Abstract
Traumatic brain injury (TBI) is a major health problem worldwide. In addition to its high mortality (35–40%), survivors are left with cognitive, behavioral, and communicative disabilities. While little can be done to reverse initial primary brain damage caused by trauma, the secondary injury of cerebral tissue due to cerebromicrovascular alterations and dysregulation of cerebral blood flow (CBF) is potentially preventable. This review focuses on functional, cellular, and molecular changes of autoregulatory function of CBF (with special focus on cerebrovascular myogenic response) that occur in cerebral circulation after TBI and explores the links between autoregulatory dysfunction, impaired myogenic response, microvascular impairment, and the development of secondary brain damage. We further provide a synthesized translational view of molecular and cellular mechanisms involved in cortical spreading depolarization-related neurovascular dysfunction, which could be targeted for the prevention or amelioration of TBI-induced secondary brain damage.
Keywords: autoregulation, neurovascular coupling, brain damage, myogenic, cerebral blood flow
traumatic brain injury (TBI) occurs when the head is hit directly or indirectly by an object (e.g., during a fall or a traffic accident) or by blast waves, or when an object (e.g., a projectile) pierces the skull and enters the brain parenchyma. Each year ∼1.7 million people in the United States (81, 123, 136) and another 2.5 million patients in the European Union (136) suffer TBI. In addition to a high mortality rate (35–40%), survivors of severe TBI and patients suffering mild but repetitive trauma are left with significant cognitive, behavioral, and communicative disabilities imparting an even larger burden to the health care systems (123) and the families of these victims. Epidemiological studies show that ∼5.3 million people live with TBI-related disabilities in the United States (81) and 7.7 million in the European Union (136). Pediatric and elderly populations are the most vulnerable, and specific subpopulations (e.g., military workers, athletes, such as boxers, football and hockey players, jumpers, skaters) are frequently exposed to repetitive head trauma (24). TBI can be mild, moderate, or severe defined by clinical appearance (decrease or loss of consciousness, loss of memory before or after an event, neurological deficit, alteration in mental state at the time of the injury), imaging findings, and various biomarkers (99).
While little can be done to reverse the initial primary brain damage caused by trauma, the secondary brain injury (in part) due to vascular/microvascular alterations, and dysregulation of cerebral blood flow (CBF) initiated by TBI is potentially preventable. TBI affects practically all tissue and cell types directly or indirectly involved in the regulation of CBF [endothelial cells, astrocytes, pericytes, the blood-brain barrier (BBB), structure of the vascular wall and perivascular innervation, onset of microhemorrhages, etc.], leading to traumatic cerebrovascular injury (73, 76). A detailed description of these divergent cerebrovascular consequences of TBI is beyond the scope of the present discussion. In this review the effect of TBI is considered for two unique mechanisms of regulation of CBF: 1) autoregulation of CBF and 2) neurovascular coupling in terms of potential mechanisms and pathophysiological consequences. The possible benefits of emerging therapeutic strategies that have the potential to restore vascular and microvascular function and prevent secondary brain ischemia are also briefly discussed.
IMPAIRED AUTOREGULATION OF CBF AFTER TBI
Autoregulation of CBF and Pathophysiological Consequences of Autoregulatory Dysfunction
The regulation of cerebral circulation has to comply with special requirements. First, cerebral tissue is very sensitive to hypoxia/ischemia and therefore requires a stable and continuous supply of nutrients and oxygen for normal neuronal function. Second, because the brain is enclosed in the cranium, uncontrolled vasodilation/engorgement would lead to pressure and volume overload of the circulation and an increases in intracranial pressure (ICP). Among others, the two main regulatory mechanisms responsible for meeting these requirements are: 1) pressure-induced vasomotor autoregulation preventing free transmission of changes in systemic blood pressure to changes in CBF and 2) neurovascular coupling adjusting CBF to the metabolic needs of active neuronal/glial tissues.
CBF has to be relatively constant to provide a continuous and stable blood flow to brain tissue despite changes in systemic blood pressure and thus cerebral perfusion pressure (CPP; = systemic blood pressure − ICP), yet allowing heterogeneity in CBF distribution and local functional hyperemia according to the increased neural/glial function. The mechanism fulfilling these requirements is called autoregulation of CBF. Autoregulation of CBF is the integration of myogenic (pressure-induced), metabolic, and sympathetic mechanisms (details provided in Mechanisms of Autoregulatory Dysfunction after TBI) (78, 82). These mechanisms adjust the diameter of cerebral resistance vessels and thus cerebrovascular resistance (CVR) to the changes of perfusion pressure: they increase CVR in case of increasing perfusion pressure and decrease it when blood pressure drops. In other words, cerebral autoregulation is a negative feedback process maintaining stable and constant blood flow when perfusion pressure changes: 1) in hypotension an intact autoregulation prevents hypoperfusion and ischemia of cerebral tissue; and 2) in hypertension it protects the cerebral microvascular bed against hyperemia and hypervolemia (Fig. 1). Mechanisms of CBF autoregulation include static and dynamic components. Static autoregulation of CBF adjusts vascular resistance (thus blood flow) to a steady-state perfusion pressure value, and it dictates how large changes in perfusion pressure can be compensated. Dynamic cerebral autoregulation restores CBF after rapid transient changes in perfusion pressure and thus determines how fast the autoregulatory compensation can be implemented. Dynamic and static cerebral autoregulation act on a continuum to maintain CBF when blood pressure changes. Regarding methods to measure autoregulation we refer to other detailed reviews and classical studies of the field (1, 83–85, 113, 129, 143, 151).
