Abstract
Compensatory reserve was measured in baboons (n = 13) during hemorrhage (Hem) and lower-body negative pressure (LBNP) using a machine-learning algorithm developed to estimate compensatory reserve by detecting reductions in central blood volume during LBNP. The algorithm calculates compensatory reserve index (CRI) from normovolemia (CRI = 1) to cardiovascular decompensation (CRI = 0). The hypothesis was that Hem and LBNP will elicit similar CRI values and that CRI would have higher specificity than stroke volume (SV) in predicting decompensation. Blood was removed in four steps: 6.25%, 12.5%, 18.75%, and 25% of total blood volume. Four weeks after Hem, the same animals were subjected to four levels of LBNP that was matched on the basis of their central venous pressure. Data (mean ± 95% confidence interval) indicate that CRI decreased (P < 0.001) from baseline during Hem (0.69 ± 0.10, 0.57 ± 0.09, 0.36 ± 0.10, 0.16 ± 0.08, and 0.08 ± 0.03) and LBNP (0.76 ± 0.05, 0.66 ± 0.08, 0.36 ± 0.13, 0.23 ± 0.11, and 0.14 ± 0.09). CRI was not different between Hem and LBNP (P = 0.20). Linear regression analysis between Hem CRI and LBNP CRI revealed a slope of 1.03 and a correlation coefficient of 0.96. CRI exhibited greater specificity than SV in both Hem (92.3 vs. 82.1) and LBNP (94.8 vs. 83.1) and greater ROC AUC in Hem (0.94 vs. 0.84) and LBNP (0.94 vs. 0.92). These data support the hypothesis that Hem and LBNP elicited the same CRI response, suggesting that measurement of compensatory reserve is superior to SV as a predictor of cardiovascular decompensation
Keywords: blood loss, central hypovolemia, stroke volume, blood pressure, compensatory mechanisms
in military and civilian trauma patients, the primary cause of death within the first hour of injury is hemorrhage (2, 4, 13, 14). As such, early assessment of those bleeding patients at greatest risk for developing shock due to reduced central blood volume is critical for effective triage decisions and optimal clinical outcomes. Lower-body negative pressure (LBNP) has provided an experimental model for the study of the physiology of human hemorrhage, especially during the early compensatory stages of hypovolemia (12). The validity of LBNP as an experimental model of hemorrhage has recently been demonstrated by comparing LBNP to actual hemorrhage in baboons (17) and humans (19).
An experimental advantage to using LBNP is the capability of safely inducing a reproducible clinical outcome in each human subject in the form of decompensation (presyncope). This approach has resulted in the identification of individuals with high and low tolerance to central hypovolemia (6, 8–10, 26). On the basis of our large database of more than 200 human LBNP experiments, a machine-learning algorithm based on analysis of arterial waveform features was developed to detect the reserve to compensate for reductions in central blood volume (8). We call this physiological measurement the compensatory reserve; the algorithm calculates a compensatory reserve index (CRI), which reflects the proportion of intravascular volume remaining before the onset of decompensation (21) and can distinguish those individuals with low tolerance to central hypovolemia (6). CRI ranges from 1 to 0, where CRI = 1 represents normovolemia and CRI = 0 represents hypovolemia at the point of decompensation (6, 21).
More recently, the measurement of compensatory reserve has been shown to be more specific than standard vital signs in the tracking of circulating blood volume in humans during actual mild (∼10%) to moderate (∼20%) hemorrhage (7, 22, 27). However, for obvious ethical reasons, human hemorrhage experiments cannot be designed with decompensation endpoints to demonstrate the capability of the CRI algorithm to distinguish individuals with high and low tolerance to actual blood loss. As such, there exists no validation of the compensatory reserve response during LBNP with actual hemorrhage.
Using the arterial blood pressure waveform data obtained during our previous study to compare hemorrhage and LBNP in baboons (17), we took the opportunity to perform the first head-to-head direct comparison between actual hemorrhage and matching LBNP applications in which decompensation was elicited in low-tolerant animals (i.e., animals who could not tolerate 25% hemorrhage without experiencing decompensation during LBNP and hemorrhage). With this retrospective analysis, we were able to test the hypothesis that the measurement of the compensatory reserve can be used to accurately distinguish individuals with low vs. high tolerance to hemorrhage due to higher specificity.
METHODS
Animals.
