Abstract
Mini-tyrosyl-tRNA synthetase (mini-TyrRS), the N-terminal domain of tyrosyl-tRNA synthetase, is a recently identified protein released by endothelial cells that exhibits angiogenic and leukocyte chemoattractant, ELR-motif (Glu-Leu-Arg)-dependent activities in vitro. We sought to determine whether exogenous mini-TyrRS exerts these and other cytokine-like actions in physiological and pathological settings in vivo. High-dose mini-TyrRS (600 μg·kg−1·day−1) augmented while low-dose mini-TyrRS (3 μg·kg−1·day−1), unexpectedly, inhibited angiogenesis in the ischemic mouse ear. Enhanced angiogenesis was associated with increased CD45- and CD4-positive leukocyte accumulation. Mini-TyrRS also had biphasic actions on both basal and mustard oil-evoked and VEGF-evoked leakage of Evan's blue dye-albumin in nonischemic ear and in endothelial cell monolayers, that is, low-dose inhibited and high-dose augmented leakage. Mutation of the ELR motif of mini-TyrRS abolished the above activities. Mini-TyrRS was reduced (immunoblot) in extracts of ischemic calf muscle and in thoracic aorta explants exposed to hypoxia or VEGF. Inhibition of VEGF with a soluble Flt1 “trap” protein abolished this hypoxic-induced reduction in mini-TyrRS in aorta explants. These data show that mini-TyrRS has dose-dependent biphasic effects on ischemic angiogenesis and vascular permeability in vivo, that is, antiangiogenic and antipermeability activities at low concentration and proangiogenic, propermeability activities at high concentrations.
Keywords: hypoxia, ischemia, vascular endothelial growth factor
aminoacyl-trna synthetases, which catalyze the aminoacylation of tRNA molecules, are essential for encoding genetic information during translation (22, 32–35). In higher eukaryotes, aminoacyl-tRNA synthetases associate with other polypeptides to form supramolecular multienzyme complexes. The eukaryotic tRNA synthetases consist of a core enzyme, which is closely related to the prokaryotic counterpart of the tRNA synthetase, and an additional domain that is appended to the amino-terminal or carboxyl-terminal end of the core enzyme. Human tyrosyl-tRNA synthetase (TyrRS), for example, has a carboxyl-terminal domain that is not part of the prokaryotic and lower eukaryotic TyrRS molecules. The synthetase also has an N-terminal domain that is cleaved by several endogenous enzymes, yielding mini-tyrosyl tRNA synthetase (mini-TyrRS).
Mini-TyrRS has recently been shown to possess cytokine-like actions, leading to its inclusion in a growing family of aminoacyl tRNA synthetase (AARS) multifunction cytokine-like proteins and peptides (22, 32–35). Mini-TyrRS stimulates neutrophil activation and chemotaxis in vitro and is angiogenic in endothelial cell cultures and in chick chorioallantoic membrane (CAM) and mouse matrigel implants (22, 32–35). Like CXC chemokines, such as IL-8, mini-TyRS has an ELR motif (Glu-Leu-Arg) that confers its chemokine and angiogenic activities. Mutation of this motif inhibits binding and abolishes stimulation of leukoctyes and induction of angiogenesis. Monocytes/macrophages, T-lymphocytes, and endothelial progenitor cells are important in angiogenesis, where they are recruited into and around new capillary sprouts and secrete growth factors and cytokines that promote endothelial cell proliferation and migration (9, 13).
Despite these intriguing in vitro actions, no studies have examined the mini-TyrRS in physiological or pathological settings in vivo. Therefore, the purpose of this study was to determine whether exogenous mini-TyrRS augments angiogenesis and leukocyte recruitment in ischemic tissue in vivo. We also investigated it for other key actions exhibited by angiogenic factors, namely, the induction of vasodilation and increased vascular permeability. Our findings show that mini-TyrRS augments angiogenesis and leukocyte adhesion in a mouse ear model of ischemic angiogenesis, and it increases permeability but lacks vasoactive actions. Intriguingly, at low concentrations mini-TyrRS had opposite effects, i.e., angiostatic activity, as well as reduced baseline and evoked increases in permeability. These novel biphasic actions of mini-TyrRS have potential therapeutic and physiological implications.
METHODS
Reagents.
Rabbit anti-human mini-TyrRS antibody and human recombinant mini-TyrRS were from aTyr Pharma (La Jolla, CA). mFlt-trap (soluble VEGF-A receptor decoy) was kindly provided by Napoleon Ferrara and Stuart Bunting (Genentech, South San Francisco, CA). Bovine coronary venular endothelial cells were a gift from Cynthia Meininger, Texas A&M University. Four- to five-month-old mice were used in ear artery ligation (C57BL/6) and permeability models (sv129); a total of 285 animals were used to obtain the in vivo data shown in the figures. Procedures were performed aseptically and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Unilateral ear artery ligation.
One- to two-millimeter incisions at the base of the pinna were made overlying the central and peripheral ear artery trunks. Each artery was transected between two 7.0 ligatures placed 1 mm apart (1). The central ear artery was ligated distal to its lateral branch to prevent ear necrosis. Twenty microliters of PBS containing mini-TyrRS at 4 doses, mutant mini-TyrRS, or PBS (vehicle) was injected subcutaneously every 12 h (Fig. 1).
Fig. 1.
