The retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) houses a conditional expiratory oscillator that emerges during metabolic challenges and generates active expiration. Herein we identify a 5-HT2-dependent mechanism in the RTN/pFRG that triggers active expiration at resting conditions. This mechanism is recruited during intermittent hypoxia exposure and essential for the development of expiratory long-term facilitation. These findings help to understand the central mechanisms underpinning the development of cardiorespiratory adaptations associated with hypoxia exposure.
Keywords: active expiration, 5-hydroxytryptamine, retrotrapezoid nucleus/parafacial respiratory group, intermittent hypoxia
Abstract
Abdominal expiratory activity is absent at rest and is evoked during metabolic challenges, such as hypercapnia and hypoxia, or after the exposure to intermittent hypoxia (IH). The mechanisms engaged during this process are not completely understood. In this study, we hypothesized that serotonin (5-HT), acting in the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG), is able to generate active expiration. In anesthetized (urethane, ip), tracheostomized, spontaneously-breathing adult male Holtzman rats we microinjected a serotoninergic agonist and antagonist bilaterally in the RTN/pFRG and recorded diaphragm and abdominal muscle activities. We found that episodic (3 times, 5 min apart), but not single microinjections of 5-HT (1 mM) in the RTN/pFRG elicited an enduring (>30 min) increase in abdominal activity. This response was amplified in vagotomized rats and blocked by previous 5-HT receptor antagonism with ketanserin (10 µM). Episodic 5-HT microinjections in the RTN/pFRG also potentiated the inspiratory and expiratory reflex responses to hypercapnia. The antagonism of 5-HT receptors in the RTN/pFRG also prevented the long-term facilitation (>30 min) of abdominal activity in response to acute IH exposure (10 × 6–7% O for 45 s every 5 min). Our findings indicate the activation of serotoninergic mechanisms in the RTN/pFRG is sufficient to increase abdominal expiratory activity at resting conditions and required for the emergence of active expiration after IH in anesthetized animals.
NEW & NOTEWORTHY
The retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) houses a conditional expiratory oscillator that emerges during metabolic challenges and generates active expiration. Herein we identify a 5-HT2-dependent mechanism in the RTN/pFRG that triggers active expiration at resting conditions. This mechanism is recruited during intermittent hypoxia exposure and essential for the development of expiratory long-term facilitation. These findings help to understand the central mechanisms underpinning the development of cardiorespiratory adaptations associated with hypoxia exposure.
in mammals, the contraction of inspiratory pumping muscles (mainly the diaphragm) generates the inspiratory flow. The expiratory airflow, however, occurs passively at resting conditions consequent to the recoil forces of the chest and the lungs (9, 20). The coordination of muscle contraction and relaxation relies on interacting brain stem neurons of the central respiratory rhythm and pattern generator (21, 57). Inspiratory neurons of the pre-Bötzinger complex (pre-BötC) in the ventrolateral medulla play a central role in the respiratory rhythmogenesis (58). Mutual interactions of the pre-BötC with other respiratory compartments, including the Bötzinger complex (BötC), the dorsolateral pons, and the dorsal respiratory group, are essential for the generation of the three-phase breathing pattern (7, 13, 57).
During metabolic challenges, such as physical exercise, hypoxia, and hypercapnia, the respiratory rhythm and pattern modify and increase ventilation (2, 29, 34). In addition to inspiratory activation, expiration transforms into an active process and abdominal contractions emerge, contributing to elevate tidal volume and respiratory frequency (32, 34). The parafacial respiratory group (pFRG) is suggested to be at the core of the neural circuitry generating the active expiration pattern (28, 30). This region is located ventral to the facial nucleus and partially overlapped with the chemosensitive neurons of the retrotrapezoid nucleus (RTN) (60, 62). The pFRG was originally described as a group of preinspiratory neurons that contribute to respiratory rhythmogenesis in neonates (49), a finding not confirmed in adult rats (22). Later studies reported that the pFRG houses conditional expiratory neurons that are silent at normocapnic/normoxic conditions, but fire rhythmically in conditions of hypercapnia (1), peripheral chemoreceptor stimulation (46), or reduced inhibitory drive (50). The fact that the hypercapnia-driven abdominal activity was abolished either by the pharmacological inhibition (1, 46) or the selective silencing of the phox2B-positive neurons of the RTN/pFRG (40) further supports the hypothesis that this region is essential for the generation of active expiration. However, the mechanisms required to stimulate the pFRG and bring about active expiration are not elucidated.
Serotonin (5-HT) is an important transmitter involved with the respiratory chemoreflex control (55). Activation of serotoninergic mechanisms also elicits synaptic plasticity that leads to persistent neuronal activation (3, 12, 64). Such serotonin-dependent plasticity is observed in medullary and motor inspiratory neurons after repetitive episodes of brief hypoxia, or intermittent hypoxia (IH), and underlies the IH-induced progressive increase in the inspiratory motor activity (4, 10, 27, 39). IH also promotes active expiration at resting conditions (33, 45, 66), which has been suggested to be dependent on the hyperactivity of the RTN/pFRG neurons (33, 43). However, it remains to be elucidated the mechanisms underpinning the development of active expiration following IH. Based upon the role of 5-HT in the scenario of respiratory adaptations induced by IH, in the present study we investigated the effects of 5-HT in the RTN/pFRG on the abdominal expiratory activity at resting conditions. We also explored whether the activation of serotoninergic mechanisms of the RTN/pFRG is required to generate active expiration after IH.
MATERIAL AND METHODS
Animals and ethical approval.
Experiments were performed on male adult Holtzman rats (280–290 g) obtained from the Animal Breeding Centre of the São Paulo State University, Araraquara. The animals were kept at 22 ± 1°C on a 12:12-h light/dark cycle (lights on 0700 – lights off 1900) with access to food and water ad libitum. All experimental procedures followed the Guide for the Care and Use of Laboratory Animals published by the Brazilian National Council for Animal Experimentation Control (CONCEA) and by the National Institutes of Health (NIH publication No. 85–23 revised 1996) and were approved by the Local Ethical Committee in Animal Experimentation (protocol 21/2012).
Experimental preparation.
