Abstract
The collecting duct endothelin-1 (ET-1), endothelin B (ETB) receptor, and nitric oxide synthase-1 (NOS1) pathways are critical for regulation of fluid-electrolyte balance and blood pressure control during high-salt feeding. ET-1, ETB receptor, and NOS1 are highly expressed in the inner medullary collecting duct (IMCD) and vasa recta, suggesting that there may be cross talk or paracrine signaling between the vasa recta and IMCD. The purpose of this study was to test the hypothesis that endothelial cell-derived ET-1 (paracrine) and collecting duct-derived ET-1 (autocrine) promote IMCD nitric oxide (NO) production through activation of the ETB receptor during high-salt feeding. We determined that after 7 days of a high-salt diet (HS7), there was a shift to 100% ETB expression in IMCDs, as well as a twofold increase in nitrite production (a metabolite of NO), and this increase could be prevented by acute inhibition of the ETB receptor. ETB receptor blockade or NOS1 inhibition also prevented the ET-1-dependent decrease in ion transport from primary IMCDs, as determined by transepithelial resistance. IMCD were also isolated from vascular endothelial ET-1 knockout mice (VEETKO), collecting duct ET-1 KO (CDET-1KO), and flox controls. Nitrite production by IMCD from VEETKO and flox mice was similarly increased twofold with HS7. However, IMCD NO production from CDET-1KO mice was significantly blunted with HS7 compared with flox control. Taken together, these data indicate that during high-salt feeding, the autocrine actions of ET-1 via upregulation of the ETB receptor are critical for IMCD NO production, facilitating inhibition of ion reabsorption.
Keywords: collecting duct, nitric oxide, ETB receptor, endothelin-1, high-salt diet
the renal endothelin-1 (ET-1) and nitric oxide (NO) systems have many commonalities. Both act as local factors (autocrine and/or paracrine) to promote natriuresis and diuresis (see Refs. 21 and 29), and both are highly expressed in the inner medullary collecting duct (IMCD) (17, 26, 46–48). The IMCD has relatively high expression of ET-1, endothelin B (ETB) receptors, NO synthase-1 (NOS1), and NOS3 (26, 47, 48), and studies have determined that ET-1 stimulates IMCD NO production via the ETB receptor (19, 43). The physiological importance of the collecting duct (CD), ET-1, and NO systems has been demonstrated through a series of studies with CD-specific genetic deletions of ET-1, the ETB receptor, or NOS1 in mice. Collecting duct ET-1 knockout (CDET-1KO), as well as CD ETB knockout (CDETBKO) mice, are hypertensive and have blunted natriuresis during salt loading. Similarly, CD NOS1 knockout (CDNOS1KO) mice have salt-sensitive blood pressure with blunted natriuresis during the first few days of consuming a high-salt diet (17). Both the ET-1/ETB and NOS1/NO signaling pathways are critical for regulating natriuresis through the inhibition of epithelial sodium channels (ENaC) (4, 5, 18). Thus, CD ET-1 and NO signaling cascades are a critical part of maintaining whole body fluid-electrolyte balance during high-salt feeding.
ET-1 is classically described as a peptide produced by the endothelium and acts in a paracrine manner on ET receptors of the vascular smooth muscle cells leading to constriction (see Ref. 28). The kidney is highly vascularized, and it is the vasa recta that surround the loops of Henle and collecting ducts. The vasa recta are composed of endothelial cells and contractile cells known as pericytes and ET-1, ET receptors, NOS1, and NOS3 are all expressed in these cells (32). Previous studies have determined that both extrinsic (35) and intrinsic (7, 9, 11, 39) NOS activity leads to dilation of the vasa recta and an increase in medullary blood flow. On the contrary, ET-1 leads to constriction of the vasa recta (41) and a reduction in medullary blood flow. Thus, the vasa recta tone, as in all vascular beds, is determined by the actions of dilatory and constrictor signaling cascades that maintain medullary blood flow at a level that matches metabolic demand in medullary tissue. Evidence presented above, suggests that both extrinsic (paracrine) and intrinsic (autocrine) actions of ET-1 and NO are part of maintaining vasa recta tone. However, whether there is cross talk, whereby vasa recta endothelium-derived ET-1 acts as a paracrine factor on the IMCD leading to NOS activation, remains to be determined.
High-salt diets upregulate renal ET-1 production, and they also induce medullary ETB receptor activation, facilitating natriuresis (24). It is unknown whether high-salt feeding also upregulates ETB receptor expression. Furthermore, high-salt diets stimulate IMCD NO production (22, 31). Inner medullary NOS activity and urinary excretion of NO metabolites are reduced in CDET-1KO mice on both normal- and high-salt diets without changes in NOS expression (40). These data suggest that CD-derived ET-1 regulates CD NOS activity (but not expression) in an autocrine manner.
The purpose of this study was to determine the putative contribution of vasa recta endothelial cell-derived ET-1 compared with the contribution of CD-derived ET-1 on CD NOS activation and NO production. The focus of this study was on the IMCD, as this segment of the nephron has the highest total NOS activity, and we previously reported that high-salt feeding leads to a significant increase in IMCD NO production (22). Cross talk between the vasa recta endothelial cells and IMCD via ET-1 could be a novel regulatory pathway in high-salt-induced IMCD NOS activation. It was hypothesized that high-salt feeding upregulates expression of the ETB receptor leading to ETB-dependent IMCD NO. We further hypothesized that endothelial-derived ET-1 and CD-derived ET-1 promotes IMCD NOS activation and NO production. To test these hypotheses, C57BL/6J mice, the vascular endothelial specific ET-1 knockout (VEETKO) mouse, CDET-1KO mouse, and respective flox control mice were utilized. VEETKO mice have lower circulating ET-1 (25) and decreased renal ET-1 expression compared with flox control mice (15, 25). We reasoned that if endothelium-derived ET-1 was critical for HS-dependent IMCD NOS activation, then mice lacking endothelial ET-1 (VEETKO) would present with reduced IMCD NO production. Likewise, if HS-dependent CD-derived ET-1 stimulates IMCD NO production, then CDET-1KO mice would present with reduced IMCD NO.
METHODS
Animals.