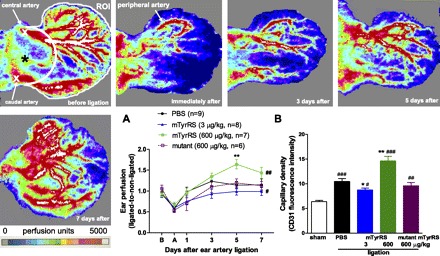
Mini-tyrosyl-tRNA synthetase (TyrRS) has biphasic actions on recovery of blood flow and angiogenesis in a model of ear ischemia. Top: Doppler perfusion was determined for the ear in an anatomically defined region of interest (ROI; see materials and methods) before and at indicated times after ligation of the central and peripheral ear arteries (“X” in first panel); same mouse (PBS control) used in each panel. Pseudocolor bar spans 0–5,000 perfusion units. A: perfusion values normalized to nonligated contralateral ear. Recovery of ear perfusion was inhibited by low- and augmented by high-dose mini-TyrRS (mTyrRS), while “mutant” mini-TyrRS had no effect (“mutant” here and elsewhere is mini-TyrRS with the ELR motif mutated to EYR). Doses of 0.05 and 30 μg·kg−1·day−1 had no effect (n = 8 mice/dose, data not shown), underscoring the biphasic activity. Ear doses are the total daily dose from two injections given subcutaneously into the base of ear (“*” in first panel) 12 h apart. #P < 0.05, ##P < 0.01, ANOVA; **P < 0.01, Bonferroni t-test; 2-tailed tests here and other figures. B: angiogenesis (capillary density, measured at day 7 after ligation) was inhibited by low-dose and augmented by high-dose mini-TyrRS, while mutant mini-TyrRS had no effect compared with PBS. Fluorescence intensity of CD31 antibody was averaged for eight fields spanning the entire ear cross section (see supplmental data for representative histological images). #P < 0.05, ##P < 0.01, ###P < 0.001 vs. sham ligation; *P < 0.05, **P < 0.01 vs. PBS. n = 6–9/bar. Values are expressed as means ± SE for “n” number of animals for this and subsequent figures.
Laser-Doppler perfusion imaging.
Animals were anesthetized with 1.125% isoflurane supplemented with 2:3 oxygen:air. Perfusion was obtained before ligation, immediately after, and on 1, 3, 5, and 7 days using a scanning laser-Doppler perfusion imager (LDI2-IR; Moor Instruments, Devon, UK) modified for high resolution (10). Regions of interest (ROIs) were drawn by an investigator blind to drug treatment using Moor software to delineate a region between the ear margin and a circle extending from a line drawn to connect the two pinna notches. Unless otherwise indicated, perfusion was measured at 38°C rectal temperature to reduce vasomotor tone.
Vascular albumin leakage.
Albumin leakage (permeability) was measured with Evans blue dye (T1824) (18) in the ear or dorsolateral back skin. Mini-TyrRS, mutant mini-TyrRS or PBS was injected (10 μl, 32-gauge needle here and elsewhere) subcutaneously into the ear dorsum at the base of the pinna. Thirty minutes was observed to allow absorption of the volume and resolution of any effects on local interstitial fluid pressure. Then, 25 μl T1824 (30 mg/kg) was administered via the jugular vein. Immediately afterward, allyl isothiocyanate [the active ingredient in mustard oil; Sigma; diluted with mineral oil to 5% (vol/vol)] or mineral oil (control) was applied topically (5 μl) to the dorsal and ventral surfaces of both ears with a cotton-tip applicator. Thirty minutes later, the vasculature was perfusion-fixed [1% paraformaldehyde (PFA) in 50 mM citrate buffer, pH 3.5] for 1 min at 120 mmHg. Ears were removed, dried at 55°C for 24 h, and weighed. Vascular leakage was indicated as T1824 content extracted by incubation in 1 ml formamide for 48 h at 55°C, and measured with a spectrophotometer at 610 nm against a standard curve (31).
VEGF-induced permeability was examined in the shaved back skin. Twenty microliters of PBS containing mini-TyrRS or PBS alone was injected subcutaneously. Thirty minutes later, T1824 was injected intravenously as above, followed by VEGF-A165 (100 ng in 20 μl PBS; R&D Systems, Minneapolis, MN) or PBS injected subcutaneously at the same location. Thirty minutes later, a skin circle, identified with a stereomicroscope circumscribing the extent of blue dye, was excised and T1824 content was determined as above.
Bovine coronary venule endothelial cell culture and monolayer permeability.
Bovine coronary venule endothelial cells (BCVECs) (passages 10–15) were seeded onto culture dishes or onto 0.4-μm transwell inserts (Corning, Corning, NY) (3 × 105 cells/insert), both precoated with 1.5% gelatin, and maintained in Dulbecco's modified Eagle's medium (DMEM) with 20% FBS at 10% CO2 until a tight confluent monolayer was achieved. Cells were then pretreated with mini-TyrRS for 10 min, followed by 100 ng/ml VEGF for 30 min. Monolayers were then treated with Evans blue-BSA complex (0.67 g/l and 40 g/l) in HEPES-buffered saline for 30 min. Evans blue-albumin in the lower well was measured at 610-nm absorbance. Transendothelial albumin flux is expressed as percent clearance of albumin, compared with untreated controls.
Thoracic aorta explants.