Fig. 1.
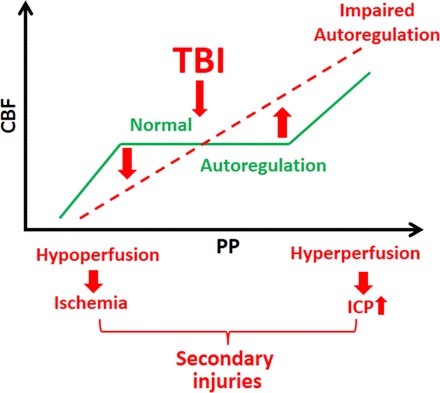
Scheme illustrating the consequences of autoregulatory dysfunction after traumatic brain injury (TBI). When autoregulatory function is intact (green line) despite changes of perfusion pressure [PP: systemic blood pressure − intracranial pressure (IP)] cerebral blood flow (CBF) is maintained at a near constant level. (Please note that the transient drop in basal CBF following TBI is not depicted.) The proposed model predicts that, following TBI, autoregulation of CBF is compromised; thus, CBF passively changes as a function of PP. When PP decreases, autoregulatory dysfunction results in significant hypoperfusion and cerebral ischemia. In contrast, when PP increases, autoregulatory dysfunction results in cerebral hyperperfusion, leading to increased intracranial pressure. These mechanisms lead to the formation of cytotoxic and vasogenic edema. The resulting decline in oxygen and nutrient supply contributes to secondary injury of cerebral tissue, increasing mortality and compromising functional recovery after TBI.
As mentioned above, when autoregulatory mechanisms, due to their impaired capacity to decrease cerebrovascular resistance, cannot maintain approximately unchanged cerebral perfusion when blood pressure decreases, then autoregulatory dysfunction leads to ischemia of cerebral tissue. On the contrary, at high pressure values, the significance of autoregulatory mechanisms responsible for maintaining constant blood flow can be appreciated when taking into account the anatomical fact that the brain is situated in the closed cranium consisting of three main volume compartments: cerebral tissue, cerebrospinal fluid, and intravascular blood. Volume expansion of one of the compartments can only be compensated by a decrease in the others, as stated in the Kellie-Monroe doctrine and its modified versions (101). Thus, in case of low intracranial compliance (when compensatory capacity of CSF dynamics is attenuated), an uncontrolled increase in cerebral blood volume (which is the only compartment with higher pressure than normal or even pathological ICP) would lead to sudden increases in ICP and may damage the cerebral microcirculation (25, 26). This pathophysiological mechanism can be observed, for example, in hypertensive encephalopathy (116) and in aged mice not able to adapt to hypertension (140, 141). A similar pathophysiological role of autoregulatory dysfunction and consequent vascular engorgement after TBI in the development of edema formation and elevation of ICP is a matter of ongoing debate. In case of brain trauma, theoretically, ICP can be increased either by edema of cerebral tissue or by enhanced cerebrovascular volume (CBV).
Cerebral edema is classified to be either vasogenic or cytotoxic. In case of vasogenic edema (mostly due to BBB disruption) interstitial fluid accumulates after extravasation. Cytotoxic edema develops following TBI when the metabolic needs of cerebral tissue and nutrient delivery are not met. This situation is usually caused by neuronal hypermetabolism after TBI together with a permanent drop in basal CBF (90). Because of the lack of energy in maintaining ionic gradients, membrane potential is reduced or lost in neurons, which results in depolarization and activation of voltage-gated Ca2+ channels. This then leads to glutamate release and subsequent Ca2+ influx, which will result in K+ efflux from neurons and entering of Na+ and Cl− together with water (50).