All animal use and welfare adhered to the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals” following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of Augusta University and the University of Alabama at Birmingham. C57BL/6J male mice (12–16 wk old) were purchased from Jackson Laboratories. Vascular endothelial ET-1 knockout (VEETKO) mice (ET-1flox/flox; Tie2-cre+) and flox control mice (ET-1flox/flox) on a SVJ129×C57BL6/CBA background were bred as previously described (25). Initial studies with flox and VEETKO mice were performed with both male and female mice to determine potential sex differences in IMCD NO production. These mice were 16–20 wk old. Collecting duct ET-1 knockout (CDET-1KO, ET-1flox/flox; AQP2-cre+) and flox control (ET-1flox/flox) mice on a C57BL/6J background developed by Dr. Donald Kohan (1) were kindly provided to us from Dr. Charles Wingo (University of Florida). All CDET-1KO and respective flox mice were male, and 16–20 wk old at time of experimentation. All mice were maintained on standard pellet Teklad diets (0.49% NaCl, Teklad cat. no. 96208), water was available ad libitum, and mice were maintained on a normal 12:12-h dark-light cycle. For high-salt feeding, mice were placed on 4.0% NaCl Teklad pellet diets (cat. no. 92034), with water available ad libitum, for 7 days.
Sprague-Dawley male rats were used for primary IMCD culture experiments. Rats were purchased from Harlan (Envigo, Indianapolis, IN) at 8 wk of age, and IMCD isolations were conducted at 10 wk of age. Rats were maintained on a normal salt diet, with water ad libitum and on a 12:12-h dark-light cycle.
Tail cuff plethysmography.
Noninvasive, systolic blood pressure was measured by tail-cuff plethysmography (Visitech Systems, Apex, NC). The mice were trained to the system for 3 days before measurements were taken in a blinded fashion. Measurements were taken on normal salt or on day 7 of a high-salt diet.
Endothelin receptor radioligand binding assay.
To determine the expression of inner medullary ET receptors, radioligand binding assays were performed as previously described (44). In short, membrane preparations that are enriched in plasma membranes were isolated from the mouse inner medulla (IM). Two IM were homogenized by glass on glass in 200 μl of homogenization buffer (250 mM sucrose, 50 mM Tris pH 7.4, 5 mM EDTA, 15 μM phenylmethylsulfonyl fluoride) on ice. This sample was centrifuged at 1,000 g, 4°C for 30 min. The supernatant was then further spun at 30,000 g for 45 min to pellet membranes. The pellet was resuspended in homogenization buffer, and the protein was determined by Bradford assay (Quickstart reagent; Bio-Rad, Carlsbad, CA). The proteins were further diluted to 300 ng/50 μl in binding buffer (20 mM Tris pH = 7.4, 100 mM NaCl, 10 mM MgCl2, 3 mM EDTA, 15 μM phenylmethylsulfonyl fluoride, 5 μg/ml pepstatin A, 0.025% bacitracin, and 0.2% BSA). Wheat germ agglutinin polyvinyltoluene beads (scintillation proximity beads; Amersham Life Science, Arlington Heights, IL) were suspended in binding buffer (40 mg/ml), with 1 mg added to each well of a 96-well plate (Optiplate; Packard Instruments, Meridian, CT). Beads and proteins were incubated at room temperature for 3 h, while shaking gently, at which point, increasing concentrations of (0–0.02 nM diluted in binding buffer) I125-labeled ET-1 or I125-labeled ET-3 (Perkin Elmer) in the presence (nonspecific binding) or absence (total binding) of excess cold 10 μM ET-1 or cold 10 μM ET-3 (Bachem formerly American Peptide, Torrance, CA). The plate was covered and incubated for 18 h at room temperature while shaking (200 rpm). The plate was centrifuged 1,000 g for 5 min and then counted on a Packard TopCount microplate scintillation counter. ET-3-specific binding curves were also generated with membranes from IMCD preparations (see next paragraph for IMCD isolation protocol) to estimate ETB receptor expression. Protein concentrations from these samples were low; thus, only a 4-point curve (0–0.15 nM) was run with 100 ng protein/50 μl.
To determine specific binding, the nonspecific binding (cpm) was subtracted from the total binding (cpm) for each sample. Specific activity was then calculated by dividing the cpm by 2.22·specific activity (which is lot specific), and then normalized to milligram protein. Bmax and Kd were calculated by nonlinear regression, assuming one binding site using the least-square fitting method (Prism, v. 6, La Jolla, CA). To calculate the expression of ETA and ETB, the Bmax for ET-3 binding represents ETB receptor binding and the difference between the Bmax for the ET-1 binding and ET-3 binding represents ETA receptor binding.
IMCD isolation, nitrite measurement, and NOS expression.
IM were dissected and IMCD were isolated as previously described (13). For these studies, four IMs from two mice were pooled per isolation and represent n = 1 biological replicate. The IMs were chopped into 1-mm cubes, placed into 5 ml of digestion buffer (250 mM sucrose, 10 mM triethanolamine, pH 7.4, 10 mg collagenase type 1, 10 mg hyaluronidase Type IV) and incubated in a 37°C water bath, while shaking for 30 min. Next, 50 μg of DNase I in digestion buffer was added to each sample, and they were further digested 30 min while shaking in a 37°C water bath. Each sample was then filtered through a 100-μm mesh and gently centrifuged 300 g at room temperature for 2.5 min to pellet the IMCD. The pellet was then washed with sucrose buffer (250 mM sucrose, 10 mM triethanolamine, pH 7.4) and centrifuged 300 g at room temperature for 1 min, three times. The resulting IMCD pellet was resuspended in HBSS (with calcium, magnesium, but without phenol red, HyClone, GE Healthcare, Logan, UT). Nitrite production was determined from freshly isolated IMCDs by incubating cells in fresh HBSS + 200 U/ml superoxide dismutase + 250 μM l-arginine ± ET-1 or BQ788 (see below) for 1 h. Superoxide dismutase was used to quench any superoxide so that nitric oxide production could be accurately determined. The HBSS was reserved, snap frozen in liquid nitrogen, and stored at −80°C until analyzed for nitrite production by HPLC (ENO-30; Eicom, San Diego CA).
In a subset of experiments, freshly isolated IMCDs were stimulated with different doses of ET-1 dissolved in water (0–300 nM) for 1 h in HBSS. Furthermore, different subsets of isolated IMCDs were incubated with the ETB antagonist, BQ788 (1 μM; Sigma, St. Louis, MO), 30 min prior to the 1 h HBSS collection. BQ788 was dissolved in DMSO (final concentration 0.1%). All treatments were given to one biological replicate, as there was not enough sample to divide among all treatments.
IMCD suspensions were homogenized in kidney buffer (50 mM Tris, 0.1 mM EDTA, 0.1 mM EGTA, 10 μM leupeptin, 2 μM pepstatin A, 1 mM phenylmethylsulfonyl fluoride, PhosStop tab, pH 7.4) by sonication with 5 × 1 s bursts on ice, and centrifuged 2,000 g for 5 min. Protein concentration was determined by Bradford assay. Protein (5 μg) was separated by SDS-PAGE, as previously described (20). Primary antibodies included anti-NOS1 (R20, polyclonal; Santa Cruz Biotechnology, Dallas, TX), anti-NOS3 (monoclonal, BD Biosciences, San Jose, CA), and anti-actin (monoclonal, Sigma).