Rat thoracic aortas were isolated and maintained in serum-free medium composed of DMEM/F12, 10 mg/l insulin, 5 μg/l selenium and 5.5 mg/l transferrin in 21% or 1% O2. After exposure to VEGF for 4 days, samples were frozen in liquid nitrogen for immunoblot assay.
Immunohistochemistry.
For capillary density, ears were perfusion-fixed with 4% PFA in PBS (pH 7.4) at 120 mmHg and then postfixed in 4% PFA for 24 h and embedded in paraffin. Eight-micrometer-thick sections located 5500 μm from the distal tip of the pinna were quantified for capillary density after staining for CD31 (sc-1506, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA), followed by Cy3-conjugated secondary antibody (1:600). Vessels were imaged in eight different fields (×200 magnification) that covered the entire ear (cartilage, skin surface, and hair follicles with autofluoresence were excluded in digital images). Capillary density was derived from mean intensity of CD31 immunofluorescence (Image-J Software, National Institutes of Health, Bethesda, MD). T-cells and leukocytes were stained in adjacent sections with rat anti-mouse CD4 antibody (1:50, sc-13573; Santa Cruz Biotechnology) and CD45 antibody (1:200, 30-F11, BD Pharmingen, Franklin Lakes, NJ), respectively, followed with Cy3-conjugated secondary antibody (1:400–600). CD4- and CD45-positive cells were counted for the entire ear cross-section at ×400 magnification.
Immunoblot.
Tissues were powdered in a liquid nitrogen-cooled pulverizer. Tissues and cultured cells were lysed in 1.5% Triton-X100 lysis buffer containing protease inhibitors (30 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) and phosphatase inhibitors (1 mM sodium-orthovanadate, 2.5 mM sodium-pyrophosphate). Samples were electrophoresed through 10% SDS-polyacrylamide and transferred to nitrocellulose membranes. Membranes were probed with antibodies against mini-TyrRS (1:1,000 dilution) and tubulin (1:5,000, ab6160, Abcam) followed by Alexa Fluor-680 (Molecular Probes, Carlsbad, CA) or IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA) conjugated secondary antibodies at 1:5,000. Membranes were scanned and analyzed with the Odyssey system (LI-COR Biosciences, Lincoln, NE).
Statistics.
Data are given as means ± SE. Differences were subjected to unpaired t-tests (2-tailed) or ANOVA followed by Bonferroni tests for multiple comparisons (2-tailed). P < 0.05 was considered significant.
RESULTS
Mini-TyrRS has biphasic effects on ischemic angiogenesis.
Previous studies have reported that mini-TyrRS induced angiogenesis in cultured endothelial cell, CAM, and mouse matrigel assays (22, 32–35). To determine whether mini-TyrRS has angiogenic activity in vivo in ischemia, we examined its effects in the mouse ear made ischemic by ligation of the peripheral and central ear arteries, leaving the proximal-lateral branch of the central artery intact (Fig. 1). This is the first description of a mouse ear model for quantitative measure of perfusion, angiogenesis, and permeability. We developed it because of its ready access for these measures, plus its amenability to local administration of agents. In the PBS control group, perfusion declined 50% immediately after ligation, followed by recovery within 3–5 days mediated by angiogenesis and growth of collaterals between the above arterial trees (Fig. 1A). Local subcutaneous injection (20 μl, twice daily every 12 h) of 3 μg·kg−1·day−1 mini-TyrRS into the base of the ear inhibited, while 600 μg·kg−1·day−1 augmented, recovery of perfusion. Doses of 0.05 and 30 μg·kg−1·day−1 had no effect (n = 8 mice/dose, data not shown), underscoring the biphasic activity. Doses higher than 600 μg·kg−1·day−1 were not tested for reasons of concentration and cost. Mutant mini-TyrRS (ELR mutated to EYR) had no effect.
We next examined the effective doses in the Fig. 1A experiment for an effect on capillary density measured 7 days after ligation. The biphasic effect of mini-TyrRS on recovery of ear perfusion was accompanied by similar changes in capillary density (Fig. 1B). Capillary density, which increased in ischemia as expected, was inhibited by 3 μg·kg−1·day−1 and augmented by 600 μg·kg−1·day−1 mini-TyrRS, whereas mutant mini-TyrRS had no effect. This biphasic activity is different from in vitro, where only dose-dependent stimulation of angiogenesis occurs. Body weight did not differ between PBS or drug groups at any time point. In addition, daily subcutaneous injection of mini-TyrRS in the ear was not accompanied by erythema or edema.
Mini-TyrRS increases leukocyte accumulation in ischemia.
Monocytes/macrophages and T-lymphocytes are involved in ischemic angiogenesis in vivo (9, 17). Furthermore, mini-TyrRS stimulates monocyte adhesion and transmigration in vitro (13, 19, 22). We, therefore, examined whether mini-TyrRS affects leukocyte density in cross sections of the ear adjacent to those used for determining capillary density in Fig. 1B. As expected, CD45+ and CD4+ cells increased after ligation (Fig. 2). At low-dose mini-TyrRS, which inhibits angiogenesis, no effect on leukocyte density was observed. However, high-dose mini-TyrRS caused a further increase of both cell types, whereas mutant mini-TyrRS was without effect.
Fig. 2.