Surgical procedures and experiments were performed upon anesthetized (urethane, 1.2 g/kg ip; pH 7.4 regulated with sodium bicarbonate) rats. The level of anesthesia was confirmed by the absence of corneal and toe-pinch withdrawal reflexes. When necessary additional doses of urethane were administrated (10–20% of initial dose). Body temperature was maintained at 36–38°C. Animals were initially placed in supine position and a cervical incision was made to perform tracheostomy and bilateral vagotomy (the latter only in a subgroup of animals). Polyethylene catheters (PE-50 connected to PE-10; Clay Adams, Parsippany, NJ) were inserted into the right femoral artery and vein for arterial pressure measurements and systemic administration of drugs, respectively. Bipolar stainless steel electrodes were implanted in the diaphragm (DIA) and in the oblique abdominal muscles (ABD) to perform electromyographic (EMG) recordings of inspiratory and expiratory motor activities, respectively. After the catheter and electrodes implants, the animals were then placed in a stereotaxic apparatus (David Kopf, Tujunga, CA) to perform microinjections in the RTN/pFRG, using the following stereotaxic coordinates (52): 2.6 mm caudal from lambda; 1.8 mm lateral from the longitudinal suture, and 11.0 mm ventral from the dorsal surface. Animals breathed spontaneously and were maintained at 99.9% oxygen (O2) during surgery and experimental protocols, except when exposed to hypoxia or hypercapnia. Slow intravenous administration (3–4 ml·kg−1·h−1) of Ringer solution (in mM: 125 NaCl, 24 NaHCO3, 3 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, 10 dextrose) containing lactate (2 mM) was performed during the experiment to minimize deviations in blood pH and to maintain fluid balance (34, 63). A period of at least 30 min was allowed for stabilization before starting the experimental protocols.
Recordings of cardiorespiratory parameters.
DIA and ABD signals were amplified (P511, Grass Technologies, Middleton, WI) and band-pass filtered (0.1–1 kHz). Pulsatile arterial pressure (PAP) was recorded by connecting the arterial catheter to a pressure transducer (model MLT0380; ADInstruments) and, in turn, to an amplifier (AVS Projects, São Carlos, Brazil). The EMG and PAP signals were acquired using an A/D converter [model 1401, Cambridge Electronic Design (CED) Cambridge, UK] and recorded at 2-kHz sampling rate (Spike 2, version 7, CED). Values of mean arterial pressure (MAP, mmHg) and heart rate (HR, beats/min) were derived from PAP signals. The EMG signals were rectified and smoothed (time constant: 50 ms). DIA activity was evaluated by its burst amplitude (mV) and frequency (cycles per minute, cpm). ABD activity was assessed by its mean activity (mV). The changes in the DIA and ABD activities were expressed as percentage values (%) in relation to basal activity. The changes in the other parameters were evaluated in their raw units. All analyses were performed offline using the Spike 2 software.
Acute intermittent hypoxia (AIH) and hypercapnia.
The anesthetized animals were exposed to AIH and hypercapnia, as previously described (16, 33, 34, 63). The AIH paradigm consisted of 10 brief episodes of hypoxia (6–7% O2, balanced in N2, for 45 s) interspersed by 5 min of hyperoxia (99.9% O2). For the induction of hypercapnia, the animals were exposed to a mixture containing 10% CO2 (balanced in O2) for 5 min. The hypoxic and hypercapnic gases were initially humidified and then administrated to the animals through the tracheal cannula using a gas mixer device (AVS Projetos, São Carlos, Brazil) connected to a gas analyzer (model ML2016, ADInstruments, Bella Vista, NSW, Australia) and to cylinders of pure N2 and O2 (White Martins, São Carlos, Brasil).
In a subgroup of anesthetized animals, we measured the arterial partial pressure of O2 () and pH after AIH exposure. To this, samples of arterial blood (50 µl) were collected (through the catheter implanted into the femoral artery) and analyzed using a blood analyzer (i-SAT handheld, Abbot, IL). Measurements were performed before and at 30 and 60 min after the exposure to AIH.
Experimental protocols.
Baseline cardiorespiratory parameters were recorded initially for 15–20 min. In the first set of experiments, we explored the effects of the activation of serotoninergic mechanisms in the RTN/pFRG on the respiratory and cardiovascular parameters. Based on previous studies (38, 47), single and episodic (3 times, 5 min apart) bilateral microinjections of 5-HT (1 mM; Sigma-Aldrich, St. Louis, MO) or vehicle (Ringer’s solution) were performed in the RTN/pFRG of either vagus-intact or vagotomized animals. A 5-min time interval was given between consecutive 5-HT microinjections in the RTN/pFRG to reproduce the same time interval of hypoxic stimuli during IH exposure. Baseline cardiorespiratory parameters were monitored for 60 min after microinjections. A separate group of vagus-intact rats were exposed to hypercapnia (10% CO2 for 5 min) before, and 30 and 60 min after the microinjections of 5-HT, and the cardiorespiratory reflex responses were analyzed. In another group of vagotomized rats, the episodic microinjections of 5-HT in the RTN/pFRG were preceded (5 min) by bilateral microinjections of vehicle or ketanserin (10 µM; RBI, Natick, MA), a 5-HT2A/C antagonist (41, 53), to verify the contribution of the 5-HT2 receptors in the 5-HT-induced cardiorespiratory changes. To determine if 5-HT2 receptor antagonism in the RTN/pFRG affected baseline variables, a group of vagotomized animals received bilateral microinjections of ketanserin and were monitored for 60 min.
To investigate the involvement of 5-HT and 5-HT2 receptors in the development of AIH-induced respiratory changes, bilateral microinjections of vehicle or ketanserin were performed in the RTN/pFRG of vagus-intact rats immediately before and during the exposure to AIH (between the 3rd and 4th, and between the 6th and 7th episodes). The rationale to perform the ketanserin microinjections during the AIH was to guarantee the effectiveness of the 5-HT2 receptor antagonism throughout the period of exposure, since we observed that, in our experimental conditions, the effects of ketanserin in the RTN/pFRG on the 5-HT responses were partially reversed after 20–30 min (data not shown). All drugs were dissolved in Ringer’s solution with pH 7.4 and microinjected manually in the RTN/pFRG using a needle (30 gauge) connected to a 1-μl syringe (Hamilton Company, Reno, NV) through a P10 catheter. The volume of microinjections was 50 nl.
Histology.
