Charles River and Harlan) by administration of 40 mg/kg monocrotaline (MCT; Sigma Aldrich) subcutaneously, which reliably induces stable mild PAH after 2 wk (54). We employed a mild PAH phenotype to reflect patients in early or controlled disease stages who may be better suited to the intensity of high intervals used in traditional HIIT [~90% of V̇o2 reserve (V̇o2R)], as we have tested here (25, 60).
Control (CON) animals received subcutaneous vehicle (saline). Animals were assigned to one of the following six groups: 1) MCT plus a 6-wk protocol of continuous exercise training (MCT-CExT; n = 7), 2) MCT plus a 6-wk protocol of HIIT (MCT-HIIT; n = 8), 3) MCT but “sedentary” with no training program (MCT-SED; n = 10), 4) saline control plus continuous exercise training (CON-CExT; n = 5), 5) saline control plus HIIT (CON-HIIT; n = 6), and 6) saline control sedentary (CON-SED; n = 6).
V̇o2max Testing
Because running on treadmill is a skilled activity for rats, all rats were familiarized to the treadmill (Columbus Instruments, Columbus, OH) at speeds and inclines that would be required during subsequent testing, as previously performed by our group (9, 38). Figure 1 presents a schematic of the protocol timeline. Immediately before MCT/saline injections, exercise testing was performed for all animals to determine maximal aerobic capacity (V̇o2max; expressed relative to body weight) using indirect open-circuit calorimetry (Oxymax; Columbus Instruments) and an incremental treadmill protocol in 3-min stages (8, 59). Exercise testing was repeated for all rats at 2 and 8 wk postinjection.
Fig. 1.

Study protocol. Following a period of treadmill familiarization, maximal oxygen uptake (V̇o2max) was measured. Rats then received either monocrotaline (MCT, 40 mg/kg ip) to induce pulmonary arterial hypertension (PAH, n = 25) or saline for healthy controls (n = 17). Later (2 wk), V̇o2max testing was repeated to establish pretraining values, and echocardiography was performed on a subset of rats. A 6-wk treadmill program was then initiated for rats assigned to exercise training, performed in 5 sessions/wk. Continuous exercise training (CExT, n = 12) involved 60 min of uninterrupted steady-state running at 50% of V̇o2 reserve (V̇o2R) determined in the second exercise test. High-intensity interval training (HIIT, n = 14) involved a brief warm up and then five cycles of alternating high- and low-intensity intervals as follows: 2 min at 85–90% V̇o2R and 3 min at 30% V̇o2R, totaling 30 min. Rats assigned to remain sedentary (SED, n = 16) were placed on a stationary treadmill on a matched schedule. At the conclusion of 6 wk, final V̇o2max testing and echocardiography were performed, and, 3 days later, invasive hemodynamic measures were performed, followed by sacrifice and tissue harvest.
Treadmill Training
A 6-wk treadmill running program was initiated for rats assigned to exercise training, performed in five sessions per week. For rats assigned to CExT, uninterrupted steady-state running was performed with treadmill speed and incline set to elicit an intensity of 50% of V̇o2R determined in the second exercise test, calculated for each animal by the method of Karvonen as [(V̇o2max – V̇o2resting) × 0.50] + V̇o2resting. The intensity of 50% V̇o2R was chosen, since it is within the exercise intensity range recommended by the American College of Sports Medicine for exercise prescription in cardiopulmonary patients (42). Session duration was progressed from 30 min up to 60 min by the end of week 2.
For rats assigned to HIIT, sessions began with a 6-min warm up at 50% V̇o2R and then proceeded into five 5-min cycles of alternating high- and low-intensity intervals as follows: 2 min at 85–90% V̇o2R and 3 min at 30% V̇o2R (totaling 30 min). Training time between HIIT and CExT was intentionally unmatched, since it is an important aspect of the HIIT approach that only about one-half of the session duration of CExT is required to achieve similar work performed. Rats assigned to remain SED were placed on a stationary treadmill on a matched schedule.
Invasive Hemodynamic Measurements
Three days following the final exercise test, rats underwent nonsurvival surgery for invasive hemodynamic measurements. We waited for 3 days to avoid confounding effects of the exercise tests (9, 38). Rats were anesthetized by inhaled isofluorane, orotracheally intubated, and mechanically ventilated (rate of 68 breaths/min, volume is adjusted as necessary to keep arterial blood gases within normal parameters). Isofluorane delivery was set to 5% for induction and 2% for maintenance, with a gas mixture initially at 100% oxygen and then stabilized at room air. The left carotid artery was cannulated with PE-50 tubing, and the right internal jugular vein was cannulated with a 2-Fr Millar catheter (Millar Instruments, Houston, TX) for recordings of pulmonary and systemic pressures as described previously (37), with correct RV catheter position determined by wave form analysis indicating typical RV waveform. RV systolic pressure (RVSP) and mean arterial pressure (MAP) were assessed at room air during normocapnia and normal pH (determined via i-STAT blood gas analyzer; Abbott Point of Care, Princeton, NJ).
Implantable Telemetry
To assess dynamic changes in RV and systemic pressure during exercise, a male Sprague-Dawley rat (280 g) was instrumented with an implantable telemetry sensor-transmitting probe (model HD-S21; Data Sciences International) via laparotomy and thoracotomy as previously described (9). Following surgery, the animal recovered for 2 wk before exercise testing and MCT administration (40 mg/kg). To assess hemodynamic response to treadmill running loads required by CExT and HIIT protocols, serial exercise testing was performed for the instrumented rat over an 8-wk period (pre-MCT, and at 2-wk intervals post-MCT). The testing was conducted over three consecutive days and consisted of: 1) determination of V̇o2max as described above; 2) 30 min sampling of steady-state running at 50%V̇o2R, identical to that used for training of the CExT rats; and 3) 30 min sampling of alternating high (85–90% V̇o2R)- and low (30% V̇o2R)-intensity running, identical to that used for training of the HIIT rats. Recordings of systemic blood pressure (abdominal aorta), RV pressures, heart rate, EKG (electrodes over the right pectoral muscle and left caudal rib region), and body temperature were obtained at rest and during all treadmill testing, as well as during recovery from testing.
Echocardiography
A recent report of HIIT promoting pathological LV adaptation for pre-heart-failure hypertension rats (30) prompted us to include echocardiography to rule out potential worsening of RV function for HIIT-trained MCT rats. Echocardiography was performed before and after the 6 wk of HIIT and compared with parameters obtained for a subset of untrained (SED) MCT and CON animals. Rats were lightly anesthetized with 1–2% isoflurane via nose cone, and images were obtained by a blinded sonographer as previously described by our group (18). Upon determination of all echocardiographic end points, rats were recovered from anesthesia. Wall thicknesses, pressures, and other derived values were assessed in accordance with published methods (39), including cardiac output (derived from RV outflow tract diameter and velocity time integral), which is expressed relative to body mass as cardiac index. Index of total pulmonary vascular resistance (TPRi) was calculated as reported, which divides the RVSP by the cardiac index.
