Abstract
Widmeier E, Tan W, Airik M, Hildebrandt F. A small molecule screening to detect potential therapeutic targets in human podocytes. Am J Physiol Renal Physiol 312: F157–F171, 2017. First published October 19, 2016; doi:10.1152/ajprenal.00386.2016. Steroid-resistant nephrotic syndrome (SRNS) inevitably progresses to end-stage kidney disease, requiring dialysis or transplantation for survival. However, treatment modalities and drug discovery remain limited. Mutations in over 30 genes have been discovered as monogenic causes of SRNS. Most of these genes are predominantly expressed in the glomerular epithelial cell, the podocyte, placing it at the center of the pathogenesis of SRNS. Podocyte migration rate (PMR) represents a relevant intermediate phenotype of disease in monogenic causes of SRNS. We therefore adapted PMR in a high-throughput manner to screen small molecules as potential therapeutic targets for SRNS. We performed a high-throughput drug screening of a National Institutes of Health Clinical Collection (NCC) library (n = 725 compounds) measuring PMR by videomicroscopy. We used the Woundmaker to perform individual 96-well scratch wounds and screened compounds using a quantitative kinetic live cell imaging migration assay using IncuCyte ZOOM technology. Using a normal distribution for the average PMR in wild-type podocytes with a vehicle control (DMSO), we applied a 90% confidence interval to define “distinct” compounds (5% faster/slower PMR) and found that 12 of 725 compounds (at 10 μM) reduced PMR. Clusters of drugs that alter PMR included actin/tubulin modulators such as the azole class of antifungals and antineoplastic vinca-alkaloids. We hereby identify compounds that alter PMR. The PMR assay provides a new avenue to test therapeutics for nephrotic syndrome. Positive results may reveal novel pathways in the study of glomerular diseases such as SRNS.
Keywords: steroid-resistant nephrotic syndrome, podocyte, small molecule screen
the prevalence of end-stage kidney disease, which requires dialysis or transplantation for survival, has been increasing over the last few decades (27). Total healthcare cost for chronic kidney disease (CKD) now exceeds >$40 billion dollars annually (26) and continues to rise as renal replacement therapy and transplant survival have improved. Although there have been advancements in treatment regimens, there has been little to no drug development to address any of the primary causes of CKD, including steroid-resistant nephrotic syndrome (SRNS) and its histological hallmark, focal segmental glomerulosclerosis (FSGS). SRNS represents the second most frequent cause of CKD that manifests before 25 yr of age (104). Currently, there are more than 30 monogenic genes that, if mutated, cause SRNS. All of them are relevantly expressed in the glomerular epithelial cell, the podocyte, placing it at the center of the pathogenesis of SRNS (71). The pathophysiology of nephrotic syndrome is characterized by structural alteration of cytoskeleton and molecular reorganization of slit diaphragm components leading to foot process effacement (FP). Previous work has demonstrated the well-established concept that FP effacement is a migratory event, making the podocyte migration rate (PMR) an important functional assay for studying pathological condition in vitro (93). Within the podocyte, there are several pathway-specific mechanisms that are essential for disease pathogenesis. Previous work has established small Rho-like GTPase signaling to play a central role in the pathogenesis of SRNS (39–41, 102). In studying the effect of small Rho-like GTPase signaling, the PMR was demonstrated to represent a relevant intermediate phenotype of disease in monogenic causes of SRNS (38, 40, 41). As we have shown in SRNS, PMR can be increased or decreased, therefore implying that the balance of RhoA/Rac1/Cdc42 signaling, not overall PMR, is relevant to disease (9, 39, 40). In the last 10–15 yr advances in high-throughput screening techniques have opened the way for drug discovery (51, 122). In addition, several groups have recently attempted to adapt high-content screening to the field of nephrology by using podocyte based assays (68). We therefore hypothesized that using PMR in a live cell-based assay could be adapted in a high-throughput fashion to identify pathways integral to the pathogenesis of nephrotic syndrome as well as potential novel therapeutics. We have identified 12 compounds that reduced PMR.
METHODS AND STUDY DESIGN
Drug library.
The National Institutes of Health Clinical Collection (NIHCC) is a library of 725 compounds spread across ten 96-well plates (8 plates have 80 compounds, 1 plate has 45 compounds, and 1 plate has 40 compounds). The drugs represented by this library have been selected because of their purity, solubility, and commercial availability for resupply (NCC; National Center for Advancing Translational Sciences, Bethesda, MD). The compounds were prepared and shipped by Evotec as part of the National Institutes of Health Small Molecule Repository (NIHSMR). The compounds were shipped as a 10-mM stock solution diluted in DMSO solvent. Other compounds tested include Rho Activator II (CN03; Cytoskeleton), Rho Pathway Inhibitor I (ROCK Y-27632, CN06; Cytoskeleton), Rac1 inhibitor (553502; Millipore), and Rac1 Inhibitor II (553511; Millipore).
Cell culture.
The immortalized human podocyte cell line was a kind gift from M. Saleem (University of Bristol) and was cultured as previously described (96). Human podocytes were plated at 35,000 cells/well and incubated at 33°C 12 h before making the scratch wound. Cells were grown at 37°C after the scratch wound was made and allowed to migrate. Cells were tested for mycoplasma contamination on a biweekly basis.
Human podocyte cell line expressing stable nuclear mKate2 (red fluorophore).
IncuCyte NucLight Red lentivirus reagent was purchased from Essen Bioscience. The human podocyte cell line (gift from M. Saleem) was transduced according to manufacturer’s instructions per protocol, and 48 h after transduction, puromycin at a final concentration of 4 µg/ml was added to the medium for selection of transduced cells, which stably express nuclear mKate2.
Scratch wound assay.
Cells were plated on 96-well Image-lock plates (Essen Bioscience). Podocytes were examined for confluency as a monolayer via light microscopy before initiation of any scratch wound. Scratches were made by using a 96-pin tool (Woundmaker) as per protocol.
Proliferation assay.
Cells were plated on 96-well Image-lock plates (Essen Bioscience). Before initiation of any scratch wound, podocytes were examined for confluence as a monolayer via light microscopy. Scratches were made by using a 96-pin tool (Woundmaker) as per protocol. Whole well images were acquired at the beginning (t0) and at the end (tx) of the experiment.
Videomicroscopy.
Podocyte cell proliferation was assessed using the whole well assay format. Cells were monitored automatically via live cell imaging using the IncuCyte videomicroscopy system at the beginning (t0) and at the end (tx) of the experiment. Whole well images were automatically acquired and recorded by the IncuCyte software (Controller version 2015A Rev 1). Podocyte cell migration was assessed using the scratch-wound assay format. Cells were monitored automatically via live kinetic cell imaging using the IncuCyte videomicroscopy system at 60-min intervals. Wound images were automatically acquired and recorded by the IncuCyte software (Controller version 2015A Rev 1).
Data analysis.
Data processing and analysis for proliferation assay were done using the IncuCyte 96-well Basic Analyzer software module. Videomicroscopy was performed with a ×4 objective. Individual whole wells were analyzed with the IncuCyte GUI software. Data processing and analysis for migration assay were done using the IncuCyte 96-well Kinetic Cell Migration and Invasion Assay software module. Data were then exported to Excel for further analysis. Wound width is defined as the area of the wound at any time t, as determined by the processing software. Wound confluence is expressed as a percentage of the scratch wound that is filled with cells at any given time t, when compared with when the scratch was initially performed. Wound closure, as a measurement of PMR, was monitored at 60-min intervals for at least 20 h. Videomicroscopy was performed with a ×10 objective. Migration was performed at 37°C to minimize the effects of cell proliferation. Individual scratch wounds were analyzed with the Incucyte GUI software and inspected visually for cell viability. All wells that did not demonstrate a confluent monolayer after the scratch wound/washing process were discarded from analysis (Fig. 1A).
Fig. 1.
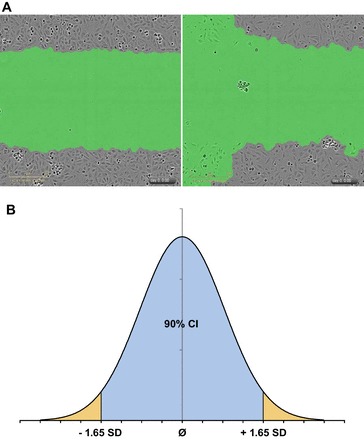
Methods to determine positive hits with videomicroscopy based scratch wound assay. Wells for the podocyte migration rate (PMR) assay were chosen for quality depending on wound width, confluence (A), and characteristics of a normal distribution curve (B). A: 2 representative images are shown of human podocytes at time point t0, after a scratch-wound. Left: adequate scratch with a confluent monolayer above and below the wound with no debris/cellular remains within the wound to confound analysis. Right: inadequate wound width and cell confluency due to poor podocyte coverage above and below the scratch wound. B: normal distribution curve is displayed with demarcations at 1.65 SD from the mean [90% confidence interval (CI)]. In the first part of our evaluation, outliers (outside the 90% CI) in wound width were deemed inadequate and discarded from analysis. In the second part of our evaluation, wound confluence was used to identify wells with “distinct” PMR outside the 90% CI and kept as positive results.
Drug screen.
Each drug plate of the NIHCC was plated at 10 μM into an individual well for scratch wound assay analysis. Each compound was screened in triplicate to ensure reproducibility. Every plate screened also had a 16 replicates for DMSO as a vehicle control, and this was used as a control to compare wound closure rates. After the preliminary screen was completed, all preliminary hits were then screened again in quadruplicate at differing concentrations (1, 5, and 10 μM) to assess for a dosage effect.
Statistical analysis.