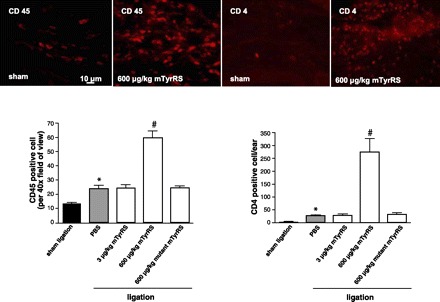
High-dose mini-TyrRS augments accumulation of CD45- and CD4-positive cells 7 days after ear artery ligation. Representative 8-μm-thick sections of ear stained with anti-CD45 and anti-CD4 antibodies. CD45-positive cells are the average of 10 high-power fields. CD4-positive cells are for the entire ear cross section. *P < 0.01 vs. sham ligation; #P < 0.001 vs. PBS. n = 6–9/bar.
Mini-TyrRS has biphasic effects on baseline and evoked increase in permeability.
Increased permeability of the endothelium is an important step in the initial phase of sprouting angiogenesis, and several angiogenic factors, e.g., VEGF, regulate angiogenesis, in part, through alterations in permeability (2, 7, 9, 20, 25). We thus examined whether mini-TyrRS modulates leakage (“permeability”), using extravasation of Evans blue-conjugated albumin in normal, nonligated ears. Similar to its biphasic effects on angiogenesis (Fig. 1), mini-TyrRS also had biphasic effects on permeability (Figs. 3 and 4). Low-dose mini-TyrRS (30 μg/kg) reduced baseline leakage by 50%, while high-dose (600 μg/kg) increased leakage greater than twofold (Fig. 3A); mutant mini-TyrRS had no effect. We also tested mini-TyrRS on mustard oil-induced increase in permeability. Mini-TyrRS caused dose-dependent inhibition of induced leakage at low doses, with maximal inhibition at 3 μg/kg, whereas 600 μg/kg slightly augmented induced permeability but not significantly (Fig. 3B). Mutant mini-TyrRS had no effect, suggesting—like its actions on angiogenesis, leukocyte accumulation, and baseline permeability—that the ELR motif is required for mini-TyrRS's modulation of mineral oil-induced permeability. Similar results were obtained with VEGF-induced leakage (Fig. 4A). The biphasic action of mini-TyrRS on VEGF-induced leakage was confirmed in endothelial cell monolayers (Fig. 4B).
Fig. 3.
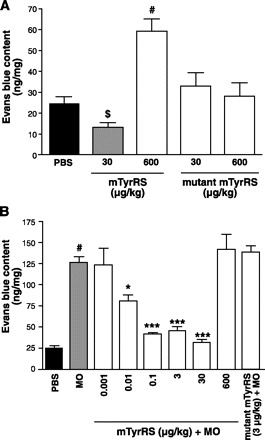
Mini-TyrRS has biphasic actions on baseline and mustard oil (MO)-evoked increase in macromolecular leakage in ear. A: mini-TyrRS, alone, at low-dose (sc) reduced and at high-dose augmented leakage, while mutant mini-TyrRS had no effect. B: low-dose mini-TyrRS inhibited MO-induced ear leakage, while high-dose or mutant mini-TyrRS had no effect. Trans-endothelial albumin flux expressed as a percentage of clearance of Evans blue-conjugated BSA, compared with untreated controls. $P < 0.05, #P < 0.01 vs. PBS, *P < 0.05, ***P < 0.001 vs. MO. n = 4–7/bar.
Fig. 4.
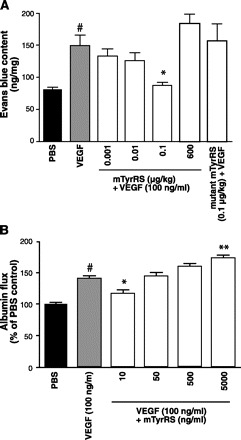
Mini-TyrRS has biphasic actions on vascular endothelial growth factor (VEGF) evoked increase in macromolecular permeability. A: low-dose mini-TyrRS (sc) inhibited VEGF-induced leakage in dorsolateral trunk skin. B: mini-TyrRS had biphasic actions on VEGF-induced leakage in endothelial cell monolayers. Trans-endothelial albumin flux expressed as a percentage of clearance of Evans blue-conjugated BSA, compared with untreated controls. #P < 0.01 vs. PBS, *P < 0.05, **P < 0.01 vs. VEGF; n = 4–7/bar.
Mini-TyrRS lacks vasoactive actions.
Because some angiogenic factors, such as VEGF and basic fibroblast growth factor (bFGF), exhibit vasodilator and vasoconstrictor activity, respectively (9), and because such activity of mini-TyrRS could alter perfusion in the ear ligation and permeability in the Evans blue experiments, we evaluated the effect of mini-TyrRS on perfusion of the normal (nonligated) mouse ear. The following protocols (conducted in separate animals) reflect two requirements in these in vivo experiments: First, 10 min were required to obtain the laser scanning-Doppler perfusion measurement. Second, 20–30 min were allowed after a local injection (20 μl for all agents tested) to permit any elevation of interstitial pressure to subside.