IMCD plus vasa recta were also analyzed for nitrite production.
Flox, VEETKO, and CDET-1KO male mice were placed on a HS7 diet, and the IMs were dissected, chopped into 1-mm cubes, and incubated in HBSS + 200 U/ml superoxide dismutase + 250 μM l-arginine for 1 h at 37°C. The HBSS was snap frozen until analyzed by HPLC, and the diced IMs were homogenized in kidney buffer for protein determination.
Primary IMCD culture and transepithelial resistance measurements.
The IMs from 1 rat were dissected, and the IMCD was isolated as stated above. After pelleting the IMCD in HBSS, the cells were resuspended in DMEM: F12 + 10% FBS, 1% penicillin/streptomycin, 0.01× insulin/transferrin/selenium and seeded onto 24-well transwell inserts coated in collagen (24-well, 0.4-μm pore, 2.0 ± 0.2 × 106 pores/cm2; Corning, Corning, NY). A single IMCD isolation was seeded onto 12 transwells. The cells were grown to confluency (5 days), and transepithelial resistance measurements were taken daily using the EVOM (epithelial voltohmmeter) with an STX2 chopstick electrode (World Precision Instruments, Sarasota, FL). Only wells that produced resistances >300 Ohms/cm2 were used in experiments. On the day of experimentation, the cells were acclimated to room temperature for 30 min in plain DMEM-F12 media. Basal transepithelial resistance (TER) measurements were taken for each well. Next, one well from each animal received a pretreatment of either vehicle (water), ETA antagonist (30 nM; A127722), the ETB antagonist (30 nM; A192621), the total NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 5 mM), NOS1 inhibitor VNIO (1 μM), or NOS2 inhibitor 1400 W (100 nM) to the basolateral compartment of the well. All drugs were water-soluble and purchased from Sigma, except that ET receptor antagonists were provided as a gift from Abbvie. After 30 min of incubation, the TER was determined again. Next, each well was incubated with 100 nM ET-1 for 5 min, again on the basolateral side, and TER was measured. Finally, 40 μM amiloride was added to the apical side as a positive control for inhibition of ion transport processes. The change in resistance was calculated as the % change from basal TER.
Quantitative real-time PCR. Total RNA was extracted from IMs using Tri-Reagent following the manufacturer's instructions (Sigma). RNA (5 μg) was reverse transcribed using Invitrogen's superscript III RT kit, and relative quantitative expression of EDNRA, EDNRB, and GAPDH (as a housekeeping gene) were determined using Bio-Rad's SsoAdvanced Universal SYBR Green Supermix. The following QuantiTect quantitative RT-PCR primers from Qiagen (Valencia, CA) were used: Ednra (cat. no. QT00121625) spanned exons 4 and 5, Ednrb (cat. no. QT00139384) spanning exons 4 and 5, and Gapdh (cat. no. QT01658692) spanning exons 2 and 3.
Statistics.
All data are expressed as means ± SE. For the IMCD nitrite production experiments, a one-way ANOVA with Dunnett's post hoc test were performed. For NOS expression, IMCD nitrite production, and systolic blood pressure, a two-factor ANOVA (for genotype and diet) was performed with Sidak's multiple-comparison post hoc test. Relative mRNA expression was calculated using the 2delta delta CT with Gapdh as the housekeeping gene, and statistical significance was determined with an unpaired, two-tailed, Student's t-test. For the % change in TER, a repeated-measures two-factor ANOVA (for ET-1 and drug pretreatment) was performed with Sidak's multiple-comparison post hoc test. Significance was considered with a P < 0.05.
RESULTS
A high-salt diet increases ETB receptor expression in the inner medulla and IMCD.
To determine the ET receptor expression profile in the IM, radio-ligand binding studies on IM membrane preparations were performed. In C57BL/6J mice on a normal salt (NS) diet (Fig. 1A), there was significantly more ET-1-specific binding (Bmax: 637 ± 124 fmol/mg protein, Kd: 0.06 ± 0.03 nM), than ET-3 specific binding (Bmax: 310 ± 44 fmol/mg protein, Kd: 0.03 ± 0.02 nM; n = 5, P < 0.05). Yet, after 7 days of a high-salt diet (HS7) (Fig. 1B), IM ET-1 and ET-3 specific binding were similar (ET-1 Bmax: 763 ± 126 fmol/mg protein, Kd: 0.08 ± 0.02 nM; ET-3 Bmax: 706 ± 152 fmol/mg protein, Kd: 0.08 ± 0.04 nM, n = 4, P > 0.05). Total ET-1 binding between NS (Bmax: 637 ± 124 fmol/mg protein) and HS7 (Bmax: 763 ± 126 fmol/mg protein) was not statistically different (P = 0.24). From these curves, it was determined that with NS feeding, the male mouse IM expressed 63 ± 7% ETB receptors and 37 ± 7% ETA receptors (Fig. 1, C and D). However, with HS feeding there is a significant loss of the IM ETA receptor and significant increase in expression of the ETB receptor (Fig. 1, C and D).
Fig. 1.
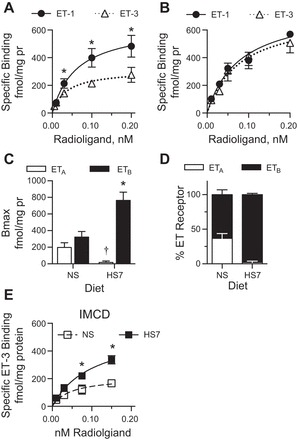
Membrane preparations from mouse inner medullae (IM) or inner medullary collecting ducts (IMCD) were used in the radio-ligand binding assay. A: endothelin (ET)-1 (●, solid line) and ET-3 (△, dashed line), specific binding curves for IM membrane homogenates from mice on a normal-salt diet (NS). There was significantly greater ET-1 specific binding than ET-3. Nonlinear regression lines are plotted; n = 5, *P < 0.05. B: ET-1 (●, solid line) and ET-3 (△, dashed line) specific binding curves for IM membrane homogenates from mice on a 7-day high-salt diet (HS7). ET-1 and ET-3 specific bindings were similar. See text for Bmax and Kd values. Nonlinear regression lines are plotted; n = 4, P > 0.05. C: Bmax for IM ETA and ETB receptors from NS- or HS7-fed mice. HS fed mice have significantly more ETB receptors and significantly less ETA receptors compared with NS fed mice (†P < 0.01 compared with NS ETA, *P < 0.01 compared with NS ETB). D: percent expression of ETA (white bars) and ETB (black bars) in the IM from mice on a NS or HS7 diets. E: ET-3 specific binding curves from IMCD membrane homogenates from mice on NS (□, dashed line; n = 7) or HS7 diets (■, solid line; n = 10). A high-salt diet resulted in a significant increase in ET-3 specific binding. See text for Bmax and Kd values. Nonlinear regression lines are plotted.