Student's t-test was used to determine the statistical significance between two interventions, and one-way ANOVA followed by Bonferroni’s correction was used for multiple comparisons (GraphPad Prism software). A statistically significant difference was defined as P < 0.05 and is marked as follows: **P < 0.01, ***P < 0.001, ****P < 0.0001.
RESULTS
We first performed a proliferation assay to distinguish the proliferation rate of wild-type podocytes under migration conditions using the control condition containing DMSO vehicle control (0.1%). We demonstrate that the proliferation rate of human podocytes significantly decreases over time by 23.2% over 26 h predominantly due to DMSO toxicity (Fig. 2) (88). Additionally, we performed a proof of principle experiment with RhoA and Rac1 signaling pathway effectors (Fig. 3) replicating previous data (1, 2). The proof of principle experiment with the established microtubule modulators had shown a reduction in PMR (Fig. 4 and Fig. 5) replicating previous data (111, 112). Additionally, our data showed that microtubule modulators impair the cell viability reducing the cell count, however, without compromising the PMR (Fig. 6). In these experiments we demonstrate that PMR can be reproducibly measured and that pharmaceutical compounds can be assayed in a high throughput fashion. We then extended the live cell based kinetic videomicroscopy based scratch wound assay to a National Insitutes of Health small molecule therapeutic library to assess for pharmaceutical modifiers of PMR.
Fig. 2.
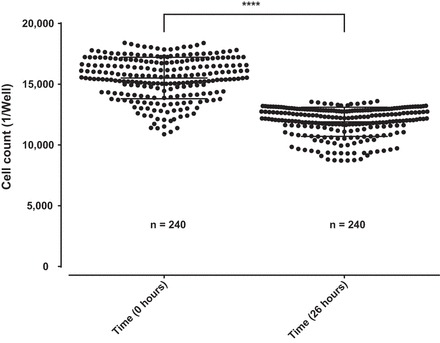
Proliferation assay in immortalized undifferentiated human podocytes. Stably nuclear mKate2 expressing wild-type podocytes from an immortalized human podocytes cell line were seeded 12 h before the beginning of the experiment on a 96-well image-lock plate. Images were captured at the beginning and at the end of the experiment. A significant reduction of cell count in average of 23.2% on 240 replicates is shown over a period of 26 h in cultured media containing DMSO vehicle control (0.1%). Data are expressed as means ± SD for 3 independent experiments, 80 replicates each.
Fig. 3.
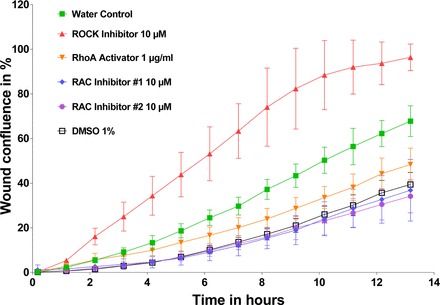
Proof of principle experiment with RhoA and Rac1 effectors. Wild-type podocytes were seeded 12 h before scratch wound analysis on a 96-well image-lock plate. After scratch wound was made in the confluent podocyte monolayer, podocytes were exposed to different compounds including ROCK inhibitor (CN06; Cytoskeleton), RhoA activator (CN03; Cytoskeleton), and Rac1 inhibitor #1 (553502; Millipore), and Rac1 inhibitor #2 (553511; Millipore). Concentrations are as follows: RhoA inhibitor: 10 μM; RhoA activator: 1 μg/ml; Rac1 inhibitor #1: 10 μM; and Rac1 inhibitor #2: 10 μM, as per dosing instructions. RhoA effectors were diluted in water. Rac1 effectors were diluted in DMSO. The volume of total media including drug in each well is 100 μl. Note that, whereas ROCK inhibitors increased PMR, RhoA activators as well as Rac1 inhibitors #1 and #2 decreased PMR. Data are expressed as means ± SD for 2 independent experiments.
Fig. 4.
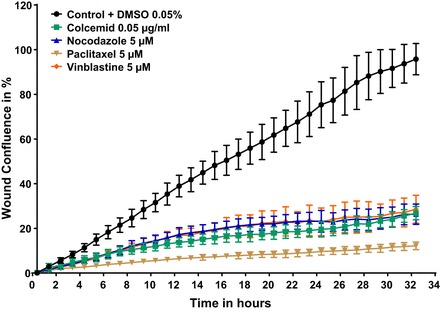
Proof-of-principle experiment with established modulators of microtubule polymerization/depolymerization such as colcemid, nocodazole, paclitaxel, and vinblastine. Stably nuclear mKate2 expressing wild-type podocytes were seeded 12 h before scratch wound analysis on a 96 well image-lock plate. After scratch wound was made in the confluent podocyte monolayer, podocytes were exposed to compounds including colcemid (no. 10295892001; Roche), nocodazole (M1404; Sigma), paclitaxel (T7402; Sigma), vinblastine (V1377; Sigma), and DMSO vehicle control (0.05%). Concentrations are as follows: 0.05 μg/ml colcemid, 5 μM nocodazole, 5 μM paclitaxel, and 5 μM vinblastine. Colcemid was delivered as ready to use solution, DMSO was added accordingly. Nocodazole, paclitaxel, and vinblastine were diluted in DMSO. All drugs significantly decreased PMR at the established concentrations.
Fig. 5.
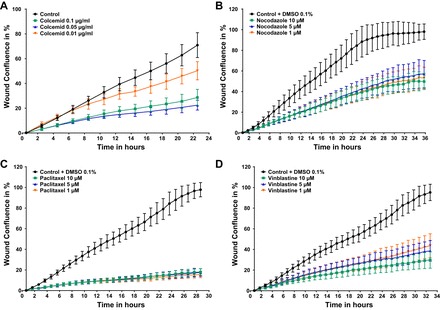
Dose response of established modulators of microtubule demonstrates decreased PMR. Stably nuclear mKate2 expressing wild-type podocytes were seeded 12 h before scratch wound analysis on a 96 well image-lock plate. After scratch wound was made in the confluent podocyte monolayer, podocytes were exposed to compounds in different concentration as indicated. A: colcemid (control, n = 47; 0.1 µg/ml colcemid, n = 47; 0.05 µg/ml colcemid, n = 32; and 0.01 µg/ml colcemid, n = 31) alters the PMR partially in a concentration-response manner. B and C: nocodazole (0.1% DMSO vehicle control, n = 45; 10 µM nocodazole, n = 48; 5 µM nocodazole, n = 3; and 1 µM nocodazole, n = 32) and paclitaxel (0.1% DMSO vehicle control, n = 46; 10 µM paclitaxel, n = 48; 5 µM paclitaxel, n = 31, 1 µM paclitaxel, n = 32) reduce PMR in a concentration-independent manner. D: whereas vinblastine (0.1% DMSO vehicle control, n = 47; 10 µM vinblastine, n = 48; 5 µM vinblastine, n = 32; and 1 µM vinblastine, n = 32) reduces PMR in a concentration-dependent manner.
Fig. 6.
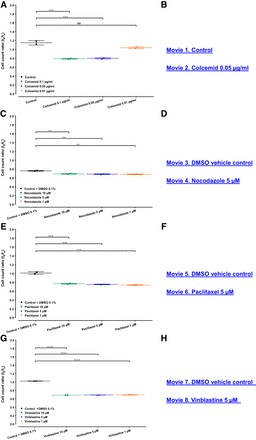
Toxicity of established microtubule modulators reduce podocytes cell count without compromizing the PMR. Wild-type podocytes from an immortalized human podocytes cell line stably expressing nuclear mKate2 were seeded 12 h before the beginning of the experiment on a 96-well image-lock plate. Whole well images were captured after drug exposure at the beginning (t0) and at the end (tx) of the experiment to perform the quantitative analysis of total cell count. Live cell imaging captured immortalized human podocytes on an hourly basis to characterize the cellular morphology and viability of podocytes, while undergoing migration in a scratch wound assay. A, C, E, and G: all compounds at indicated concentration reduce the cell count ratio and show substantial cell morphology changes vs. control condition. B, D, F, and H: all compounds significantly affected PMR as well, however, without compromising the migratory behavior (see Fig. 5; also see Supplemental Movies 1-8; Supplemental material for this article is available at the Journal website). Data are expressed as a ratio of means ± SD for 2 independent experiments. NS = not significant; **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA Bonferroni’s multiple comparison test.
All 725 compounds from the NIHCC were examined by the PMR assay. Wells with significantly different PMR as compared with vehicle control (DMSO) were defined as “distinct” and were thereby identified as positive results. These “distinct” wells were isolated from the exported IncuCyte data in a five-step process:
-
1)
After visual inspection, all wells that passed this initial filtering criterion were subject to a quantitative quality control step. Remaining wells were measured for wound width at time, t0 (at initial scratch), and all outliers outside the 90% confidence interval were removed from analysis as well (Fig. 1A). The 90% confidence interval for wound width was determined from a standard deviation calculated by using the wound width for all experimental conditions per plate at t0 (Fig. 1B). No individual compound was analyzed for wound closure rate if there were not at least two replicate conditions passing visual or wound width inspection.
-
2)
Wells that passed visual and quantitative inspection then were analyzed for wound closure rates, as a measurement of PMR. Wound closure rates were calculated using wound confluence as determined by the IncuCyte software module.
-
3)
For each individual compound, each replicate was averaged and wound confluence was measured over time. Assuming a normal distribution of wound closure in wild-type immortalized podocytes, the rate (slope of wound confluence over change in time) was calculated for each compound during the linear phase of migration and compared with the rate of wound closure of a control condition (DMSO).
-
4)
Any compound with a wound closure rate during the linear phase of migration that was outside the 90% confidence interval was classified as a positive result (Fig. 7).
