Abstract
Respiratory muscle training (RMT) improves functional capacity in chronic heart-failure (HF) patients, but the basis for this improvement remains unclear. We evaluate the effects of RMT on the hemodynamic and autonomic function, arterial baroreflex sensitivity (BRS), and respiratory mechanics in rats with HF. Rats were assigned to one of four groups: sedentary sham (n = 8), trained sham (n = 8), sedentary HF (n = 8), or trained HF (n = 8). Trained animals underwent a RMT protocol (30 min/day, 5 day/wk, 6 wk of breathing through a resistor), whereas sedentary animals did not. In HF rats, RMT had significant effects on several parameters. It reduced left ventricular (LV) end-diastolic pressure (P < 0.01), increased LV systolic pressure (P < 0.01), and reduced right ventricular hypertrophy (P < 0.01) and pulmonary (P < 0.001) and hepatic (P < 0.001) congestion. It also decreased resting heart rate (HR; P < 0.05), indicating a decrease in the sympathetic and an increase in the vagal modulation of HR. There was also an increase in baroreflex gain (P < 0.05). The respiratory system resistance was reduced (P < 0.001), which was associated with the reduction in tissue resistance after RMT (P < 0.01). The respiratory system and tissue elastance (Est) were also reduced by RMT (P < 0.01 and P < 0.05, respectively). Additionally, the quasistatic Est was reduced after RMT (P < 0.01). These findings show that a 6-wk RMT protocol in HF rats promotes an improvement in hemodynamic function, sympathetic and vagal heart modulation, arterial BRS, and respiratory mechanics, all of which are benefits associated with improvements in cardiopulmonary interaction.
Keywords: myocardial infarction, cardiovascular control, diaphragm, cardiopulmonary interaction
the hallmark symptoms of heart failure (HF) subsequent to myocardial infarction (MI) are dyspnea and early fatigue, leading to reduced exercise tolerance and functional capacity (1). Furthermore, after MI, the neurohumoral excitation, which initially helps to preserve the cardiac output of patients who have systolic dysfunction, becomes deleterious with the persistence of cardiac dysfunction (12). In HF, the neurohumoral excitation is characterized by sympathetic hyperactivity and the attenuation of parasympathetic activity (12), which is associated with blunted baroreflex sensitivity (BRS) (30). In this context, impaired short-term control of arterial pressure (AP) and decreased heart-rate variability (HRV) (30) have been associated with an increased risk of sudden death of cardiac origin (45) and postinfarct mortality, regardless of the ejection fraction or ventricular arrhythmias (30).
Together with the cardiovascular alterations, changes in the respiratory system, represented by respiratory muscle weakness (32, 34, 35), an inadequate ventilation/perfusion ratio (35), pulmonary edema, and abnormal respiratory mechanics (15, 17), have a pivotal role in the symptomatology of HF. Specifically, the respiratory muscle weakness present in HF, both in humans and animals (32, 34, 35, 47), represents an important and independent predictor of poor prognosis (34) and has also been associated with functional limitation. The pulmonary system is intimately linked with the cardiovascular system anatomically and hemodynamically and through a number of mechanisms, plays a noteworthy role in exercise intolerance. However, the association between the cardiovascular and respiratory alterations and functional limitation in HF is still not fully understood.
Modulation of the cardiovascular system by the sympathetic and parasympathetic systems is modified markedly by respiration (2, 19). The periodic ventilation, present in patients with HF, is related directly with sympathetic hyperactivity (46). Furthermore, in HF, respiratory muscle weakness contributes toward increased neurohumoral excitation due to hyperactivity of the diaphragmatic metaboreflex, resulting in increased sympathetic vasoconstrictor activity, which decreases perfusion in skeletal muscle (10, 12, 22).
Respiratory muscle training (RMT) is used frequently in clinical practice, although its benefits are still the subject of debate (20). Recently, our group demonstrated that RMT in patients with HF led to increased respiratory muscle strength and performance, thus increasing exercise capacity and quality of life (8). Additionally, there is some evidence that RMT significantly reduces inspiratory muscle metaboreflex (6, 8). A few reports (4, 42), which investigated the effects of RMT in normal rats, demonstrated that RMT increases diaphragmatic mass and leads to hypertrophy in IIa and IIb fiber types. However, the effect of RMT on neurohumoral control of the cardiovascular system and respiratory mechanics in HF rats has not been evaluated.
Therefore, we conducted the present study to test the hypothesis that a 6-wk protocol of RMT could be associated with improvement in cardiac morphometric, hemodynamic, and autonomic function; BRS; and respiratory mechanics in rats with HF.
METHODS
Animals.
Experiments were performed on 32 male Wistar rats (200 and 230 g) from the Animal Breeding Unit of the Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA; Brazil). They were housed in groups of three/cage, receiving food and water ad libitum in an animal room maintained at 22°C under a 12:12-h light-dark cycle. The investigation followed the ethical rules established by the Guide for Care and Use of Experimental Animals published by the National Institutes of Health (publication no. 85-23, revised in 1996). All procedures outlined in this study were approved by the UFCSPA Ethics and Research Committee (protocol 712/08).
Surgery to induce MI.