Tissue Harvest
On the final day of the protocol, immediately following the invasive hemodynamic measurements, rats were euthanized under anesthesia via exsanguination, and lungs, heart, and soleus were harvested as reported previously by our group (37). For consistency, tissues from the telemetry-implanted rat were not included in the morphometry and biochemical assays.
Pulmonary Vascular Remodeling
Lungs were flushed with normal saline through a catheter in the pulmonary artery (PA) until clear return was obtained from the left atrium. After excision of the right lung, the left lung was then inflated with formalin/agarose via the trachea with 10% buffered formalin in agarose under constant pressure (15 mmHg), removed from the thoracic cavity, and paraffin embedded. To characterize the pulmonary vascular phenotype in terms of pulmonary arterial wall hypertrophy, Verhoeff-Van Giesson (VVG) immunohistochemical staining was performed on lung sections of the three MCT groups and the untreated control (CON-SED) animals. Pulmonary vascular wall area was then determined from bright-field microscopy images in a blinded fashion for small- and medium-sized PAs (<200 µm diameter, 10 vessels/animal, ×20 objective) as previously described by our group (18).
Assessment of Lung Endothelial Nitric Oxide Synthase
To investigate training impact on a key regulator of pulmonary vascular tone, endothelial nitric oxide synthase (eNOS) expression and activation status were assessed in lung tissues from the three MCT groups and the untreated control animals. Measurement of lung total eNOS and eNOS phosphorylated at activating site serine-1177 or at inhibiting site threonine-495 (p-eNOSSer1177 and p-eNOSThr495, respectively) was performed via electrophoresis and immunoblot analysis of lung homogenates as previously described by our group (9). Primary antibodies were a polyclonal antibody for eNOS (1:200 dilution; Santa Cruz Biotechnology, Dallas, TX), p-eNOSSer1177 (1:500 dilution; Cell Signaling, Danvers, MA), p-eNOSThr495 (1:500 dilution; Cell Signaling), or vinculin (1:1,000 dilution; Sigma Aldrich) as loading control. The intensity of Western blotting bands was measured by densitometry using ImageJ software (NIH) and expressed normalized to vinculin band intensity and relative to untreated controls.
RV Hypertrophy
RV hypertrophy was assessed by measuring the widely used Fulton index {weight of RV divided by weight of the left ventricle plus septum [RV/(LV + S)]} as described previously (37), with a value greater than ~0.30 expected in the PH rat model. Immediately after determination of RV and LV + S weights, sections of the RV were snap-frozen for further biochemical analyses or immersed in 10% buffered formalin for immunohistochemistry studies. An additional assessment of RV hypertrophy was provided by echocardiographic measurement of RV wall thickness and change from baseline in RV wall thickness by a blinded sonographer.
RV Apelin
Because apelin is recognized as a potent inotropic, anti-apoptotic, and anti-inflammatory mediator that has been reported to be downregulated in the RV of MCT rats (16), RV apelin levels were assessed via electrophoresis and immunoblot analysis of RV homogenates of the three MCT groups and the untreated control animals. Equal amounts of protein (determined by bicinchoninic acid protein assay) were resolved by 7.5% SDS-PAGE, followed by immunoblotting with rabbit polyclonal antibody (1:500; Abcam, Cambridge, MA) followed by secondary anti-rabbit antibody (1:2,000; Abcam). The intensity of Western blotting bands was measured by densitometry using ImageJ software (NIH) and expressed normalized to vinculin band intensity and relative to sedentary MCT animals.
RV and Skeletal Muscle Metabolism
To further characterize the MCT phenotype, and to investigate potential training-induced adaptations in myocardial or skeletal muscle substrate utilization, indicators of oxidative and nonoxidative (glycolytic) metabolism were assessed in RV and soleus samples of the three MCT groups and the untreated control animals. For Western blotting of RV and soleus whole muscle homogenates, equal amounts of protein (determined by bicinchoninic acid protein assay) were resolved by 7.5% SDS-PAGE, followed by immunoblotting using a total oxidative phosphorylation antibody cocktail (OXPHOS, diluted 1:500; Abcam) and using rat heart mitochondria (Abcam) as a positive control. The intensity of Western blotting bands was measured by densitometry using ImageJ software (NIH) and expressed normalized to vinculin band intensity and relative to sedentary MCT animals. Oxidative capacity of the RV and soleus was also evaluated by assessment of glucose transporter-1 (GLUT-1) abundance as an indicator of cytoplasmic glycolysis. Immunofluorescent staining for Glut-1 (at 1:150 dilution, no. ab652; Abcam) was performed on cryofixed RV and soleus as previously described by our group (9). Mean pixel intensity of GLUT-1 staining was determined using ImageJ software (NIH) and expressed relative to sedentary MCT animals.
RV Inflammation, Fibrosis, and Apoptosis
To assess whether the high work rates encountered during intense intervals of a HIIT session may induce detrimental RV responses with chronic use, RV inflammation, apoptosis, and fibrosis were examined for the three MCT groups and the untreated control animals using immunofluorescence and histological assays. Lymphocyte infiltration was assessed as an indicator of RV inflammation via immunofluorescence staining for CD45 (1:20 dilution; Santa Cruz Biotechnology) in RV cryosections as previously described by our group (9). CD45+ counts were expressed as the number of positive-stained cells per field, averaging at least six randomly chosen fields per RV. Cardiomyocyte apoptosis was assessed with terminal deoxynucleotidyl transferase-dUTP nick end-labeling (TUNEL) of RV cryosections according to the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN) with DAPI costaining and expressed relative to nuclei count. RV fibrosis was assessed on formalin-fixed paraffin-embedded RV sections as percent positively stained area with Masson’s trichrome staining and expressed relative to MCT sedentary animals.
Statistical Analyses
Data are presented in Figs. 1-7 as means ± SE. An ANOVA by group assignment was performed with repeated measures (exercise testing and body mass data at three time points) or without repeated measures (hemodynamic data and Fulton index) as appropriate using Tukey’s multiple-comparison posttest analysis to determine between-group differences. ANOVA was also implemented for data from histological and biochemical assays performed for the three MCT groups and the untreated healthy control group (CON-SED). Echocardiographic data obtained at two time points (pre- and posttraining) for HIIT and SED animals (both MCT and CON) were evaluated using repeated-measures ANOVA. Pearson product correlations were performed to further explore relationships between dependent variables. All statistical analysis was carried out using SPSS, version 23.0, and differences at α level of 0.05 (P < 0.05) were considered statistically significant.
RESULTS
CExT and HIIT Improve Aerobic Exercise Capacity in Mild PH
Aerobic capacity.
Two weeks after MCT, rats showed a mild PH phenotype, evidenced by a reduction from baseline in aerobic capacity (by −7 ± 1.5%, Fig. 2A) and faster time to exhaustion (Fig. 2B). In contrast, there was no change (P > 0.05) from baseline for saline-injected animals. Together, these data suggest an expected impairment in physical function in MCT-injected rats before intervention, and this impairment was similar (P > 0.05) between rats assigned to HIIT vs. CExT groups. Because training workloads were set relative to each rat’s individually determined postinjection maximum, the group means ± SE for treadmill speed (m/min) and incline (deg) eliciting a relative training intensity of 50% V̇o2R used in CExT was lower as expected for MCT-CExT (9.3 ± 0.6, 2.9 ± 1) compared with CON-CExT (12 ± 1.3, 7 ± 1.2). Likewise, mean ± SE treadmill speed used for the high-intensity intervals of HIIT performed at an incline of 25° to elicit 85–90% V̇o2R was lower for MCT-HIIT (14.2 ± 0.3 incline 25°) compared with CON-HIIT (16.3 ± 0.2).