-
5)
“Distinct” wells were then confirmed again in quadruplicate with different concentrations as noted previously. The 90% confidence interval was determined from a standard deviation as calculated by using the DMSO vehicle control as the standard control condition.
Fig. 7.
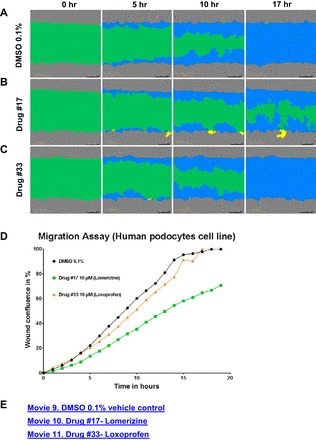
Positive drug screening hits determined by PMR as compared with vehicle control. Examples of positive and negative results in National Institutes of Health Clinical Collection (NIHCC) Plate #4. A: DMSO 0.1% vehicle control at time 0, 5, 10, and 15 h, respectively. B: Drug #17 (10 μM), lomerizine, at time 0, 5, 10, and 15 h, respectively. C: Drug #33 (10 μM), loxoprofen, at time 0 and 5, 10, and 15 h, respectively. D: graphical representation of wound confluence (y-axis) over time (x-axis) for DMSO, Drug #17 (lomerizine), and Drug #33 (loxoprofen) over an average of 3 replicates. E: see Supplemental Movies 9-11 for movies of wound confluence over time of vehicle control DMSO 0.1%, Drug #17 (lomerizine), and Drug #33 (loxoprofen) for 1 replicate.
Of the 725 compounds screened, 632 compounds passed the quality filtering steps of visual inspection (Fig. 1A) and wound width standardization for analysis of podocyte migration rate.
Of the 632 compounds analyzed for PMR via wound closure rate, 61 compounds were deemed as initial positive results (Table 1. Fifty-seven compounds reduced PMR in podocytes and 4 compounds increased PMR (Fig. 8).
Table 1.
Fifty-seven preliminary positive hits from drug screening with decreased PMR and 4 with increased PMR in 725 compounds NIHCC at 10-µM concentration
| Name | Result | Mechanism | Pubchem ID | Reference | Drug Library Plate |
|---|---|---|---|---|---|
| Topotecan | Decreased migration | Anti-neoplastic topo I inhibitor | 46386667 | (64, 99) | NIHCC Plate_1_A8 |
| SDM25N | Decreased migration | Gamma-receptor antagonist | 46387008 | (75) | NIHCC Plate_1_C5 |
| Vincristine | Decreased migration | Vinca-alkaloid-inhibitor of mitosis | 46386588 | (57, 87) | NIHCC Plate_1_G8 |
| Vindesine | Decreased migration | Vinca-alkaloid-inhibitor of mitosis | 46386586 | (87) | NIHCC Plate_1_H11 |
| Adenosine, N-(2-hydroxycyclopentyl)-, (1S-trans) | Decreased migration | Activation of purine receptors A1 and A2 | 46387015 | (110) | NIHCC Plate_2_A6 |
| Ezetimibe | Decreased migration | inhibits absorption of cholesterol | 46386640 | (2, 37) | NIHCC Plate_2_A8 |
| 8-Azaspiro[4.5]decane-7,9-dione, 8-[2-][(2,3-dihydro-1,4-benzodioxin-2-yl)methyl]amino[ethyl]-,monomethanesulfonate | Decreased migration | Anticonvulsant | 46387017 | (84) | NIHCC Plate_2_A10 |
| N,N′-diacetyl-1,6-diaminohexane | Decreased migration | Experimental compound; used in production of nylon | 46386869 | N/A | NIHCC Plate_2_B2 |
| Cefatrizine propylene glycol | Decreased migration | Cephalosporin | 46386659 | (81) | NIHCC Plate_2_B3 |
| Oxymetholone | Decreased migration | Anabolic steroid | 46386778 | (7, 80) | NIHCC Plate_2_B10 |
| Anastrozole | Decreased migration | Nonsteroidal aromatase inhibitor | 46386543 | (79, 97) | NIHCC Plate_2_C7 |
| Rimcazole | Decreased migration | Sigma-receptor antagonist | 46387003 | (42) | NIHCC Plate_2_D4 |
| Zolmitriptan | Decreased migration | Triptan-selective agonist of serotonin 1B and 1D receptor | 46386880 | (74, 89) | NIHCC Plate_2_E11 |
| Artesunate | Decreased migration | Semi-synthetic derivative of artemisinin | 46386645 | (52) | NIHCC Plate_2_H6 |
| Nimetazepam | Decreased migration | Benzodiazepine | 46386768 | (56) | NIHCC Plate_2_H8 |
| Ramipril | Increased migration* | ACE inhibitor | 46386770 | (69) | NIHCC Plate_3_B2 |
| Ampiroxicam | Increased migration* | Prodrug of piroxicam; NSAID-reversible cox-1 inhibitor | 46386688 | (17, 33) | NIHCC Plate_3_E4 |
| Glycine, N-[2-(acetylthio)methyl]-1-oxo-3-phenylpropyl-,phenylmethyl ester | Increased migration* | Amino acid; neurotransmitter in CNS | 46386930 | (8, 20, 65, 117) | NIHCC Plate_3_E6 |
| Triptolide | Decreased migration | Anti-inflammatory; podocyte protective | 46386571 | (12, 16, 46) | NIHCC Plate_3_H2 |
| Ethylestrenol | Decreased migration | Anabolic steroid (Pregnane steroids)-little androgenic effect | 46386573 | (114) | NIHCC Plate_3_H4 |
| Midazolam HCl | Increased migration* | Benzodiazepine-GABA potentiator | 46386603 | (76, 103) | NIHCC Plate_3_H6 |
| Lomerizine DiHCl | Decreased migration | Ca channel blocker | 46386707 | (45, 94) | NIHCC Plate_4_A4 |
| Pancuronium | Decreased migration | Nondepolarizing muscle relaxant; competitive acetylcholine antagonist at NMJ | 46386853 | (29) | NIHCC Plate_4_B3 |
| Trazodone HCl | Decreased migration | Antidepressant; binds 5HT2; selective reuptake inhibitor | 46386915 | (73) | NIHCC Plate_4_B8 |
| Metronidazole | Decreased migration | Nitroimidazole antibiotic; inhibiting bacterial DNA synthesis | 46386860 | (121) | NIHCC Plate_4_B9 |
| Saquinavir mesylate | Decreased migration | HIV protease inhibitor | 46386596 | (28) | NIHCC Plate_4_C6 |
| Tegaserod maleate | Decreased migration | 5HT4 agonist; used for IBS | 46386624 | (15) | NIHCC Plate_4_C7 |
| Diphenylcyclopropenone | Decreased migration | Local irritant | 46386897 | (106) | NIHCC Plate_4_C8 |
| Nifekalant HCl | Decreased migration | Class III anti-arrhythmic; inhibits hERG channel | 46386697 | (36, 50) | NIHCC Plate_4_C11 |
| Bifemelane | Decreased migration | Neuroprotective; mechanism not well understood | 46386922 | (82, 86) | NIHCC Plate_4_D6 |
| Loratidine | Decreased migration | Second generation H1 receptor antagonist | 46386837 | (14) | NIHCC Plate_4_D7 |
| Mesoridazine | Decreased migration | Phenothiazine; adrenergic blockade; hERG cell blockade | 46386921 | (22, 108) | NIHCC Plate_4_D8 |
| Irinotecan HCl | Decreased migration | Antineoplastic-topo I inhibitor | 46386616 | (18) | NIHCC Plate_4_D10 |
| Rifapentine | Decreased migration | Antibiotic; inhibits RNA polymerase in bacteria | 46386637 | (70) | NIHCC Plate_4_D11 |
| Vinorelbine bitatrate | Decreased migration | Vinca-alkaloid-inhibitor of mitosis | 46386815 | (57, 87) | NIHCC Plate_4_E4 |
| Indatraline | Decreased migration | Monoamine transporter inhibitor | 46386808 | (47, 115) | NIHCC Plate_4_E5 |
| Ketorolac | Decreased migration | NSAID; COX1 and COX2 inhibition | 46386614 | (48) | NIHCC Plate_4_E9 |
| Ethynylestradiol | Decreased migration | Synthetic estrogen derivative | 46386858 | (98) | NIHCC Plate_4_F9 |
| Cetraxate HCl | Decreased migration | Anti-ulcer cytoprotective agent (GI tract) | 46386678 | (66) | NIHCC Plate_4_F10 |
| HTMT | Decreased migration | H1/H2 agonist | 46386998 | (91) | NIHCC Plate_4_F11 |
| SR 57,227A | Decreased migration | 5HT3 agonist | 46386849 | (11) | NIHCC Plate_4_G9 |
| Tripelennamine HCl | Decreased migration | H1 antagonist | 46386917 | (119) | NIHCC Plate_4_H9 |
| 5-Methoxytryptamine | Decreased migration | Tryptamine derivative (melatonin)-agonist of 5HT1,2,4,6,7 receptors) | 46387021 | (34) | NIHCC Plate_4_H11 |
| Nifedipine | Decreased migration | Ca-channel blocker | 46386790 | (21, 77, 107a) | NIHCC Plate_5_F8 |
| Doxapram | Decreased migration | K-channel subfamily K blocker | 46386821 | (4, 90, 120) | NIHCC Plate_5_H10 |
| Nitazoxanide | Decreased migration | Pyruvate-flavodoxin oxidoreductase inhibitor | 46386689 | (13, 49) | NIHCC Plate_6_A6 |
| 5-Cyclopropyl-1-(2-methoxypropyl)-5-methyl-2-phenylpiperazine | Decreased migration | Unknown | 104170212 | N/A | NIHCC Plate_7_D5 |
| 8-tert-butyl-6-(2-methoxypropyl)-6,9-diazaspiro[4.5]decane | Decreased migration | Unknown | 104170115 | N/A | NIHCC Plate_7_A10 |