Rats were anesthetized with xylazine (12 mg/kg ip) and ketamine (90 mg/kg ip), intubated, and artificially ventilated. Ligation of the left coronary artery and sham operations were performed as described previously (40). After surgery, the animals received a single injection of monofenew (0.05 ml/100 g) and gentamicin (0.05 ml/100 g).
Experimental design.
After MI, rats were allowed a minimum of 4 wk to recover (time necessary to develop the HF state) (40) and were assigned to one of four experimental groups: sedentary sham rats (Sed-Sham; n = 8), trained sham rats (RMT-Sham; n = 8), sedentary HF rats (Sed-HF; n = 8), or trained HF rats (RMT-HF; n = 8).
RMT protocol.
Four weeks after MI or sham surgery, the rats assigned to the groups, which would include RMT, underwent a 5-day adaption protocol, as described previously (4, 42). The RMT protocol began in the 5th wk. It comprised 30 min/day, 5 days/wk for 6 wk, during which time, a progressive increase in resistance was achieved by reducing the internal diameter of the hole through which the animal breathed, as described previously (4, 42).
Conscious cardiovascular measurements.
In the week following the RMT period, under general anesthesia with xylazine (12 mg/kg ip) and ketamine (90 mg/kg ip), two catheters filled with saline (0.06 ml) and heparin (0.01 ml) were implanted into the abdominal aorta and inferior vena cava. The catheters were used for the purposes of directly measuring mean AP (MAP) and administering drugs, respectively. Awake rats were studied 1 day after catheter placement. The arterial catheter was attached to a 40-cm polyethylene tube connected to a strain-gauge pressure transducer (Miniature Pulse Transducer RP-155, Narco Bio-Systems, Houston, TX), coupled to a pressure amplifier (Stemtech, Houston, TX), and blood-pressure signals were recorded over a 15-min period (CODAS, 1-kHz sampling frequency, Dataq Instruments, Akron, OH). The recorded data were analyzed on a beat-to-beat basis to quantify the parameters of interest.
BRS.
As described above, 1 day after catheter placement and prior to the baroreflex test, HR and blood-pressure signals were recorded for 15 min to serve as baseline control recordings for each rat. Baroreflex-mediated changes were measured during peak increases or decreases in MAP due to systemic venous injection of a single dose of phenylephrine (8 μg/ml; Sigma Chemical, St. Louis, MO) or sodium nitroprusside (100 μg/ml; Sigma Chemical), respectively (41). The corresponding peak reflex changes in HR were recorded after each dose of these drugs. The changes in MAP were within the 10- to 30-mmHg range. The maximum changes in MAP and HR were measured, and BRS was determined by fitting the MAP and HR changes to a sigmoidal, logistic equation, as described previously (23).
Vagal and sympathetic control of HR.
After returning to basal values (∼15 min after baroreflex evaluation), HR and MAP were recorded again for a 15-min period to serve as a baseline measurement. Vagal and sympathetic tone and effect, together with intrinsic HR (IHR) were determined by injecting methylatropine (3 mg/kg iv; Sigma Chemical) and propranolol (4 mg/kg iv; Sigma Chemical) at a maximum volume of 0.2 ml/injection. The vagal effect was evaluated as the difference between the maximum HR after methylatropine injection and the control HR. The sympathetic effect was evaluated as the difference between the control HR and minimum HR after propranolol injection. The vagal tone was calculated as the difference between the IHR and the HR after propranolol injection. The sympathetic tone was determined as the difference between the HR after methylatropine injection and the IHR (37).
Assessment of respiratory mechanics.
Twenty-four hours after autonomic evaluation, the rats were anesthetized again, as described above, and tracheostomized, and a rigid-type cannula (2-mm ID) was inserted into the trachea and tied firmly in place. The cannula was connected to a small animal ventilator (flexiVent, Scireq, Montreal, QC, Canada). Rats were mechanically ventilated at a breath rate of 90 breaths/min, with a tidal volume (VT) of 10 ml/kg using 5 cmH2O-positive end-expiratory pressure established by a water column. No muscle relaxants were used. If necessary, additional doses of anesthetics were given as required. In addition, the breath rate was set above normal (120 breaths/min) to suppress spontaneous breathing when measuring respiratory mechanics (7, 18). Rats were allowed to stabilize on the ventilator for 5 min before the measurements were taken. Impedance of the respiratory system was measured following a forced oscillation. Impedance was measured during a 16-s vol perturbation signal. Before taking the measurements of each rat, calibration signals were collected by applying the volume oscillation through the tracheal cannula, at first, with the cannula completely closed and then, with it open to atmosphere (25). The perturbation of 16 s is composed of sinusoids with mutually prime frequencies ranging from 0.25 to 19.625 Hz, which were chosen to avoid harmonic distortion (21). The amplitudes of the sinusoids decreased hyperbolically with frequency.