The pioneering work of Marmarou et al. showed that, in patients after severe TBI, 1) increased water content of the brain is responsible for increased ICP rather than increased CBV (91) 2) and the increased cerebral water is primarily due to cytotoxic edema and only in part to vasogenic edema (92). They also demonstrated that CBV decreased after TBI. However, 1) they studied the patients a few hours after the injury, so it is not clear whether or not autoregulatory dysfunction and consequent hypervolemia contribute to the rise in ICP acutely, right after trauma. 2) It is also possible that once cytotoxic edema is developed low CBV is a consequence of the space-occupying effect of intracellular water (especially when blood pressure is in the normal range). From this point of view autoregulatory dysfunction and “vasoparalysis” can be a compensatory mechanism to preserve normal ICP. 3) It is also not known what occurs in case of severely elevated systemic blood pressure, when autoregulation should prevent high pressure and volume reaching the brain. Theoretically, in this case, uncontrolled increase in CBV should further enhance cytotoxic edema-induced high ICP. 4) Finally, lack of autoregulatory protection would lead to increased wall tension when blood pressure increases, which has been shown to exacerbate vascular oxidative stress in cerebral arteries (130). Cerebrovascular oxidative stress impairs key mechanisms of regulation of CBF, such as endothelial function and neurovascular coupling (139). The importance of this possibility is underlined by our recent study showing that neurovascular uncoupling alone, without any other circulatory or neuronal changes, is capable of causing cognitive deficit in mice (137). Neurovascular dysfunction is also considered to be a central mechanism of cognitive decline and dementia due to hypertension, aging, and even Alzheimer's disease (43, 114).
TBI-Induced Autoregulatory Dysfunction in Humans
Clinical studies provide ample evidence that both severe and mild TBI impair static and dynamic autoregulation of CBF in response to both decreasing and increasing perfusion pressure in adults and children (23, 25, 26, 38, 39, 75, 103, 104, 107, 111). In the late 1990s (25) and around the year 2000, Czosnyka et al. provided further important evidence of disturbed cerebral autoregulation following TBI (25, 26). Using a dynamic approach, they correlated blood flow velocity changes in middle cerebral arteries (MCAs) of patients with spontaneous slow fluctuations in CPP generating a correlation coefficient (Mx) indicative of autoregulatory function: positive values of Mx indicate a correlation between perfusion pressure and blood flow velocity showing passive dependence of CBF on CPP and therefore impaired autoregulation. Similarly, a moving correlation coefficient between spontaneous fluctuations in arterial blood pressure and intracranial pressure can be generated (pressure reactivity index, PRx), which indicates intact autoregulatory function when negative or zero values are shown (blood pressure fluctuations are not transmitted to fluctuations in ICP) (20). Because transmission of blood pressure to changes in ICP are determined by the changes of CVR evoked by changes in intraluminal pressure, PRx is a useful clinical measure to observe and describe pressure-induced myogenic responses of the cerebrovasculature. PRx and Mx correlate with each other being two distinct measures of the same mechanisms. In these studies, the averaged flow velocities over perfusion pressure values converged to the shape of the known autoregulatory curve with the lower limit being CPP <55 mmHg and upper limit CPP >105 mmHg. In other words, the upper limit of autoregulation in TBI patients is lower than the physiological value (∼150 mmHg). They also found that autoregulation was disturbed in patients with high ICP (>25 mmHg) and low arterial pressure (<75 mmHg). Although increased Mx, PRx, and ICP were associated with “unfavorable” outcomes (moderate disability), from these results it is not clear if disturbed autoregulation plays a causal role in the rise of ICP or vice versa. In addition, linking autoregulatory function to secondary injury PRx distinguished between fatal and nonfatal outcomes.
It is very important to note that the onset of autoregulatory dysfunction after TBI is not mandatory; autoregulatory function can be intact following TBI (∼50–90% of severe TBI patients have diminished or absent autoregulatory function) (80, 117, 144). As described above, in autoregulatory dysfunction, an optimal value of perfusion pressure can be defined, at which autoregulation functions. Further studies should place a greater emphasis in establishing and understanding the underlying mechanisms of this heterogeneity.
As mentioned above, autoregulatory dysfunction can also be caused by mild TBI (144). Its importance might be best appreciated in chronic repetitive mild brain trauma, which has been demonstrated to lead to the development of chronic traumatic encephalopathy (CTE) characterized by cognitive and psychiatric problems. The histological hallmark of the disease is the perivascular aggregation of the tau protein with prominent perivascular spaces (15, 97), in addition to chronic traumatic cerebrovascular injury (among others the phenotypic changes of the BBB including the increase of perivascular matrix proteins fibronetctin and perlecan and accumulation of amyloid β due to disturbed perivascular drainage) (72–74).
In addition to the mentioned mechanisms Bailey et al. provided important evidence that autoregulatory dysfunction is also involved in the pathology of CTE. They found that dynamic autoregulation of CBF is impaired in professional male boxers compared with age- and physical fitness-matched male nonboxers that was associated with frontotemporal neurocognitive dysfunction and the volume and intensity of sparring, during which most of the mild head trauma occurred. They also demonstrated a more marked orthostatic hypotension in boxers, which can lead to transient cerebral hypoperfusion due to the lack of autoregulatory compensation (14). Autoregulatory dysfunction may also participate in the development of postconcussive symptoms after nonrepetitive mild TBI in the pediatric population (15).