At the end of the experiments, animals received bilateral microinjections of Evans Blue dye (50 nl, 2% Vetec, Fine Chemicals, Rio de Janeiro, Brazil) in the RTN/pFRG and were perfused transcardiacally with saline and formalin solution (10%). The brain was removed, fixed in formalin solution (10%) for 48 h, frozen, and cut in 50-μm slices. The sections were stained using the Giemsa method and analyzed with light microscopy.
Statistical analyses.
The data are expressed as means ± standard error of mean (SE). The evoked responses to 5-HT microinjections in the RTN/pFRG, the effects of 5-HT2 antagonism in RTN/pFRG, and the changes elicited by AIH were analyzed using two-way ANOVA with repeated measurements followed by Bonferroni’s tests for multiple comparisons (intra- and between experimental groups). The comparisons were carried out using GraphPad Prism software (version 5, La Jolla, CA), and differences were considered significant when P < 0.05.
RESULTS
Episodic microinjections of 5-HT in the RTN/pFRG generate active expiration.
At the moment of the injection, unilateral microinjection of 5-HT (1 mM) in the RTN/pFRG of urethane-anesthetized rats did not modify the cardiorespiratory parameters of both vagus-intact and vagotomized animals. In contrast, long-term cardiorespiratory changes were observed after bilateral microinjections of 5-HT in the RTN/pFRG, which differed according to the pattern of injections. Single 5-HT microinjections in the RTN/pFRG of vagus-intact animals (n = 8, Fig. 1A) evoked a modest increase (~10%) in DIA burst amplitude at 30 min after the injections (P < 0.05, Fig. 1E). A slight increase was observed in the ABD activity (Fig. 1C), which was not statistically significant (Fig. 1G). No changes were observed in the other parameters evaluated (Figs. 1, F–I). In the group of vagus-intact rats that received episodic 5-HT in the RTN/pFRG (n = 7, Fig. 1B), the DIA amplitude and frequency remained unchanged after microinjections (Figs. 1, E and F). On the other hand, the ABD activity exhibited an irregular (not observed in every respiratory cycle) but sustained increase that lasted, at least, 60 min (P < 0.05, Fig. 1G). The MAP and HR remained the same after episodic microinjections of 5-HT in the RTN/pFRG. Microinjections of vehicle (n = 5) did not produce any cardiorespiratory changes (Fig. 1, E–I). The sites of 5-HT microinjections were centered ventromedially in relation to the caudal pole of the facial nucleus, as illustrated in Fig. 1J. The group data are summarized in the Table 1.
Fig. 1.
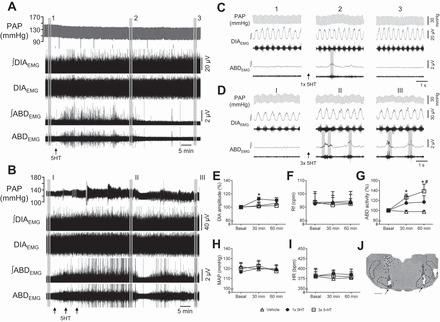
Episodic microinjections of 5-HT in the RTN/pFRG evoke abdominal expiratory activity in anesthetized vagus-intact rats. A and B: pulsatile arterial pressure (PAP), raw and integrated (∫) diaphragmatic (DIAEMG) and abdominal (ABDEMG) electromyographic recordings of vagus-intact animals, representative from their respective group, illustrating the effects of single (A, arrow) and episodic (B, arrows) bilateral microinjections of 5-HT in the RTN/pFRG. C and D: expanded tracings from A and B, respectively, illustrating the PAP recordings and the integrated DIAEMG and ABDEMG activities before (1 and I) and at 30 (2 and II) and 60 min (3 and III) after microinjections. E–I: average values of DIA burst amplitude (E) and frequency (F), ABD mean activity (G), mean arterial pressure (MAP, H), and heart rate (HR; I) before (basal) and after microinjections of vehicle (n = 5), single 5-HT (n = 8) and episodic 5-HT (n = 7) in the RTN/pFRG. J: photomicrography of a coronal slice of the brain stem from a representative rat of the group, showing the sites of microinjections in the RTN/pFRG (arrows). Abbreviations: VII, facial nucleus; py, pyramidal tract; RPa, raphe pallidus nucleus; sp5, spinal trigeminal tract. *Different from respective basal values, P < 0.05. #Different from vehicle group at the corresponding time, P < 0.05.
Table 1.
Cardiorespiratory changes elicited by microinjections of vehicle (n = 5), single 5-HT (n = 8), and episodic 5-HT (n = 7) in the RTN/pFRG of vagus-intact anesthetized rats
| Vehicle |
Single 5-HT |
Episodic 5-HT |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | 30 min | 60 min | Basal | 30 min | 60 min | Basal | 30 min | 60 min | |
| DIA, % | 100 | 105 ± 4 | 108 ± 7 | 100 | 113 ± 3* | 111 ± 5 | 100 | 101 ± 6 | 102 ± 9 |
| Rf, cpm | 93 ± 8 | 93 ± 7 | 94 ± 7 | 92 ± 2 | 93 ± 3 | 93 ± 3 | 93 ± 6 | 90 ± 6 | 92 ± 6 |
| ABD, % | 100 | 98 ± 3 | 98 ± 2 | 100 | 115 ± 13 | 117 ± 12 | 100 | 126 ± 6* | 139 ± 14*# |
| MAP, mmHg | 126 ± 1 | 123 ± 2 | 123 ± 1 | 116 ± 5 | 120 ± 6 | 118 ± 5 | 119 ± 5 | 122 ± 5 | 117 ± 5 |
| HR, beats/min | 390 ± 21 | 384 ± 24 | 386 ± 24 | 381 ± 16 | 387 ± 6 | 384 ± 7 | 382 ± 9 | 381 ± 8 | 379 ± 9 |
Values are means ± SE DIA, diaphragmatic burst amplitude; Rf, respiratory frequency; ABD, abdominal mean activity; MAP, mean arterial pressure; HR, heart rate.
Different from respective baseline, P < 0.05.
Different from vehicle group at the corresponding time, P < 0.05.