Adult male baboons (n = 13, 8–12 yr old, 25–35 kg body wt) were used to compare responses to hemorrhage and LBNP. The protocol was approved by the Institutional Animal Care and Use Committee of the Texas Biomedical Research Institute (San Antonio, TX). This study has been conducted in compliance with the Animal Welfare Act, implementing the animal welfare regulations and adhering to the principles of the “Guide for the Care and Use of Laboratory Animals”. The details of this protocol are presented in a previous publication (17) and are summarized below.
Surgical procedures.
On the day of each experimental session (hemorrhage or LBNP), the baboons were sedated and intubated to maintain an open airway and allowed to breathe spontaneously during the experiments. Arterial pressure and central venous pressure (CVP) were measured via catheters inserted in the axillary artery and vein, respectively. The CVP catheter was advanced to the right atrium. For hemorrhage experiments, two additional catheters were inserted in the femoral artery and vein for blood removal and replacement, respectively. After catheter placement, ECG leads were attached to monitor heart rate. At the completion of the hemorrhage and LBNP experiments, the catheters were removed, and the skin incisions at the catheter insertion sites were sutured closed.
Hemorrhage experiment.
Arterial blood pressure, heart rate, and CVP were recorded continuously during the experiment. Baseline values were monitored for 20 min prior to a stepwise hemorrhage of 25% blood volume via the femoral artery. As an a priori protocol termination criterion, the hemorrhage procedure was stopped prematurely when a systolic arterial pressure less than 70 mmHg was attained prior to 25% blood loss. The four steps of hemorrhage represented 6.25%, 12.5%, 18.75%, and 25% blood volume were achieved over a 28-min period (7 min/step). The maximum hemorrhage level was 25%. Hemorrhage blood volumes at each step were calculated on the basis of an assumed total blood volume of 71 ml/kg, which was measured in a separate group of animals (17). After the last step of hemorrhage, shed blood was replaced via the femoral vein.
Lower-body negative pressure experiment.
Four weeks after the hemorrhage experiment, the baboons were again sedated and instrumented for recording of arterial blood pressure, CVP, and heart rate. The animals were placed supine in an LBNP chamber. Baseline values were monitored for 20 min prior to a stepwise LBNP procedure to match the previous hemorrhage steps. LBNP levels for each step were determined by matching CVP measurements from the animal's previous hemorrhage study. As in the hemorrhage experiment, a systolic arterial pressure less than 70 mmHg was the termination criterion. However, unlike the hemorrhage experiments, LBNP was applied until this termination criterion was attained (decompensation). At the end of the procedure, the negative pressure was released, and hemodynamic variables were allowed to stabilize for 20 min.
Estimating compensatory reserve.
Arterial waveforms obtained during hemorrhage and LBNP experiments were analyzed with an algorithm developed to estimate the compensatory reserve called the CRI. The algorithm was originally developed on the basis of extensive analysis of a large body of waveform data obtained from human subjects during progressive central hypovolemia induced with LBNP (6, 21). Figure 1 (11) illustrates the different characteristics of an arterial pressure waveform during normovolemia (Fig. 1A) and hypovolemia (Fig. 1B). The arterial waveform is composed of the ejected wave (cardiac output), and the reflected wave (peripheral vascular resistance). The two waves overlap during normal conditions but separate during hypovolemic conditions. The observed or recorded total waveform is outlined in gray. The features of the total waveform are affected by the compensatory mechanisms involved in the control of cardiac output and peripheral vascular resistance during conditions of hypovolemia. The algorithm for measuring compensatory reserve evaluates the subtle changes in the features of the arterial waveform (gray outline in Fig. 1) and integrates all of the physiological compensatory mechanisms affecting these features. The algorithm can assess changes in waveform features in an individual-specific manner, such that a CRI value is capable of assessing an individual's capacity to compensate for blood loss.
Fig. 1.
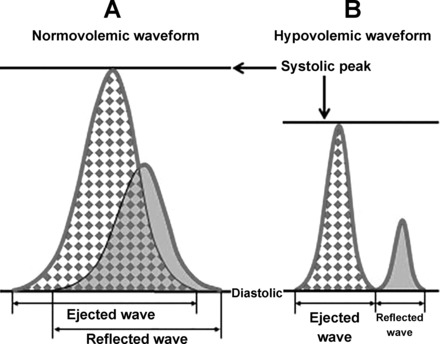
Illustration of the different characteristics of an arterial pressure waveform during normovolemia (A) and hypovolemia (B). The features of the arterial pressure waveform (gray outline) are dependent on systolic and diastolic pressure, as well as and the ejected and reflected waves of the pressure pulse. This illustration has been previously published (11).