| Felodipine | Decreased migration | Ca-channel blocker, inhibition of mineralocorticoid receptor, PDE1A/1B inhibitor | 104170219 | (21, 30, 35, 67, 100, 107a) | NIHCC Plate_7_C5 |
| Albendazole | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition | 104170113 | (23, 92, 105) | NIHCC Plate_7_C9 |
| Azathioprine | Decreased migration | Inhibitor of hypoxanthine-guanine phosphoribosyl transferase | 104170112 | (6, 32) | NIHCC Plate_7_D9 |
| Griseofulvin | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition | 104170114 | (55, 87), | NIHCC Plate_7_D10 |
| Miconazole | Decreased migration | Lanosterol 14-alpha demethylase inhibitor, K voltage-gated channel subfamily H inhibitor, Ca-activated K channel inhibitor | 104169959 | (3, 116) | NIHCC Plate_7_E3 |
| Daunorubicin | Decreased migration | DNA-topoisomerase-2 inhibitor | 104170197 | (10, 123) | NIHCC Plate_7_E7 |
| Minocycline | Decreased migration | Caspase-1 and -3 negative modulator, VEGF inhibitor, matrixmetalloproteinase-9 inhibitor, 16 rRNA inhibitor | 104169958 | (19, 31, 55, 87, 95, 109, 118) | NIHCC Plate_ |
| Mobendazole | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition | 104170137 | (72, 85) | NIHCC Plate_8_A8 |
| Digoxin | Decreased migration | Na-K-ATPase inhibitor | 104170057 | (1) | NIHCC Plate_8_G5 |
| 5-Azacytidine | Decreased migration | DNA-methyltransferase 1 inhibitor | 104170170 | (24, 83), | NIHCC Plate_9_A10 |
| Podofilox | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition, DNA-topoisomerase-2 inhibitor | 104170173 | (54, 59) | NIHCC Plate_9_C8 |
| Mitoxantrone | Decreased migration | DNA intercalation, DNA-topoisomerase-2 inhibitor | 104170122 | (21, 44) | NIHCC Plate_9_E5 |
| 1-(2-Methoxypropyl)-2,5,5-trimethyl-2-phenylpiperazine | Decreased migration | N/A | 104170092 | N/A | NIHCC Plate_10_B3 |
Preliminary hits ordered in ascending order from NIHCC plate. Fifty-seven of 61 positive results decreased migration and 4 increased (*) migration. All positive hits were screened in triplicate at 10 µM concentration and compared with a vehicle control (0.1% DMSO). NIHCC, National Institutes of Health Clinical Collection; PMR, podocyte migration rate; NMJ, neuromuscular junction; hERG, human ether-a-go-go-related gene; NSAID, nonsteroidal anti-inflammatory drugs; CNS, central nervous system; GI, gastrointestional; ACE, angiotensin-converting enzyme; COX, cyclooxygenase; Pubchem ID: https://pubchem.ncbi.nlm.nih.gov/.
Fig. 8.
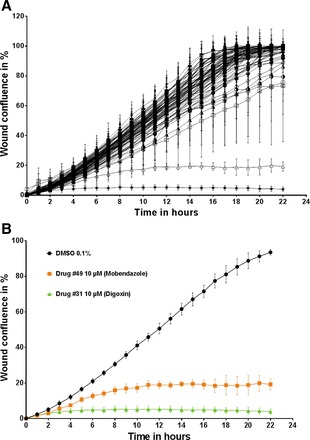
Representative example of PMR assay (NIHCC Plate #8) demonstrating wound confluence over time and positive hits. Podocytes from an immortalized human podocyte cell line was seeded 12 h before scratch wound analysis on a 96-well image-lock plate. After a scratch wound was made in the confluent podocyte monolayer, podocytes were exposed to 80 different compounds from NIHCC Drug Plate #8. Each individual drug (80 compounds) was placed in 1 individual well. DMSO vehicle control (0.1%) was seeded in the 16 remaining wells of the 96-well plate. A: wound confluence over time demonstrates podocyte migration over a period of 24 h after creation of scratch wound for all 80 individual compounds. B: 2 specific compounds with PMR outside the <90% CI are shown as positive hits. Drug #49 (mobendazole) and Drug #31 (digoxin) are highlighted in comparison to DMSO vehicle control as they are significantly outside the 90% confidence interval for Δwound confluence/Δtime, a measurement of PMR. Data are expressed as means ± SD for 3 independent experiments.
All 61 compounds were then analyzed in a secondary confirmatory screen at (1, 5, and 10 μM dissolved in 0.01, 0.05, and 0.1% DMSO, respectively) to assess for a dosage effect. In the dose-response screen, 12/61 (19.7%) of the initial positive compounds were confirmed as “distinct” at 10 μM when the podocyte migration rate was compared with the DMSO control condition (Table 2).
Table 2.
Confirmed positive hits by PMR assay from small molecule screen of 725 compounds from NIHCC with decreased PMR
| Name | Result on PMR | Mechanism | Pubchem ID | Reference | Drug Library Plate | Concentration, µM |
|---|---|---|---|---|---|---|
| Topotecan (Top) | Decreased migration | Anti-neoplastic topo I inhibitor | 46386667 | (64, 99) | NIHCC Plate_1_A8 | 10, 5, 1 |
| Vincristine (VA) | Decreased migration | Vinca-alkaloid-inhibitor of mitosis | 46386588 | (57, 87) | NIHCC Plate_1_G8 | 10, 5, 1 |
| Vindesine (VA) | Decreased migration | Vinca-alkaloid-inhibitor of mitosis | 46386586 | (87) | NIHCC Plate_1_H11 | 10, 5, 1 |
| Digoxin (Dig) | Decreased migration | Na-K-ATPase inhibitor | 104170057 | (1) | NIHCC Plate_8_G5 | 10, 5, 1 |
| Podofilox (Top/TI) | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition, DNA-topoisomerase-2 inhibitor | 104170173 | (54, 59) | NIHCC Plate_9_C8 | 10, 5, 1 |
| Albendazole (TI) | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition | 104170113 | (23, 92, 105) | NIHCC Plate_7_C9 | 10, 5 |
| Lomerizine DiHCl (CaB) | Decreased migration | Ca channel blocker | 46386707 | (45, 94) | NIHCC Plate_4_A4 | 10 |
| Pancuronium (MR) | Decreased migration | Nondepolarizing muscle relaxant; competitive acetylcholine antagonist at NMJ | 46386853 | (29) | NIHCC Plate_4_B3 | 10 |
| Trazodone hydrochloride (SSRI) | Decreased migration | Antidepressant; binds 5HT2; selective reuptake inhibitor | 46386915 | (73) | NIHCC Plate_4_B8 | 10 |
| Tegaserod maleate (5HT) | Decreased migration | 5HT4 agonist; used for IBS | 46386624 | (15) | NIHCC Plate_4_C7 | 10 |
| Irinotecan HCl (Top) | Decreased migration | Antineoplastic-topo I inhibitor | 46386616 | (18) | NIHCC Plate_4_D10 | 10 |
| Mobendazole (TI) | Decreased migration | Tubulin-alpha/beta chain polymerization inhibition | 104170137 | (72, 85) | NIHCC Plate_8_A8 | 10 |
| Mitoxantrone (Top) | Decreased migration | DNA intercalation; DNA-topoisomerase-2 inhibitor | 104170122 | (21, 44) | NIHCC Plate_9_E5 | 5, 1 |
| Adenosine, N-(2-hydroxycyclopentyl)-,(1S-trans)-(AR) | Decreased migration | Activation of purine receptors A1 and A2 | 46387015 | (110) | NIHCC Plate_2_A6 | 5 |
| N,N′-diacetyl-1,6-diaminohexane (Syn) | Decreased migration | experimental compound; used in production of nylon | 46386869 | N/A | NIHCC Plate_2_B2 | 5 |
| Zolmitriptan (Trip) | Decreased migration | Triptan-selective agonist of serotinin 1B and 1D receptor | 46386880 | (74, 89) | NIHCC Plate_2_E11 | 5 |
| Vinorelbine bitatrate (VA) | Decreased migration | Vinca-alkaloid-inhibitor of mitosis | 46386815 | (57, 87) | NIHCC Plate_4_E4 | 5 |
| Artesunate (AM) | Decreased migration | Semi-synthetic derivative of artemisinin | 46386645 | (52) | NIHCC Plate_2_H6 | 1 |
Sixty-one preliminary positive results (see Table 1) were screened in serial concentrations of 1, 5, and 10 µM. Confirmed results are above with respective concentrations at which the result was confirmed. All positive hits were screened in quadruplicate at their respective concentration and compared with a vehicle control (0.01, 0.05, and 0.1% DMSO, respectively). NIHCC, National Institutes of Health Clinical Collection; PMR, podocyte migration rate; AM, anti-microbial; AR, antiarrhythmic; CaB, calcium channel blocker; Dig, digitalis glycoside; IBS, irritable bowel syndrome; MR, muscle relaxant; NMJ, neuromuscular junction; Pubchem ID: https://pubchem.ncbi.nlm.nih.gov/; SSRI, selective serotonin reuptake inhibitor; Syn, synthetic compound; Top, topoisomerase inhibitor; Trip, triptan agonist; TI, tubulin inhibitor; VA, vinca-alkaloid.