By the fact that vagal afferent inputs may exert an inhibitory influence on the abdominal expiratory activity in anesthetized rats (34), 5-HT microinjections were also performed in the RTN/pFRG of vagotomized rats to better visualize its excitatory effects on the control of abdominal expiratory activity (Fig. 2). Episodic 5-HT (n = 6) in the RTN/pFRG evoked a substantial increase in ABD activity (P < 0.01) accompanied by a marked reduction in the DIA burst amplitude (P < 0.0001), with no changes in burst frequency (Figs. 2, E–G). No significant changes were observed in the MAP and HR (Figs. 2, H and I). Prior injections of the 5-HT2 receptor antagonist, ketanserin, in the RTN/pFRG of vagotomized rats (n = 5) prevented the increase in ABD activity as well as the depression in DIA amplitude elicited by episodic 5-HT microinjections (Fig. 2, E–G). Microinjections of vehicle in the RTN/pFRG of vagotomized rats (n = 4) did not promote changes in the cardiorespiratory parameters analyzed (Fig. 2, E–I). The sites of 5-HT, ketanserin, and vehicle microinjections in vagotomized groups were centered ventromedially in relation to the facial nucleus, as illustrated in Fig. 2J. The group data are summarized in the Table 2.
Fig. 2.
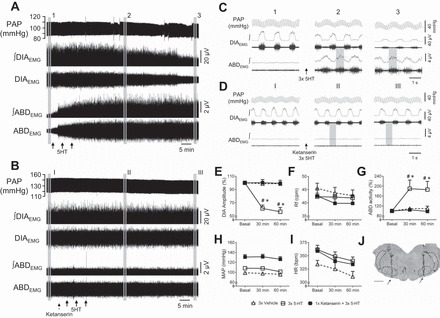
The antagonism of 5-HT2 receptors blocks the emergence of abdominal activity in response to episodic microinjections of 5-HT in the RTN/pFRG of anesthetized vagotomized rats. Pulsatile arterial pressure (PAP), raw and integrated (∫) diaphragmatic (DIAEMG) and abdominal (ABDEMG) electromyographic recordings of vagotomized animals, representative from their respective group, illustrating the cardiorespiratory changes elicited by episodic 5-HT microinjections in the RTN/pFRG (A, solid arrows) that were prevented by previous 5-HT2 receptor antagonism with ketanserin (B, dashed arrow). C and D: expanded tracings from A and B, respectively, illustrating the PAP recordings and the integrated DIAEMG and ABDEMG activities before (1 and I) and at 30 (2 and II) and 60 min (3 and III) after microinjections. E–I: average values of DIA burst amplitude (E) and frequency (F), ABD mean activity (G), mean arterial pressure (MAP, H) and heart rate (HR, I) before (basal) and after microinjections of vehicle (n = 4), episodic 5-HT (n = 6) and ketanserin + episodic 5-HT (n = 5) in the RTN/pFRG. J: photomicrography of a coronal slice of the brain stem from a representative rat of the group, showing the sites of microinjections in the RTN/pFRG (arrows). VII, facial nucleus; py, pyramidal tract; RPa, raphe pallidus nucleus; sp5, spinal trigeminal tract. *Different from respective basal values, P < 0.05. #Different from vehicle group at the corresponding time, P < 0.05.
Table 2.
Cardiorespiratory changes induced by microinjections of vehicle (n = 4), episodic 5-HT (n = 6) and ketanserin + episodic 5-HT (n = 5) in the RTN/pFRG of vagotomized anesthetized rats
| Vehicle |
Episodic 5-HT |
Ketanserin + Episodic 5-HT |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | 30 min | 60 min | Basal | 30 min | 60 min | Basal | 30 min | 60 min | |
| DIA, % | 100 | 99 ± 4 | 97 ± 2 | 100 | 61 ± 5*# | 56 ± 6*# | 100 | 100 ± 5 | 99 ± 5 |
| Rf, cpm | 45 ± 1 | 43 ± 2 | 42 ± 2 | 42 ± 1 | 42 ± 1 | 41 ± 1 | 43 ± 3 | 40 ± 3 | 40 ± 3 |
| ABD, % | 100 | 109 ± 5 | 110 ± 9 | 100 | 189 ± 35*# | 186 ± 33*# | 100 | 104 ± 4 | 98 ± 2 |
| MAP, mmHg | 99 ± 1 | 97 ± 1 | 95 ± 2 | 108 ± 1 | 108 ± 1 | 101 ± 2 | 131 ± 5 | 131 ± 5 | 126 ± 4 |
| HR, beats/min | 334 ± 7 | 325 ± 11 | 310 ± 9 | 361 ± 8 | 348 ± 8 | 340 ± 7 | 359 ± 5 | 340 ± 7 | 334 ± 6 |
Values are means ± SE. DIA, diaphragmatic burst amplitude; Rf, respiratory frequency; ABD, abdominal mean activity; MAP, mean arterial pressure; HR, heart rate.
Different from respective baseline, P < 0.05.
Different from vehicle group at the corresponding time, P < 0.05.
Bilateral microinjections of ketanserin in the RTN/pFRG of rats (n = 4) did not promote changes in baseline DIA burst amplitude (100 vs. 95 ± 6 vs. 94 ± 6%, respectively, basal, 30 and 60 min after microinjections) and frequency (92 ± 5 vs. 91 ± 6 vs. 91 ± 6 cpm, respectively, basal, 30 and 60 min after microinjections), ABD activity (100 vs. 99 ± 4 vs. 98 ± 2%, respectively, basal, 30 and 60 min after microinjections), MAP (119 ± 4 vs. 116 ± 3 vs. 117 ± 4 mmHg, respectively, basal, 30 and 60 min after microinjections) and HR (379 ± 12 vs. 381 ± 11 vs. 381 ± 11 beats/min, respectively, basal, 30 and 60 min after microinjections).
In a separate group of vagotomized animals (n = 4) with off-target injections located rostral to the RTN/pFRG, close to the rostral pole of the facial nucleus, episodic microinjections of 5-HT did not alter the following variables 30 and 60 min later from those at baseline: 1) normalized DIA burst amplitude (Δ: 4 ± 7 and 7 ± 3%); 2) respiratory frequency (Δ: 0 ± 2 and −1 ± 1 cpm); 3) normalized ABD activity (Δ: −7 ± 5 and −6 ± 6%); 4) MAP (Δ: 4 ± 2 and 1 ± 2 mmHg); and 5) HR (Δ: −6 ± 15 and −10 ± 12 beats/min). These data suggest that the observed cardiorespiratory changes elicited by 5-HT were mostly due to its actions in the RTN/pFRG.