In the first experiment, rectal temperature was lowered to 35°C to increase vascular tone. Baseline perfusion was then obtained, followed by injection of mini-TyrRS or PBS into the base of the ear. Rectal temperature was then raised to 37.5°C over 10 min to cause warming-induced vasodilation, and perfusion was obtained again. Mini-TyrRS had no effect on warming-induced dilation (Fig. 5A). In a second experiment, baseline perfusion was obtained at 35°C, and mini-TyrRS or PBS was then injected. Thirty minutes later, the vasodilator papaverine was injected in the same location, and perfusion was obtained again 30 min later. Although there was a slight suggestion that mini-TyrRS might modestly reduce the dilation (Fig. 5B), the peptide had no significant statistical effect on papavarine-mediated dilation. If mini-TyrRS has vasoconstrictor or vasodilator actions, dilator response to the superimposed warming period in the first experiment or to papavarine in the second experiment should have been altered. In a third experiment to investigate possible progressive vasoactive actions with repeated exposure, mini-TyrRS was administered locally on six consecutive days. Ear perfusion was measured at 36.5°C 24 h after each administration and just before repeat dosing. Mini-TyrRS had no effect on perfusion on day 6 (Fig. 5C) nor at any of the five earlier days (data not shown). Absence of vasoactive effects in these experiments is consistent with the absence of erythema noted at any times or dosages, either immediately after or 24 h after mini-TyrRS administration. Edema was not evident at the 600 μg/kg dosages in any of the experiments, even though this dose increased baseline permeability (Fig. 3A). This may reflect low interstitial compliance and/or efficient lymphatic drainage in the ear.
Fig. 5.
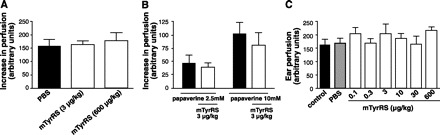
Mini-TyrRS lacks vasoactive actions. A: increase in perfusion (Doppler) induced in nonligated ear by raising rectal temperature from 35°C to 37.5°C was unaffected by mini-TyrRS (20-μl subcutaneous administration into ear immediately after 35°C measurement, followed by measurement 10 min later at 37.5°C). B: increase in perfusion induced by papavarine (adductor area sc) was unaffected by mini-TyrRS injected into same site 30 min earlier. C: baseline (control) ear perfusion was unaffected after 6 days of daily sc mini-TyrRS (daily dose given as two 20-μl injections 12 h apart); n = 4–6/bar.
Ischemia, hypoxia, and VEGF reduce mini-TyrRS in calf and isolated aorta.
Angiogenesis in response to tissue hypoxia and ischemia is achieved through upregulation of angiogenic factors such as VEGF, which, in turn, or through other mechanisms, downregulate angiostatic factors (9, 30). We thus reasoned that if endogenous mini-TyrRS normally exerts angiostatic actions at low concentrations, as suggested by the above findings showing that low-dose mini-TyrRS inhibited recovery of flow, angiogenesis, and permeability, then tissue levels of mini-TyrRS might be regulated negatively in ischemia and in response to VEGF. We used mouse hindlimb ischemia to examine this question because it is a widely used model of neovascularization and because it is difficult to extract sufficient protein of high quality from the cartilaginous and fibrous ear. In addition, this model allowed measurement of mini-TyrRS by immunoblot in muscle that experiences ischemia (gastrocnemius) vs. little or no ischemia (adductor) after femoral artery ligation (10, 18). Tissue samples were harvested on days 2, 5, and 10 after ligation of the femoral artery, as detailed elsewhere (7). Mini-TyrRS decreased in the gastrocnemius but not adductor of the ligated leg, compared with the gastrocnemius from sham animals (no surgery) or from the contralateral nonligated leg (Fig. 6, A and B). The specificity of the mini-TyrRS band identified with rabbit anti-human antibody was verified using a second goat anti-rabbit antibody that we raised against a different epitope of mini-TyrRS. To test possible involvement of hypoxia and VEGF in reduced mini-TyrRS, we examined rat thoracic aorta maintained in organ culture. Four days of exposure to VEGF or hypoxia (1% O2) caused similar reduction of mini-TyrRS (Fig. 6, C and D). Moreover, hypoxic reduction was abolished by VEGF neutralizing trap. These data suggest that hypoxic induction of VEGF may mediate reduced mini-TyrRS in ischemic tissue.
Fig. 6.
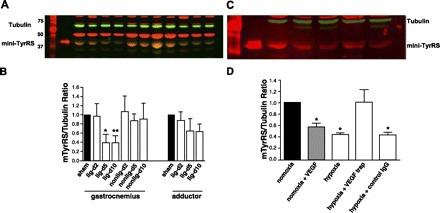
Ischemia/hypoxia and VEGF reduce mini-TyrRS levels in vivo and in vitro. A and B: Western blot analysis of mini-TyrRS in gastrocnemius and adductor of sham-surgery mice, in gastrocnemius and adductor of leg with femoral artery ligation (lig), and in gastrocnemius of the contralateral nonligated leg at the indicated days (d) after surgery. Twenty micrograms (A and B) and 30 μg (C and D) protein per lane; normalized to tubulin; n = 4/bar. C and D: Western blot analysis of mini-TyrRS in rat thoracic aorta maintained 4 days in organ culture with 100 ng/ml VEGF or 1% oxygen (hypoxia) ± VEGF trap or IgG control (0.2 mg/ml). *P < 0.05, **P < 0.01 vs. sham or normoxia; n = 3 or 4/bar.