Fig. 2.
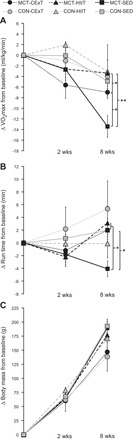
Aerobic exercise capacity and body mass. A: for rats with MCT (40 mg/kg)-induced PAH (black symbols), decrement in V̇o2max (expressed relative to body mass) was ameliorated by both a HIIT approach (gray triangles, n = 8) and a CExT (gray circles, n = 7) vs. untrained MCT (SED, black squares, n = 10), with change after 6 wk of training (8-wk time point) compared with pretraining (2-wk time point) not different (P > 0.05) from that in healthy HIIT, CExT, and SED control rats (CON, gray symbols, n = 5–6 ea). B: MCT-induced decrement in treadmill run time (time to V̇o2max) was also significantly improved by CExT and HIIT. C: body mass was significantly impacted by time for all groups but not differently affected by group assignment. *P < 0.05 and **P < 0.01.
Importantly, MCT rats treated with either HIIT (P < 0.01 vs. MCT-SED) or CExT (P < 0.05 vs. MCT-SED) exhibited more preserved V̇o2max and time to V̇o2max, with pre- to posttraining change (Fig. 2, A and B) not different (P > 0.05) from that for CON-SED over the same time period. Although the total number of training minutes for HIIT was one-half as much as for CExT, calculated cumulative work performed for MCT rats over the 6 wk was comparable between HIIT and CExT groups as intended (4,573 ± 218, 3,745 ± 616 J, P = 0.4). Change in body mass over the study period (Fig. 2C) was not differently affected by group assignment (P > 0.05).
HIIT Is Associated with Lower RV Pressures
At 8 wk post-MCT, unexercised (MCT-SED) rats exhibited resting RVSP values (Fig. 3A) that were ~60% greater compared with CON rats. MCT-HIIT rats, on the other hand, exhibited RVSP values that were similar to CON and significantly lower than both MCT-CExT and MCT-SED. However, continuous exercise had no salutary effect on RVSP, since MCT-CExT exhibited RVSP values that were similar to MCT-SED and significantly higher than both MCT-HIIT and CON rats. Aortic blood pressures were similar (P > 0.05) among groups, with mean arterial pressure values of 92 ± 4.4, 100 ± 8.7, 99 ± 6.7, 105 ± 7.3, 113 ± 7.0, and 97 ± 5.5 mmHg for MCT-CExT, MCT-HIIT, MCT-SED, CON-CExT, CON-HIIT, and CON-SED, respectively.
Fig. 3.
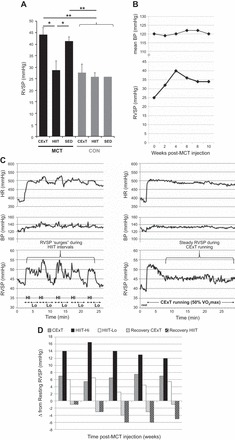
Pulmonary hypertension and exercise hemodynamics. A: for rats with MCT (40 mg/kg)-induced PAH (black bars), MCT-induced elevation in resting RV systolic pressure (RVSP) was attenuated by a HIIT (n = 8) but not CExT (n = 7) approach, with RVSP of MCT-HIIT similar to that in healthy HIIT, CExT, and SED control rats (CON, gray bars, n = 5–6 ea). *P < 0.05 and **P < 0.01. B: serial measures of simultaneous RV systolic and mean systemic resting pressures (BP) in an awake freely moving rat instrumented for implantable telemetry. Recordings are shown for pre- and 2, 4, 6, 8, and 10 wk post-MCT (40 mg/kg) injection. C: real-time tracings of heart rate (HR, top) and pulmonary (RVSP, bottom) and systemic (mean BP, middle) pressures recorded via implantable telemetry are shown during HIIT running (left) and CExT running (right) for a rat at 4 wk post-MCT (40 mg/kg). Note the intermittent surges in RVSP that correspond to high (HI)- and low (Lo)-intensity intervals during HIIT that are absent during CExT. D: change in RVSP relative to resting values during a session of CExT (solid bars) and during the high-intensity (“Hi,” black bars) and low-intensity (“Lo,” white bars) intervals of a HIIT session recorded in the instrumented rat at baseline (pre-MCT) and at 2, 4, 6, and 8 wk post-MCT. Change in RVSP from resting values during recovery from both the CExT and HIIT run sessions are also indicated at each time point as hatched gray and black bars, respectively.
Larger Postexercise Reduction in RVSP with HIIT
Figure 3, B-D, shows hemodynamics in the chronically instrumented rat at rest (B) and responses to exercise (C and D). RVSP at rest is increased by MCT injection in this animal by ~60% by week 4 with unchanged systemic pressures (Fig. 3B). Most importantly, exercise data reveal that, in contrast to the steadier hemodynamics observed during CExT running (Fig. 3C, right), pronounced “surges” in RVSP occurred during HIIT running, which corresponded to the high-intensity run intervals (Fig. 3C, left). Furthermore, hemodynamic recordings collected during recovery from these run bouts (striped bars, Fig. 3D) reveal a more marked postexercise reduction in RVSP from resting values following a HIIT session compared with a CExT session, suggesting heightened provocation of acute vasodilation by HIIT.
Larger Postexercise Reduction in RVSP with HIIT Is Associated with Increased Lung eNOS Expression
Because the telemetry studies suggested a more pronounced vasodilation effect after HIIT, we measured eNOS expression in lung homogenates of HIIT-, CExT-, and SED-MCT rats and CON-SED. Interestingly, MCT-HIIT exhibited greater fold increase in total eNOS protein than MCT-SED (P < 0.05) and CON-SED (P < 0.01) (Fig. 4A). Phosphorylation of eNOS at activation (serine) and inhibitory (threonine) sites was not different with training (data not shown). Taken together, the telemetric exercise pressure recordings and biochemistry data indicate that HIIT running prompts an acute pulmonary hemodynamic response distinct from CExT running and is associated with higher lung eNOS abundance and lower resting RVSP in MCT-PH.
Fig. 4.