For this experiment, the CRI model was calibrated to the waveform data from the baboon LBNP trials. The original model was developed from waveform analog data collected from a finger infrared photoplethysmogram during progressive LBNP experiments conducted on 100 human subjects and validated on 101 different subjects (6). Additional experimentation revealed a minimal requirement of 10 LBNP experiments using a stepwise profile similar to the original protocol for translation of the original human model to a baboon model. As such, the CRI model used for waveform analysis in the present study was calibrated to waveform analog data collected from the baboon LBNP trials, during which the decompensation criterion was attained. Estimates of compensatory reserve for each baboon were based on a separate calibration from which that test animal's data were excluded, to provide statistically unbiased data analysis. The algorithm estimates the proportion of physiological reserve available to compensate for changes in effective circulating blood volume by comparing waveform features to those in the model. The values of CRI range from 1 to 0, where CRI = 1 represents 100% capacity of physiological compensatory mechanisms to respond to changes in blood volume and CRI = 0 represents 0% capacity of compensatory mechanisms. Thus, during progressive central hypovolemia, CRI is theoretically expected to start at a value of 1 prior to volume loss (maximum compensatory reserve) and decrease during hypovolemia to a value of 0, at which point, all compensatory mechanisms responding to the hypovolemia are exhausted, resulting in cardiovascular collapse and shock.
Data analysis.
Continuous ECG, CVP, and arterial blood pressure waveform recordings were sampled at 500 Hz using LabView data acquisition software during hemorrhage and LBNP experiments. Beat-to-beat stroke volume (SV) was derived from the arterial pressure waveform using the pulse contour method (18), and beat-to-beat compensatory reserve was estimated using the algorithm described above. These beat-to-beat SV and compensatory reserve estimations were averaged to provide one value for the last 3 min of each hemorrhage and LBNP step using a commercially available software program (WinCPRS, Absolute Aliens, Turku, Finland).
Statistical analysis was conducted with commercially available software packages (SigmaStat; Systat Software, Richmond, CA and SAS v9.2; SAS Institute, Cary, NC). The Pearson product correlation coefficient was used to assess the relationship between changes in SV and changes in CRI. The probabilities of observing chance effects that changes in the dependent variables over changing circulating blood volumes (time) were different from “zero” change are presented as exact P values obtained from two-way repeated-measures analysis-of-variance tests.
Generalized estimating equations were used for repeated-measures logistic regression. The criterion for decompensation (systolic blood pressure <70 mmHg) is assessed at each LBNP step in the experiment, and at each step, animals are classified as either 1) decompensated or 2) not decompensated. The repeated-measures logistic regression procedure assesses how changes in SV and CRI are associated with this binary classification of decompensation at each step (i.e., SV and CRI are predictors of decompensation at each step of the experiment). Output from the logistic regression analyses for CRI and SV produced measures of the receiver operating characteristic area under the curve (ROC AUC), sensitivity, and specificity. Sensitivity (true positives) and specificity (true negatives) were calculated on the basis of logistic regression predicted probability cut-offs, which optimized both values giving equal weight to sensitivity and specificity (15, 16). The ability of CRI and SV to correctly predict decompensation during progressive hemorrhage was compared using the χ2-test for the difference in ROC AUC.
RESULTS
The levels of LBNP, which corresponded with 6.25%, 12.5%, 18.75%, and 25% hemorrhage were −22 ± 4, −41 ± 4, −54 ± 6, and −71 ± 5 mmHg, respectively. Figure 2 shows that CRI decreased during each step of hemorrhage and LBNP (P < 0.001). CRI values were statistically similar at baseline and responded equally to hemorrhage and LBNP (P = 0.20).
Fig. 2.
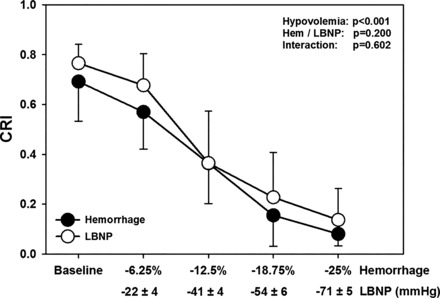
Compensatory reserve index (CRI) during baseline and four steps of hemorrhage (●) and lower-body negative pressure (LBNP; ○) corresponding to 6.25%, 12.5%, 18.75%, and 25% total blood volume loss. CRI values during hemorrhage or LBNP were not statistically different from one another. Data are expressed as means ± 95% confidence interval. P values from two-way repeated ANOVA are shown.