Five compounds, topotecan, vincristine, vindesine, digoxin, and podofilox, demonstrated significantly slower PMRs when compared with control at all three dosing concentrations. Two compounds, albendazole and mitoxantron, demonstrated a dosage effect with significantly slower migration at two of the three dosing concentrations.
All compounds that were confirmed reduced PMR as compared with the DMSO vehicle control. No compounds in the drug screen significantly increased PMR as compared with control.
The positive hits clustered into two classes of compounds: vinca-alkaloids (vincristine and vindesine) and the azole class of antifungals (albendazole and mobendazole) (Table 2). When inspected visually, both classes of medications alter migration rate as well as cell morphology (Fig. 9). These drugs commonly affect microtubule assembly formation as destabilizing agents. Furthermore, podofilox has microtubule depolymerization activity as well.
Fig. 9.
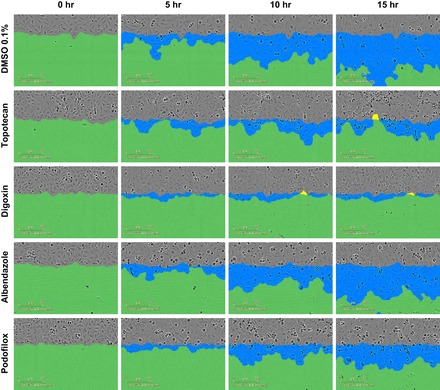
Podocyte cellular morphology at sequential time points after drug exposure. Human podocytes were qualitatively examined for podocyte morphology changes during the podocyte migration assay. Live cell imaging captured immortalized human podocytes at different time points (0, 5, 10, and 15 h) after drug exposure, while undergoing migration in a scratch wound assay. Compounds shown include DMSO vehicle control (0.1%), topotecan (10 μM), digoxin (10 μM), albendazole (10 μM), and podofilox (10 μM). All compounds significantly affected PMR as well (see Table 2).
Other antineoplastic agents that reduced PMR significantly include the topoisomerase inhibitors (topotecan and irinotecan). Other single compounds that decrease PMR include ligands for serotonin receptors (trazodone and tegaserod), a calcium channel blocker (lomerizine), a nondepolarizing muscle relaxant (pancuronium), and digoxin, a glycoside used for cardiac arrhythmias (Table 2).
DISCUSSION
Implications of positive results.
Recent technological advances have allowed high throughput screening of small molecules and compounds in kidney diseases, which has traditionally been lacking in comparison with other medical fields such as oncology. Although other publications have established potential screens for drug therapy in kidney diseases (68), live cell imaging has not previously been employed to screen for potential therapeutics. In this drug screen, we used PMR as a relevant surrogate phenotype of disease to confirm 12/61 (19.6%) of compounds at 10 μM that we initially screened as positive hits.
Of the five compounds that demonstrated a PMR that was significantly reduced compared with vehicle control at all concentrations (1, 5, and 10 μM), three of the compounds (vincristine, vindesine, and podofilox) alter microtubule formation by acting as depolymerization agents. Furthermore, two other compounds, albendazole and mobendazole, that confirmed at 10 μM act as microtubule inhibitors as well. These compounds demonstrate that drugs that affect microtubule assembly are robust effectors of PMR. Although many of the podocytic genes that cause SRNS alter actin regulation, microtubules are a critical component in the podocyte and functionally cooperate with the actin cytoskeleton (43, 61, 63). Made from sets of 13 protofilaments of α- and β-tubulin subunits, microtubules form the primary processes in the podocyte and have been shown to be important in supporting structural development of the podocyte cytoskeleton (5, 107). It was shown previously that podocytes express several microtubule-associated proteins (MAPs) such as MAP3 and MAP4 (60). It is also known that CHO1/MKLP1 and protein phosphatase 2a (PP2A) microtubule-associated motor proteins (MAPs-MP) play an important role in podocyte primary process formation and in establishing of cell polarity in cultured podocytes (62, 63). In addition, microtubules (MT) are involved in positioning and organization cell organelles inside the cell and are responsible for intracellular transport of vesicles and proteins between cell domains (113). It is also known that impairment of these functions results in a transient nephrotic syndrome in cell culture (58). Knowing that the MT and the actin cytoskeleton interact, we can assume that impairment of one component can lead to a dysfunction of the other. This is supported by cell culture studies showing that the microtubule-associated guanine nucleotide exchange factor GEF-H1 regulates actin cytoskeleton dynamics through activation of RhoA (78). In fact, there are multiple genes (WDR73, INF2, and TTC21B) that, if mutated, implicate abnormal microtubule assembly in the pathogenesis of nephrotic syndrome (25, 53, 101) confirming pathogenic relevance of our findings. Therefore, this screen confirms that targeting the pathway of microtubule assembly dynamics may be worth pursuing in the search for novel therapeutics in nephrotic syndrome.
Limitations.
One limitation to our assay is the fact that we only screened compounds of the NIHCC in wild-type immortalized podocytes. Drugs may have an effect as a therapy only in diseased states. Another issue in using wild-type podocytes is that identification of drugs that increase PMR significantly is difficult to detect. As immortalized cultured wild-type cells migrate rapidly at baseline, our assay may not have the sensitivity to detect drugs that increase PMR in a biologically significant manner with our defined criteria. It would be potentially interesting to use the assay we established in this study to screen compounds affecting relevant pathways in cell lines expressing different monogenic defects of SRNS.
Furthermore, the drug library obtained may not have been targeting the right pathways for finding compounds that affect nephrotic syndrome. It has been demonstrated that small Rho-like GTPases alter PMR and that their function is altered in nephrotic syndrome (39–41) and we demonstrated an effect of Rho and Rac1 activators/inhibitors on PMR (Fig. 3). The NIHCC small molecule library was curated due to its clinical availability for resupply and not for a specific pathway such as small Rho-like GTPases; hence, we expected a low hit rate in the screen. Future studies would be strengthened if specific libraries were used to target nephrotic syndrome relevant pathways in the podocyte such as those that regulate the small Rho-like GTPases RhoA/Rac1/Cdc42.
Future directions.
Confirmed hits in our screen are reliable and reproducible, and the results can be further applied to screen for pathway specific therapies of nephrotic syndrome. In establishing the scratch-wound assay as a relevant screening method for treatment of nephrotic syndrome, several potential applications are apparent. One application includes the study of pathway specific defects in nephr otic syndrome by using targeted small molecule libraries rather than large nonspecific small molecule screens. Another possibility includes screening cell lines with defects in monogenic causes of nephrotic syndrome. In the era of personalized medicine, this assay could potentially be used in conjunction with CRISPR/Cas9 technology to generate cell culture lines with genes that have allele-specific loss of function to screen for therapeutics. In summary, in this study, we have established a high-throughput assay that uses PMR and live cell videomicroscopy to identify modifiers of microtubule dynamics as a potential pathway for treatment in nephrotic syndrome. This assay also opens a new avenue for growth in research of drug discovery in kidney disease.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-076683 (to F. Hildebrandt). W. Tan is supported by NIH T32 Training Grant T32-DK-007726–31A1. E. Widmeier is supported by the German National Academy of Sciences Leopoldina (LPDS-2015–07).
DISCLOSURES
F. Hildebrandt receives royalties from CLARITAS.
AUTHOR CONTRIBUTIONS
E.W., W.T., M.A., and F.H. conception and design of research; E.W. and W.T. performed experiments; E.W. and W.T. analyzed data; E.W., W.T., and F.H. interpreted results of experiments; E.W. and W.T. prepared figures; E.W. and W.T. drafted manuscript; E.W., W.T., M.A., and F.H. edited and revised manuscript; E.W., W.T., M.A., and F.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Saleem for the immortalized human podocyte cell line and the NIHSMR for providing the NIHCC stock for our small molecule screen. F. Hildebrandt is the Warren E. Grupe Professor of Pediatrics.