Enhanced inspiratory and expiratory responses to hypercapnia after episodic 5-HT microinjections in the RTN/pFRG.
Before microinjections of vehicle (n = 5), vagus-intact rats exposed to hypercapnia presented an increase in the DIA amplitude (Δ = 80 ± 10%, P < 0.001, Fig. 3B) accompanied by a slight reduction in the respiratory frequency (Δ = −9 ± 2 cpm, P < 0.05, Fig. 3C), no changes in the ABD activity (Δ = −1 ± 3%, Fig. 3D) and MAP (Δ = 4 ± 2 mmHg, Fig. 3E), and bradycardia (Δ = −23 ± 6 bpm, P < 0.05, Fig. 3F). Similar responses were observed in the rats from the other experimental groups before microinjections of 5-HT in the RTN/pFRG (Fig. 3, B–F). Bilateral microinjections of vehicle or single 5-HT (n = 5) in the RTN/pFRG did not promote significant changes in the magnitude and pattern of the cardiorespiratory responses to hypercapnia (Fig. 3, B–F). On the other hand, episodic 5-HT in the RTN/pFRG (n = 6) amplified the magnitude of CO2-induced change in the DIA amplitude at 30 (Δ = 111 ± 17%, P < 0.01) and 60 min (Δ = 118 ± 11%, P < 0.05) after microinjections (Fig. 3, A and B). The ABD response, which was absent before microinjections, was evident at 30 min (Δ = 62 ± 21%), although not statistically significant (P > 0.05), and markedly potentiated 60 min (Δ = 137 ± 26%, P < 0.01) after the episodic microinjections (Fig. 3, A and D). At 60 min, a very mild hypotensive response to hypercapnia (Δ = −2 ± 1 mmHg; P < 0.05) was also observed after episodic 5-HT microinjections in the RTN/pFRG (Fig. 3E). The magnitude of changes in the DIA frequency and HR were not modified after microinjections (Fig. 3, C and F).
Fig. 3.
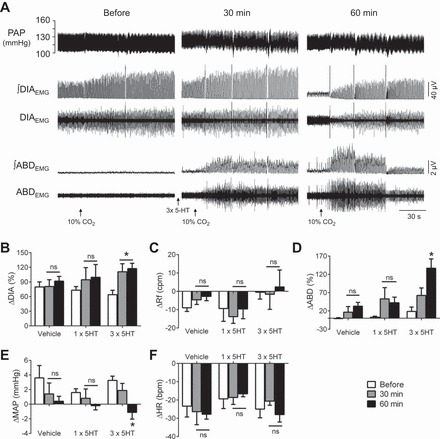
Episodic microinjections of 5-HT in the RTN/pFRG potentiate inspiratory and expiratory responses to hypercapnia. A: pulsatile arterial pressure (PAP), raw and integrated (∫) diaphragmatic (DIAEMG) and abdominal (ABDEMG) electromyographic recordings of a vagus-intact rat, representative from the group, illustrating the cardiorespiratory reflex responses to hypercapnia (10% CO2, exposure period initiation indicated by the arrow) before and at 30 and 60 min after the episodic microinjections of 5-HT in the RTN/pFRG. B–F: average values of the magnitude of the reflex changes in DIA burst amplitude (B) and frequency (C), ABD mean activity (D), mean arterial pressure (MAP, E) and heart rate (HR, F) before (basal) and after microinjections of vehicle (n = 5), single 5-HT (n = 5) and episodic 5-HT (n = 6) in the RTN/pFRG. *Different from respective basal values, P < 0.05.
5-HT2 antagonism in the RTN/pFRG prevented the emergence of active expiration induced by acute intermittent hypoxia.
As previously described (34), anesthetized vagus-intact rats exposed to acute hypoxia (6–7% O2 for 45 s) exhibited increases in DIA burst frequency and amplitude accompanied by hypotension and no significant changes in ABD activity and HR (Fig. 4A). In the group of animals that received microinjections of vehicle in the RTN/pFRG (n = 4), AIH exposure did not elicit significant changes in basal DIA burst amplitude (Δ = 3 ± 1 and 3 ± 2%, respectively, 30 and 60 min after AIH; Fig. 4E) and frequency (90 ± 8, 94 ± 7, and 93 ± 8 cpm, respectively, before, 30 and 60 min after AIH; Fig. 4F). On the other hand, AIH evoked a progressive and persistent increase in basal ABD activity (Δ = 28 ± 5 and 40 ± 9%, respectively, 30 and 60 min after AIH, P < 0.05; Fig. 4G), characterized by episodic rather than rhythmic expiratory bursts (Fig. 4B). This AIH-induced ABD hyperactivity was not associated with changes in arterial Po2 and pH. Their values at baseline were 460 ± 26 mmHg, pH 7.35 ± 0.01, and were not statistically different at 30 min (488 ± 9 mmHg, 7.33 ± 0.02) or at 60 min (435 ± 43 mmHg, 7.35 ± 0.02) after AIH. With respect to basal cardiovascular parameters, MAP levels did not modify after AIH (135 ± 3, 136 ± 3, and 132 ± 4 mmHg, respectively, before, 30 and 60 min after AIH, Fig. 4H) while HR exhibited a significant increase at 60 min after the exposure (365 ± 20, 371 ± 19, and 376 ± 17 beats/min, respectively, before, 30 and 60 min after AIH, Fig. 4I).
Fig. 4.
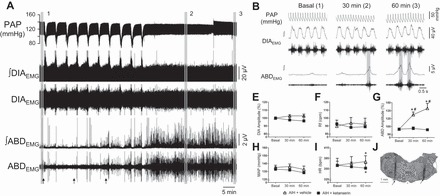
The acute intermittent hypoxia-induced long-term facilitation of abdominal activity is prevented by the antagonism of 5-HT2 receptors in the RTN/pFRG of anesthetized rats. A: pulsatile arterial pressure (PAP), raw and integrated (∫) diaphragmatic (DIAEMG) and abdominal (ABDEMG) electromyographic recordings of a vagus-intact animal, representative from the group, illustrating the changes elicited by the exposure to acute intermittent hypoxia (AIH) on basal cardiorespiratory parameters. The arrows indicate the time of vehicle injections. B: expanded tracings from A, illustrating the PAP recordings and the integrated DIAEMG and ABDEMG activities before (1 and I) and at 30 (2 and II) and 60 min (3 and III) after AIH. E–I: average values of DIA burst amplitude (E) and frequency (F), ABD mean activity (G), mean arterial pressure (MAP, H) and heart rate (HR, I) before (basal) and after AIH combined with microinjections of vehicle (n = 4) or ketanserin (n = 5) in the RTN/pFRG. J: photomicrography of a coronal slice of the brain stem from a representative rat of the group, showing the sites of microinjections in the RTN/pFRG (arrows). Abbreviations: VII, facial nucleus; py, pyramidal tract; RPa, raphe pallidus nucleus; sp5, spinal trigeminal tract. *Different from respective basal values, P < 0.05. #Different from vehicle group at the corresponding time, P < 0.05.