The ability of LBNP to mimic the compensatory reserve response during hemorrhage was supported by correlation coefficient between CRI during hemorrhage and LBNP to be 0.96 ± 0.04, slope = 1.03 (Fig. 3).
Fig. 3.
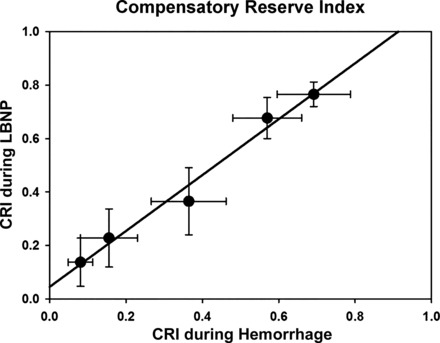
Correlation between compensatory reserve index (CRI) during hemorrhage and LBNP for baseline, 6.25%, 12.5%, 18.75%, and 25% total blood volume loss. Linear regression analysis revealed an amalgamated correlation coefficient between CRI during hemorrhage and LBNP to be 0.96 ± 0.04. Data are expressed as means ± 95% confidence interval.
CRI correlated with SV during both hemorrhage (r = 0.96 ± 0.08) and LBNP (r = 0.89 ± 0.24). The ROC AUC was statistically greater (P = 0.0006) for CRI compared with SV during actual hemorrhage, but not LBNP (Table 1). Whereas CRI and SV displayed similar sensitivities during hemorrhage, CRI provided greater specificity (92.3 and 94.8) than SV (82.1 and 83.1) as a predictor of reduced central blood volume in both hemorrhage and LBNP.
Table 1.
Comparison of receiver operating characteristics area under the curve, sensitivity, and specificity between stroke volume, and compensatory reserve index during actual hemorrhage and lower body negative pressure hemorrhage simulation experiments
| Vital Sign | Baseline | Onset of Decompensation | ROC AUC (95% CI) | χ2-Test | P Value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Hemorrhage | |||||||
| SV | 56.8 (13.3) | 26.6 (5.4) | 0.84 (0.74–0.95) | 11.75 | 0.0006 | 80.0 | 82.1 |
| CRI | 0.74 (0.18) | 0.05 (0.05) | 0.94 (0.88–1.00) | 80.0 | 92.3 | ||
| LBNP | |||||||
| SV | 60.2 (11.4) | 25.0 (4.4) | 0.92 (0.86–0.98) | 0.369 | 0.5436 | 90.9 | 83.1 |
| CRI | 0.74 (0.11) | 0.07 (0.04) | 0.94 (0.88–0.99) | 81.8 | 94.8 | ||
Values for baseline and onset of decompensation are median ± interquartile range; n = 13. ROC AUC, receiver operating characteristics area under the curve; SV, stroke volume; CRI, compensatory reserve index.
Five baboons failed to complete the entire 28-min hemorrhage period because of the onset of decompensation (i.e., SBP <70 mmHg) before the target 25% blood withdrawal could be reached. Two animals completed 18.75% (21 min), two animals completed 23% (26 min), and one animal completed 24% (27 min) blood loss. Subsequently, these same five animals experienced early decompensation during the LBNP protocol that was conducted 4 wk after the hemorrhage experiment. These five animals that did not complete 25% hemorrhage were classified as having low tolerance to hypovolemia, and the remaining eight baboons that completed the entire hemorrhage protocol were classified as having a high tolerance to hypovolemia. Analysis of SV responses comparing animals with low and high tolerance revealed similar reductions in SV (Fig. 4A). The slopes of the reduction in SV were not different between animals with low and high tolerance. In contrast, animals with low tolerance displayed a more rapid reduction in CRI (i.e., steeper slope) than those with high tolerance to hypovolemia (Fig. 4B).
Fig. 4.
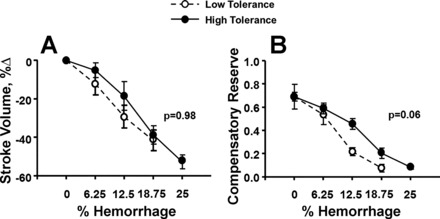
Changes in stroke volume (A) and compensatory reserve (B) during baseline, 6.25%, 12.5%, 18.75%, and 25% total blood volume loss in animals with low tolerance (○, broken lines) and high tolerance (●, solid lines) to hemorrhage. Data are expressed as means ± 95% confidence interval. P values are reported for tests of difference in slopes between low and high tolerance for stroke volume and compensatory reserve index.