REFERENCES
- 1.Aizman O, Uhlén P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci USA 98: 13420–13424, 2001. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201–1204, 2004. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez J, Montero M, Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem 267: 11789–11793, 1992. [PubMed] [Google Scholar]
- 4.Anderson-Beck R, Wilson L, Brazier S, Hughes IE, Peers C. Doxapram stimulates dopamine release from the intact rat carotid body in vitro. Neurosci Lett 187: 25–28, 1995. doi: 10.1016/0304-3940(95)11328-T. [DOI] [PubMed] [Google Scholar]
- 5.Andrews PM. The effect of vinblastine-induced microtubule loss on kidney podocyte morphology. Am J Anat 150: 53–61, 1977. doi: 10.1002/aja.1001500104. [DOI] [PubMed] [Google Scholar]
- 6.Anstey A, Lear JT. Azathioprine: clinical pharmacology and current indications in autoimmune disorders. BioDrugs 9: 33–47, 1998. doi: 10.2165/00063030-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Aramwit P, Kobpipat N, Satirapoj B, Kopple JD, Supasyndh O. Oxymetholone ameliorates insulin sensitivity in maintenance hemodialysis patients: a randomized controlled trial. Clin Nephrol 71: 413–422, 2009. doi: 10.5414/CNP71413. [DOI] [PubMed] [Google Scholar]
- 8.Aroeira RI, Ribeiro JA, Sebastião AM, Valente CA. Age-related changes of glycine receptor at the rat hippocampus: from the embryo to the adult. J Neurochem 118: 339–353, 2011. doi: 10.1111/j.1471-4159.2011.07197.x. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf S, Gee HY, Woerner S, Xie LX, Vega-Warner V, Lovric S, Fang H, Song X, Cattran DC, Avila-Casado C, Paterson AD, Nitschké P, Bole-Feysot C, Cochat P, Esteve-Rudd J, Haberberger B, Allen SJ, Zhou W, Airik R, Otto EA, Barua M, Al-Hamed MH, Kari JA, Evans J, Bierzynska A, Saleem MA, Böckenhauer D, Kleta R, El Desoky S, Hacihamdioglu DO, Gok F, Washburn J, Wiggins RC, Choi M, Lifton RP, Levy S, Han Z, Salviati L, Prokisch H, Williams DS, Pollak M, Clarke CF, Pei Y, Antignac C, Hildebrandt F. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest 123: 5179–5189, 2013. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubel-Sadron G, Londos-Gagliardi D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie 66: 333–352, 1984. doi: 10.1016/0300-9084(84)90018-X. [DOI] [PubMed] [Google Scholar]
- 11.Bachy A, Héaulme M, Giudice A, Michaud JC, Lefevre IA, Souilhac J, Manara L, Emerit MB, Gozlan H, Hamon M, Keane PE, Soubrié P, Le Fur G Sr. SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur J Pharmacol 237: 299–309, 1993. doi: 10.1016/0014-2999(93)90282-M. [DOI] [PubMed] [Google Scholar]
- 12.Bai S, Hu Z, Yang Y, Yin Y, Li W, Wu L, Fang M. Anti-Inflammatory and Neuroprotective Effects of Triptolide via the NF-κB Signaling Pathway in a Rat MCAO Model. Anat Rec (Hoboken) 299: 256–266, 2016. doi: 10.1002/ar.23293. [DOI] [PubMed] [Google Scholar]
- 13.Ballard TE, Wang X, Olekhnovich I, Koerner T, Seymour C, Hoffman PS, Macdonald TL. Biological activity of modified and exchanged 2-amino-5-nitrothiazole amide analogues of nitazoxanide. Bioorg Med Chem Lett 20: 3537–3539, 2010. doi: 10.1016/j.bmcl.2010.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baroody FM, Naclerio RM. Antiallergic effects of H1-receptor antagonists. Allergy 55, Suppl 64: 17–27, 2000. doi: 10.1034/j.1398-9995.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M. Tegaserod. Aliment Pharmacol Ther 15: 277–289, 2001. doi: 10.1046/j.1365-2036.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Huang X, Fan Y, Chen X. Protective Effect of Triptolide against Glomerular Mesangial Cell Proliferation and Glomerular Fibrosis in Rats Involves the TGF- β 1/Smad Signaling Pathway. Evid Based Complement Alternat Med 2015: 814089, 2015. doi: 10.1155/2015/814089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carty TJ, Marfat A, Moore PF, Falkner FC, Twomey TM, Weissman A. Ampiroxicam, an anti-inflammatory agent which is a prodrug of piroxicam. Agents Actions 39: 157–165, 1993. doi: 10.1007/BF01998969. [DOI] [PubMed] [Google Scholar]
- 18.Chabot GG. Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet 33: 245–259, 1997. doi: 10.2165/00003088-199733040-00001. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6: 797–801, 2000. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Okabe A, Sun H, Sharopov S, Hanganu-Opatz IL, Kolbaev SN, Fukuda A, Luhmann HJ, Kilb W. Activation of glycine receptors modulates spontaneous epileptiform activity in the immature rat hippocampus. J Physiol 592: 2153–2168, 2014. doi: 10.1113/jphysiol.2014.271700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Ji ZL, Chen YZ. TTD: Therapeutic Target Database. Nucleic Acids Res 30: 412–415, 2002. doi: 10.1093/nar/30.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi S, Haggart D, Toll L, Cuny GD. Synthesis, receptor binding and functional studies of mesoridazine stereoisomers. Bioorg Med Chem Lett 14: 4379–4382, 2004. doi: 10.1016/j.bmcl.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 23.Chu SW, Badar S, Morris DL, Pourgholami MH. Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole. Anticancer Res 29: 3791–3796, 2009. [PubMed] [Google Scholar]
- 24.Cihák A. Biological effects of 5-azacytidine in eukaryotes. Oncology 30: 405–422, 1974. doi: 10.1159/000224981. [DOI] [PubMed] [Google Scholar]
- 25.Colin E, Huynh Cong E, Mollet G, Guichet A, Gribouval O, Arrondel C, Boyer O, Daniel L, Gubler MC, Ekinci Z, Tsimaratos M, Chabrol B, Boddaert N, Verloes A, Chevrollier A, Gueguen N, Desquiret-Dumas V, Ferré M, Procaccio V, Richard L, Funalot B, Moncla A, Bonneau D, Antignac C. Loss-of-function mutations in WDR73 are responsible for microcephaly and steroid-resistant nephrotic syndrome: Galloway-Mowat syndrome. Am J Hum Genet 95: 637–648, 2014. doi: 10.1016/j.ajhg.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59, Suppl 1: A7, 2012. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 28.Dandache S, Coburn CA, Oliveira M, Allison TJ, Holloway MK, Wu JJ, Stranix BR, Panchal C, Wainberg MA, Vacca JP. PL-100, a novel HIV-1 protease inhibitor displaying a high genetic barrier to resistance: an in vitro selection study. J Med Virol 80: 2053–2063, 2008. doi: 10.1002/jmv.21329. [DOI] [PubMed] [Google Scholar]
- 29.Diaz LL, Zhang J, Heerdt PM. Comparative pharmacodynamics of pancuronium, cisatracurium, and CW002 in rabbits. J Am Assoc Lab Anim Sci 53: 283–289, 2014. [PMC free article] [PubMed] [Google Scholar]
- 30.Dietz JD, Du S, Bolten CW, Payne MA, Xia C, Blinn JR, Funder JW, Hu X. A number of marketed dihydropyridine calcium channel blockers have mineralocorticoid receptor antagonist activity. Hypertension 51: 742–748, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103580. [DOI] [PubMed] [Google Scholar]
- 31.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience 121: 619–628, 2003. doi: 10.1016/S0306-4522(03)00558-X. [DOI] [PubMed] [Google Scholar]
- 32.Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol 2: 731–743, 2004. doi: 10.1016/S1542-3565(04)00344-1. [DOI] [PubMed] [Google Scholar]
- 33.Falkner FC, Twomey TM, Borgers AP, Garg D, Weidler D, Gerber N, Browder IW. Disposition of ampiroxicam, a prodrug of piroxicam, in man. Xenobiotica 20: 645–652, 1990. doi: 10.3109/00498259009046880. [DOI] [PubMed] [Google Scholar]
- 34.Farías JG, Zepeda AB, Calaf GM. Melatonin protects the heart, lungs and kidneys from oxidative stress under intermittent hypobaric hypoxia in rats. Biol Res 45: 81–85, 2012. doi: 10.4067/S0716-97602012000100011. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa T, Yamakawa T, Midera T, Sagawa T, Mori Y, Nukada T. Selectivities of dihydropyridine derivatives in blocking Ca(2+) channel subtypes expressed in Xenopus oocytes. J Pharmacol Exp Ther 291: 464–473, 1999. [PubMed] [Google Scholar]
- 36.Furutani K, Yamakawa Y, Inanobe A, Iwata M, Ohno Y, Kurachi Y. A mechanism underlying compound-induced voltage shift in the current activation of hERG by antiarrhythmic agents. Biochem Biophys Res Commun 415: 141–146, 2011. doi: 10.1016/j.bbrc.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O’neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gee HY, Ashraf S, Wan X, Vega-Warner V, Esteve-Rudd J, Lovric S, Fang H, Hurd TW, Sadowski CE, Allen SJ, Otto EA, Korkmaz E, Washburn J, Levy S, Williams DS, Bakkaloglu SA, Zolotnitskaya A, Ozaltin F, Zhou W, Hildebrandt F. Mutations in EMP2 cause childhood-onset nephrotic syndrome. Am J Hum Genet 94: 884–890, 2014. doi: 10.1016/j.ajhg.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gee HY, Sadowski CE, Aggarwal PK, Porath JD, Yakulov TA, Schueler M, Lovric S, Ashraf S, Braun DA, Halbritter J, Fang H, Airik R, Vega-Warner V, Cho KJ, Chan TA, Morris LG, ffrench-Constant C, Allen N, McNeill H, Büscher R, Kyrieleis H, Wallot M, Gaspert A, Kistler T, Milford DV, Saleem MA, Keng WT, Alexander SI, Valentini RP, Licht C, Teh JC, Bogdanovic R, Koziell A, Bierzynska A, Soliman NA, Otto EA, Lifton RP, Holzman LB, Sibinga NE, Walz G, Tufro A, Hildebrandt F. FAT1 mutations cause a glomerulotubular nephropathy. Nat Commun 7: 10822, 2016. doi: 10.1038/ncomms10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, Beck BB, Gribouval O, Zhou W, Diaz KA, Natarajan S, Wiggins RC, Lovric S, Chernin G, Schoeb DS, Ovunc B, Frishberg Y, Soliman NA, Fathy HM, Goebel H, Hoefele J, Weber LT, Innis JW, Faul C, Han Z, Washburn J, Antignac C, Levy S, Otto EA, Hildebrandt F. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest 123: 3243–3253, 2013. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125: 2375–2384, 2015. doi: 10.1172/JCI79504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmore DL, Liu Y, Matsumoto RR. Review of the pharmacological and clinical profile of rimcazole. CNS Drug Rev 10: 1–22, 2004. doi: 10.1111/j.1527-3458.2004.tb00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol 12: 63–71, 2000. doi: 10.1016/S0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 44.Hajihassan Z, Rabbani-Chadegani A. Studies on the binding affinity of anticancer drug mitoxantrone to chromatin, DNA and histone proteins. J Biomed Sci 16: 31, 2009. doi: 10.1186/1423-0127-16-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hara H, Toriu N, Shimazawa M. Clinical potential of lomerizine, a Ca2+ channel blocker as an anti-glaucoma drug: effects on ocular circulation and retinal neuronal damage. Cardiovasc Drug Rev 22: 199–214, 2004. doi: 10.1111/j.1527-3466.2004.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 46.He L, Peng X, Liu G, Tang C, Liu H, Liu F, Zhou H, Peng Y. Anti-inflammatory effects of triptolide on IgA nephropathy in rats. Immunopharmacol Immunotoxicol 37: 421–427, 2015. doi: 10.3109/08923973.2015.1080265. [DOI] [PubMed] [Google Scholar]
- 47.Heo JC, Jung TH, Jung DY, Park WK, Cho H. Indatraline inhibits Rho- and calcium-mediated glioblastoma cell motility and angiogenesis. Biochem Biophys Res Commun 443: 749–755, 2014. doi: 10.1016/j.bbrc.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 48.Höcherl K, Kammerl MC, Schumacher K, Endemann D, Grobecker HF, Kurtz A. Role of prostanoids in regulation of the renin-angiotensin-aldosterone system by salt intake. Am J Physiol Renal Physiol 283: F294–F301, 2002. doi: 10.1152/ajprenal.00347.2001. [DOI] [PubMed] [Google Scholar]
- 49.Hoffma n PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51: 868–876, 2007. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosaka Y, Iwata M, Kamiya N, Yamada M, Kinoshita K, Fukunishi Y, Tsujimae K, Hibino H, Aizawa Y, Inanobe A, Nakamura H, Kurachi Y. Mutational analysis of block and facilitation of HERG current by a class III anti-arrhythmic agent, nifekalant. Channels (Austin) 1: 198–208, 2007. doi: 10.4161/chan.4691. [DOI] [PubMed] [Google Scholar]
- 51.Hulkower KI, Herber RL. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics 3: 107–124, 2011. doi: 10.3390/pharmaceutics3010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutterer C, Niemann I, Milbradt J, Fröhlich T, Reiter C, Kadioglu O, Bahsi H, Zeitträger I, Wagner S, Einsiedel J, Gmeiner P, Vogel N, Wandinger S, Godl K, Stamminger T, Efferth T, Tsogoeva SB, Marschall M. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa B (NF-κB) pathway by targeting RelA/p65. Antiviral Res 124: 101–109, 2015. doi: 10.1016/j.antiviral.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Huynh Cong E, Bizet AA, Boyer O, Woerner S, Gribouval O, Filhol E, Arrondel C, Thomas S, Silbermann F, Canaud G, Hachicha J, Ben Dhia N, Peraldi MN, Harzallah K, Iftene D, Daniel L, Willems M, Noel LH, Bole-Feysot C, Nitschké P, Gubler MC, Mollet G, Saunier S, Antignac C. A homozygous missense mutation in the ciliary gene TTC21B causes familial FSGS. J Am Soc Nephrol 25: 2435–2443, 2014. doi: 10.1681/ASN.2013101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iida A, Kano M, Kubota Y, Koga K, Tomioka K. Podophyllotoxin aza-analogue, a novel DNA topoisomerase II inhibitor. Chem Pharm Bull (Tokyo) 48: 486–489, 2000. doi: 10.1248/cpb.48.486. [DOI] [PubMed] [Google Scholar]
- 55.Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov 5: 821–834, 2006. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- 56.Inotsume N, Nakano M. Reversible ring-opening reactions of nimetazepam and nitrazepam in acidic media at body temperature. J Pharm Sci 69: 1331–1334, 1980. doi: 10.1002/jps.2600691124. [DOI] [PubMed] [Google Scholar]
- 57.Johnson IS, Armstrong JG, Gorman M, Burnett JP Jr. The Vinca Alkaloids: A New Class of Oncolytic Agents. Cancer Res 23: 1390–1427, 1963. [PubMed] [Google Scholar]
- 58.Kim JH, Konieczkowski M, Mukherjee A, Schechtman S, Khan S, Schelling JR, Ross MD, Bruggeman LA, Sedor JR. Podocyte injury induces nuclear translocation of WTIP via microtubule-dependent transport. J Biol Chem 285: 9995–10004, 2010. doi: 10.1074/jbc.M109.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim ND, Park ES, Kim YH, Moon SK, Lee SS, Ahn SK, Yu DY, No KT, Kim KH. Structure-based virtual screening of novel tubulin inhibitors and their characterization as anti-mitotic agents. Bioorg Med Chem 18: 7092–7100, 2010. doi: 10.1016/j.bmc.2010.07.072. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi N, Heid HW, Sakai T, Kriz W, Huber G, Mundel P. Molecular characterization reveals identity of microtubule-associated proteins MAP3 and MAP4. Biochem Biophys Res Commun 268: 306–309, 2000. doi: 10.1006/bbrc.2000.2126. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi N, Mundel P. A role of microtubules during the formation of cell processes in neuronal and non-neuronal cells. Cell Tissue Res 291: 163–174, 1998. doi: 10.1007/s004410050988. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi N, Reiser J, Kriz W, Kuriyama R, Mundel P. Nonuniform microtubular polarity established by CHO1/MKLP1 motor protein is necessary for process formation of podocytes. J Cell Biol 143: 1961–1970, 1998. doi: 10.1083/jcb.143.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi N, Reiser J, Schwarz K, Sakai T, Kriz W, Mundel P. Process formation of podocytes: morphogenetic activity of microtubules and regulation by protein serine/threonine phosphatase PP2A. Histochem Cell Biol 115: 255–266, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C. Topotecan - A novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology 56: 1–12, 1999. doi: 10.1159/000011923. [DOI] [PubMed] [Google Scholar]
- 65.Kubota H, Alle H, Betz H, Geiger JR. Presynaptic glycine receptors on hippocampal mossy fibers. Biochem Biophys Res Commun 393: 587–591, 2010. doi: 10.1016/j.bbrc.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 66.Kurebayashi Y, Ikeda T, Osada Y. Cytoprotective action of cetraxate against HCl ethanol-induced gastric lesion in rats. Jpn J Pharmacol 46: 17–25, 1988. doi: 10.1254/jjp.46.17. [DOI] [PubMed] [Google Scholar]
- 67.Lamers JM, Cysouw KJ, Verdouw PD. Slow calcium channel blockers and calmodulin. Effect of felodipine, nifedipine, prenylamine and bepridil on cardiac sarcolemmal calcium pumping ATPase. Biochem Pharmacol 34: 3837–3843, 1985. doi: 10.1016/0006-2952(85)90432-0. [DOI] [PubMed] [Google Scholar]
- 68.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, Elshabrawy HA, Mangos S, Quick KL, Sever S, Reiser J, Gupta V. A Podocyte-Based Automated Screening Assay Identifies Protective Small Molecules. J Am Soc Nephrol 26: 2741–2752, 2015. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levitt DG, Schoemaker RC. Human physiologically based pharmacokinetic model for ACE inhibitors: ramipril and ramiprilat. BMC Clin Pharmacol 6: 1, 2006. doi: 10.1186/1472-6904-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lounis N, Roscigno G. In vitro and in vivo activities of new rifamycin derivatives against mycobacterial infections. Curr Pharm Des 10: 3229–3238, 2004. doi: 10.2174/1381612043383287. [DOI] [PubMed] [Google Scholar]
- 71.Lovric S, Ashraf S, Tan W, Hildebrandt F. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant 31: 1802–1813, 2016. doi: 10.1093/ndt/gfv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald LM, Armson A, Thompson AR, Reynoldson JA. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol Biochem Parasitol 138: 89–96, 2004. doi: 10.1016/j.molbiopara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Marek GJ, McDougle CJ, Price LH, Seiden LS. A comparison of trazodone and fluoxetine: implications for a serotonergic mechanism of antidepressant action. Psychopharmacology (Berl) 109: 2–11, 1992. doi: 10.1007/BF02245475. [DOI] [PubMed] [Google Scholar]
- 74.Martin GR. Pre-clinical pharmacology of zolmitriptan (Zomig; formerly 311C90), a centrally and peripherally acting 5HT1B/1D agonist for migraine. Cephalalgia 17, Suppl 18: 4–14, 1997. [DOI] [PubMed] [Google Scholar]
- 75.McLamore S, Ullrich T, Rothman RB, Xu H, Dersch C, Coop A, Davis P, Porreca F, Jacobson AE, Rice KC. Effect of N-alkyl and N-alkenyl substituents in noroxymorphindole, 17-substituted-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy-6,7:2′,3′-indolomorphinans, on opioid receptor affinity, selectivity, and efficacy. J Med Chem 44: 1471–1474, 2001. doi: 10.1021/jm000511w. [DOI] [PubMed] [Google Scholar]
- 76.Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300: 2–8, 2002. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 77.Morel N, Buryi V, Feron O, Gomez JP, Christen MO, Godfraind T. The action of calcium channel blockers on recombinant L-type calcium channel alpha1-subunits. Br J Pharmacol 125: 1005–1012, 1998. doi: 10.1038/sj.bjp.0702162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mouawad F, Aoudjit L, Jiang R, Szaszi K, Takano T. Role of guanine nucleotide exchange factor-H1 in complement-mediated RhoA activation in glomerular epithelial cells. J Biol Chem 289: 4206–4218, 2014. doi: 10.1074/jbc.M113.506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy MJ., Jr Molecular Action and Clinical Relevance of Aromatase Inhibitors. Oncologist 3: 129–130, 1998. [PubMed] [Google Scholar]