DISCUSSION
This is the first characterization of the effects of exogenous mini-TyrRS on ischemic angiogenesis, leukocyte trafficking, permeability, and vasoactivity in vivo. We confirm several properties previously observed in vitro, identify novel biphasic actions on angiogenesis and permeability, and find evidence suggesting that tissue levels of mini-TyrRS may be regulated by hypoxia.
In the mouse ear model of ischemia, low-dose mini-TyrRS (3 μg·kg−1·day−1) inhibited, while high-dose mini-TyrRS (600 μg·kg−1·day−1) augmented angiogenesis. Intermediate doses of 0.05 and 30 μg·kg−1·day−1 had no effect. Mutant mini-TyrRS had no effect, confirming the requirement of an intact ELR. The angiostatic-like action of low-dose mini-TyrRS was not observed in previous in vitro, matrigel and CAM implant studies, in which only angiogenic actions were observed. For example, mini-TyrRS was angiogenic at 2.4–24 μg/ml (60–600 nmol/l) in matrigel implants and induced migration of cultured endothelial cells at 2 μg/ml (50 nmol/l) (33). These differences could arise for several reasons, including the context of ischemia in our study, inherent differences in conditions in vivo, in vitro, and “in matrigel”, and because the concentrations used previously are undoubtedly higher than achieved in our low-dose groups, where dilution and degradation over time would be considerable. For example, assuming mini-TyrRS injected locally into the ear distributes into the extracellular space and that degradation reduces the concentration by 10-fold (actual degradation is likely to be a least 1 order of magnitude more than this), our low- and high-dose regimens would achieve average extracellular concentrations of 0.006 μg/ml and 1.2 μg/ml. Thus, although concentrations in the ear's extracellular fluid would clearly be higher for some duration after injection, it is likely that time-averaged levels achieved in our low-dose groups were significantly lower than those in previous studies.
Recruitment of leukocytes and endothelial progenitor cells (EPCs) contributes importantly to angiogenesis in ischemia, inflammation and tumor growth (2, 9, 13, 20). These cells exhibit heterogeneous phenotypes, expressing markers for macrophages, T-cells, smooth muscle cells, fibroblasts, pericytes, and EPCs, and secrete growth factors and cytokines, which act directly or indirectly to augment endothelial cell migration, proliferation, and capillary sprouting. CD4-positive T-lymphocytes play an important role in angiogenesis by secreting angiogenic growth factors, such as VEGF (14) and bFGF (4). In the present study, high-dose mini-TyrRS further increased leukocyte (CD45-positive) accumulation in the ischemic ear by ∼2.5-fold compared with PBS control and CD4-positive cells by ∼10-fold, while low-dose mini-TyrRS had no effect. This action may contribute to the angiogenic effect of mini-TyrRS.
Besides the novel biphasic effects of mini-TyrRS on angiogenesis, mini-TyrRS also had biphasic effects on both basal and evoked permeability. In most vascular beds, including the skin, permeability to plasma proteins and smaller molecules is normally low. Ischemia, inflammation, and tumor growth are accompanied by increased vascular permeability, which is an important early step in angiogenesis in these conditions (2, 9, 27) The resulting leakage of plasma proteins and other circulating macromolecules helps to convert the normally anti-angiogenic stroma into a proangiogenic provisional stroma (9, 11). Many angiogenic factors, such as VEGF (16), bFGF (7), IL-8 (25), and thrombin (16) increase endothelial permeability (2). On the other hand, antagonism of increased permeability reduces angiogenesis (5, 29, 30). For example, the angiostatic proteins angiostatin (28), caveolin-1 (5) and TNP-470 (26) reduce evoked increases in permeability. However, to our knowledge, no endogenous angiostatic factor has been reported to reduce basal permeability like that observed in the present study for mini-TyrRS. On the other hand, high-dose mini-TyrRS increased basal permeability. We also found that mini-TyrRS at low doses caused dose-dependent inhibition of evoked leakage by mustard oil and VEGF, while high-dose mini-TyrRS tended to augment evoked leakage. This positive regulatory effect of mini-TyrRS on basal and evoked permeability may contribute to the angiogenic action of mini-TyrRS. Although the mechanisms underlying the biphasic effect await future studies, estrogen has similar biphasic effects on permeability in ECs in vitro (36).
The specificity of our findings regarding angiogenesis, leukocyte accumulation, and increased permeability are supported by their dependence on an intact ELR (Glu-Leu-Arg) motif, i.e., mini-TyrRS with this motif mutated had no effects. This motif is required for binding, neutrophil activation/adhesion, and angiogenesis induced by mini-TyrRS in vitro and other CXC ELR-containing chemokines, such as IL-8 (15, 16, 22, 32, 33, 35). However, the ELR motif need not be angiogenic. For example, the ELR motif-containing chemokine GRO-β has antiangiogenic properties (8), and members of the CXC subfamily, induced by interferons, lack the ELR motif but are potent inhibitors of angiogenesis (19). In addition—and of potential relevance to our findings—22–26-amino acid peptides derived from proangiogenic ELR-containing CXC chemokines exhibit potent antiproliferative and antimigratory activity in vitro (19). The mini-TyrRS receptor has not yet been identified, nor is it known whether mini-TyrRS binds to different receptors at low vs. high concentrations. Multiple receptors and/or processing of the protein after export from endothelial and other vascular cells (15, 22, 35) could underlie the biphasic properties we observed. For example, the AARS cytokines mini- and T2-TrpRS, thought to be derived from proteolysis and/or alternative splicing of TrpRS, inhibit VEGF signaling and are angiostatic (21, 22, 34, 35). It has recently been reported in endothelial cells that mini-TyrRS release is induced by TNF-α, undergoes binding, and induces phosphorylation of Src, Akt, ERK, and VEGF-receptor 2, which is required for tube formation (15). These factors are also involved in signaling angiogenesis, angiostatic activity, leukocyte adhesion, and changes in vascular permeability (2, 9, 20, 23, 30) and thus could be involved in the biphasic action of mini-TyrRS on these processes identified herein. Knowledge of the signaling pathways activated at low vs. high concentrations of mini-TyrRS in vivo or in intact vessel organ culture preparations will be required to understand the basis for the biphasic activities identified in this study.