DISCUSSION
In the present investigation, baboons were exposed to a progressive controlled hemorrhage and matched levels of LBNP that resulted in an average maximal reduction of ∼25% of their estimated circulating blood volume. Similar to our previous findings on hemodynamic, metabolic, and neuroendocrine responses (17), we found that the compensatory reserve response was similar during both hemorrhage and LBNP (Fig. 2). We hypothesized that a model developed with state-of-the-art feature-extraction and machine-learning techniques would provide earlier and more accurate estimates of blood volume loss in individual animals with varying compensatory responses. To test this hypothesis, we compared stroke volume responses and compensatory reserve measurements in a group of baboons who were able to tolerate the entire 25% hemorrhage (High-Tolerance Group) to a group who experienced decompensation prior to completing the entire protocol (Low-Tolerance Group). Since stroke volume and compensatory reserve are both calculated from features of the arterial waveform, there was a high correlation between the two measures. However, consistent with previous observations in humans (6, 9, 25), data from this study reaffirmed that stroke volume failed to distinguish low- from high-tolerant baboons (Fig. 4A).
In contrast, the compensatory reserve as measured by CRI was progressively reduced at a faster rate in low- compared with high-tolerant baboons (Fig. 4B). This finding was further supported by lower specificity in the stroke volume measurements during both hemorrhage (82.1) and LBNP (83.1) compared with specificities of 92.3 (hemorrhage) and 94.8 (LBNP) for the CRI. Specificity is an important component in differentiating CRI from other measures because it corresponds to its ability to distinguish high-tolerant animals, which tend to be more prevalent than low-tolerant animals. Equally important is the fact that CRI exhibited greater ROC AUC values, indicating that when considering sensitivity and specificity simultaneously, CRI was a better predictor of decompensation than SV, particularly in the actual hemorrhage experiment. Recognizing that CRI's performance was the same in both hemorrhage and LBNP experiments also suggests that it is a more consistent measurement of an individual's response to blood loss than SV, which was more responsive in LBNP than in the actual hemorrhage experiment. As such, measuring the magnitude of the reserve to compensate for blood loss proved to be the most robust marker of individual variation between subjects because it distinguishes those individuals at highest risk for early decompensation.
Several unique features of measuring the compensatory reserve are demonstrated by the data presented in Fig. 4. The ability to distinguish a faster rate of reduced compensatory reserve in low-tolerant animals with the same volume of blood loss indicates the ability of the model to provide individualized assessment of compensatory status (Fig. 4B). Differences in slopes of the stimulus-response (blood volume-CRI) relationship have been shown to represent the individual reserve required to compensate for the reduction in circulating blood volume (7). Previous experiments using LBNP (6, 23) support the notion that individuals with relatively low tolerance to reductions in central blood volume display more rapid depletion of their compensatory reserve. The results of this investigation are unique in that they extend previous data by demonstrating for the first time that a subgroup of animals who demonstrated early decompensation could be described by a steeper (faster) depletion in their compensatory reserve (slope difference = 1.43; P = 0.06) during both progressive LBNP and actual hemorrhage.
The algorithm developed to calculate compensatory reserve was based on a LBNP protocol in conscious humans in which the initial baseline stage was executed under controlled experimental conditions (e.g., adequate hydration, sleep, food intake, etc.) with subjects in the supine posture (i.e., optimum central blood volume). Although a CRI of 1.0 represents an optimum reserve for compensation, humans have consistently demonstrated an average baseline CRI value of ∼0.9 (6, 7). In the present study, the median value for CRI in baboons prior to either LBNP or blood loss experiments (baseline) was only 0.74. It is likely that this relatively low baseline value for CRI reflects the variability inherent in the baboon CRI algorithm derived for a relatively small cohort of animals. The algorithm for baboons was produced based on a model in which N−1 animals were used to evaluate data from the Nth animal. In the current study of 13 baboons, the baboon CRI algorithm was constructed using LBNP data from 12 baboons to produce a valid estimate for the 13th animal. This procedure was repeated 13 times so that CRI values for each animal were generated on the basis of the algorithm model from the remaining 12 animals. Therefore, depending on how each animal presented at baseline relative to its peers (perhaps related to various factors that can compromise compensatory mechanisms such as the level of tolerance, sedation, or hydration), it is possible that the model yielded a lower baseline CRI because that particular Nth baboon was more compromised at baseline. Although this method is valid, it produced an algorithm that exhibited greater variability compared with our previous experience with the CRI algorithm derived from human data (7, 27). As a result, the baboon CRI algorithm provided a wide range of baseline CRI values (0.9 to 0.4) that contributed to a low median value (0.74) relative to humans. However, comparison of changes in CRI to progressive LBNP and hemorrhage should not be altered by relatively low baseline CRI, since the experiment was conducted as a repeated-measures design with the animals under the same experimental conditions (e.g., sedation). Finally, the slower rate of diminution of compensatory reserve in the high- compared with the low-tolerant animals during controlled hemorrhage in the present study is consistent with the notion that a faster rate of fall in CRI during progressive reduction in central blood volume is associated with earlier development of hypotension, and reducing the rate of CRI reduction can delay cardiovascular decompensation (23).