- 80.National Toxicology Program NTP Toxicology and Carcinogenesis Studies of Oxymetholone (CAS NO. 434-07-1) in F344/N Rats and Toxicology Studies of Oxymetholone in B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser 485: 1–233, 1999. [PubMed] [Google Scholar]
- 81.Neu HC, Fu KP. Cefatrizine activity compared with that of other cephalosporins. Antimicrob Agents Chemother 15: 209–212, 1979. doi: 10.1128/AAC.15.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishibayashi S, Ogawa N, Asanuma M, Kondo Y, Mori A. Tubulin and actin mRNAs in the young-adult and the aged rat brain: effects of repeated administration with bifemelane hydrochloride. Arch Gerontol Geriatr 19: 265–272, 1994. doi: 10.1016/0167-4943(94)00572-9. [DOI] [PubMed] [Google Scholar]
- 83.O’Dwyer K, Maslak P. Azacitidine and the beginnings of therapeutic epigenetic modulation. Expert Opin Pharmacother 9: 1981–1986, 2008. doi: 10.1517/14656566.9.11.1981. [DOI] [PubMed] [Google Scholar]
- 84.Obniska J, Kamiński K. Synthesis and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane, 2-azaspiro[4.5]decane-1,3-dione and 3-cyclohexylpyrrolidine-2,5-dione. Part IV. Acta Pol Pharm 63: 101–108, 2006. [PubMed] [Google Scholar]
- 85.Ochola DO, Prichard RK, Lubega GW. Classical ligands bind tubulin of trypanosomes and inhibit their growth in vitro. J Parasitol 88: 600–604, 2002. doi: 10.1645/0022-3395(2002)088[0600:CLBTOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 86.Ogawa N, Mizukawa K, Haba K, Asanuma M, Mori A. Chronic bifemelane hydrochloride administration enhances muscarinic cholinergic receptor binding in the senescent rat brain. J Med 22: 17–27, 1991. [PubMed] [Google Scholar]
- 87.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov 5: 993–996, 2006. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 88.Pal R, Mamidi MK, Das AK, Bhonde R. Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells. Arch Toxicol 86: 651–661, 2012. doi: 10.1007/s00204-011-0782-2. [DOI] [PubMed] [Google Scholar]
- 89.Pascual J. [Mechanism of action of zolmitriptan]. Neurologia 13, Suppl 2: 9–15, 1998. [PubMed] [Google Scholar]
- 90.Peers C. Effects of doxapram on ionic currents recorded in isolated type I cells of the neonatal rat carotid body. Brain Res 568: 116–122, 1991. doi: 10.1016/0006-8993(91)91386-F. [DOI] [PubMed] [Google Scholar]
- 91.Qiu R, Melmon KL, Khan MM. Effects of histamine-trifluoromethyl-toluidide derivative (HTMT) on intracellular calcium in human lymphocytes. J Pharmacol Exp Ther 253: 1245–1252, 1990. [PubMed] [Google Scholar]
- 92.Ramírez T, Benítez-Bribiesca L, Ostrosky-Wegman P, Herrera LA. In vitro effects of albendazole and its metabolites on the cell proliferation kinetics and micronuclei frequency of stimulated human lymphocytes. Arch Med Res 32: 119–122, 2001. doi: 10.1016/S0188-4409(01)00259-4. [DOI] [PubMed] [Google Scholar]
- 93.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832, 2004. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 94.Ren Y, Liu T, Song G, Hu Y, Liang J. Determination of lomerizine in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 947-948: 96–102, 2014. doi: 10.1016/j.jchromb.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 95.Rocchetti R, Talevi S, Margiotta C, Calza R, Corallini A, Possati L. Antiangiogenic drugs for chemotherapy of bladder tumours. Chemotherapy 51: 291–299, 2005. doi: 10.1159/000088950. [DOI] [PubMed] [Google Scholar]
- 96.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002. [DOI] [PubMed] [Google Scholar]
- 97.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev 30: 343–375, 2009. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 98.Sarna MA, Hollenberg NK, Seely EW, Ahmed SB. Oral contraceptive progestins and angiotensin-dependent control of the renal circulation in humans. J Hum Hypertens 23: 407–414, 2009. doi: 10.1038/jhh.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmidt F, Rieger J, Wischhusen J, Naumann U, Weller M. Glioma cell sensitivity to topotecan: the role of p53 and topotecan-induced DNA damage. Eur J Pharmacol 412: 21–25, 2001. doi: 10.1016/S0014-2999(00)00923-7. [DOI] [PubMed] [Google Scholar]
- 100.Sharma RK, Wang JH, Wu Z. Mechanisms of inhibition of calmodulin-stimulated cyclic nucleotide phosphodiesterase by dihydropyridine calcium antagonists. J Neurochem 69: 845–850, 1997. doi: 10.1046/j.1471-4159.1997.69020845.x. [DOI] [PubMed] [Google Scholar]
- 101.Shaye DD, Greenwald I. The disease-associated formin INF2/EXC-6 organizes lumen and cell outgrowth during tubulogenesis by regulating F-actin and microtubule cytoskeletons. Dev Cell 32: 743–755, 2015. doi: 10.1016/j.devcel.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 102.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 103.Skerritt JH, Johnston GA. Enhancement of GABA binding by benzodiazepines and related anxiolytics. Eur J Pharmacol 89: 193–198, 1983. doi: 10.1016/0014-2999(83)90494-6. [DOI] [PubMed] [Google Scholar]
- 104.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 11: 366–373, 2007. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 105.Solana HD, Sallovitz JM, Lanusse CE, Rodriguez JA. Enantioselective binding of albendazole sulphoxide to cytosolic proteins from helminth parasites. Methods Find Exp Clin Pharmacol 24: 7–13, 2002. doi: 10.1358/mf.2002.24.1.677121. [DOI] [PubMed] [Google Scholar]
- 106.Spedding M, Gittos M, Mir AK. Calcium antagonist properties of diclofurime isomers. I. Functional aspects. J Cardiovasc Pharmacol 9: 461–468, 1987. doi: 10.1097/00005344-198704000-00011. [DOI] [PubMed] [Google Scholar]
- 107.Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev 31: 443–481, 2011. doi: 10.1002/med.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107a.Striessnig J. Ca 2+ channel blockers. In Encyclopedic reference of molecular pharmacology, (ed. by Offermanns S and Rosenthal W). Berlin, Germany: Springer, 2004, p. 201–207. [Google Scholar]
- 108.Su Z, Martin R, Cox BF, Gintant G. Mesoridazine: an open-channel blocker of human ether-a-go-go-related gene K+ channel. J Mol Cell Cardiol 36: 151–160, 2004. doi: 10.1016/j.yjmcc.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 109.Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288: F91–F97, 2005. doi: 10.1152/ajprenal.00051.2004. [DOI] [PubMed] [Google Scholar]
- 110.Trincavelli ML, Daniele S, Martini C. Adenosine receptors: what we know and what we are learning. Curr Top Med Chem 10: 860–877, 2010. doi: 10.2174/156802610791268756. [DOI] [PubMed] [Google Scholar]
- 111.Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol 24: 625–640, 1970. [PubMed] [Google Scholar]
- 112.Vasiliev JM, Gelfand IM, Domnina LV, Rappoport RI. Wound healing processes in cell cultures. Exp Cell Res 54: 83–93, 1969. doi: 10.1016/0014-4827(69)90296-1. [DOI] [PubMed] [Google Scholar]
- 113.Vasiliev JM, Samoylov VI. Regulatory functions of microtubules. Biochemistry (Mosc) 78: 37–40, 2013. doi: 10.1134/S0006297913010045. [DOI] [PubMed] [Google Scholar]
- 114.Verheul HA, Schot LP, Deckers GH, Schuurs AH. Effects of tibolone, lynestrenol, ethylestrenol, and desogestrel on autoimmune disorders in NZB/W mice. Clin Immunol Immunopathol 38: 198–208, 1986. doi: 10.1016/0090-1229(86)90138-8. [DOI] [PubMed] [Google Scholar]
- 115.Vilums M, Heuberger J, Heitman LH, IJzerman AP. Indanes–Properties, Preparation, and Presence in Ligands for G Protein Coupled Receptors. Med Res Rev 35: 1097–1126, 2015. doi: 10.1002/med.21352. [DOI] [PubMed] [Google Scholar]
- 116.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11: 382–402, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winkelmann A, Maggio N, Eller J, Caliskan G, Semtner M, Häussler U, Jüttner R, Dugladze T, Smolinsky B, Kowalczyk S, Chronowska E, Schwarz G, Rathjen FG, Rechavi G, Haas CA, Kulik A, Gloveli T, Heinemann U, Meier JC. Changes in neural network homeostasis trigger neuropsychiatric symptoms. J Clin Invest 124: 696–711, 2014. doi: 10.1172/JCI71472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res 95: 364–371, 2004. doi: 10.1161/01.RES.0000138581.04174.2f. [DOI] [PubMed] [Google Scholar]
- 119.Yeh SY. Metabolic profile of tripelennamine in humans. J Pharm Sci 80: 815–819, 1991. doi: 10.1002/jps.2600800902. [DOI] [PubMed] [Google Scholar]
- 120.Yost CS. A new look at the respiratory stimulant doxapram. CNS Drug Rev 12: 236–249, 2006. doi: 10.1111/j.1527-3458.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol 23: 1039–1047, 2012. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zock JM. Applications of high content screening in life science research. Comb Chem High Throughput Screen 12: 870–876, 2009. doi: 10.2174/138620709789383277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zunino F, Capranico G. DNA topoisomerase II as the primary target of anti-tumor anthracyclines. Anticancer Drug Des 5: 307–317, 1990. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