Autoregulatory Dysfunction in Animal Models of TBI
Experimental studies provided further evidence for TBI-induced autoregulatory dysfunction, corroborating the clinical findings. Lewelt et al. (88) applied low (1.5–2.2 atmospheres) and severe (2.8–4.8 atmospheres) fluid percussion injuries on cats and measured CBF after impact using the hydrogen clearance technique in response to decreasing blood pressure achieved by bleeding of the animals. They observed intact autoregulatory responses in three out of eight cats in the low-pressure trauma group. In the severe group, three out of eight cats had no autoregulatory function: CBF decreased as a function of blood pressure. Two animals maintained CBF until 100 mmHg, and the remaining maintained CBF until 80 mmHg, indicating impaired but partly preserved autoregulatory function (heterogeneity of autoregulatory dysfunction after TBI). DeWitt et al. also confirmed these findings in cats (30). Similar results were obtained in rats (40) using laser Doppler flowmetry demonstrating impaired autoregulatory response to bleeding-induced hypotension 24 h after impact-acceleration injury.
A rat study from Fujita et al. observing direct arteriolar dilation also showed that the autoregulatory response to reduction in blood pressure is impaired after impact acceleration and lateral fluid percussion injuries (48). Extending these findings, Nawashiro et al. showed that impact acceleration injury also disrupts autoregulatory function in response to increases in blood pressure (106). On the contrary, however, Bedell et al. reported (16) that CBF response to hypotension after a parasagittal fluid percussion injury in Sprague-Dawley rats does not change and is not affected by hypothermia. Although the presented data show no significant difference between the groups, the mean arterial pressure-CBF curve of the injured rats trended toward a steeper curve than control rats, and the use of isoflurane anesthesia (known to dilate cerebral vessels) may have influenced the results.
In summary, human and experimental studies provided evidence that TBI heterogeneously impairs autoregulation of CBF, which is associated with both unfavorable and fatal outcomes (20, 25, 26, 71). Dysfunctional autoregulation has bidirectional consequences: it results in ischemia with relatively small reductions in blood pressure (thus increasing cytotoxic edema) and permits marked increases in CBF with modest increases in blood pressure (see Fig. 1). Although cytotoxic edema seems to be the primary factor in TBI-related development of cerebral edema formation and in the increases of ICP, autoregulatory dysfunction-related increase in CBV may increase microvascular volume and exacerbate extravasation when the BBB is disrupted (BBB disruption is most likely permissive for the development of autoregulatory dysfunction-related edema formation because an intact BBB is capable of preventing the extravasation of intraluminal fluid by active osmotic compensatory mechanisms, even during severely increased hydrostatic pressure in the arteriolar and capillary bed) (54). It is also possible that a bidirectional pathological link exists: autoregulatory dysfunction likely promotes pressure-induced injury to the microvascular endothelial cells, leading to disruption of the BBB (140). This concept is supported by the findings that mannitol is less effective in reducing ICP when autoregulation is impaired (103). Regarding the cellular and molecular mechanisms of TBI-induced microvascular injury and BBB disruption, we refer to other recent reviews (2, 3). When blood pressure is above the normal range, autoregulatory dysfunction, by allowing greater blood volume and pressure to enter the brain, may enhance cytotoxic edema-related increased ICP. Pathological consequences of the dysfunction of mechanisms maintaining the upper part of the autoregulatory curve after TBI need further studies both in humans and animal models.
Mechanisms of Autoregulatory Dysfunction after TBI
Role of impaired pressure-induced myogenic response in autoregulatory dysfunction associated with TBI.
The purpose of autoregulation is to adjust CVR in response to changes in perfusion pressure to maintain a nearly constant CBF. Cerebral arterial vessels actively dilate and constrict when blood pressure decreases or increases, respectively. This pressure-induced myogenic response plays a central role in CBF autoregulation (11, 17, 18, 22, 42, 45, 47, 49, 57, 59, 95, 96, 98, 102, 110, 140, 146). Several studies demonstrated that TBI impairs both myogenic dilation and constriction of cerebral resistance vessels (Fig. 2). The myogenic function of cerebral vessels can be studied in vivo by observing the responses of pial arteries and arterioles through a cranial window in response to changes in blood pressure (48) or ex vivo by using pressure myography (138). The latter approach when using isolated vessels has the advantage that primary impairment of pressure-induced intrinsic vascular responses can be identified without the confounding effects of factors produced by neurons and glia after TBI.
Fig. 2.