The constant-phase model described by Hantos et al. (21) was used to partition impedance into components representing the mechanical properties of the airway and parenchyma. The constant-phase model was fitted as: Zrs (f) = Raw + i2πf Iaw + [Gti − iHti]/(2π.f)α, where Zrs is respiratory system impedance, Raw is airway resistance, Iaw is the inertance, Gti is tissue resistance, Hti is lung elastance (Est), i is the imaginary unit, f is frequency, and α = (2/π)arctan(Hti/Gti) (7). Quasistatic Est reflects the static elastic recoil pressure of the lungs at a given lung volume and was measured by applying the pressure-volume curve technique using the Salazar-Knowles equation, as described by the flexiVent manufacturer.
Cardiac hemodynamic evaluation.
After evaluating the respiratory mechanics, while under anesthesia, as described previously, a polyethylene catheter (PE-50) was inserted into the right carotid artery for hemodynamic evaluation. The AP was recorded first during a 5-min period. Then, the catheter was positioned inside the left ventricle (LV), and the pulse wave was monitored using the typical graphic registration of ventricular pressure and recorded for 5 min. These data were used to determine LV systolic pressure (LVSP), LV maximum change in pressure over time (+dP/dtmax) and LV minimum change in pressure over time (−dP/dtmax), and LV end-diastolic pressure (LVEDP).
Infarct size, heart hypertrophy, and pulmonary and hepatic congestion.
The animals were killed with an overdose of anesthetic (thiopental 80 mg/kg ip), and the heart, lungs, and liver were removed and weighed. The right ventricle (RV) and LV were dissected and weighed. The LVs were filled with an insufflating latex balloon and placed in 10% formaldehyde for a minimum of 3 days before being cut into two equal transverse sections. These sections were embedded in paraffin for subsequent analysis of the infarct size. The ventricles were sectioned (4 μm) using a RM-2255 rotary microtome (Leica, Germany). The sections were stained with hematoxylin and eosin and magnified using a stereomicroscope (Stemi SV6, Zeiss, Germany). The percentage of the infarcted area was determined as described previously (40).
The heart weight-to-body weight ratio (HW/BW), LV/BW, and RV/BW values were determined. Lungs and liver were dehydrated (80°C) for 48 h and then weighed again to evaluate the water percentage.
Cardiopulmonary interaction.
To test the effects of RMT on the association between cardiovascular and pulmonary parameters—a process known as cardiopulmonary interaction—we tested the correlation between cardiovascular and pulmonary variables.
Statistical analysis.
All data are expressed as mean ± SD. The intergroup data were analyzed using two-way ANOVA, followed by the Bonferroni post hoc test to compare the group (HF or Sham) and intervention (RMT or Sed) effects. Pearson's correlation analysis was performed to test associations. A P < 0.05 was considered statistically significant. The GraphPad Prism 5 program (GraphPad Software, San Diego, CA) and SigmaPlot 11.0 for Windows (Systat Software, Chicago, IL) were used in the data analysis.
RESULTS
Mortality, BW, infarct size, heart hypertrophy, and pulmonary and hepatic congestion.
Mortality in MI-induced HF rats, during or after surgery, was 34%. In the sham groups, there were no deaths during the study. The Sed-HF group presented pulmonary and hepatic congestion compared with sham groups; RMT reduced the pulmonary (P < 0.001 for group and training, and P < 0.01 for interaction effects) and hepatic congestion (P < 0.01 for group, and P < 0.001 for training effects). The HW/BW, LV/BW, and RV/BW were higher in the Sed-HF group compared with the Sed-Sham group. RMT decreased cardiac hypertrophy (HW/BW; P < 0.001 for group, and P < 0.01 for training effects) and RV hypertrophy (RV/BW; P < 0.001 for group, and P < 0.01 for training and interaction effects) compared with the Sed-HF group. All of these data are summarized in Table 1.
Table 1.
Body weight, morphometric cardiac characteristics, infarct area, and lung and hepatic congestion of sham-operated rats and rats with left ventricle dysfunction
| Groups | Initial BW, g | Final BW, g | Infarcted Area, % | HW/BW, mg/g | LV/BW, mg/g | RV/BW, mg/g | Pulmonary Congestion, % | Hepatic Congestion, % |
|---|---|---|---|---|---|---|---|---|
| Sed-Sham | 216 ± 13 | 312 ± 31 | [en] | 2.65 ± 0.24 | 2.22 ± 0.29 | 0.43 ± 0.16 | 72.43 ± 1 | 71.73 ± 1 |
| RMT-Sham | 212 ± 9 | 313 ± 33 | [en] | 2.56 ± 0.21 | 2.08 ± 0.26 | 0.49 ± 0.14 | 71.28 ± 2 | 71.92 ± 1 |
| Sed-HF | 220 ± 7 | 311 ± 24 | 44.05 ± 5 | 3.66 ± 0.43a | 2.59 ± 0.17b | 1.07 ± 0.29a | 76.01 ± 1c | 73.64 ± 1c |
| RMT-HF | 217 ± 9 | 307 ± 26 | 45.57 ± 5 | 3.17 ± 0.22b | 2.45 ± 0.37d | 0.72 ± 0.22b | 71.49 ± 2 | 71.12 ± 1 |
Values are means ± SD; n = 8 for all groups. BW, body weight; HW/BW, heart weight-to-BW ratio; LV/BW, left ventricle-to-BW ratio; RV/BW, right ventricle-to-BW ratio; Sed-Sham, sedentary sham rats; RMT-Sham, respiratory muscle training sham rats; Sed-HF, sedentary heart failure rats; and RMT-HF, respiratory muscle training heart failure rats.