Next, radioligand studies with IMCD membrane preparations from male C57BL/6J were performed. Because these samples provided a limited amount of protein, only radiolabeled ET-3 curves were generated to estimate specific ETB receptor expression. Similar to what we reported with the IM membrane preparations (Fig. 1, A–D), mice on a HS7 diet had significantly greater ET-3 specific binding in the membrane of the IMCD (HS7: Bmax = 552.5 ± 102.6 fmol/mg protein; Kd: 0.1 ± 0.04 nM, n = 10 biological replicates; NS: Bmax = 210.9 ± 48.6 fmol/mg protein, Kd: 0.05 ± 0.03 nM, n = 7 biological replicates) (Fig. 1E).
Relative mRNA expression for both Ednra and Ednrb were determined from the IM of C57BL/6J mice on NS or HS7 diets. Ednra expression was similar between NS (1.0 ± 0.2 AU) and HS7 (1.4 ± 0.2 AU) feeding (n = 3–5, P = 0.27). Likewise, Ednrb mRNA expression was similar between NS (1.0 ± 0.2 AU) and HS7 (1.2 ± 0.3 AU)-fed mice (n = 3–5, P = 0.53).
High-salt diet mediates ETB-dependent increased NO production in IMCD.
IMCD were isolated from C57BL/6J male mice on a NS or HS7 diet, and basal nitrite (an index of NO) production was measured. We verified that acute 100 nM and 300 nM ET-1 increased IMCD nitrite production significantly compared with basal nitrite production (Fig. 2A; 0 nM, n = 8, 50 nM, n = 4, 100 nM n = 8, and 300 nM, n = 4, P = 0.02) in C57BL/6J mice fed NS. High-salt feeding in C57BL/6J mice led to a significant twofold increase in basal nitrite production (Fig. 2B) that was abolished by acute treatment of IMCDs with BQ788, a selective ETB receptor antagonist (Fig. 2B; n = 9, P > 0.05).
Fig. 2.
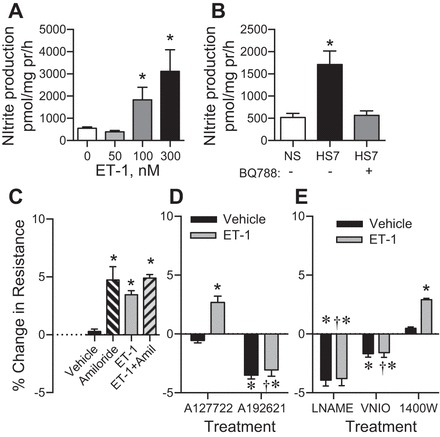
Nitrite production from freshly isolated IMCD from C57BL/6J mice. A: acute, 1-h ET-1 treatment leads to a concentration-dependent increase in nitrite production. n = 4–8, *P = 0.02 compared with 0 nM ET-1. B: IMCD from HS7-fed C57BL/6J mice produce significantly more nitrite than NS-fed mice that is blocked by acute (1.5 h) ETB receptor antagonism with BQ788; n = 9. *P < 0.05 compared with NS. C–E: changes in transepithelial resistance (TER) from primary cultures of rat IMCDs. All treatments were performed on a different well from cultures derived from the same animal. For simplicity, the treatments are separated into three panels. C: acute amiloride, ET-1, or amiloride + ET-1 treatment results in a significant increase in % change in TER compared with vehicle-treated IMCD; n = 6. *P < 0.05 compared with vehicle. D: ETA blockade with A12772 had no significant effect on basal TER, and ET-1 stimulated an increase in TER. ETB blockade with A192621 significantly reduced resting TER, and ET-1 failed to change TER in these cells. E: total NOS inhibition with l-NAME or NOS1 inhibition with VNIO resulted in a significant reduction in TER. This reduction was not significantly affected by treatment with ET-1. Inhibition of NOS2 by 1400 W had no significant effect on basal TER, with ET-1 resulting in a significant increase in TER in the 1400 W-treated cells. *P < 0.05 compared with vehicle in C; †P < 0.05 compared with ET-1 treatment in D.
ET-1 via ETB and NOS1 mediate an inhibition of IMCD ion transport. Primary cultures of IMCDs from male Sprague-Dawley rats on a NS diet were grown to confluency and allowed to polarize. Rats were used because from 1 rat, we can culture enough cells to be subjected to each treatment (repeated-measures design). Acute amiloride or ET-1 treatment resulted in a significant increase in transepithelial resistance (TER), indicating an inhibition of ion transport (Fig. 2C; n = 6, P < 0.01). Pretreatment with an ETB antagonist (A192621), but not an ETA antagonist (A127722), resulted in a significant decrease in TER (PA192621 < 0.01, PA12772 > 0.05) (Fig. 2D). This suggests that basal ETB activation inhibits ion transport in the IMCD. Application of ET-1 led to a significant increase in TER in the ETA-antagonized cells (P < 0.01) but did not significantly change TER in the ETB-antagonized cells (P > 0.05). (PET-1 < 0.0001, Pantagonist < 0.001, Pinteraction = 0.003, Psubject matching = 0.58, number of comparisons per family = 3).
Cells were also pretreated with either l-NAME to inhibit all NOS isoforms, VNIO to inhibit NOS1, or 1400 W to inhibit NOS2 (Fig. 2E). Both l-NAME and VNIO treatment alone, resulted in a significant decrease in TER (PL-NAME < 0.001, PVNIO < 0.01), suggesting basal NO production inhibits ion transport in the IMCD. NOS2 inhibition did not have a significant effect on TER (P > 0.05). Next, application of ET-1 failed to significantly change TER in the l-NAME and VNIO-treated cells, but it led to a significant increase in TER in the 1400 W-treated cells (P < 0.05). These data suggest that NOS1 and possibly NOS3 are critical for inhibiting ion transport from the IMCD, but not NOS2. (PET-1 < 0.0001, Pinhibitor < 0.001, Pinteraction < 0.001, Psubject matching < 0.01; number of comparisons per family = 6).
Deletion of vascular endothelial ET-1 has no significant effect on IMCD NO production.