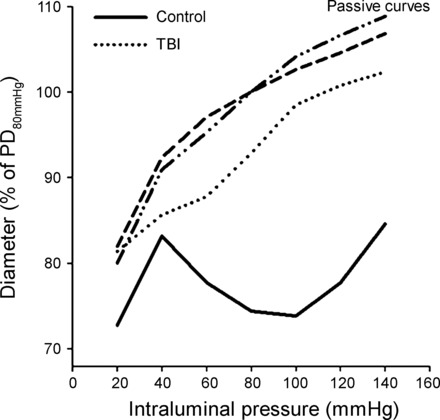
TBI impairs myogenic response of cerebral arteries. Redrawn original recordings of changes of inner diameters of isolated middle cerebral arteries (MCA) from sham-operated rats and rats 24 h after severe TBI (caused by the weight-drop method) using pressure myography (138). Please note that the MCA develops an active tone in response to increasing PP in control animals, and this myogenic response overlaps the range of autoregulation of CBF. Myogenic response is absent 24 h after the animal suffered severe TBI. Passive properties of the vessels did not differ between control and TBI rats. PD80mmHg, passive diameter at 80 mmHg.
Previous studies using pressure myography showed that isolated MCA of rats exhibit impaired myogenic dilation in response to decreases in perfusion pressure after severe controlled cortical impact. This observation supports the concept that TBI primarily impairs vasomotor mechanisms intrinsic to the vascular wall. The findings that TBI-induced impairment is present on both the ipsilateral (2 and 24 h after impact) and the contralateral side (24 h after the impact) (51) suggest that TBI leads to a generalized vasomotor dysfunction in the cerebral microcirculation. Similarly, decreased myogenic dilation can also be demonstrated in isolated rat MCAs shortly after fluid percussion injury (93).
Recent studies (145) showed that, in isolated rat MCAs, myogenic constriction is also decreased after moderate fluid percussion and severe weight-drop injury (Fig. 2). Although the clinical importance of these observations was demonstrated by Budohoski et al. showing that negative or zero pressure reactivity index [which is a surrogate of intact cerebrovascular myogenic function (vide supra)] correlates with both intact autoregulatory function and better outcome in TBI patients (20), the molecular mechanisms of the TBI-related impairment of cerebral myogenic function are less known.
Cellular mechanisms of impaired myogenic dilation of cerebral vessels after TBI.
The mechanisms of impaired myogenic dilation of cerebral vessels after TBI are likely multifaceted. TBI induces excessive production of vascular nitric oxide (NO) (126, 145) and accumulation of (in part NADPH oxidase-derived) reactive oxygen species (ROS), such as O2− (77) (Fig. 3). These processes together lead to the production of peroxynitrite (ONOO−) (29). In both endothelial and smooth muscle cells, ONOO− was shown to inhibit vascular Ca2+-activated K+ (BK) channels (19), thus causing constriction of cerebral arteries (37). Because BK channels were demonstrated to contribute to myogenic dilation (13), this mechanism is likely to be involved in TBI-related impairment of myogenic dilation. An interesting study from Yu et al. showed that scavenging TBI-induced production of ONOO− restores myogenic dilation of rat MCAs via improving gap junction communication between smooth muscle cells (150). Prostaglandins, especially PGE2, have been demonstrated to contribute to dilation of pial arteries in response to hypotension, which is disturbed by fluid-percussion injury in newborn pigs (7). However, findings of Dabertrand et al. make these results controversial, providing substantial evidence that isolated cerebral arterioles of rats and mice constrict, rather than dilate, to PGE2 administration (27). It has to be noted that responses of human cerebral arterioles to PGE2 administration are not known.
Fig. 3.
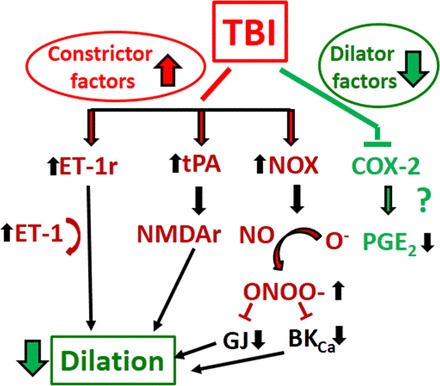
Proposed mechanisms underlying impaired myogenic autoregulatory protection after TBI: TBI-induced decreased myogenic dilation. TBI impairs the capability of cerebral vessels to dilate (thus to decrease cerebrovascular resistance) when PP drops. For detailed description of these pathways we refer to the text. BKCa, large-conductance Ca2+-activated K+ channel; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; NADPH ox, NADPH oxidase; GJ, gap junction; ONOO−, peroxynitrite; iNOS, inducible nitric oxide synthase; NO, nitric oxide; NMDA, N-methyl-d-aspartate; NMDAr, N-methyl-d-aspartate receptor; ET-1, endothelin-1; ET-1r, endothelin-1 receptor; tPA, tissue plasminogen activator.