P < 0.01 compared with RMT-HF, RMT-Sham, and Sed-Sham;
P < 0.05 compared with RMT-Sham and Sed-Sham;
P < 0.001 compared with RMT-HF, RMT-Sham, and Sed-Sham; b and dP < 0.05 compared with RMT-Sham.
BRS.
The baroreceptor-mediated reflex showed that the values related to the highest slope point of the MAP (MAP50) were lower in the Sed-HF group compared with the Sed-Sham group. The RMT-HF group showed a significant increase in the MAP50 compared with the Sed-HF group (P < 0.01 for group, and P < 0.05 for training effects). BRS (gain) was also higher (P < 0.05 for training effect) in the RMT-HF group than in the Sed-HF group. These data are summarized in Table 2 and Fig. 1.
Table 2.
Baroreceptor reflex responses and HR of sham-operated rats and rats with left ventricle dysfunction
| Groups | MAP50, mmHg | Maximum gain, beats · min−1 · mmHg−1 |
|---|---|---|
| Sed-Sham | 112.8 ± 11.7 | −7.7 ± 0.8 |
| RMT-Sham | 115.6 ± 10.2 | −8.4 ± 2.6 |
| Sed-HF | 94.8 ± 9.7* | −6.5 ± 1.3 |
| RMT-HF | 110.3 ± 16.4 | −10.2 ± 3.3† |
Values are means ± SD; n = 8 for all groups. HR, heart rate; and MAP50, values related to the highest slope point of the mean arterial pressure.
P < 0.05 compared with RMT-HF, RMT-Sham, and Sed-Sham; and
P < 0.05 compared with Sed-HF.
Fig. 1.
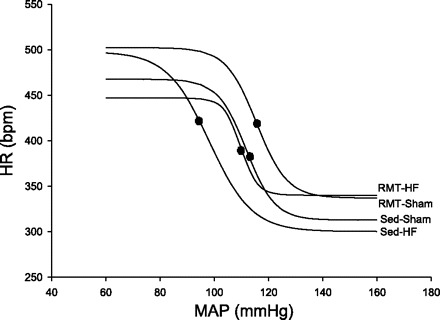
Values are means ± SD; n = 8 for all groups. Two-way ANOVA and Bonferroni post hoc test. Sed-Sham, sedentary sham rats; RMT-Sham, respiratory muscle training-Sham rats; Sed-HF, sedentary heart failure rats; RMT-HF, RMT HF rats. Plots showing the average values of the relationship between mean arterial pressure (MAP) and heart rate (HR) using logistic sigmoidal baroreceptor curve analysis. P < 0.05 comparing RMT-HF with the Sed-HF group. ∙, values related to the highest slope point of the MAP (MAP50; corresponding to the value found at 1/2 of the HR range evoked by the baroreflex response).
Vagal and sympathetic control of HR.
The results for autonomic function are summarized in Table 3. Vagal effect and vagal tone were lower in the Sed-HF than in the Sed-Sham group. After RMT, the vagal effect was greater in the RMT-HF group (P < 0.001 for group, and P < 0.05 for training effects). The sympathetic effect and tone were greater in the Sed-HF group than in the Sed-Sham group. After RMT, in the HF group, there was a decrease in sympathetic tone (P < 0.01 for group, training, and interaction effects). Resting HR was greater in the Sed-HF than in Sed-Sham rats. In HF rats, RMT reduced resting HR (P < 0.001 for group, and P < 0.05 for training and interaction effects). Overall, therefore, RMT improved all autonomic function indices.
Table 3.
Cardiovascular, autonomic, and systemic arterial pressure evaluations of sham-operated rats and rats with left ventricle dysfunction
| Groups | Vagal Effect, bpm | Vagal Tone, bpm | Sympathetic Effect, bpm | Sympathetic Tone, bpm | IHR, bpm | HR, bpm | MAP, mmHg | SAP, mmHg | DAP, mmHg |
|---|---|---|---|---|---|---|---|---|---|
| Sed-Sham | 119 ± 20 | 70 ± 19 | 32 ± 13 | 64 ± 18 | 380 ± 26 | 343 ± 26 | 121 ± 10 | 144 ± 10 | 101 ± 9 |
| RMT-Sham | 119 ± 27 | 81 ± 27 | 53 ± 18 | 64 ± 9 | 381 ± 14 | 356 ± 19 | 119 ± 9 | 139 ± 12 | 101 ± 8 |
| Sed-HF | 51 ± 22a | 32 ± 14b | 61 ± 14c | 91 ± 20d | 352 ± 23 | 399 ± 35a | 102 ± 8a | 118 ± 9a | 87 ± 8a |
| RMT-HF | 82 ± 22b | 43 ± 30e | 45 ± 21 | 56 ± 19 | 350 ± 29 | 362 ± 24 | 119 ± 18 | 135 ± 18 | 105 ± 17 |
Values are means ± SD; n = 8 for all groups. IHR, intrinsic HR; SAP, systolic AP; and DAP, diastolic AP.