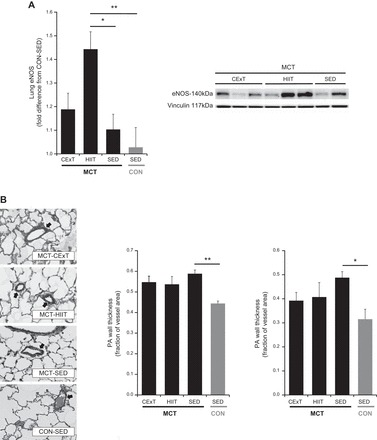
Lung endothelial nitric oxide synthase (eNOS) expression and vascular remodeling. A: for rats with MCT (40 mg/kg)-induced PAH (black bars), only HIIT (n = 8) and not CExT (n = 7) significantly increased lung eNOS abundance vs. untrained animals (SED, n = 10) as assessed by immunoblotting (expressed as fold difference from mean value for CON-SED, gray bar, n = 6). A representative immunoblot showing the band corresponding to eNOS and to vinculin (loading control) is also shown. B: MCT-induced thickening of pulmonary arterial walls (PA wall thickness as a fraction of vessel area) was not significantly different following either HIIT or CExT compared with SED, for either small-diameter (<100 µm, left) or medium-diameter (100–200 µm, right) vessels in MCT; however, PA wall thickness in both small and medium vessels was only significantly greater for untrained MCT (MCT-SED) compared with untreated healthy controls (CON-SED). Representative images of Verhoeff-Van Giesson (VVG)-stained elastin surrounding arteries (arrows) in lung sections are also provided. *P < 0.05 and **P < 0.01.
Preserved Pulmonary Vascular Structure in Exercised MCT Rats
Pulmonary arterial wall thickness (by VVG staining, Fig. 4B) was greater for MCT animals in both small-diameter (<100 µm, Fig. 4B, left) vessels, by 30% (MCT-CExT and HIIT) to 45% (MCT-SED), and medium-diameter (100-200 µm, Fig. 4B, right) vessels, by 26% (MCT-CExT and HIIT) to 50% (MCT-SED). Although there was no significant difference in MCT rats between trained vs. sedentary animals, no significant increase in pulmonary arterial wall thickness was observed for the trained animals (both HIIT and CExT), whereas MCT-SED was significantly increased compared with healthy controls (P < 0.01 for small-diameter vessels, P < 0.05 for medium-diameter vessels, vs. CON-SED). This suggests potential beneficial effects of exercise training on pulmonary artery remodeling.
Less RV Hypertrophy and Better RV Function with HIIT
MCT animals treated with HIIT had ratio of RV to LV + S mass (Fig. 5A) similar to that of healthy (CON-CExT, -HIIT, and -SED) animals; this observation was absent in MCT animals treated with CExT. Given the beneficial effects of HIIT on cardiopulmonary hemodynamics and RV hypertrophy, we performed echocardiography to further characterize RV function in these animals. We confirmed the findings suggested by the Fulton index by demonstrating that HIIT-trained MCT animals exhibited an attenuated increase in RV free wall thickness over 6 wk (Fig. 5B, top) compared with sedentary MCT (P < 0.05), resulting in lower final wall thickness compared with sedentary MCT (P < 0.05). Furthermore, echocardiography indicated improvement in RV function in HIIT-trained MCT, including better-maintained cardiac output over 6 wk (cardiac index, Fig. 5C, top), P < 0.05), lower final index of total pulmonary vascular resistance (TPRi, Fig. 5D, top), and tendency for better ratio of pulmonary artery acceleration time over pulmonary artery ejection time (P = 0.07) vs. MCT-SED. Correlation analysis indicated that final RVSP was related to RV hypertrophy as determined by Fulton index (R = 0.64, P < 0.01) as well as by wall thickness in echocardiography (R = 0.65, P < 0.01). A differential effect of HIIT on echocardiographic parameters was not observed for CON animals (Fig. 5, B-D, bottom) except in cardiac output and cardiac index where SED animals exhibited increase over 6 wk, contrary to that observed in MCT-SED.
Fig. 5.
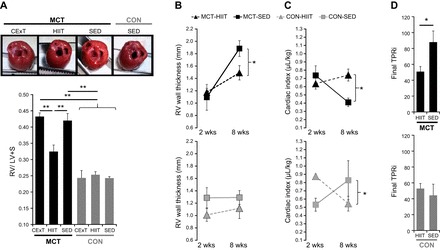
Right ventricle (RV) hypertrophy and function. A: for rats with MCT (40 mg/kg)-induced PAH (black bars), elevation in the ratio of RV to the left ventricle (LV) + septum (S) mass (Fulton index, a measure of RV hypertrophy) was attenuated by a HIIT (n = 8) but not CExT (n = 7) approach, with values for MCT-HIIT significantly lower (P < 0.01) than MCT-CExT and untrained (SED) MCT (n = 10) and similar to that for healthy HIIT, CExT, and SED CON rats (gray bars, n = 5–6 ea). Representative images show a top-down view of hearts (great vessels and atria removed) from MCT-CExT, MCT-HIIT, MCT-SED, and CON-SED where increased thickness of RV (far right wall) relative to LV (far left wall) for MCT-CExT and MCT-SED can be appreciated. B–D: echocardiography performed pre (“2-wk”)- and post (“8-wk”)-intervention for HIIT [gray triangles, n = 8 MCT (black, top), n = 4 CON (gray, bottom)] and SED (black squares, n = 8 MCT, n = 4 CON) demonstrate that, in HIIT-trained MCT rats, increase in RV wall thickness (B) was ameliorated, and cardiac output (expressed normalized by body mass as cardiac index, C) was better maintained. Calculated final total pulmonary vascular resistance index (TPRi, D) was also lower for MCT-HIIT vs. MCT-SED. *P < 0.05 and **P < 0.01.
Training Impact on RV and Skeletal Muscle Metabolism
Untreated MCT rats (MCT-SED) exhibited greater abundance of Glut-1 in immunofluorescence staining of RV (Fig. 6A) and soleus (Fig. 6B) compared with untreated healthy controls (CON-SED), suggesting a shift toward glycolytic (nonoxidative) metabolism in both cardiac and skeletal muscle. Interestingly, both HIIT- and CExT-trained MCT rats exhibited less Glut-1 (P < 0.01) compared with MCT-SED in the RV (Fig. 6A). However, only HIIT was associated with increased RV expression of an electron transport chain complex, cytochrome IV (Fig. 6C). Training effects were also observed for soleus but, in contrast to those observed in the RV, occurred only with a CExT approach. Only MCT-CExT exhibited similar soleus Glut-1 (Fig. 6C) to CON-SED, with a tendency (P = 0.08) for increased soleus expression of an electron transport chain complex, cytochrome III, vs. MCT-HIIT and MCT-SED (Fig. 6D). Taken together, these data indicate that exercise training, in either a HIIT or CExT regimen, may positively impact MCT-induced glycolytic shift in RV and skeletal muscle.
Fig. 6.