Nitrite production was measured in IMCD freshly isolated from VEETKO and flox control male and female mice on NS or HS7. High-salt feeding led to a significant twofold to three-fold increase in basal nitrite production in both the male VEETKO and flox mice (Fig. 3A, Flox NS: n = 9, HS: n = 5, VEETKO NS: n = 6, HS7: n = 10, Pdiet < 0.001, Pgenotype = 0.7, Pinteraction = 0.3). Likewise, in the IMCD from female VEETKO and flox mice, there was a significant twofold increase in nitrite production with high-salt feeding (Fig. 3B, flox NS and HS7: n = 6 each, VEETKO NS and HS7: n = 12 each, Pdiet = 0.009, Pgenotype = 0.5, Pinteraction = 0.5). There was no significant effect of sex on HS-induced IMCD NO production. Furthermore, systolic blood pressure was determined in male and female flox and VEETKO mice on a normal salt and 7-day HS diets. Both male (Fig. 3C, NS: n = 9, HS: n = 9) and female (Fig. 3D, NS: n = 10, HS: n = 4) mice had similar systolic blood pressures on normal and high-salt diets (all P from two-factor ANOVAs were >0.05). There was no significant effect of sex on systolic blood pressure in either the flox control of VEETKO mice. Experiments from this point were conducted in only male mice.
Fig. 3.
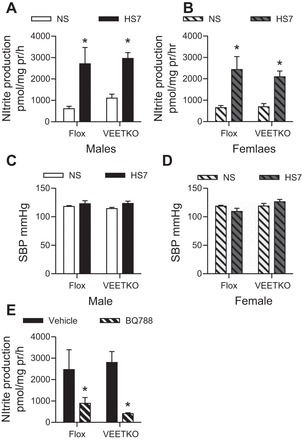
IMCD nitrite production from IMCDs of vascular endothelial ET-1 knockout (VEETKO) and flox control male and female mice on NS or HS7 diets. A: high-salt feeding led to a significant increase in male flox and VEETKO IMCD nitrite production (Flox NS: n = 9, HS7: n = 5, VEETKO NS: n = 6, HS7: n = 10. *P < 0.001 compared with NS). B: high-salt feeding led to a significant increase in female flox and VEETKO IMCD nitrite production. (Flox NS and HS7: n = 6 each, VEETKO NS and HS7: n = 12 each, *P < 0.001 compared with NS). C and D: systolic blood pressure of male (n = 9 per group) (C) and female flox (n = 10 per group) (D) and VEETKO mice (n = 4 per group). E: IMCDs from male flox and VEETKO mice on a HS7 diet treated acutely, ex vivo with the ETB antagonist, BQ788. Antagonism of the ETB receptor resulted in an attenuation of the HS-mediated increase in IMCD nitrite production (n = 4 per group, *P < 0.001 compared with vehicle).
IMCD from both NS and HS7 mice were also acutely treated with the ETB antagonist, BQ788. ETB receptor blockade with BQ788 had no significant effect on basal nitrite production from flox mice (vehicle = 711.5 ± 135.2 pmol·mg protein−1·h−1; BQ788 = 829.2 ± 45.3 pmol·mg protein−1·h−1) on a NS diet (n = 4, P = 0.44) or VEETKO mice (vehicle = 666.0 ± 241.5 pmol·mg protein·h−1; BQ788 = 564.9 ± 80.0 pmol/mg protein/h) on a NS diet (n = 4, P = 0.7). Similar to IMCD isolated from C57BL/6J mice, acute ETB receptor antagonism prevented the HS7-dependent increase in nitrite in IMCD of both VEETKO and flox mice (Fig. 3E; n = 4, P < 0.001).
Deletion of CD ET-1 results in reduced IMCD NO production in HS-fed mice.
IMCD were also isolated from male CDET-1KO and flox mice on normal- and high-salt diets. After 7 days of HS feeding, flox control mice had a significant twofold increase in IMCD nitrite production (Fig. 4A; n = 8, P < 0.05). However, CDET-1KO mice had similar IMCD nitrite production on NS diets as flox control mice, but failed to increase their IMCD nitrite production with high-salt feeding (Fig. 4A, NS: n = 5, HS: n = 7, P > 0.05).
Fig. 4.
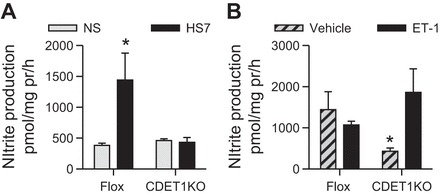
IMCD nitrite production from CDET-1KO and flox control male mice. A: high-salt diets for 7 days (HS7) results in a significant increase in IMCD nitrite production in flox control mice, but not CDET-1KO mice. Flox NS and HS n = 7 and 8, respectively. CDET-1KO NS and HS n = 5 and 7. *P < 0.05 compared with NS. B: acute 1-h stimulation with 100 nM ET-1 to isolated IMCDs from flox and CDET-1KO mice on HS7 diet. Vehicle groups are the same groups from A. Exogenous ET-1 did not significantly increase IMCD nitrite production in flox control mice. However, exogenous ET-1 resulted in a significant increase in IMCD nitrite production from CDET-1KO mice. ET-1 treatment of flox, n = 3; CDET-1KO, n = 3. *Pinteraction = 0.04.
To determine whether exogenous ET-1 stimulates IMCD NO production, IMCD from flox and CDET-1KO mice on a HS7 diet were acutely treated with ET-1 for 1 h. Acute, exogenous ET-1 had no significant effect on flox IMCD nitrite production, suggesting that a HS diet leads to maximal IMCD NO production. However, IMCD from CDET-1KO on HS7 diet treated acutely with ET-1 presented with a significant twofold to three-fold increase in IMCD nitrite production (Fig. 4B; n = 3; Pgenotype = 0.8, PET-1 = 0.8, Pinteraction = 0.04).
Nitrite production from IMCD+vasa recta samples is reduced in CDET-1KO mice.
Male VEETKO, CDET-1KO, and their respective flox controls were placed on a HS7 diet. Next, the IMs were dissected, minced into 1-mm cubes, and analyzed for nitrite production. This crude preparation contains IMCD, vasa recta and thin limbs of Henle. Nitrite production was determined from these samples. VEETKO and flox control samples produced similar levels of nitrite (n = 4 animals/group, P = 0.39) (Fig. 5A). Conversely, this preparation from CDET-1KO mice produced a significant 50% reduction in nitrite production compared with their respective flox control (Flox: n = 4 mice, CDET-1KO: n = 3 mice, P = 0.02) (Fig. 5B).
Fig. 5.
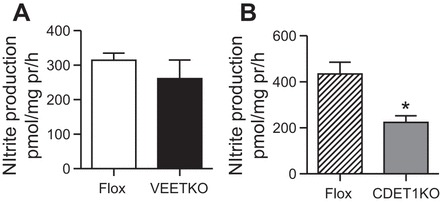
Nitrite production from samples containing IMCD, vasa recta, and thin limb of Henle. All mice were on a HS7 diet. A: nitrite production was similar between VEETKO and flox control male mice. n = 4 animals/group, Student's t-test, P = 0.39. B: nitrite production was significantly reduced in samples from male CDET-1KO compared with their flox control. n = 4 flox and 3 CDET-1KO, Student's t-test, *P = 0.02.