P < 0.05 compared with RMT-HF, RMT-Sham, and Sed-Sham;
P < 0.05 compared with RMT-Sham and Sed-Sham;
P < 0.05 compared with Sed-Sham;
P < 0.01 compared with RMT-HF, RMT-Sham, and Sed-Sham; b and eP < 0.05 compared with RMT-Sham.
Respiratory mechanics.
The respiratory system resistance (Rrs) showed an increase in the Sed-HF group (Fig. 2A). Gti, which has a noteworthy role in Rrs, was also increased in Sed-HF rats (Fig. 2B). RMT was able to decrease Rrs (P < 0.001 for group, training, and interaction effects) and Gti (P < 0.001 for group and interaction, and P < 0.01 for training effects) in HF rats. Respiratory system Est (Ers) was greater in Sed-HF rats; RMT restored the Ers (P < 0.001 for group, and P < 0.01 for training and interaction effects; Fig. 3A). Tissue Est (Hti), which has a main role in Ers, was also increased in HF rats; RMT restored Hti (P < 0.001 for group, and P < 0.05 for training and interaction effects; Fig. 3B). Additionally, quasistatic Est was greater in the Sed-HF group; RMT reduced Est in HF rats (P < 0.01 for group, training, and interaction effects; Fig. 3C).
Fig. 2.
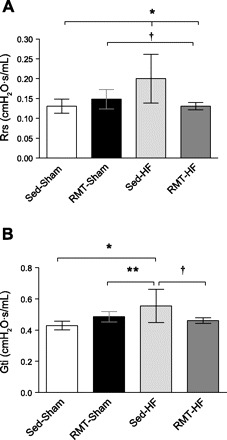
Values are means ± SD; n = 8 for all groups. Two-way ANOVA and Bonferroni post hoc test. A: Rrs, respiratory system resistance. *P < 0.001 compared with RMT-HF and Sed-Sham; †P < 0.01 compared with RMT-Sham. B: Gti, tissue resistance. *P < 0.001 compared with Sed-Sham; †P < 0.01 compared with Sed-HF; **P < 0.05 compared with Sed-HF.
Fig. 3.
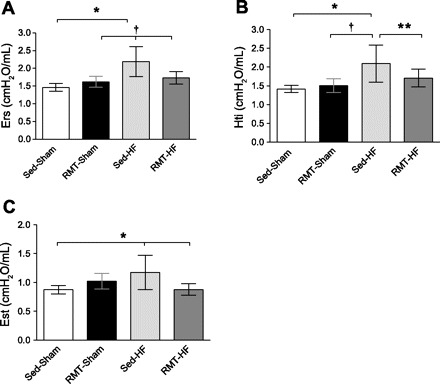
Values are means ± SD; n = 8 for all groups. Two-way ANOVA and Bonferroni post hoc test. A: Ers, respiratory system elastance (Est). *P < 0.001 compared with Sed-Sham; †P < 0.01 compared with RMT-HF and RMT-Sham. B: Hti, tissue Est. *P < 0.001 compared with Sed-Sham; †P < 0.01 compared with RMT-Sham; **P < 0.05 compared with Sed-HF. C: Est, quasistatic Est. *P < 0.01 compared with Sed-Sham and RMT-HF.
Hemodynamic variables.
In sham rats, RMT had no effect on LVEDP, LVSP, +dP/dtmax, or −dP/dtmax but improved all four parameters in infarcted rats (Table 4). RMT also normalized systolic pressure, diastolic pressure, and MAP (Table 3).
Table 4.
Hemodynamic variables of sham-operated rats and rats with left ventricle dysfunction
| Groups | LVEDP (mmHg) | LVSP (mmHg) | +dP/dtmax (mmHg/s) | −dP/dtmax (mmHg/s) |
|---|---|---|---|---|
| Sed-Sham | 4.6 ± 1 | 120.1 ± 13.5 | 6113 ± 1389 | −4175 ± 932 |
| RMT-Sham | 5.5 ± 1.6 | 122 ± 15.1 | 6644 ± 982 | −4247 ± 472 |
| Sed-HF | 21.9 ± 5.2* | 93.1 ± 8.5* | 4071 ± 678† | −2602 ± 432† |
| RMT-HF | 14.5 ± 6.7‡ | 113.4 ± 11 | 5237 ± 1630‡ | −2921 ± 852‡ |
Values are means ± SD; n = 8 for all groups. LVEDP, LV end-diastolic pressure; LVSP, LV systolic pressure; +dP/dtmax, LV maximum change in pressure over time; and −dP/dtmax, LV minimum change in pressure over time.
P < 0.01 compared with RMT-HF, RMT-Sham, and Sed-Sham;
P < 0.001 compared with RMT-Sham and Sed-Sham; and
P < 0.01 compared with RMT-Sham and Sed-Sham.
Correlations.
To study cardiopulmonary interaction, we examined the relationship between cardiovascular and pulmonary parameters. Positive correlations were found among RV hypertrophy and pulmonary congestion (r = 0.40, P < 0.05), sympathetic tone (r = 0.58, P < 0.001), and effect (r = 0.43, P < 0.05). Negative correlations were found between RV hypertrophy and vagal tone (r = −0.46, P < 0.05) and effect (r = −0.59, P < 0.01). Moreover, there were positive correlations between pulmonary congestion and sympathetic tone (r = 0.39, P < 0.05) and negative correlations between pulmonary congestion and vagal effect (r = −0.42, P < 0.05).