In animals that received microinjections of ketanserin in the RTN/pFRG before and during the exposure to AIH (n = 5), the development of augmented ABD activity (Δ = 1 ± 3 and −1 ± 1%, respectively, 30 and 60 min after AIH; Fig. 4G) and tachycardia (365 ± 11, 361 ± 13 and 357 ± 13 beats/min, respectively, before, 30 and 60 min after AIH, Fig. 4I) was completely prevented. The other cardiorespiratory parameters analyzed were not modified either by AIH or by the 5-HT2 antagonism (Fig. 4, E, F, and H). The sites of bilateral ketanserin microinjections in the RTN/pFRG are illustrated on Fig. 4J.
DISCUSSION
Abdominal expiratory contractions are observed in conditions of metabolic challenges as a reflex mechanism to increase ventilation (32, 34). Neurons of the RTN/pFRG region are suggested to compose the conditional expiratory oscillator, which plays a central role in the generation of active expiratory pattern (1, 28, 30). In the present study, we provide evidence that 5-HT, acting on 5-HT2 receptors, stimulates the RTN/pFRG oscillator and transforms expiration into an active process in anesthetized animals at resting conditions (hyperoxia/normocapnia). We also demonstrate that the blockade of this 5-HT2-dependent mechanism in the RTN/pFRG is able to prevent the emergence of long-term abdominal hyperactivity elicited by acute IH. Our data indicate the presence of a relevant serotoninergic mechanism at the level of RTN/pFRG, which plays an excitatory role and contributes to the generation of active expiration, especially in conditions of acute IH.
Excitatory effects of 5-HT in the RTN/pFRG and generation of abdominal activity.
Serotoninergic neurons, in particular those located within the medulla oblongata, innervate several nuclei involved with the control of respiratory activity and exert a potent modulatory effect on breathing (11, 14, 36, 55, 56). Previous studies have shown that 5-HT, acting on chemosensitive neurons of the RTN, causes an increase in phrenic nerve activity by a mechanism involving 5-HT2 Gq-mediated inhibition of KCNQ channels (26) and 5-HT7 Gs-mediated activation of HCN channels (25). In our experimental conditions, 5-HT microinjections in the RTN/pFRG did not evoke any acute respiratory response at the moment of injections. By the fact that 5-HT stimulates chemosensitive neurons by a pH-independent mechanism (47), the basal activity of RTN neurons may determine the impact of 5-HT on respiratory activity. In this regard, we speculate that in our experimental conditions (ip urethane anesthesia, spontaneously breathing rats), basal activity of the RTN neurons might be different compared with previous studies, which may explain the absent of acute respiratory responses to 5-HT.
Despite the lack of acute cardiorespiratory changes, we verified that episodic, but not single 5-HT microinjections in the RTN/pFRG promoted sustained increase in the abdominal activity of vagus-intact animals. This effect is in agreement with the notion that 5-HT may induce short- and long-term plasticity that causes persistent neuronal activation (10, 64). Interestingly, the magnitude of this response was markedly amplified in vagotomized rats due to the absence of vagal-dependent inhibition on the mechanisms generating abdominal activity (34). There is evidence suggesting that serotonin-dependent plasticity is mediated, at least in part, by the activation of 5-HT2 receptors (10). We verified that abdominal hyperactivity elicited by episodic 5-HT in the RTN/pFRG was fully prevented by prior microinjections of ketanserin, suggesting the involvement of 5-HT2 receptors (41, 53). Accordingly, we propose that 5-HT in the RTN/pFRG, acting on 5-HT2 receptors, may cause persistent activation of the neurons that compose the expiratory oscillator. By the fact that single 5-HT microinjections in the RTN/pFRG did not cause effects of the same magnitude and duration, we hypothesize that the modulatory effect of 5-HT on the expiratory oscillator might be pattern dependent.
In addition to the increased basal abdominal activity, episodic microinjections of 5-HT in the RTN potentiated the inspiratory and expiratory responses to hypercapnia. Our findings agree with Mulkey et al. (47) demonstrating that 5-HT sensitizes RTN neurons and enhances their response to acidification by a 5-HT2-mediated, pH-independent mechanism. By the fact that chemosensitive Phox2-b neurons of the RTN are essential for the emergence of active expiration in conditions of hypercapnia (40), the sensitization of chemosensitive cells in the RTN by episodic 5-HT microinjections may be considered as a potential mechanism leading to the emergence of abdominal hyperactivity at resting conditions. In this regard, it might be intuitive to expect that inspiratory motor activity would also increase. In contrast, we noticed that diaphragmatic activity slightly increased after single 5-HT microinjections and did not change after episodic 5-HT microinjections in vagus-intact animals. In vagotomized rats, episodic 5-HT in the RTN/pFRG promoted a marked decrease in diaphragm burst amplitude, an effect that was completely prevented by prior 5-HT2 antagonism. It has been demonstrated that the activation of expiratory neurons of the RTN/pFRG leads to the stimulation of the inhibitory augmenting-expiratory neurons (aug-E) of the BötC (1). These neurons are suggested to establish inhibitory connections with inspiratory neurons of the pre-BötC, the rostral ventral respiratory group and phrenic motor nucleus (19, 57). Thereby, the reduction in diaphragm burst amplitude after episodic 5-HT microinjections in the RTN/pFRG of vagotomized rats may be consequent to the exaggerated activation of RTN/pFRG expiratory neurons that, in turn, lead to the activation of inspiratory-inhibiting BötC neurons, a hypothesis that requires additional experiments to be elucidated. By the fact that the level of excitation of RTN/pFRG neurons may have been smaller in vagus-intact animals that received episodic or single microinjections of 5-HT [due to the presence of inhibitory feedback from pulmonary afferents (34)], the changes in diaphragm activity were absent or even rapidly evident, respectively.