Measurement of stroke volume has proven to provide useful information about circulating volume status because of the relationship between cardiac filling and central blood volume (i.e., sensitivity to changing volume). Since stroke volume is associated with features of the arterial pulse waveform (5, 20, 24), it is not surprising that stroke volume showed a high correlation with blood loss similar to that of CRI. However, the rate of change in stroke volume during blood loss is similar in individuals regardless of their tolerance to hypovolemia (8, 21). This is demonstrated in the present study by the similar rate of stroke volume decrease (slope) in Fig. 4A. At decompensation, humans with a high tolerance to blood loss have relatively lower stroke volume than individuals with low tolerance (8, 21), which was also observed in the baboons (Fig. 4A). As a result, stroke volume as an independent measure does not predict decompensation. In contrast, changes in compensatory reserve occurred at a faster rate in baboons with low tolerance (steeper slope) compared with those with high tolerance (Fig. 4B). At decompensation, compensatory reserve was similar in baboons with low and high tolerance, thus accurately predicting decompensation in all baboons. This ability of changes in compensatory reserve to differentiate individuals with high compared with low tolerance to reduced central blood volume is reflected by the higher ROC AUC and specificity for the CRI compared with stroke volume in the present investigation. As such, our findings support the notion that, compared with measurements of stroke volume, advanced machine-learning techniques designed to identify real-time subtle changes in the patterns and features of arterial waveforms can improve the fidelity of decision support related to diagnosis and care of patients who are at greatest risk of early onset of shock in an emergency medical setting.
Traditional approaches designed to provide potential early markers of imminent cardiovascular instability have been limited by algorithms based on population averages with significant interindividual and intraindividual variance and requirements for demographic information such as age, sex, height, and weight. The algorithm for determining compensatory reserve is unique in that it captures the status of “individuals” by estimating the relative amount of reserve remaining for compensation during blood loss without having to “know” demographics or any other information. Within this construct, it is noteworthy that compensatory reserve responses of baboons during either LBNP or hemorrhage are similar to those previously reported in humans during LBNP (6, 21, 23) or hemorrhage (7, 22, 27). This detailed model, which captures individual status, is best demonstrated by the different slopes of regression for baboons with low compared with high tolerance to progressive reductions in central blood volume (Fig. 4B).
The measurement of CRI reflects the status of compensation reserve for a particular central blood volume and is based on the fundamental biophysics of pressure-flow relationships within the cardiovascular system that dictate changes in features of the arterial waveform with inputs from compensatory mechanisms (e.g., reflex-mediated autonomic nerve activity, tissue metabolism). The results of the present investigation demonstrate the physiological relationship between significant progressive central blood volume reduction and the underlying absolute reserve capacity that dictates differences among individuals.