IMCD NOS expression is not regulated by high-salt, endothelium-derived ET-1, or IMCD-derived ET-1.
We sought to determine whether a high-salt diet, endothelium-derived ET-1 and/or IMCD-derived ET-1 regulates IMCD NOS expression. As shown in Fig. 6A, NOS1 expression was similar between VEETKO and flox control mice on either a NS or HS diet (n = 10/genotype/group; P > 0.05). Similar to wild-type mice (22), VEETKO and flox mice express a single NOS1 splice variant, NOS1β (130 kDa). VEETKO mice express similar levels of NOS3 in the IMCD compared with flox control mice either on a NS or HS diet (Fig. 6, A–C, n = 10/genotype/group, P > 0.05). Furthermore, IMCD expression of NOS1β or NOS3 from CDET-1KO mice was also similar to flox controls on a HS7 diet (n = 4 for Flox, n = 6 for CDET-1KO, P > 0.05) (Fig. 6, D–F).
Fig. 6.
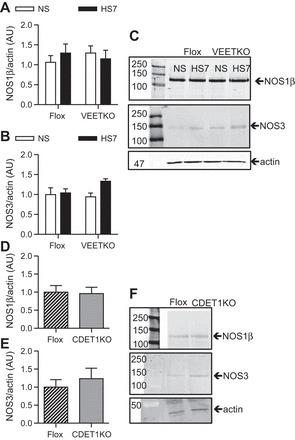
IMCD expression of NOS1β and NOS3 expression. A: VEETKO and flox control mice have similar expression of NOS1β on both normal salt (NS) and 7-day high-salt (HS7) diets. n = 10/genotype/group, P > 0.05. B: VEETKO and flox mice have similar levels of IMCD NOS3 on both NS and HS7 diets. n = 10/group and genotype, P > 0.05. C: representative Western blots used in the analysis of A and B, for NOS1β, NOS3, and the loading control, actin. D: CDET-1KO mice (n = 6) and flox control mice (n = 4 preparations) have similar IMCD NOS1β (Student's t-test: P = 0.9) and E: NOS3 expression while on a HS7 diet (Student's t-test: P = 0.6). F: representative Western blots used in the analysis of A and B, for NOS1β, NOS3, and the loading control, actin. The top panel molecular ladder is discontinuous with the rest of the blot, as indicated by the white space.
DISCUSSION
ET-1 and NO are local autocrine/paracrine factors that are critical for regulating natriuresis and diuresis in the kidney. This study yielded a number of novel findings. First, chronic (7 day) high-salt feeding leads to a shift in the ET receptor profile, with the IM expressing almost exclusively the ETB receptor. Moreover, there was a significant increase in ETB receptors specifically in the IMCD with high-salt feeding. Second, acute ET-1 treatment or chronic high-salt feeding leads to a significant increase in IMCD NO production, and this is mediated by the IMCD ETB receptor. Third, ET-1 inhibition of IMCD ion transport is mediated through the ETB, NOS1, and NOS3. Fourth, although endothelium-derived ET-1 of the vasa recta may act in a paracrine manner to regulate IMCD physiology, our data indicate that it is the autocrine actions of ET-1 from the IMCD that regulate IMCD NO production. The IMCD from mice lacking vascular endothelial ET-1 (VEETKO) produces similar amounts of NO with NS feeding, while they have a twofold increase in NO with high-salt feeding. However, mice lacking CD ET-1 (CDET-1KO) fail to increase their IMCD NO production with high-salt feeding. Finally, samples with IMCD, vasa recta, and thin limbs from CDET-1KO mice produce significantly less NO compared with controls. Thus, we conclude that it is the autocrine actions of IMCD ET-1 that predominantly regulate IMCD NO production and that high salt induces an upregulation of ETB receptors facilitating the increase in NO production and inhibition of ion transport.
Both the ET-1 and NO systems are highly expressed in the IMCD (8, 17, 26, 27, 46–48). Furthermore, disruption of either the CD ET-1 (1), CD ETB receptor (12), or CD NOS1 (17) results in a salt-sensitive blood pressure phenotype, which is likely ENaC dependent (4, 5, 18). In males, the CD ETA receptor plays a limited role in the regulation of sodium homeostasis, especially under high-salt conditions (4), and male CDETAKO mice do not display a salt-sensitive phenotype (13). Likewise, recent work with male rats determined that the high-salt diet led to activation of medullary ETB receptors to promote natriuresis (24). Data presented in this study further demonstrated that there is a shift to solely ETB expression in the IM (and likely IMCD) from mice on a high-salt diet. Thus, during high-salt feeding, there is an upregulation of the ETB receptor and activation of NO production that promotes natriuresis and so maintains fluid-electrolyte balance.
There are a number of possible mechanisms to explain the high-salt diet-dependent increase in ETB expression and decrease in ETA expression. First, there could be differences in transcription. However, this appears unlikely, because we found mRNA expression of both Ednra and Ednrb to be similar in the IM between mice on NS and HS7 diets. Second, there could be differences in protein turnover rates. It is established that ETA and ETB receptors have divergent COOH-terminal sequences. The ETA has a COOH-terminal, internal PDZ ligand critical for regulating recycling of the ETA receptor (36), and the ETB has a cluster of serines in the COOH terminus that likely interact with arrestins and target ETB for lysosomal degradation (3, 37). Truncation of the ETA (or ETB) COOH terminus results in predominantly lysosomal localization and degradation (37). Moreover, ETA interactions with the small GTPases (rab proteins) results in recycling, while it has been demonstrated that rab mutants lead to retardation of the recycling pathway and cause entrapment in endosomes and lysosomal degradation (37). ETA has been determined to interact with rab11, a marker of recycling endosomes, while ETB receptors interact with rab7 and lysosomes (45). Furthermore, ETA interactions with Tip60, an acetyltransferase, promote recycling (30). Whether a high-salt diet inhibits arrestin signaling and promotes ETB accumulation in the membrane, and/or inhibits rab or Tip60 signaling, resulting in a degradation of ETA, remains to be determined. Third, posttranslational modifications, such as ubiquitination of ETA and ETB, may affect their turnover. Recently, jun activation domain-binding protein-1 (JAB1) was identified as increasing ETA and ETB degradation (34) via the ubiquitination pathway (45). However, ETB receptors were found to be highly ubiquitinated at lysines in the COOH terminus that were unique to ETB receptors (45) and had greater association with JAB1 compared with ETA receptors (34), resulting in faster degradation. Thus, it is plausible that with high-salt feeding, there is a shift resulting in a decrease in ETB ubiquitination and an increase in JAB1/ETA interactions and ubiquitination. The mechanism(s) involved in dietary salt regulation of ET receptor expression remains to be determined.