We cannot exclude the possibility that the effects induced by episodic microinjections of 5-HT were consequent to the spread of the drug to areas adjacent to the RTN/pFRG, such as the raphe nucleus and the ventral respiratory column (VRC). We verified that microinjections of 5-HT in the rostral portion of the RTN (close to the rostral pole of facial nuclei) did not evoke changes in the cardiorespiratory parameters evaluated. The centers of those microinjections in the rostral RTN were located at the same level of the raphe magnus nucleus, which has been demonstrated to be involved in the processing of ventilatory responses to hypercapnia (15). Based upon these observations, we suggest that the changes in basal and chemoreflex abdominal activity induced by microinjections in the RTN/pFRG may have not been related to the spread of 5-HT to the adjacent raphe nucleus. With respect to the VRC, previous studies documented that 5-HT, acting at the level of BötC and pre-BötC, promotes sympathoinhibition (42) and inspiratory excitation (10, 54), responses that were not observed in the present study. Therefore, the modulatory effects of 5-HT on the expiratory oscillator may be mainly dependent on the activation of serotoninergic mechanisms in the RTN/pFRG.
Long-term facilitation of expiratory motor activity induced by IH depends on the serotoninergic mechanisms in the RTN/pFRG.
Acute IH elicits a compensatory increase in basal ventilation that is suggested to be associated with the long-term facilitation of inspiratory motor outputs, including phrenic, hypoglossal, and intercostal nerves (5, 16, 24). Different from these studies, we did not observe significant increases in diaphragm burst amplitude and frequency after IH. It is possible that this contradictory result relies on differences in methodological procedures, such as the presence of intact vagus nerves, which may have limited the IH-induced facilitation of inspiratory motor activity due to the activation of pulmonary feedback afferents. We may also consider the fact that our experiments were performed in spontaneously breathing rats, which may have exhibited differences at baseline and poststimulus arterial partial pressure of CO2 in comparison to paralyzed artificially ventilated animals (31). Despite the lack of changes in inspiratory activity, we observed that acute IH promoted a progressive and sustained increase in the abdominal motor activity, indicating that the mechanisms responsible for the generation of active expiration were stimulated persistently after IH. This is in agreement with previous studies reporting that animals submitted to chronic IH (for 10 days) exhibit active expiration at rest (45, 66), and support the notion that a long-term facilitation of expiratory motor activity is an important ventilatory component underpinning the compensatory increase in ventilation after IH. The fact that the exposure to acute IH did not promote changes in the and pH supports the concept that the induced expiratory long-term facilitation was mediated by plastic changes in central mechanisms controlling breathing pattern.
Accumulating evidence indicates that augmented ventilation after IH is dependent on the activation of serotoninergic mechanisms (4, 6, 37). Previous studies showed that either systemic or site-specific (e.g., phrenic motor nucleus) antagonism of the serotoninergic receptors prevents the inspiratory long-term facilitation after IH (6, 16, 23). In the present study, we show for the first time that serotoninergic mechanisms in the RTN/pFRG are essential for the development of expiratory long-term facilitation induced by IH. By the fact that microinjections of ketanserin in the RTN/pFRG inhibit the effects of IH on abdominal activity, we propose that IH causes a long-lasting activation of the RTN/pFRG expiratory oscillator through a 5-HT2-mediated mechanism. The serotoninergic neurons of the caudal raphe (17, 51), particularly from raphe magnus and raphe obscurus (46), may be an important source of 5-HT to the RTN/pFRG during IH. This possibility agrees with studies showing that hypoxia activates neurons in the caudal raphe (17, 18) and the antagonism of serotoninergic receptors in this region prevents the respiratory changes induced by IH (61). However, additional experiments are required to confirm the source of 5-HT and the molecular mechanisms leading to the persistent activation of the RTN/pFRG after IH.
The exposure to IH is also associated with the development of high levels of sympathetic activity and elevation of arterial pressure levels (16, 35, 63, 65), which is coupled to the emergence of active expiratory pattern (45, 66). In our experimental conditions, the anesthetized rats exposed to IH did not show significant changes in the arterial pressure levels, but exhibited elevated heart rate. This IH-elicited tachycardia was prevented by the microinjections of ketanserin in the RTN/pFRG, which might suggest that the activation of RTN/pFRG expiratory oscillator by 5-HT during IH also contributes to increase sympathetic activity to the cardiovascular system. However, this possibility must be investigated by additional experiments involving direct recordings of sympathetic activity.
Concluding remarks.
In the present study, we reported for the first time that 5-HT, applied episodically in the RTN/pFRG, promotes a progressive and sustained increase in abdominal motor activity, through the activation of 5-HT2 receptors. These findings extend previous observations about the role of serotoninergic mechanisms in the RTN/pFRG in the control of breathing and indicate that 5-HT is an important transmitter involved with the activation of the expiratory conditional oscillator. Moreover, this 5-HT2 dependent mechanism of the RTN/pFRG is engaged during IH and is essential for the development of long-term facilitation of expiratory motor activity. Accumulating clinical and experimental evidence indicates that I H is an important risk factor for the development of cardiorespiratory dysfunctions, including sympathetic-mediated arterial hypertension, respiratory dysfunctions, and chemoreflex hyperactivity (8, 43, 44, 48, 59, 66). The maintenance of higher levels of sympathetic activity after the chronic exposure to IH has been associated with strengthened respiratory-sympathetic coupling consequent to the generation of active expiration (45, 66). Therefore, the identification of 5-HT and the 5-HT2 receptors in the RTN/pFRG as a key mechanism for the emergence of abdominal hyperactivity might help to understand the development of the cardiorespiratory dysfunctions associated with IH.
GRANTS
This study was supported by São Paulo Research Foundation (FAPESP, Grant 2013/17251-6 to D. B. Zoccal and Grant 2009/54888-7 to E. Colombari; and PhD fellowship 2014/06976-2 to E. V. Lemes), the National Council for Scientific and Technological Development (CNPq, Grant 478640/2013-7), and the National Institutes of Health (Grant R01-AT-008632).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.V.L. performed experiments; E.V.L. and D.B.Z. analyzed data; E.V.L., E.C., and D.B.Z. interpreted results of experiments; E.V.L., E.C., and D.B.Z. edited and revised manuscript; E.V.L., E.C., and D.B.Z. approved final version of manuscript; D.B.Z. conception and design of research; D.B.Z. prepared figures; D.B.Z. drafted manuscript.