DISCUSSION
In the present report, we have demonstrated for the first time that RMT in rats with HF induced: 1) improvement in cardiovascular function, as demonstrated by the decrease in LVEDP, increase in LVSP, and decrease in RV hypertrophy and lung and hepatic congestion; 2) decreased sympathetic tone, increased vagal effect, and improved arterial baroreceptor sensitivity; and 3) improvement in respiratory mechanics, which was verified by the decrease in the Rrs and Gti and also by the decrease in Ers, Hti, and Est. These benefits result in improved cardiopulmonary interaction in HF. Interestingly, in the present report, there are no differences between the RMT-Sham and Sed-Sham groups in any studied parameter. This may be explained by the fact that in the Sham groups, there are no impairments in the studied variables, which therefore, showed no response to the RMT protocol. In addition, the intensity of RMT may have been insufficient to produce improvements in normal rats.
The beneficial effects of RMT on the contractile properties and morphological characteristics of the diaphragm have been described previously in rats (4, 42). Human studies aiming to test the impact of RMT in HF patients have shown benefits, such as improved functional capacity, symptomatology, and quality of life (6, 8). However, there is no consensus regarding the responses to RMT in the physiopathology of HF due to the complex inter-relationship between the pulmonary and cardiovascular systems. To the best of our knowledge, the present report is the first to show that RMT has potential beneficial effects on cardiovascular and pulmonary parameters and consequently, on cardiopulmonary interaction in HF rats.
The experimental model of MI in rats results in HF (14, 40). In this model, MI areas >30% represent a severe myocardial loss, which results in sustained hemodynamic dysfunction with high LVEDP, lower LVSP, and heart hypertrophy. In the present study, the infarcted area was ∼45%, which is associated with severe impairment of the hemodynamic function, respiratory system mechanics, and neurohumoral excitation.
In a previous study developed in our laboratory (38), we demonstrated a reduction of 12% in LVEDP in rats with MI submitted to a swimming protocol compared with sedentary MI rats. However, regarding RMT, the few existing studies used only normal rats and showed positive effects of RMT on morphometric and functional characteristics of the diaphragm (4, 42). In HF, the reduced cardiac reserve, more labored breathing, and increased blood flow demand from respiratory muscle may result in competition for the blood distribution between respiratory and locomotor vascular beds (39). The redirection of blood flow into the diaphragm is, in part, due to local metabolic accumulation during overload. This activates group IV phrenic afferent fibers, which leads to an increase in sympathetic vasoconstrictor activity in the limbs, increasing peripheral vascular resistance and decreasing oxygen supply to limb muscles (10, 22). This reflex is known as the inspiratory muscle metaboreflex, which is hyperactivated in HF (10, 12). The inspiratory muscle metaboreflex hyperactivity may be worsened by respiratory muscle weakness and is directly associated with exercise intolerance. In humans with HF, RMT reduces the inspiratory muscle metaboreflex and diminishes the influence of respiratory fatigue on the reduction in peripheral blood flow, both at rest and during exercise (6). In addition, a reduction in the inspiratory muscle work via inspiratory pressure unloading results in a significant increase in active limb-muscle blood flow (39). In the present study, we found that a 6-wk RMT protocol reduced LVEDP by 33% and increased LVSP by 21% in the HF-trained group compared with the Sed-HF group. Also, the RMT-HF group reduced the RV compensatory hypertrophy found in the Sed-HF group by 32% and led to decreased lung and hepatic congestion. The improved cardiovascular function observed after RMT results in increased LVSP and reduced LVEDP. In turn, this may produce peripheral vascular adaptations, which are translated into decreased hepatic and lung congestion. If these adaptations are present, the work of the RV decreases, minimizing the RV hypertrophy, as also demonstrated in this study.
On the other hand, the sympathetic hyperactivity, vagal attenuation, and decreased BRS observed in this report and by others (9, 30) lead to impaired HRV, which promotes important alterations to the hemodynamic function and tissue perfusion. Here, we have demonstrated that RMT reduces neurohumoral excitation and improves BRS, which can result in the recovery of blood flow in limb muscles, reducing cardiac overload. In this context, decreased sympathetic hyperactivity and improved parasympathetic attenuation are associated with increased functional capacity and long-term survival in HF patients (29).
As mentioned above, changes in respiration are known to modulate cardiovascular activity (2). A reduction in breathing rate together with a higher VT can increase the sensitivity of arterial baroreceptors, both in healthy individuals and those with cardiovascular diseases. This response seems to occur due to the increase in vagal activity and reduction in sympathetic activity (3).
RMT in healthy individuals promotes an increase in cardiac vagal tone and improves exercise capacity (24). In the experimental model of HF rats, electrical vagal stimulation improved long-term survival by preventing hemodynamic deterioration, as shown by the decreased LVEDP values and increased +dP/dtmax (33). Evidence from studies involving electrical vagal stimulation in humans with HF suggests it is beneficial in such individuals (44).