Paracrine signals from the renal epithelium to the endothelium may be an important regulator of sodium homeostasis. For example, NO (10, 35) and superoxide (33) produced by the medullary thick ascending limb (mTAL) modulates descending vasa recta tone, thereby regulating medullary blood flow. This is an important physiological process that if disrupted leads to renal dysfunction. Dahl salt-sensitive rats have greater mTAL-derived reactive oxygen species (likely superoxide) that diffuses into the renal interstitium. This results in decreased medullary blood flow, sodium retention, hypertension, and renal injury via reduced vasa recta NO bioavailability (33). It is unknown whether CD ET-1 or NO regulates vasa recta tone and medullary blood flow similar to the mTAL. Part of the salt-sensitive blood pressure phenotype of the CDET-1KO or CDNOS1KO mice may be due to impaired tubulovascular crosstalk, leading to vasa recta dysfunction in the IM.
In the current study, we provide a number of lines of evidence that inner medullary endothelium-derived ET-1 is not a critical regulator of IMCD NOS activity. First, high-salt-induced IMCD NO production was similar between control and VEETKO mice (in both males and females), and it was mediated via the ETB receptor. Systolic blood pressure, as determined by noninvasive tail cuff, was similar between the genotypes and sexes regardless of the salt in the diet. This is the first report of female VEETKO blood pressure. Although, it was originally reported that male VEETKO mice have a small, but significant, reduction in blood pressure on a NS diet (25), our study is in agreement with Heiden et al. (14) and Speed et al. (42), who recently reported that male VEETKO and flox mice have similar blood pressures with NS feeding. Speed et al. (42) also determined (by telemetry) that a week of high-salt diet resulted in a small, but significant, reduction in male VEETKO blood pressure. These data suggest that the stress of the tail cuff used in the current study may mask the high-salt-mediated reduction in blood pressure observed in VEETKO mice. But despite this likely reduction in blood pressure of VEETKO mice on a high-salt diet, there was a similar increase in IMCD NO production between the sexes. Second, NO production from samples of IMCD, vasa recta, and thin limbs were again similar between flox control and VEETKO mice on a high-salt diet. Third, IMCD NO production from CDET-1KO mice was significantly reduced, and this reduction was maintained in samples that also contained vasa recta. These results confirm previous work that demonstrated that CD ET-1 knockout mice have similar NOS1 and NOS3 expression, but have reduced NOS1 and NOS3 activity and urinary NOx (nitrite+nitrate) excretion (40). Interestingly, it was determined that exogenous ET-1-stimulated IMCD NO from CDET-1KO mice. These data suggest that endothelin receptors are expressed and can be activated on the IMCD of CDET-1KO mice. However, these data further suggest that the ET-1 produced by the vasa recta cannot compensate for the lack of CD ET-1 to stimulate IMCD NO production (although there may be some contribution to other IMCD functions). From these data, we conclude that it is the autocrine actions of ET-1 that promote IMCD NO production.
The mechanism(s) involved in IMCD ET-1 activation of NO production during high-salt feeding remains speculative. NOS activity is complex and regulated via a number of mechanisms. These include expression levels, phosphorylation or other posttranslational modifications, subcellular localization, protein-protein interactions, and substrate or cofactor availability (e.g., see Ref. 2). IMCD expression of NOS1 and NOS3 was similar among flox control, VEETKO, and CDET-1KO mice, regardless of dietary sodium intake. Thus, regulation is more complex than simply expression. We recently reported that dynamin-2 is a novel NOS-interacting protein that in the kidney is induced by high salt and regulates IMCD NO production (16). Whether dynamin-2 is the link between ET-1 stimulation of NOS remains to be determined. Another candidate is the extracellular signal-regulated kinases pathway (Erk1/2). ET-1 via ETB stimulates phosphorylation of Erk1/2 (p4/42); however, inhibition of Erk1/2 does not prevent the ET-1/ETB-mediated increase in IMCD NO production (19). With high-salt feeding, there is an increase in fluid intake to maintain hydration, leading to increased urine flow through the nephron. This increase in fluid flow is also a potent stimulator of IMCD ET-1 (38) and NO production (6, 18). With an increase in fluid flow, intracellular calcium concentrations are increased, and this is a critical regulatory pathway for IMCD ET-1 production (38). NOS activity is also stimulated by increases in intracellular calcium, making intracellular calcium a top candidate as the second messenger between ET-1 and NO production. In freshly isolated IMCDs, exogenous ET-1 stimulates an increase in intracellular calcium in both male and female rats (23). However, the increase in intracellular calcium was greater in males and was via both the ETA and ETB receptors. In the current study, we did not observe a sex difference in the IMCD NO production induced by high-salt feeding in mice. Whether this difference is due to the species being studied or differences in intracellular calcium signaling remain to be tested. Thus, the direct link between ET-1/ETB activation and NO production remains to be elucidated.
Perspectives and Significance
Our laboratory previously showed that CD NO signaling is a critical part of maintaining whole body fluid-electrolyte balance during high-salt feeding. The current study indicates that it is the autocrine, and not the paracrine, actions of ET-1 via the ETB receptor that are critical for IMCD NO production. In addition, this study shows that the high-salt diet also induces increased expression of IMCD ETB receptors, while IMCD NO production is increased without an increase in NOS expression. Many Americans and the world's population consume high levels of salt in their diets; thus, delineation of the IMCD-specific intracellular signaling pathway(s) of ETB receptor-mediated activation of NO production is important to reveal potential targets for maintenance of fluid-electrolyte balance.