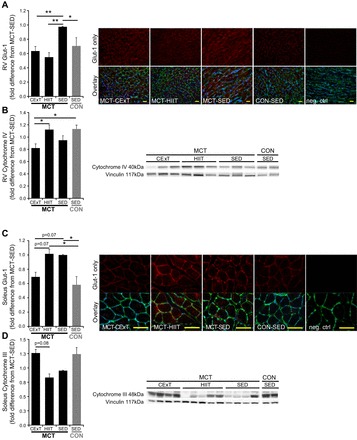
RV and soleus metabolism. Abundance of glucose transporter Glut-1 was greater for RV (A) and soleus (C) in sedentary rats with MCT (40 mg/kg)-induced PAH (black bars, MCT-SED, n = 10) vs. sedentary healthy controls (gray bars, CON-SED, n = 6). In the RV (A), trained MCT had less Glut-1 compared with SED MCT whether they followed either a HIIT (n = 8) or CExT (n = 7) approach. Immunoblotting for oxidative phosphorylation proteins (OXPHOS) in RV homogenates (B) indicated that training also increased expression of electron transport chain cytochrome IV (expressed as fold difference from MCT-SED for loading control-normalized densitometry value) but only for rats trained with a HIIT approach. For the soleus (C and D), a training effect toward attenuating MCT-induced dependence on glycolytic metabolism was also observed but, opposite to the RV, only occurred for CExT and not HIIT. CExT-trained MCT tended to express less Glut-1 (C) and more cytochrome III in OXPHOS immunoblots of soleus homogenates (D) compared with MCT-HIIT, with mean values not significantly different from that for CON-SED. Abundance of Glut-1 was measured by mean pixel intensity of red immunofluorescent staining shown in representative images, with green representing wheat-germ agglutin-stained myocyte membrane and blue representing nuclei. A representative immunoblot showing the band corresponding to cytochrome III and to vinculin (loading control) is also shown. Results depicted in bar graphs on the left are expressed as fold difference from MCT-SED in mean pixel intensity. *P < 0.05 and **P < 0.01.
RV Inflammation, Fibrosis, and Apoptosis
To investigate potential adverse effects of training, biochemical assays were performed to evaluate RV fibrosis, inflammation, and apoptosis for all trained and sedentary MCT rats and untreated CON-SED. Immunoblotting experiments revealed a training effect on RV apelin, an anti-apoptotic and anti-inflammatory mediator and positive inotropic regulator that is inducible by exercise. Much higher RV apelin was observed with HIIT vs. CExT (increased by ~75%, P < 0.01) (Fig. 7A). Neither HIIT nor CExT increased RV inflammatory or apoptotic cells as indicated by similar levels (P > 0.05 vs. MCT-SED) of CD45+ (Fig. 7B) and TUNEL+ (Fig. 7C) cells. Interestingly, MCT-induced increase in RV fibrosis (trichrome staining, Fig. 7D) was attenuated by HIIT (by approximately one-third, P < 0.05 vs. MCT-SED), which supports echo data of improved RV function in these animals.
Fig. 7.
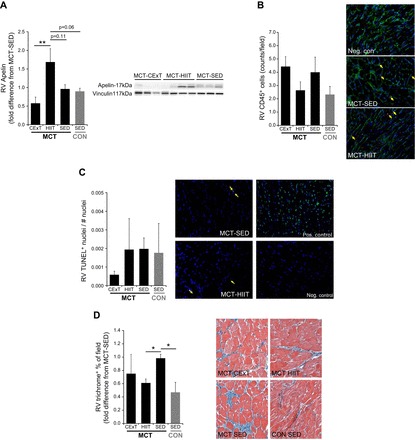
RV inflammation, apoptosis, fibrosis, and apelin expression. A: immunoblotting of RV homogenates for the anti-apoptotic/anti-inflammatory mediator and positive inotropic regulator apelin in rats with MCT-induced (40 mg/kg) PAH (MCT, black bars) and untreated healthy CON rats (gray bars) revealed a higher protein abundance in MCT that were trained with HIIT (n = 8) but not with CExT (n = 7). Values are expressed as fold difference from untrained (SED) MCT (n = 8). In fixed RV sections of MCT rats (black bars) and untreated healthy CON rats (gray bars), infiltration of CD45+ cells (lymphocytes, B) measured by immunofluorescent staining (count/field, mean ± SE) was not different from untrained animals (SED), either after a HIIT or CExT approach. Adjacent panels are representative images, with arrows indicating examples of CD45+ (red) cells, green representing wheat-germ agglutin-stained myocyte membrane, and blue representing nuclei. Terminal deoxynucleotidyl transferase-dUTP nick end-labeling (TUNEL) staining for myocyte apoptosis was also performed (% of TUNEL+ cells, mean ± SE, C). Adjacent panels are representative images, including a positive and negative control slide, with arrows indicating examples of TUNEL+ (bright green) cells and blue representing nonapoptotic nuclei. RV sections were additionally assessed for fibrosis with Masson’s trichrome (blue, in images) staining (D), and MCT-induced increase in RV fibrosis (expressed as fold difference from MCT-SED in %positively stained field) was less for HIIT-trained MCT. *P < 0.05 and **P < 0.01.
DISCUSSION
Our most important finding was that, while both HIIT and CExT attenuated MCT-induced decrement in aerobic capacity, only HIIT lowered pulmonary pressures and attenuated RV hypertrophy and dysfunction. Previous studies of chronic exercise effects in animals and in patients with PAH revealed mixed impact on hemodynamics, with one study demonstrating a small but statistically significant training-induced lowering of resting pulmonary pressures (23) but all other studies indicating no effect (17, 22, 24, 43). To our knowledge, this is the first exercise intervention associated with robust favorable impact on hemodynamics and the RV in PAH.
Our data show for the first time the chronic exercise-induced upregulation of endogenous pulmonary eNOS expression in PAH, which has previously been demonstrated in healthy animals and models of systemic vascular disease (34, 36, 52, 65). While immunoblotting data may not necessarily reflect nitric oxide (NO) bioavailability, increased eNOS increases NO production (44) and NO-dependent arterial relaxation (21, 33, 36), both typically impaired in PAH (35). We found an increase in total eNOS protein with HIIT but no change in phosphorylation pattern. This was not surprising since final exercise bout and tissue harvest were separated by 3 days to mitigate confounding effects of acute exercise on chronic adaptation. In our previous work, a single bout of moderate-intensity exercise (75% V̇o2max) induced acute pulmonary eNOS activation (as determined in serine and threonine phosphorylation) in lung tissue collected 1 h later and transiently normalized pulmonary pressure in a MCT (50 mg/kg) rat model of moderate PAH (9). In that work, telemetric recordings during running also indicated a running-induced acute pulmonary pressure reduction, as we have seen here (Fig. 3D) and, concomitancy with unchanged stroke volume [estimated by O2 pulse (V̇o2/heartbeat)], supported a mechanism of acute pulmonary resistance reduction (e.g., vasodilation) and not an acutely failing RV. We believe this also to be the case for the present telemetric observations of postrunning pulmonary pressure reduction (Fig. 3D). However, because assumptions about the relationship between oxygen consumption and heart rate are not upheld during non-steady-state running (precluding calculation of O2 pulse), it is not possible to ascertain relative contribution of acute changes in pulmonary resistance vs. RV contractility underlying the more pronounced acute pulmonary pressure relief evoked by HIIT.