In the present study, the vagal effect in HF rats improved after RMT. Furthermore, these rats showed greater BRS. In rabbits, exercise training normalizes baroreflex control and sympathetic activity (16, 36). We observed that RMT, after HF, decreased resting HR, a response probably due to the 38% reduction in sympathetic tone. Physical exercise has several effects in patients with HF. It reduces muscle sympathetic nervous activity and consequently, peripheral vascular resistance, promoting greater perfusion of peripheral muscles (13, 43). There is also an improvement in functional capacity, as indicated by an increase in peak maximal oxygen consumption (1). Increases in resting HR are associated with high mortality, regardless of cause, in cardiovascular diseases, and particularly, in cardiac sudden death (27, 31). Reduction of resting HR with the use of β-blockers after MI decreases the mortality among HF patients (28); in this context, the present report has demonstrated a reduction in resting HR.
Chronic HF is associated with a number of pulmonary-related disorders, including restrictive and to a lesser extent, obstructive changes, reduced carbon monoxide diffusion capacity, reduced respiratory muscle strength and oxygenation, and an overall increased work of breathing (17, 34, 39). These alterations are associated with pulmonary congestion and edema (26), due to LV dysfunction (35), and are exacerbated during exercise, which contributes to dyspnea and fatigue in HF patients (5). Hydrostatic pulmonary edema is caused by an increase in microvascular pulmonary pressure after increased LVEDP (11); pulmonary edema promotes an increase in the tissue component of the resistance in the respiratory system and decreases the airway cross-sectional area (5), leading to increased work of breathing, which activates diaphragm metaboreflex.
Regarding the effects of RMT on respiratory mechanics in rats with MI, we found a decrease in tissue and consequently, in Rrs. We also found a reduction in static tissue and static Ers. These results are consistent with the results observed in the hemodynamic variables, such as a reduction in LVEDP and in RV hypertrophy, as well as with the improvement in the pulmonary congestion, shown clearly in this study. In addition, the tissue mechanical parameters reported in the present study correspond to the total respiratory system, and hence, they may reflect changes in the chest-wall compartment. It is possible that RMT resulted in diaphragm thickening and a consequent hidden, opposite change—an elevation in chest-wall Est, which counteracted the observed, overall decrease in total Est.
There are some limitations regarding the present report. First, there is no histological evaluation of the adaptations in the lungs or diaphragm nor any measurement of respiratory muscle performance; both would help identify the effects of RMT in muscle fiber and lung parenchyma adaptations. However, the effects of RMT on diaphragmatic characteristics using this model in normal rats have been described previously (4, 42). Second, the lack of a functional capacity evaluation may limit the understanding of the effects of RMT on peripheral skeletal muscle in this model. Finally, diaphragm functional tests, such as citrate synthase activity, respiratory muscle fatigue, and endurance, which would provide additional information about the effects of RMT, were not performed.
In conclusion, RMT results in improved sympathetic and parasympathetic modulation on HR and BRS. In addition, there is improvement in lung congestion, heart hypertrophy, hemodynamic function, and respiratory system mechanics, resulting in a better cardiopulmonary interaction in a rat model of HF, subsequent to MI. These findings determine a noteworthy contribution for using RMT as a new, nonpharmacological treatment modality in HF. Additionally, clinical studies may be done to test the effects of this intervention on cardiopulmonary interaction and other variables in patients with HF.
GRANTS
Support for this work was provided by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brasília, Brazil) and the Universidade Federal de Ciências da Saúde de Porto Alegre (Brazil).
DISCLOSURES
The authors have no financial relationship with any commercial entity with an interest in the subject of this paper.
AUTHOR CONTRIBUTIONS
Author contributions: R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. conception and design of research; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. performed experiments; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. analyzed data; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. interpreted results of experiments; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. prepared figures; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. drafted manuscript; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. edited and revised manuscript; R.B.J., V.S.H., E.Q., P.R.C., L.A.S., L.L.X., and P.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We are very grateful to Deise Mota Pacheco, Ramiro Barcos Nunes, and Alberto Antonio Rasia-Filho for their support during the development of this study.