GRANTS
American Heart Association Post Doctoral Fellowship to K. A. Hyndman; Preparation for Graduate and Medical Education National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI)-funded summer program (NIH/NHLBI R25HL120883) A. M. Arguello; World Premier International Research Center Initiative from MEXT, Japan to M. Yanagisawa. NIH Program Project Grant on Endothelin Control of Renal Excretory and Hemodynamic Function (P01 HL-95499) to J. S. Pollock.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.H., T.T.G., and J.S.P. conception and design of research; K.A.H., C.D., A.M.A., T.T.G., K.M.B., and M.B. performed experiments; K.A.H., A.M.A., and T.T.G. analyzed data; K.A.H., T.T.G., and J.S.P. interpreted results of experiments; K.A.H. prepared figures; K.A.H. and J.S.P. drafted manuscript; K.A.H., C.D., A.M.A., T.T.G., K.M.B., M.B., M.Y., and J.S.P. edited and revised manuscript; K.A.H., C.D., A.M.A., T.T.G., K.M.B., M.B., M.Y., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
M. Yanagisawa is a former Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem 275: 17,596-17,604, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cao C, Edwards A, Sendeski M, Lee-Kwon W, Cui L, Cai CY, Patzak A, Pallone TL. Intrinsic nitric oxide and superoxide production regulates descending vasa recta contraction. Am J Physiol Renal Physiol 299: F1056–F1064, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Todd-Turla K, Wang WH, Cao X, Smart A, Brosius FC, Killen PD, Keiser JA, Briggs JP, Schnermann J. Endothelin-1 mRNA in glomerular and epithelial cells of kidney. Am J Physiol Renal Fluid Electrolyte Physiol 265: F542–F550, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Crawford C, Kennedy-Lydon T, Sprott C, Desai T, Sawbridge L, Munday J, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron Physiol 120: 17–31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickhout JG, Mori T, Cowley AW Jr.. Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Edwards A, Cao C, Pallone TL. Cellular mechanisms underlying nitric oxide-induced vasodilation of descending vasa recta. Am J Physiol Renal Physiol 300: F441–F456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Ge Y, Strait KA, Stricklett PK, Yang T, Kohan DE. Role of prostaglandins in collecting duct-derived endothelin-1 regulation of blood pressure and water excretion. Am J Physiol Renal Physiol 293: F1805–F1810, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Heiden S, Vignon-Zellweger N, Masuda S, Yagi K, Nakayama K, Yanagisawa M, Emoto N. Vascular endothelium derived endothelin-1 is required for normal heart function after chronic pressure overload in mice. PLos One 9: e88730, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimlich JB, Speed JS, Bloom CJ, O'Connor PM, Pollock JS, Pollock DM. ET-1 increases reactive oxygen species following hypoxia and high-salt diet in the mouse glomerulus. Acta Physiol (Oxf) 213: 722–730, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyndman KA, Arguello AM, Morsing SK, Pollock JS. Dynamin-2 is a novel NOS1β interacting protein and negative regulator in the collecting duct. Am J Physiol Regul Integr Comp Physiol 310: R570–R577, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62: 91–98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyndman KA, Bugaj V, Mironova E, Stockand JD, Pollock JS. NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct. Am J Physiol Renal Physiol 308: F244–F251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyndman KA, MacDonell AH, Pollock JS. Extracellular signal-regulated kinases 1/2 signaling pathways are not involved in endothelin regulation of mouse inner medullary collecting duct nitric oxide production. Life Sci 91: 578–582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol 301: F118–F124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyndman KA, Pollock JS. Nitric oxide and the A and B of endothelin of sodium homeostasis. Curr Opin Nephrol Hypertens 22: 26–31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyndman KA, Xue J, MacDonell A, Speed JS, Jin C, Pollock JS. Distinct regulation of inner medullary collecting duct nitric oxide production from mice and rats. Clin Exp Pharmacol Physiol 40: 233–239, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin C, Speed JS, Hyndman KA, O'Connor PM, Pollock DM. Sex differences in ET-1 receptor expression and Ca2+ signaling in the IMCD. Am J Physiol Renal Physiol 305: F1099–F1104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin C, Speed JS, Pollock DM. High salt intake increases endothelin B receptor function in the renal medulla of rats. Life Sci pii: S0024–3205(15)30131-4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kisanuki YY, Emoto N, Ohuchi T, Widyantoro B, Yagi K, Nakayama K, Kedzierski RM, Hammer RE, Yanagisawa H, Williams SC, Richardson JA, Suzuki T, Yanagisawa M. Low blood pressure in endothelial cell-specific endothelin 1 knockout mice. Hypertension 56: 121–128, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura K, Tanaka T, Kato J, Ogawa T, Eto T, Tanaka K. Immunoreactive endothelin in rat kidney inner medulla: marked decrease in spontaneously hypertensive rats. Biochem Biophys Res Commun 162: 38–44, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Kohan DE. Endothelin synthesis by rabbit renal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 261: F221–F226, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compr Physiol 1: 883–919, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HJ, Chun M, Kandror KV. Tip60 and HDAC7 interact with the endothelin receptor a and may be involved in downstream signaling. J Biol Chem 276: 16,597-16,600, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension 27: 688–692, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension 35: 337–341, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Mori T, O'Connor PM, Abe M, Cowley AW Jr.. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 49: 1336–1341, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Nishimoto A, Lu L, Hayashi M, Nishiya T, Horinouchi T, Miwa S. Jab1 regulates levels of endothelin type A and B receptors by promoting ubiquitination and degradation. Biochem Biophys Res Commun 391: 1616–1622, 2010. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor PM, Cowley AW Jr.. Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep 12: 86–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, Dahl SG, Attramadal H. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol 67: 1581–1590, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Paasche JD, Attramadal T, Sandberg C, Johansen HK, Attramadal H. Mechanisms of endothelin receptor subtype-specific targeting to distinct intracellular trafficking pathways. J Biol Chem 276: 34,041-34,050, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Pandit MM, Gao Y, van Hoek A, Kohan DE. Osmolar regulation of endothelin-1 production by the inner medullary collecting duct. Life Sci pii: S0024–3205(15)30063-1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhinehart KL, Pallone TL. Nitric oxide generation by isolated descending vasa recta. Am J Physiol Heart Circ Physiol 281: H316–H324, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silldorff EP, Yang S, Pallone TL. Prostaglandin E2 abrogates endothelin-induced vasoconstriction in renal outer medullary descending vasa recta of the rat. J Clin Invest 95: 2734–2740, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speed JS, Heimlich JB, Hyndman KA, Fox BM, Patel V, Yanagisawa M, Pollock JS, Titze JM, Pollock DM. Endothelin-1 as a master regulator of whole-body Na+ homeostasis. FASEB J 29: 4937–4944, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol 290: F1315–F1319, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Unique endothelin receptor binding in kidneys of ETB receptor deficient rats. Am J Physiol Regul Integr Comp Physiol 284: R674–R681, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Terada K, Horinouchi T, Fujioka Y, Higashi T, Nepal P, Horiguchi M, Karki S, Hatate C, Hoshi A, Harada T, Mai Y, Ohba Y, Miwa S. Agonist-promoted ubiquitination differentially regulates receptor trafficking of endothelin Type A and Type B receptors. J Biol Chem 289: 35,283-35,295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchida S, Takemoto F, Ogata E, Kurokawa K. Detection of endothelin-1 mRNA by RT-PCR in isolated rat renal tubules. Biochem Biophys Res Commun 188: 108–113, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest 90: 1043–1048, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu F, Park F, Cowley AW Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999. [DOI] [PubMed] [Google Scholar]