Tissue hypoxia in ischemia and tumor growth induces many of the steps involved in angiogenesis, e.g., increased permeability, inflammation, endothelial cell proliferation and migration, and matrix degradation (2, 3, 6, 9, 12, 20, 23, 24). A number of the proteins that mediate these processes, such as endothelial nitric oxide synthase, VEGF, angiopoietin-2, Akt, and bFGF are regulated by hypoxia. Mini-TyrRS levels detected by immunoblot of tissue lysates were significantly reduced in gastrocnemius muscle when examined at 5 and 10 days after ligation of the femoral artery. Similar reductions were also observed in thoracic aorta explants exposed to either hypoxia or VEGF. Moreover, in the latter model, a VEGF-trap-blocking protein abolished reduction in mini-TyrRS during hypoxia. These findings in explants suggest that the reduction of mini-TyrRS in calf muscle after femoral ligation in vivo may be mediated by hypoxia and VEGF. This supports our hypothesis from the findings in Fig. 1 that the physiological effects of low concentrations of mini-TyrRS are angiostatic. It is possible that the decline in mini-TyrRS detected in tissue extracts of calf and aorta explants reflects a regulated reduction in cleavage of the protein from full-length TyrRS to cause withdrawal of its angiostatic effect produced at low concentrations (Fig. 1). On the other hand, it has recently been shown that mini-TyrRS is released by stimulation of endothelial cells with TNF-α in vitro (15). Thus, it is also possible that export from endothelial cells (and possibly other cell types) in response to ischemia, hypoxia, and VEGF [conditions tested herein; however, VEGF did not induce release of mini-TyrRS in vitro (15)] could have reduced intracellular levels. This plus proteolysis of any released mini-TyrRS may have resulted in lower levels detected in tissue extracts. Additional studies and development of assays to measure released mini-TyrRS will be required to determine whether the decline in mini-TyrRS with the models and stimuli examined herein reflects increased export and achievement of angiogenic concentrations or is due to a regulated reduction in levels to withdraw the angiostatic activity of low mini-TyrRS levels. If evidence for the latter is obtained, the angiogenic and related actions of high-dose mini-TyrRS that we observed may reflect a potential therapeutic use of the protein in ischemia, while the low-dose angiostatic and barrier-increasing actions may reflect physiological activities as well as a potential therapeutic use to inhibit pathological angiogenesis.“ Clearly, more work needs to be done to understand expression/proteolysis of TyrRS under different physiological conditions.
In summary, we report that low-dose mini-TyrRS inhibits basal and evoked permeability and ischemic angiogenesis. High-dose has opposite effects and, in addition, augments recruitment of CD45-positive and CD4-positive cells in ischemic tissue. To our knowledge, mini-TyrRS is the first factor observed to inhibit angiogenesis at low and stimulate it at high concentrations. We also provide in vitro evidence in aorta that mini-TyrRS levels are reduced by VEGF-dependent signaling in hypoxia, suggesting a similar mechanism may underlie its reduction in ischemic tissue in vivo. These findings suggest that endogenous mini-TyrRS may serve an angiostatic function in certain physiological settings. Moreover, administration of it at low vs. high levels could provide a therapeutic approach to limit vs. augment angiogenesis in certain diseases. Important areas of future study include determining whether stimulation of leukocyte accumulation by mini-TyrRS reflects induction of adhesion molecules on endothelial cells, leukocytes, or both, or release of inflammatory cytokines from these or other cell types, and similar questions regarding the biphasic effects of mini-TyrRS on baseline and induced permeability and ischemic angiogenesis. Besides a number of tools that need to be developed, strategies to antagonize endogenous production/cleavage/secretion and block the responsible receptor(s) will be needed to test the potential physiological roles suggested by the findings in this study.