Another interesting possibility is the role of TBI-induced production of tissue plasminogen activator (tPA). Armstead et al. showed that, in a newborn pig, an inactive tPA variant (tPA-S481A) prevents the impairment of autoregulation when administered 30 min after fluid percussion injury (FPI) by inhibiting overactivation of N-methyl-d-aspartate (NMDA) receptors. TBI results in increased production of the vasoconstrictor endothelin-1 (ET-1), and tPA-S481A also inhibits the ET-1 receptor (8). In addition, ET-1 antagonizes NMDA receptor-mediated vasodilation (6) and leads to impaired dilation of cerebral vessels mediated by K+ channel agonists after FPI, via release of superoxide anion. The role of ROS is also suggested by studies of Kim et al. showing that arteriolar dilation to hypotension after FPI was restored by both 1) the transfer of human copper-zinc superoxide dismutase in rats via intracisternal administration of recombinant adenoviruses, and 2) treatment of the pial circulation with the NADPH oxidase inhibitor diphenyleneiodonium (77). On the other hand, ROS production may play a beneficial role in preserving dilator capacity of cerebral vessels: an interesting study of Sullivan et al. demonstrated that NADPH oxidase 2-derived ROS activate Ca2+-permeable transient receptor potential ankyrin 1 (TRPA1) channels on the cerebrovascular endothelium leading to Ca2+ influx. These Ca2+ “sparklets” activated intermediate-conductance Ca2+-sensitive K+ channels leading to hyperpolarization of vascular smooth muscle and dilation of vessels (134). Future studies should examine the effects of the pharmacological and/or genetic activation of TRPA1/intermediate-conductance Ca2+-sensitive K+ channels on cerebrovascular tone following TBI.
Cellular mechanisms of impaired myogenic constriction of cerebral vessels after TBI.
The vascular processes leading to the impairment of myogenic constriction of cerebral vessels following brain trauma are less understood (Fig. 4). As mentioned above, TBI induces excessive production of NO both in cerebral/glial tissue and in the cerebrovasculature (126, 145). NO was shown to contribute to the initial increase in CBF 30 min after trauma (126), and inhibition of NO with VAS203 decreased ICP in mice (125). Based on these initial findings, Villalba et al. (145) tested the hypothesis that increased vascular NO is responsible for the absence of myogenic constriction of cerebral vessels after TBI. They found that 24 h after FPI of rats myogenic constrictions were diminished in both the ipsilateral and contralateral MCAs studied in a myograph chamber. The authors also demonstrated that production of NO was greatly increased in both the endothelial and smooth muscle layers of MCAs after TBI and that inhibition of NO production with NG-nitro-l-arginine increased myogenic tone of the impacted vessels.
Fig. 4.
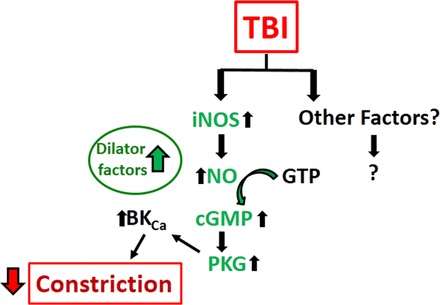
Proposed mechanisms underlying impaired myogenic autoregulatory protection after TBI: TBI-induced impaired myogenic constriction. The mechanisms of the dysfunction of pressure-induced constriction are less known. For detailed description of these pathways, we refer to the text.
Along with these functional data they showed that TBI enhanced vascular expression of inducible nitric oxide synthase (iNOS). They also showed that constrictions after inhibition of guanylyl cyclase, protein kinase G (PKG), and BK channels were decreased in animals after trauma. Based on these findings, the authors proposed that TBI triggers endothelial production of iNOS-derived NO, which then decreases pressure-induced smooth muscle Ca2+ concentration due to hyperpolarization after the cGMP/PKG-dependent activation of BK channels (145). Although the effect of inhibition of NO production on the myogenic response of MCAs is convincing, after the treatment, myogenic tone seems to be lower at 80 and 100 mmHg compared with control vessels, and the pressure range over 100 mmHg is not covered by the study. Thus it is not clear if NO is responsible for the loss of myogenic constriction at higher pressure values and whether NO has a permissive or a direct role in the impairment of the pressure-induced constriction. In other words: is NO produced in response to pressure after TBI?
Although the vascular endothelium is capable of modulating vascular myogenic tone (for example, flow-induced dilation attenuates pressure-induced constriction when flow and pressure change simultaneously) (142), it is not thought to contribute to myogenic constriction, and this would support a permissive role (for a review on the topic, please see Ref. 28). The vessels did not constrict to the thromboxane analog U-46619 or to K+, as they did in controls, indicating that MCAs lost their ability to constrict to agonists beyond the effect of pressure-induced constriction. This would further support the permissive indirect role of NO in the defective myogenicity of cerebral vessels after TBI. It also further complicates the notion that, after TBI, ONOO− is formed from NO, which is a known constrictor of cerebral vessels. Downstream signaling from NO in myogenic constriction after TBI is also in question, since the authors showed constriction to the inhibitors of the mentioned targets instead of the restoration of the myogenic response in the presence of these drugs.
Neurogenic and metabolic effects on autoregulatory function following TBI.