REFERENCES
- 1.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539–3559, 2009. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham KA, Feingold H, Fuller DD, Jenkins M, Mateika JH, Fregosi RF. Respiratory-related activation of human abdominal muscles during exercise. J Physiol 541: 653–663, 2002. doi: 10.1113/jphysiol.2001.013462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aira Z, Buesa I, Gallego M, García del Caño G, Mendiable N, Mingo J, Rada D, Bilbao J, Zimmermann M, Azkue JJ. Time-dependent cross talk between spinal serotonin 5-HT2A receptor and mGluR1 subserves spinal hyperexcitability and neuropathic pain after nerve injury. J Neurosci 32: 13568–13581, 2012. doi: 10.1523/JNEUROSCI.1364-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 5.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bautista TG, Dutschmann M. Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience 267: 22–29, 2014. doi: 10.1016/j.neuroscience.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Beaudin AE, Waltz X, Pun M, Wynne-Edwards KE, Ahmed SB, Anderson TJ, Hanly PJ, Poulin MJ. Human intermittent hypoxia-induced respiratory plasticity is not caused by inflammation. Eur Respir J 46: 1072–1083, 2015. doi: 10.1183/09031936.00007415. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA 101: 4292–4295, 2004. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol 101: 219–230, 1995. doi: 10.1016/0034-5687(95)00045-F. [DOI] [PubMed] [Google Scholar]
- 12.Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus 22: 790–801, 2012. doi: 10.1002/hipo.20940. [DOI] [PubMed] [Google Scholar]
- 13.Costa-Silva JH, Zoccal DB, Machado BH. Glutamatergic antagonism in the NTS decreases post-inspiratory drive and changes phrenic and sympathetic coupling during chemoreflex activation. J Neurophysiol 103: 2095–2106, 2010. doi: 10.1152/jn.00802.2009. [DOI] [PubMed] [Google Scholar]
- 14.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31: 1981–1990, 2011. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol (1985) 103: 1780–1788, 2007. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- 16.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 17.Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res 567: 11–24, 1991. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- 18.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348: 161–182, 1994. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 19.Ezure K, Tanaka I, Saito Y. Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci Res 45: 41–51, 2003. doi: 10.1016/S0168-0102(02)00197-9. [DOI] [PubMed] [Google Scholar]
- 20.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci 28: 2506–2515, 2008. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics 4: 175–181, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS, Mulkey DK. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J Neurophysiol 113: 1195–1205, 2015. doi: 10.1152/jn.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci 32: 16943–16952, 2012. doi: 10.1523/JNEUROSCI.3043-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci 35: 1052–1067, 2015. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iscoe S. Control of abdominal muscles. Prog Neurobiol 56: 433–506, 1998. doi: 10.1016/S0301-0082(98)00046-X. [DOI] [PubMed] [Google Scholar]
- 30.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol (1985) 89: 1345–1351, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Jenkin SE, Milsom WK. Expiration: breathing’s other face. Prog Brain Res 212: 131–147, 2014. doi: 10.1016/B978-0-444-63488-7.00008-2. [DOI] [PubMed] [Google Scholar]
- 33.Lemes EV, Aiko S, Orbem CB, Formentin C, Bassi M, Colombari E, Zoccal DB. Long-term facilitation of expiratory and sympathetic activities following acute intermittent hypoxia in rats. Acta Physiol (Oxf) 217: 254–266, 2016. doi: 10.1111/apha.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemes EV, Zoccal DB. Vagal afferent control of abdominal expiratory activity in response to hypoxia and hypercapnia in rats. Respir Physiol Neurobiol 203: 90–97, 2014. doi: 10.1016/j.resp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Leuenberger UA, Brubaker D, Quraishi SA, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121: 87–93, 2005. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol 461: 213–233, 1993. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci 30: 12466–12473, 2010. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol 286: R334–R341, 2004. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- 42.Miyawaki T, Goodchild AK, Pilowsky PM. Rostral ventral medulla 5-HT1A receptors selectively inhibit the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 280: R1261–R1268, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol 105: 3080–3091, 2011. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moraes DJ, Bonagamba LG, Costa KM, Costa-Silva JH, Zoccal DB, Machado BH. Short-term sustained hypoxia induces changes in the coupling of sympathetic and respiratory activities in rats. J Physiol 592: 2013–2033, 2014. doi: 10.1113/jphysiol.2013.262212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 33: 19223–19237, 2013. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of the retrotrapezoid nucleus/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J Neurophysiol 108: 882–890, 2012. doi: 10.1152/jn.00193.2012. [DOI] [PubMed] [Google Scholar]
- 47.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narkiewicz K, Wolf J, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea and hypertension. Curr Cardiol Rep 7: 435–440, 2005. doi: 10.1007/s11886-005-0061-z. [DOI] [PubMed] [Google Scholar]
- 49.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31: 2895–2905, 2011. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlinac Dodig I, Pecotic R, Valic M, Dogas Z. Acute intermittent hypoxia induces phrenic long-term facilitation which is modulated by 5-HT1A receptor in the caudal raphe region of the rat. J Sleep Res 21: 195–203, 2012. doi: 10.1111/j.1365-2869.2011.00948.x. [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press, 1998. [Google Scholar]
- 53.Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576: 289–295, 2006. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333: 637–642, 2011. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience 115: 1247–1259, 2002. doi: 10.1016/S0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 57.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valic M, Pecotic R, Pavlinac I, Valic Z, Peros K, Dogas Z. Microinjection of methysergide into the raphe nucleus attenuated phrenic long-term facilitation in rats. Exp Brain Res 202: 583–589, 2010. doi: 10.1007/s00221-010-2161-2. [DOI] [PubMed] [Google Scholar]
- 62.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33: 7756–7761, 2013. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol 588: 3075–3088, 2010. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu H, Wu F, Schacher S. Site-specific and sensory neuron-dependent increases in postsynaptic glutamate sensitivity accompany serotonin-induced long-term facilitation at Aplysia sensorimotor synapses. J Neurosci 17: 4976–4986, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol 94: 972–983, 2009. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- 66.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]