Supplementary Material
Acknowledgments
The authors thank Young Greenberg for dialyzing mini-TyrRS, K Kirk McNaughton for histological assistance, and John Reader for helpful discussion.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.AhnSTAhn ST, Mustoe TA. Effects of ischemia on ulcer wound healing: a new model in the rabbit ear. Ann Plast Surg 24: 17–23, 1990. [DOI] [PubMed] [Google Scholar]
- 2.BazzoniGBazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84: 869–901, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Ben-YosefYBen-Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A. Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res 90: 784–791, 2002. [DOI] [PubMed] [Google Scholar]
- 4.BlotnickSBlotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci USA 91: 2890–2894, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BucciMBucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000. [DOI] [PubMed] [Google Scholar]
- 6.CalvaniMCalvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 107: 2705–2712, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CaoRCao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res 94: 664–670, 2004. [DOI] [PubMed] [Google Scholar]
- 8.CaoYCao Y, Chen C, Weatherbee JA, Tsang M, Folkman J. Gro-beta, a -C-X-C- chemokine, is an angiogenesis inhibitor that suppresses the growth of Lewis lung carcinoma in mice. J Exp Med 182: 2069–2077, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CarmelietPCarmeliet P. Angiogenesis in health and disease. Nat Med 9: 653–660, 2003. [DOI] [PubMed] [Google Scholar]
- 10.ChalothornDChalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics 30: 179–191, 2007. [DOI] [PubMed] [Google Scholar]
- 11.DvorakHFDvorak HF, Senger DR, Dvorak AM, Harvey VS, McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science 227: 1059–1061, 1985. [DOI] [PubMed] [Google Scholar]
- 12.FischerSFischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol Cell Physiol 276: C812–C820, 1999. [DOI] [PubMed] [Google Scholar]
- 13.FrantzSFrantz S, Vincent KA, Feron O, Kelly RA. Innate immunity and angiogenesis. Circ Res 96: 15–26, 2005. [DOI] [PubMed] [Google Scholar]
- 14.FreemanMRFreeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, Peoples GE, Klagsbrun M. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res 55: 4140–4145, 1995. [PubMed] [Google Scholar]
- 15.GreenbergYGreenberg Y, King M, Ewalt K, Yang X, Schimmel P, Reader J, Tzima E. The novel fragment of tyrosyl tRNA synthetase, mini-TyrRS, is secreted to induce an angiogenic response in endothelial cells. FASEB J 25: 1597–1605, 2008. [DOI] [PubMed] [Google Scholar]
- 16.HebertCAHebert CA, Vitangcol RV, Baker JB. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem 266: 18989–18994, 1991. [PubMed] [Google Scholar]
- 17.HeilMHeil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 10: 45–55, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ItoWDIto WD, Arras M, Scholz D, Winkler B, Htun P, Schaper W. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am J Physiol Heart Circ Physiol 273: H1255–H1265, 1997. [DOI] [PubMed] [Google Scholar]
- 19.KaragiannisEDKaragiannis ED, Popel AS. Novel anti-angiogenic peptides derived from ELR-containing CXC chemokines. J Cell Biochem 104: 1356–1363, 2008. [DOI] [PubMed] [Google Scholar]
- 20.MehradBMehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost 97: 755–762, 2007. [PMC free article] [PubMed] [Google Scholar]
- 21.OtaniAOtani A, Slike BM, Dorrell MI, Hood J, Kinder K, Ewalt KL, Cheresh D, Schimmel P, Friedlander M. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci USA 99: 178–183, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ParkSPark S, Kim S. Do aminoacyl-tRNA synthetases have biological functions other than in protein biosynthesis? IUBMB Life 58: 556–558, 2006. [DOI] [PubMed] [Google Scholar]
- 23.PredescuSAPredescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 293: L823–L842, 2007. [DOI] [PubMed] [Google Scholar]
- 24.PughCWPugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684, 2003. [DOI] [PubMed] [Google Scholar]
- 25.RampartMRampart M, Van Damme J, Zonnekeyn L, Herman AG. Granulocyte chemotactic protein/interleukin-8 induces plasma leakage and neutrophil accumulation in rabbit skin. Am J Pathol 135: 21–25, 1989. [PMC free article] [PubMed] [Google Scholar]
- 26.Satchi-FainaroRSatchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, Feng D, Dvorak AM, Dvorak HF, Puder M, Mukhopadhyay D, Folkman J. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell 7: 251–261, 2005. [DOI] [PubMed] [Google Scholar]
- 27.SengerDRSenger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol 149: 293–305, 1996. [PMC free article] [PubMed] [Google Scholar]
- 28.SimaJSima J, Zhang SX, Shao C, Fant J, Ma JX. The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett 564: 19–23, 2004. [DOI] [PubMed] [Google Scholar]
- 29.StabileEStabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 108: 205–210, 2003. [DOI] [PubMed] [Google Scholar]
- 30.TabruynSPTabruyn SP, Griffioen AW. Molecular pathways of angiogenesis inhibition. Biochem Biophys Res Commun 355: 1–5, 2007. [DOI] [PubMed] [Google Scholar]
- 31.ThurstonGThurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514, 1999. [DOI] [PubMed] [Google Scholar]
- 32.WakasugiKWakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284: 147–151, 1999. [DOI] [PubMed] [Google Scholar]
- 33.WakasugiKWakasugi K, Slike BM, Hood J, Ewalt KL, Cheresh DA, Schimmel P. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol Chem 277: 20124–20126, 2002. [DOI] [PubMed] [Google Scholar]
- 34.WakasugiKWakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, Friedlander M, Cheresh DA, Schimmel P. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci USA 99: 173–177, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.YangXLYang XL, Schimmel P, Ewalt KL. Relationship of two human tRNA synthetases used in cell signaling. Trends Biochem Sci 29: 250–256, 2004. [DOI] [PubMed] [Google Scholar]
- 36.YeLYe L, Martin TA, Parr C, Harrison GM, Mansel RE, Jiang WG. Biphasic effects of 17-β-estradiol on expression of occludin and transendothelial resistance and paracellular permeability in human vascular endothelial cells. J Cell Physiol 196: 362–369, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