In addition to the pressure-induced myogenic response of cerebral vessels, other factors are involved in setting CVR during changes in perfusion pressure. Accordingly, neurogenic (sympathetic) and metabolic factors were suggested to contribute to CBF autoregulation, but their role in TBI-induced autoregulatory dysfunction is less known (55). In newborn pigs following FPI, administration of norepinephrine prevented CBF reductions in response to decreasing blood pressure via the inhibition of mitogen-activated protein kinases (MAPKs) and probably via increasing perfusion pressure (10). Another interesting study also from Armstead et al. (9) demonstrated that, in piglets, glucagon treatment (known to decrease neuronal glutamate release after TBI) together with the mentioned inhibition of tPA fully restored hypotension-induced pial arterial dilation after FPI via decreased ERK MAPK and NMDA and increased PGE2-PGI2 levels. Neuronal metabolism is increased after trauma and, together with the acutely decreased basal blood flow, leads to a mismatch between the increased metabolic demand and nutrient supply (46). Although the increased lactate levels are capable of modulating cerebrovascular tone and thus autoregulatory function, the consequent direct ischemic insult and cytotoxic edema formation play a more important role in TBI-related brain swelling and ICP increase than vascular engorgement and increase in CBF (90).
SPREADING DEPOLARIZATION-INDUCED CEREBROMICROVASCULAR DYSFUNCTION: ROLE IN SECONDARY INJURY IN TBI
Characteristics of Spreading Depolarization Events and Their Role in Dysregulation of CBF
There is growing evidence that spreading depolarizations (SD), propagating waves of depolarization across the gray matter, contribute to cerebral microvascular dysfunction and pathogenesis of secondary neuronal injury after TBI. SDs recurrent in injured tissue cause a dramatic disruption of ionic homeostasis (58, 128), dendritic beading and swelling of neurons and astrocytes (119, 120, 131), glucose depletion and accumulation of lactate leading to tissue acidosis (44, 67, 122, 124), the release of glutamate together with related neuronal calcium load (69), and typical changes in local CBF (12). Regarded originally as an experimental curiosity, spontaneously generating SD has proven to be a potent pathogenic mechanism in neurological diseases such as migraine with aura, subarachnoid hemorrhage, ischemic stroke, and TBI (32).
It has been recognized that SDs establish the initial damage of the ischemic core and secondary lesion growth in the penumbra of focal ischemia (62). The initial mass tissue depolarization that follows the drop of CBF below a critical threshold (5–10 ml/100 g/min) with a short delay (i.e., 2–5 min) is a persistent SD, which defines the primary infarction. Subsequently, additional repetitive SDs recur spontaneously for hours or days in the penumbra region (35, 61, 133), where insufficient perfusion sets the scene for a critical supply-demand mismatch to trigger SDs (147). These later SDs propagate slowly (1–8 min/mm) across the cortex and convert electrically silent but viable penumbra tissue into the core region, thereby expanding the infarcted zone. Recent clinical studies have shown that SD emerges as a potent pathomechanism of the progression of secondary injury in TBI, as well (63, 65, 68).
The characteristic features of SD are a large transient negative shift in the slow electrical or direct current (DC) potential and the simultaneous silencing of brain electrical activity termed spreading depression (Fig. 5A) (52, 87). The SD-related negative shift of the DC potential represents the complete loss of resting membrane potential to a near 0 mV, reflecting an increase of extracellular K+ from 3–4 to 30–60 mM, and the concomitant decrease of the extracellular concentration of Na+ from 140–150 to 50–70 mM and of Ca2+ from 1–1.5 to 0.2–0.8 mM (115). At the level of the brain tissue, SD is an intense self-igniting local depolarization of a critical mass of cells, which propagates to adjacent cell populations in the cerebral gray matter by means of increasing extracellular K+ or glutamate concentration (128).
Fig. 5.
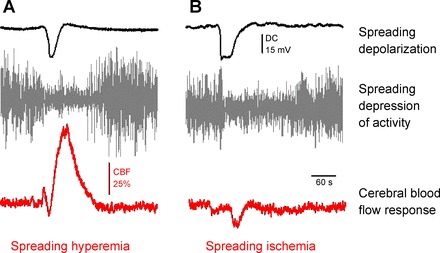
The spreading depolarization (SD) phenomenon as assessed at a single recording site by direct current (DC) potential recording (black trace) and alternating current (AC)-electrocorticography (gray trace); and two distinct types of associated CBF response (red trace) acquired by laser Doppler flowmetry. Representative traces are original recordings from the rat brain. SD was elicited by the topical application of high-concentration K+ in the young intact (A) and the old ischemic (B) parietal cortex.