REFERENCES
- 1. Antunes-Correa LM, Melo RC, Nobre TS, Ueno LM, Franco FGM, Braga AMW, Rondon MUPB, Brum PC, Barretto ACP, Middlekauff HR, Negrao CE. Impact of gender on benefits of exercise training on sympathetic nerve activity and muscle blood flow in heart failure. Eur J Heart Fail 12: 58–65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. Modulatory effects of respiration. Auton Neurosci 90: 47–56, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, Yeung LY, Sanderson JE, Pedretti R, Tramarin R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 105: 143–145, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Bisschop A, Gayan-Ramirez G, Rollier H, Gosselink R, Dom R, de Bock V, Decramer M. Intermittent inspiratory muscle training induces fiber hypertrophy in rat diaphragm. Am J Respir Crit Care Med 155: 1583–1589, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Cabanes LR, Weber SN, Matran R, Regnard J, Richard MO, Degeorges ME, Lockhart A. Bronchial hyperresponsiveness to methacholine in patients with impaired left ventricular function. N Engl J Med 320: 1317–1322, 1989. [DOI] [PubMed] [Google Scholar]
- 6. Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 51: 1663–1671, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Collins RA, Gualano RC, Zosky GR, Atkins CL, Turner DJ, Colasurdo GN, Sly PD. Hyperresponsiveness to inhaled but not intravenous methacholine during acute respiratory syncytial virus infection in mice. Respir Res 6: 142, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dall'Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 47: 757–763, 2006. [DOI] [PubMed] [Google Scholar]
- 9. De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol 50: 2285–2290, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Dixon DL, De Pasquale CG, De Smet HR, Klebe S, Orgeig S, Bersten AD. Reduced surface tension normalizes static lung mechanics in a rodent chronic heart failure model. Am J Respir Crit Care Med 180: 181–187, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrão CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 9: 630–636, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281: R1734–R1745, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Frank NR, Lyons HA, Siebens AA, Nealon TF. Pulmonary compliance in patients with cardiac disease. Am J Med 22: 516–523, 1957. [DOI] [PubMed] [Google Scholar]
- 16. Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 125: 669–682, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol 89: 908–916, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, Nozawa T, Takashima S, Umeno K, Inoue H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation 104: 418–423, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Gosselink R, Decramer M. Inspiratory muscle training: where are we? Eur Respir J 7: 2103–2105, 1994. [DOI] [PubMed] [Google Scholar]
- 21. Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 22. Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 85: 609–618, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Head GA, McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst 21: 203–213, 1987. [DOI] [PubMed] [Google Scholar]
- 24. Hepburn H, Fletcher J, Rosengarten TH, Coote JH. Cardiac vagal tone, exercise performance and the effect of respiratory training. Eur J Appl Physiol 94: 681–689, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Hirai T, McKeown KA, Gomes RF, Bates JH. Effects of lung volume on lung and chest wall mechanics in rats. J Appl Physiol 86: 16–21, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Jessup M, Brozena S. Heart failure. N Engl J Med 348: 2007–2018, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 352: 1951–1958, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol 57: 43F–49F, 1986. [DOI] [PubMed] [Google Scholar]
- 29. La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002. [DOI] [PubMed] [Google Scholar]
- 30. La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351: 478–484, 1998. [DOI] [PubMed] [Google Scholar]
- 31. Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol 51: 1725–1733, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Lecarpentier Y, Chemla D, Blanc FX, Pourny JC, Joseph T, Riou B, Coirault C. Mechanics, energetics, and crossbridge kinetics of rabbit diaphragm during congestive heart failure. FASEB J 12: 981–989, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, Haass M. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 103: 2153–2158, 2001. [DOI] [PubMed] [Google Scholar]
- 35. Meyer FJ, Zugck C, Haass M, Otterspoor L, Strasser RH, Kubler W, Borst MM. Inefficient ventilation and reduced respiratory muscle capacity in congestive heart failure. Basic Res Cardiol 95: 333–342, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Mousa TM, Liu D, Cornish KG, Zucker IH. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J Appl Physiol 104: 616–624, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Negrao CE, Moreira ED, Santos MC, Farah VM, Krieger EM. Vagal function impairment after exercise training. J Appl Physiol 72: 1749–1753, 1992. [DOI] [PubMed] [Google Scholar]
- 38. Nunes RB, Tonetto M, Machado N, Chazan M, Heck TG, Veiga AB, Dall'Ago P. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol 104: 1641–1647, 2008. [DOI] [PubMed] [Google Scholar]
- 39. Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979. [DOI] [PubMed] [Google Scholar]
- 41. Quagliotto E, Neckel H, Riveiro DF, Casali KR, Mostarda C, Irigoyen MC, Dall'ago P, Rasia-Filho AA. Histamine in the posterodorsal medial amygdala modulates cardiovascular reflex responses in awake rats. Neuroscience 157: 709–719, 2008. [DOI] [PubMed] [Google Scholar]
- 42. Rollier H, Bisschop A, Gayan-Ramirez G, Gosselink R, Decramer M. Low load inspiratory muscle training increases diaphragmatic fiber dimensions in rats. Am J Respir Crit Care Med 157: 833–839, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Roveda F, Middlekauff HR, Rondon MUPB, Reis SF, Souza M, Nastari L, Barretto ACP, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure—a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Schwartz PJ, De Ferrari GM. Vagal stimulation for heart failure: background and first in-man study. Heart Rhythm 6: S76–S81, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Schwartz PJ, La Rovere MT. ATRAMI: a mark in the quest for the prognostic value of autonomic markers. Autonomic Tone and Reflexes After Myocardial Infarction. Eur Heart J 19: 1593–1595, 1998. [DOI] [PubMed] [Google Scholar]
- 46. van de Borne P, Oren R, Abouassaly C, Anderson E, Somers VK. Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 81: 432–436, 1998. [DOI] [PubMed] [Google Scholar]
- 47. van Hees HW, Ottenheijm CA, Granzier HL, Dekhuijzen PN, Heunks LM. Heart failure decreases passive tension generation of rat diaphragm fibers. Int J Cardiol 141: 275–283, 2010. [DOI] [PubMed] [Google Scholar]