We have previously demonstrated that hemorrhage and LBNP elicit similar hemodynamic responses, but a difference was observed in how vascular fluid shifts affect hematocrit (17). During hemorrhage, transcapillary fluid flux causes fluid shifts from extravascular to intravascular compartments, resulting in hemodilution (1, 17). In contrast, the external negative pressure during LBNP reverses transcapillary fluid flux causing fluid shifts from intravascular to extravascular compartments, which results in hemoconcentration (17, 29). Another difference between hemorrhage and LBNP was that hemorrhage caused a decrease in central venous saturation, while LBNP did not affect it (17). Importantly, the CRI values were similar between hemorrhage and LBNP despite these differences between the two models. The similar CRI values can be explained by the recruitment of compensatory mechanisms in the two models. On the basis of estimates of average total blood volume, maximal hemorrhage volumes, and hematocrit values in our previous study (17), we estimated a 7% plasma volume increase during hemorrhage and a 10% plasma volume decrease during LBNP using the equations of van Beaumont (28). This difference in circulating blood volume between the hemorrhage and LBNP model was not physiologically impactful to cardiac filling and central hemodynamics, as evidenced by similar responses in the measured hemodynamic parameters (17). The similarity of hemodynamic responses between hemorrhage and LBNP most likely indicates that increasing circulating blood volume with transcapillary refill during hemorrhage acted to compensate for a greater reduction in mixed venous oxygen saturation. As such, during hemorrhage, transcapillary fluid flux acted as a compensatory mechanism to offset blood loss. In contrast, during LBNP, the compensatory mechanisms responding to the central hypovolemia did not include transcapillary refill. Thus, emphasizing that the CRI algorithm recognizes the sum of compensatory responses that are reflected by specific changes in the features of arterial waveform and explains why the CRI values were similar between the two models despite a slightly different combination of compensatory responses used during hemorrhage and LBNP. This notion is supported by our most recent work, demonstrating that feature changes in the arterial waveform represents the sum total of all individual compensatory mechanisms, which can be quite different across individuals (3). Comparisons of CRI and SV between low- and high-tolerant animals during hemorrhage (or LBNP) would not be affected by transcapillary fluid flux since changes in hematocrit were similar between groups within each experimental condition. The similarity in responses of compensatory reserve between LBNP and hemorrhage in the present study corroborates earlier findings that LBNP represents a valid physiological model of blood loss (17, 19), particularly for the study of the human response during the compensatory phase of hemorrhage.
In summary, the results of the present investigation demonstrated that LBNP mimics the response of the compensatory reserve that results from actual hemorrhage. Furthermore, the results of this investigation demonstrate for the first time that a novel measurement of compensatory reserve based on advanced machine learning and feature extraction analysis of arterial waveforms overcomes the limited specificity of stroke volume by accurately differentiating those individuals at greatest risk for early decompensation (i.e., low tolerance to blood loss).
Perspectives and Significance
Monitoring standard vital signs in the prehospital setting (civilian or military) is ineffective for the assessment of impending cardiovascular shock in a bleeding patient. The CRI algorithm has been developed to measure compensatory reserve from the features of the arterial wave form as a predictor of cardiovascular decompensation. The existing capability to integrate the CRI algorithm into any standard monitor that generates an arterial waveform (including a finger pulse oximeter available in the medical kits of U.S. Army combat medics and civilian first responders) provides a dramatic “leap forward” in prehospital triage decision support.
DISCLOSURES
Jane Mulligan and Greg Grudic are employed by Flashback Technologies.
AUTHOR CONTRIBUTIONS
C.H.-L. and V.A.C. conception and design of research; C.H.-L. and V.A.C. performed experiments; C.H.-L., J.T.H., J.M., G.Z.G., and V.A.C. analyzed data; C.H.-L., J.T.H., J.M., G.Z.G., and V.A.C. interpreted results of experiments; C.H.-L. and V.A.C. prepared figures; C.H.-L. and V.A.C. drafted manuscript; C.H.-L., J.T.H., J.M., and V.A.C. edited and revised manuscript; C.H.-L., J.T.H., J.M., G.Z.G., and V.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This research was supported by funding from the U.S. Army Medical Research and Materiel Command Combat Casualty Care Research Program and in part by a postdoctoral fellowship provided by the Oak Ridge Institute of Science and Education. We thank Gary Muniz from U.S. Army Institute of Surgical Research; and Dr. Robert E. Shade and Dr. Cassondra Bauer from the Texas Biomedical Research Institute for their technical contributions to this study.
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense. C. Hinojosa-Laborde, J.T. Howard, and V.A. Convertino have disclosed no conflicts of interest. G. Z. Grudic and J. Mulligan developed the CRI model used in this study and are cofounders of Flashback Technologies.