The augmented exercise-induced pulmonary eNOS activation and enhanced acute postexercise pulmonary vasodilatory response in MCT-PAH rats (9) may be a consequence of higher flow-mediated shear forces. Only when applied as HIIT did chronic exercise result in greater total pulmonary eNOS protein and alleviation of pulmonary hypertension (Figs. 3 and 4) while mild intensity continuous exercise stimulus (50% V̇o2max, CExT) did not. The induction of eNOS protein and improved pulmonary pressures following HIIT but not CExT may be explained by a difference in the pulmonary vascular stimulation provided by the two training approaches. Telemetric recordings during HIIT and CExT sessions revealed very different exercise hemodynamic profiles generated by the two approaches where HIIT running was accompanied by quick-changing high-magnitude pulmonary pressures (Fig. 3C, left) in contrast to an elevated but relatively unchanging pulmonary pressure in CExT running (Fig. 3C, right). The ability for shear forces at the vessel wall to prompt bursts of NO production acutely and, when applied repeatedly, to enhance NO signaling machinery is enhanced in the presence of quick-changing high-magnitude shear as opposed to statically applied shear (6). This is in agreement with multiple studies demonstrating superior improvement in vascular function as assessed via brachial artery flow-mediated dilation with a HIIT approach compared with traditional continuous training (27, 58). Abnormal microvascular shear adaptation has been reported for a rat model of PAH induced by vascular endothelial growth factor receptor blocker sugen 5416 + hypoxia (57), as well as in patients (57), promoting lung endothelial injury and vessel remodeling (57). Therefore, future work should directly investigate if the quick-changing high-magnitude pulmonary pressures we observed during HIIT help to restore microvascular shear responses in PAH and how this relates to disease progression.
Improved pulmonary hemodynamics in HIIT-trained MCT may explain the pronounced reduction in resting RVSP measures (Fig. 3A) and RV hypertrophy (Fig. 5, A and B), which we interpret as evidence of benefit. Serial echocardiographic measures revealed lower pulmonary resistance and higher cardiac output (TPRi and cardiac index, Fig. 5, C and D) for HIIT-trained animals. Superior outcomes with HIIT may have also been derived from direct effects on the RV. Histological and biochemical assessment of the RV indicated a healthier myocardium for HIIT-trained MCT, including greater apelin expression (Fig. 7A), less fibrosis (Fig. 7D), and potentially less metabolic dependence on cytoplasmic glycolysis (Fig. 6, A and B). Apelin has been recognized as a potent inotropic substance and strong vasodilator, hence its increased interest as a potential treatment/biomarker of PAH (5) and its response to treatment, including exercise (63). We previously identified apelin to be an important contributor to RV function in our laboratory, which prompted us to include RV apelin expression as a secondary end point. To our knowledge, ours is the first report of training-induced changes in RV apelin. The higher RV apelin observed for MCT rats following 6 wk of HIIT may have contributed to the improved RV function observed in these animals via direct effects on myocardial contractility.
Our observations regarding training impact on indicators of glycolytic vs. oxidative metabolism are particularly relevant in light of the growing evidence that PAH is associated with an inefficient metabolic shift (56) in both cardiac and skeletal muscle mitochondrial substrate utilization from aerobic to anaerobic metabolism that contributes to diminished exercise tolerance (41, 49). Direct measures of myocyte metabolism were beyond the scope of this work; however, immunostaining and immunoblotting data (Fig. 6, A and B) indicate an interesting possible differential response of skeletal and cardiac muscle to training approach. More robust oxidative metabolic adaptations were observed in the RV myocardium in response to HIIT, whereas training-induced metabolic adaptations in the soleus were only observed for the CExT approach. This is in agreement with other studies that have reported divergent adaptive responses by muscle types, for example, in a Dahl sodium-sensitive rat model of hypertension, not only did CExT prompt greater oxidative metabolic adaptations compared with HIIT in skeletal muscle but findings in white vs. red gastrocnemius indicated a fiber-type specificity to this response (29). The absence of a robust skeletal muscle metabolic training adaptation in our HIIT rats may be a consequence of the lower absolute workloads and session duration of our protocol compared with that in other studies (46, 64) using more physically able disease models, and workloads set relative to calculated critical power (11), and thus may not have provided a sufficient stimulus to achieve these effects.
Exercise may worsen RV inflammation in PAH if coincident with excessive RV wall stress (28), as reported with high LV wall stress in an LV overload rodent model (55) and systemic hypertension rodent models (12, 51). Despite the high work rate run intervals, HIIT-treated rats had no evidence of heightened RV proinflammatory/apoptotic signaling for MCT, nor was this observed for CExT-trained MCT (Fig. 7, A-D), which is in contrast to that reported after 6 wk of treadmill (CExT-type) training for rats that received 60 mg/kg MCT (28). The reasons we did not observe increased inflammation may result from a less severe model of PAH and because we adjusted the treadmill workload relative to each animal’s postdisease V̇o2max, to avoid greater relative strain on animals with worse disease, and more closely reflect individualized exercise prescription for patients.
Limitations and Future Directions
First, the relationship between higher exercise pressures of HIIT and the positive adaptations we observed (enhancement of hemodynamics and exercise capacity, attenuation of RV maladaptive remodeling) is indirect and remains associative until future mechanistic experiments directly interrogate causation of these adaptations by HIIT’s higher exercise pressures. Second, we tested the effect of exercise on male rats with a mild PH phenotype. Although this may suggest the translation of our findings to clinical exercise interventions may be focused on early disease, it remains to be determined if prescribed training applications as we have tested here will have similar benefit with no detrimental RV effects in more angioproliferative severe PH, as well as in females. Given the known sexual dimorphism in PAH incidence (4), outcomes (31, 32), and acute exercise responses (38), sex differences in the training adaptations we have described here remain to be investigated. Last, the exercise sessions of the HIIT and CExT groups were performed with identical session frequency but were not time matched, with CExT sessions lasting 60 min but HIIT sessions only one-half of that duration. Whereas this more accurately reflects how HIIT is performed in clinical practice, it introduces the possibility that superior outcomes with HIIT are resultant from briefer session durations provoking less cumulative training-induced RV wall stress. Because calculated cumulative work performed over the 6 wk was comparable with HIIT and CExT, we believe that this alternative explanation is not as likely and chose to keep HIIT session durations unmatched to facilitate translation of findings to a customary HIIT prescription for patients. In terms of time cost, the shorter training sessions of HIIT may provide additional appeal to patients, since time constraints are commonly cited as barriers to exercise adherence (20). Not only may short bouts of higher-intensity exercise be more enjoyable than longer steady-state effort of continuous exercise (7), the anaerobic energy utilization required in HIIT may better mimic the physiological requirements of activities of daily living in those with cardiopulmonary disease (10).
Perspectives and Significance
Our report identifies for the first time that exercise training using a HIIT approach is superior to customary CExT for improving hemodynamics and RV remodeling and dysfunction in a rat model of PAH and does not promote RV inflammation or cardiomyocyte apoptosis. These outcomes with HIIT may be explained by pulsatile pulmonary vascular exposure to flow-shear stimulus promoting pulmonary eNOS upregulation, coupled with reduced fibrosis, apelin upregulation, and improved metabolic profile in the RV muscle. The pulmonary pressure- reducing and RV-preserving effect observed only with HIIT encourages further investigation of this alternative training approach in other models and in patients as a potentially more optimal exercise regimen for PAH.
GRANTS
This work was supported in part by the American Heart Association Midwest Affiliates Scientist Development Grant No. 13SDG17140066 (to M. B. Brown).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.B.B. and T.L. conception and design of research; M.B.B., E.N., G.M.L., J.B.G., B.A.G., A.W., M.O., and A.J.F. performed experiments; M.B.B., E.N., G.M.L., J.B.G., B.A.G., A.W., M.O., and T.L. analyzed data; M.B.B., E.N., G.M.L., A.J.F., R.G.P., I.P., J.A.K., and T.L. interpreted results of experiments; M.B.B. prepared figures; M.B.B. and G.M.L. drafted manuscript; M.B.B., E.N., G.M.L., I.P., J.A.K., and T.L. edited and revised manuscript; M.B.B., E.N., G.M.L., J.B.G., B.A.G., A.W., M.O., A.J.F., R.G.P., I.P., J.A.K., and T.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Andrea Frump for assistance with RV apelin assays. We additionally thank Marjorie Albrecht for assistance with lung and soleus biochemical assays.
REFERENCES
- 1.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985) 119: 753–758, 2015. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 121: 2045–2066, 2010. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu AS, Arena R, Myers J, Padmakumar R, Maiya AG, Cahalin LP, Waxman AB, Lavie CJ. Exercise intolerance in pulmonary hypertension: mechanism, evaluation and clinical implications. Expert Rev Respir Med 10: 979–990, 2016. doi: 10.1080/17476348.2016.1191353. [DOI] [PubMed] [Google Scholar]
- 4.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137: 376–387, 2010. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 5.Baker PN, Johnson IR. The use of the hand-grip test for predicting pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol 56: 169–172, 1994. doi: 10.1016/0028-2243(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 6.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JD, Close GL, MacLaren DP, Gregson W, Drust B, Morton JP. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci 29: 547–553, 2011. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]
- 8.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol 47: 1278–1283, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Brown MB, Chingombe TJ, Zinn AB, Reddy JG, Novack RA, Cooney SA, Fisher AJ, Presson RG, Lahm T, Petrache I. Novel assessment of haemodynamic kinetics with acute exercise in a rat model of pulmonary arterial hypertension. Exp Physiol 100: 742–754, 2015. doi: 10.1113/EP085182. [DOI] [PubMed] [Google Scholar]
- 10.Butcher SJ, Jones RL. The impact of exercise training intensity on change in physiological function in patients with chronic obstructive pulmonary disease. Sports Med 36: 307–325, 2006. doi: 10.2165/00007256-200636040-00003. [DOI] [PubMed] [Google Scholar]
- 11.Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588: 5077–5087, 2010. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Costa Rebelo RM, Schreckenberg R, Schlüter KD. Adverse cardiac remodelling in spontaneously hypertensive rats: acceleration by high aerobic exercise intensity. J Physiol 590: 5389–5400, 2012. doi: 10.1113/jphysiol.2012.241141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Man FS, Handoko ML, Groepenhoff H, van ’t Hul AJ, Abbink J, Koppers RJ, Grotjohan HP, Twisk JW, Bogaard HJ, Boonstra A, Postmus PE, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 34: 669–675, 2009. doi: 10.1183/09031936.00027909. [DOI] [PubMed] [Google Scholar]
- 14.Dubach P, Myers J, Dziekan G, Goebbels U, Reinhart W, Vogt P, Ratti R, Muller P, Miettunen R, Buser P. Effect of exercise training on myocardial remodeling in patients with reduced left ventricular function after myocardial infarction: application of magnetic resonance imaging. Circulation 95: 2060–2067, 1997. doi: 10.1161/01.CIR.95.8.2060. [DOI] [PubMed] [Google Scholar]
- 15.Dubé BP, Laveneziana P. Exploring cardio-pulmonary interactions by examining the ventilatory, pulmonary gas exchange, and heart rate kinetics response to high-intensity cycle exercise in COPD patients. Respir Physiol Neurobiol 219: 103–105, 2015. doi: 10.1016/j.resp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Falcão-Pires I, Gonçalves N, Henriques-Coelho T, Moreira-Gonçalves D, Roncon-Albuquerque R Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 296: H2007–H2014, 2009. doi: 10.1152/ajpheart.00089.2009. [DOI] [PubMed] [Google Scholar]
- 17.Fox BD, Kassirer M, Weiss I, Raviv Y, Peled N, Shitrit D, Kramer MR. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail 17: 196–200, 2011. doi: 10.1016/j.cardfail.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 308: L873–L890, 2015. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 62, Suppl: D60–D72, 2013. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590: 1077–1084, 2012. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gielen S, Sandri M, Erbs S, Adams V. Exercise-induced modulation of endothelial nitric oxide production. Curr Pharm Biotechnol 12: 1375–1384, 2011. doi: 10.2174/138920111798281063. [DOI] [PubMed] [Google Scholar]
- 22.Grünig E, Ehlken N, Ghofrani A, Staehler G, Meyer FJ, Juenger J, Opitz CF, Klose H, Wilkens H, Rosenkranz S, Olschewski H, Halank M. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 81: 394–401, 2011. doi: 10.1159/000322475. [DOI] [PubMed] [Google Scholar]
- 23.Grünig E, Lichtblau M, Ehlken N, Ghofrani HA, Reichenberger F, Staehler G, Halank M, Fischer C, Seyfarth HJ, Klose H, Meyer A, Sorichter S, Wilkens H, Rosenkranz S, Opitz C, Leuchte H, Karger G, Speich R, Nagel C. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 40: 84–92, 2012. doi: 10.1183/09031936.00123711. [DOI] [PubMed] [Google Scholar]
- 24.Grünig E, Maier F, Ehlken N, Fischer C, Lichtblau M, Blank N, Fiehn C, Stöckl F, Prange F, Staehler G, Reichenberger F, Tiede H, Halank M, Seyfarth HJ, Wagner S, Nagel C. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 14: R148, 2012. doi: 10.1186/ar3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 42: 587–605, 2012. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Hafstad AD, Boardman NT, Lund J, Hagve M, Khalid AM, Wisløff U, Larsen TS, Aasum E. High intensity interval training alters substrate utilization and reduces oxygen consumption in the heart. J Appl Physiol (1985) 111: 1235–1241, 2011. doi: 10.1152/japplphysiol.00594.2011. [DOI] [PubMed] [Google Scholar]
- 27.Hallmark R, Patrie JT, Liu Z, Gaesser GA, Barrett EJ, Weltman A. The effect of exercise intensity on endothelial function in physically inactive lean and obese adults. PLoS One 9: e85450, 2014. doi: 10.1371/journal.pone.0085450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handoko ML, de Man FS, Happé CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 120: 42–49, 2009. doi: 10.1161/CIRCULATIONAHA.108.829713. [DOI] [PubMed] [Google Scholar]
- 29.Holloway TM, Bloemberg D, da Silva ML, Quadrilatero J, Spriet LL. High-intensity interval and endurance training are associated with divergent skeletal muscle adaptations in a rodent model of hypertension. Am J Physiol Regul Integr Comp Physiol 308: R927–R934, 2015. doi: 10.1152/ajpregu.00048.2015. [DOI] [PubMed] [Google Scholar]