REFERENCES
- 1.Arnauld E, Czernichow P, Fumoux F, Vincent JD. The effects of hypotension and hypovolaemia on the liberation of vasopressin during hemorrhage in the unanesthetized monkey (Macaca mulatta). Pflügers Arch 371: 193–200, 1977. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med 149: 55–62, 1984. [PubMed] [Google Scholar]
- 3.Carter R III, Hinojosa-Laborde C, Convertino VA. Variability in integration of mechanisms associated with tolerance to progressive reductions in central blood volume: the compensatory reserve. Physiol Rep In press, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma 54: S13–S19, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Convertino VA, Cooke WH, Holcomb JB. Arterial pulse pressure and its association with reduced stroke volume during progressive central hypovolemia. J Trauma 61: 629–634, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Convertino VA, Grudic GZ, Mulligan J, Moulton SL. Estimation of individual-specific progression to cardiovascular instability using arterial waveforms. J Appl Physiol 115: 1196–1202, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Convertino VA, Howard JT, Hinojosa-Laborde C, Cardin S, Batchhelder P, Mulligan J, Grudic GZ, Moulton SL, MacLeod DB. Individual-specific, beat-to-beat trending of significant human blood loss: the compensatory reserve. Shock 44: 27–32, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Convertino VA, Moulton SL, Grudic GZ, Rickards CA, Hinojosa-Laborde C, Gerhardt RT, Blackbourne LH, Ryan KL. Use of advanced machine-learning techniques for noninvasive monitoring of hemorrhage. J Trauma 71: S25–S32, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart reate and vasoconstrictor reserves. Clin Auton Res 22: 123–130, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Convertino VA, Sather TM. Vasoactive neuroendocrine responses associated with tolerance to lower body negative pressure in humans. Clin Physiol 20: 177–184, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Convertino VA, Wirt MD, Glenn JF, Lein BC. The compensatory reserve for early and accurate prediction of hemodynamic compromise: a review of the underlying physiology. Shock In press. doi: 10.1097/SHK.0000000000000559, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol 96: 1249–1261, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, Bolenbaucher R, Blackbourne LH. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma 71: S4–S8, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Eastridge BJ, Mabry R, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK Jr, Kotwal RS, Holcomb J, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 73: S431–S437, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. Can J Emerg Med 8: 19–20, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Int Med 4: 627–635, 2013. [PMC free article] [PubMed] [Google Scholar]
- 17.Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, Chung KK, Cap AP, Convertino VA. Validation of lower body negative pressure as an experimental model of hemorrhage. J Appl Physiol 116: 406–415, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J 11 Suppl I: 26–32, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, van Helmond N, Curry TB, van Buskrik CM, Convertino VA, Joyner MJ. Reductions in central venous pressure by lower body negative pressure or blood loss elicit similar hemodynamic responses. J Appl Physiol 117: 131–141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath SP, Ryan KL, Wendelken SM, Rickards CA, Convertino VA. Pulse oximeter plethysmographic waveform changes in awake, spontaneously breathing, hypovolemic volunteers. Anesth Analg 112: 368–374, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Moulton SL, Mulligan J, Grudic GZ, Convertino VA. Running on empty: The compensatory reserve index. J Trauma Acute Care Surg 75: 1053–1059, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Nadler R, Convertino VA, Gendler S, Lending G, Lipsky AM, Cardin S, Lowenthal A, Glassberg E. The value of noninvasive measurement of the compensatory reserve index in monitoring and triage of patients experiencing minimal blood loss. Shock 42: 93–98, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Poh PYS, Carter R, Hinojosa-Laborde C, Mulligan J, Grudic GZ, Convertino VA. Respiratory pump contributes to increased physiological reserve for compensation during simulated haemorrhage. Exp Physiol 99: 1421–1426, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Reisner AT, Xu D, Ryan KL, Convertino VA, Rickards CA, Mukkamala R. Monitoring non-invasive cardiac output and stroke volume during experimental human hypovolaemia and resuscitation. Br J Anaesth 106: 23–30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickards CA, Ryan KL, Cooke WH, Convertino VA. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity. J Appl Physiol 111: 1048–1058, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Sather TM, Goldwater DJ, Montgomery LD, Convertino VA. Cardiovascular dynamics associated with tolerance to lower body negative pressure. Aviat Space Environ Med 57: 413–419, 1986. [PubMed] [Google Scholar]
- 27.Stewart CL, Mulligan J, Grudic GZ, Convertino VA, Moulton SL. Detection of low-volume blood loss: compensatory reserve versus traditional vital signs. J Trauma Acute Care Surg 77: 892–897, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. J Appl Physiol 32: 712–713, 1972. [DOI] [PubMed] [Google Scholar]
- 29.Ward KR, Tiba MH, Ryan KL, Filho IP, Rickards CA, Witten T, Soller BR, Ludwig DA, Convertino VA. Oxygen transport characterization of a human model of progressive hemorrhage. Resuscitation 81: 987–993, 2010. [DOI] [PubMed] [Google Scholar]