- 30.Holloway TM, Bloemberg D, da Silva ML, Simpson JA, Quadrilatero J, Spriet LL. High intensity interval and endurance training have opposing effects on markers of heart failure and cardiac remodeling in hypertensive rats. PLoS One 10: e0121138, 2015. doi: 10.1371/journal.pone.0121138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jaïs X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A, Vonk Noordegraaf A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 145: 1230–1236, 2014. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol (1985) 90: 1102–1110, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res 67: 187–197, 2005. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Konduri GG, Ou J, Shi Y, Pritchard KA Jr. Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 285: H204–H211, 2003. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 36.Kuru O, Sentürk UK, Koçer G, Ozdem S, Bașkurt OK, Cetin A, Yeșilkaya A, Gündüz F. Effect of exercise training on resistance arteries in rats with chronic NOS inhibition. J Appl Physiol (1985) 107: 896–902, 2009. doi: 10.1152/japplphysiol.91180.2008. [DOI] [PubMed] [Google Scholar]
- 37.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahm T, Frump AL, Albrecht ME, Fisher AJ, Cook TG, Jones TJ, Yakubov B, Whitson J, Fuchs RK, Liu A, Chesler NC, Brown MB. 17β-Estradiol mediates superior adaptation of right ventricular function to acute strenuous exercise in female rats with severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L375–L388, 2016. doi: 10.1152/ajplung.00132.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Rigel DF. Echocardiographic examination in rats and mice. Methods Mol Biol 573: 139–155, 2009. doi: 10.1007/978-1-60761-247-6_8. [DOI] [PubMed] [Google Scholar]
- 40.Mainguy V, Maltais F, Saey D, Gagnon P, Martel S, Simon M, Provencher S. Effects of a rehabilitation program on skeletal muscle function in idiopathic pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 30: 319–323, 2010. doi: 10.1097/HCR.0b013e3181d6f962. [DOI] [PubMed] [Google Scholar]
- 41.Mainguy V, Maltais F, Saey D, Gagnon P, Martel S, Simon M, Provencher S. Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 65: 113–117, 2010. doi: 10.1136/thx.2009.117168. [DOI] [PubMed] [Google Scholar]
- 42.Medicine ACoS ACSM’s Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 43.Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, Meyer FJ, Karger G, Buss J, Juenger J, Holzapfel N, Opitz C, Winkler J, Herth FF, Wilkens H, Katus HA, Olschewski H, Grünig E. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 114: 1482–1489, 2006. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 44.Miyauchi T, Maeda S, Iemitsu M, Kobayashi T, Kumagai Y, Yamaguchi I, Matsuda M. Exercise causes a tissue-specific change of NO production in the kidney and lung. J Appl Physiol (1985) 94: 60–68, 2003. doi: 10.1152/japplphysiol.00269.2002. [DOI] [PubMed] [Google Scholar]
- 45.Murray AJ. Taking a HIT for the heart: why training intensity matters. J Appl Physiol (1985) 111: 1229–1230, 2011. doi: 10.1152/japplphysiol.01078.2011. [DOI] [PubMed] [Google Scholar]
- 46.Musch TI. Effects of sprint training on maximal stroke volume of rats with a chronic myocardial infarction. J Appl Physiol (1985) 72: 1437–1443, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Osterling K, MacFadyen K, Gilbert R, Dechman G. The effects of high intensity exercise during pulmonary rehabilitation on ventilatory parameters in people with moderate to severe stable COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 9: 1069–1078, 2014. doi: 10.2147/COPD.S68011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandey A, Garg S, Khunger M, Garg S, Kumbhani DJ, Chin KM, Berry JD. Efficacy and safety of exercise training in chronic pulmonary hypertension: systematic review and meta-analysis. Circ Heart Fail 8: 1032–1043, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002130. [DOI] [PubMed] [Google Scholar]
- 49.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 88: 1011–1020, 2010. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 11: 216–222, 2004. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 51.Schultz RL, Swallow JG, Waters RP, Kuzman JA, Redetzke RA, Said S, de Escobar GM, Gerdes AM. Effects of excessive long-term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension 50: 410–416, 2007. doi: 10.1161/HYPERTENSIONAHA.106.086371. [DOI] [PubMed] [Google Scholar]
- 52.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 74: 349–353, 1994. doi: 10.1161/01.RES.74.2.349. [DOI] [PubMed] [Google Scholar]
- 53.Spruijt OA, de Man FS, Groepenhoff H, Oosterveer F, Westerhof N, Vonk-Noordegraaf A, Bogaard HJ. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 191: 1050–1057, 2015. doi: 10.1164/rccm.201412-2271OC. [DOI] [PubMed] [Google Scholar]
- 54.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 55.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 115: 1398–1407, 2007. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 56.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab 19: 558–573, 2014. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Szulcek R, Happé CM, Rol N, Fontijn RD, Dickhoff C, Hartemink KJ, Grünberg K, Tu L, Timens W, Nossent GD, Paul MA, Leyen TA, Horrevoets AJ, de Man FS, Guignabert C, Yu PB, Vonk-Noordegraaf A, van Nieuw Amerongen GP, Bogaard HJ. Delayed microvascular shear-adaptation in pulmonary arterial hypertension: role of PECAM-1 cleavage. Am J Respir Crit Care Med 93: 1410–1420, 2016. doi: 10.1164/rccm.201506-1231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tjønna AE, Rognmo Ø, Bye A, Stølen TO, Wisløff U. Time course of endothelial adaptation after acute and chronic exercise in patients with metabolic syndrome. J Strength Cond Res 25: 2552–2558, 2011. doi: 10.1519/JSC.0b013e3181fb4809. [DOI] [PubMed] [Google Scholar]
- 59.Weissmann N, Peters DM, Klöpping C, Krüger K, Pilat C, Katta S, Seimetz M, Ghofrani HA, Schermuly RT, Witzenrath M, Seeger W, Grimminger F, Mooren FC. Structural and functional prevention of hypoxia-induced pulmonary hypertension by individualized exercise training in mice. Am J Physiol Lung Cell Mol Physiol 306: L986–L995, 2014. doi: 10.1152/ajplung.00275.2013. [DOI] [PubMed] [Google Scholar]
- 60.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48: 1227–1234, 2014. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 61.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 62.Zafrir B. Exercise training and rehabilitation in pulmonary arterial hypertension: rationale and current data evaluation. J Cardiopulm Rehabil Prev 33: 263–273, 2013. doi: 10.1097/HCR.0b013e3182a0299a. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Ren CX, Qi YF, Lou LX, Chen L, Zhang LK, Wang X, Tang C. Exercise training promotes expression of apelin and APJ of cardiovascular tissues in spontaneously hypertensive rats. Life Sci 79: 1153–1159, 2006. doi: 10.1016/j.lfs.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 64.Zhang LQ, Zhang XQ, Musch TI, Moore RL, Cheung JY. Sprint training restores normal contractility in postinfarction rat myocytes. J Appl Physiol (1985) 89: 1099–1105, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Zhou M, Widmer RJ, Xie W, Jimmy Widmer A, Miller MW, Schroeder F, Parker JL, Heaps CL. Effects of exercise training on cellular mechanisms of endothelial nitric oxide synthase regulation in coronary arteries after chronic occlusion. Am J Physiol Heart Circ Physiol 298: H1857–H1869, 2010. doi: 10.1152/ajpheart.00754.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