In the rat, the physiological pattern of SD-associated CBF response includes four sequential components: 1) an initial brief drop of CBF; 2) a marked transient peak hyperemia; 3) a less obvious late hyperemia; and 4) a sustained hypoperfusion also known as spreading oligemia (12). The share of these four elements in the CBF response is variable, with the peak hyperemic element being the most conspicuous. The hyperemic component is comparable to functional hyperemia of physiological neurovascular coupling, since it supplies the brain tissue with energy substrates to be used by ion exchange pumps for the restoration of resting membrane potential. Comprehensive analysis and comparison of the CBF response in the rat and patients revealed a good correspondence (109). Therefore, data obtained from rat models have an accepted relevance for human disease states.
In the ischemic brain, the CBF response to SD is more dominated by vasoconstrictor mechanisms, and low perfusion pressure further limits the extent of blood flow increase (44, 70). Thus, hyperemia is diminished while ischemic components are more pronounced (100, 149). In the most severe form, the vasoconstriction completely overrides dilatation (Fig. 5B) and causes pathological inverse neurovascular coupling, known as spreading ischemia (32). This atypical SD-associated CBF variation during ischemia aggravates metabolic supply-demand mismatch in the tissue and can delay recovery from SD. Longer cumulative SD duration, in turn, increases the probability of neuronal cell death and eventually infarction (31, 32, 35).
The different phases of the SD-related CBF response are assumed to be the result of a sequence and combination of extracellular ionic, neurotransmitter, and metabolic changes (12). These include, for example, interstitial K+ elevation, variation in the release of NO or prostaglandins, or the modulation of adenosine receptors (12). Because of the complexity of these interactions, it is rather challenging to discriminate the significance of individual factors. Still, it has been suggested that a high level of extracellular K+ exceeding 20 mM concentration (vasoconstrictor stimulus) in combination with decreased NO availability (permissive vasodilator agent) promote vasoconstriction, and thereby trigger the shift to spreading ischemia with SD (33, 148). These conditions are present in TBI, since interstitial K+ is markedly elevated (118) and the availability of NO may be limited by fast reaction with superoxide to form reactive nitrogen species (56). In addition, the produced ONOO− could promote vasoconstriction by the potential inhibition of vascular Ca2+-activated K+ channels (19), as described above. Such pathophysiological conditions may favor the development of spreading ischemia as a consequence of SDs.
Evidence for the Occurrence and Injurious Potential of SD in TBI Patients
The confirmation of SD occurrence in patients was delayed for many years by the lack of an appropriate methodology. SD propagation escaped detection on regular scalp EEG possibly because bone and other tissue greatly alter the electrical signal, and the frequency filtering, amplification, and time scale display of conventional EEG are not optimal to reveal SDs (36, 66). Thus, the first evidence for SD to occur in the neocortex after TBI was obtained with invasive probes. Monitoring of extracellular K+ concentration with a surface minielectrode in the cortex, in combination with the assessment of local CBF and tissue metabolism (tissue NADH levels), revealed recurrent SDs at the sampling site about every 30 min, leading to the recording of >40 events in a comatose patient. These SDs appeared as transient increases of interstitial K+ level, coupled with typical hemodynamic responses resembling those previously seen with SDs in the rodent brain (94).
A more generalizable/applicable approach to monitoring SD was then developed by adapting electrocorticographic (ECoG) techniques used in epilepsy monitoring to the postoperative monitoring of TBI. SD was originally discovered in rabbits as transient abrupt reductions in ECoG amplitude acquired by cortical surface electrodes (87). Thus, in a groundbreaking study, Strong and colleagues placed ECoG electrode strips on the cortical surface in patients who required surgery for traumatic intracranial hematoma. This study demonstrated that spreading depression of the ECoG signal occurs commonly in TBI patients (133). Subsequent studies then showed that the spreading depressions are accompanied by the hallmark negative DC shift of SD.
DC shifts were first revealed as slow potential changes recorded with alternating current (AC)-coupled amplifiers (41), then by reconstruction of full-band DC-coupled recordings from AC-coupled recordings (63), and finally by DC-coupled electrocorticography (34). Recording the DC shift of SD is recognized now as a required standard for identification of these events, since it not only resolves ambiguous signals but also reveals a subset of SDs that occur without spreading depression of spontaneous activity (65). SD without spreading depression occurs when spontaneous activity has already been suppressed by ischemia or prior SDs and is termed isoelectric SD.
Once reliable detection of SD in the injured human brain had been established, subsequent studies aimed to determine the incidence and time course of SDs, and conditions that may favor SD elicitation (63). These studies showed that 55–60% of surgical TBI patients develop SDs and that SDs often occur in repetitive patterns through at least 7 days posttrauma (54, 56, 57). Lower levels of cerebral perfusion and high systemic temperature are factors that increase the probability of SD, likely through increased mismatch of energy supply-demand (147), although the vast majority of SDs in TBI occur when systemic variables are in normal ranges (63). Considering the damaging effects of SD in ischemia, the potential of SD to contribute to secondary injury in TBI patients was assessed. As suspected, SDs with prolonged durations and those occurring in association with isoelectricity or periodic epileptiform discharges in the ECoG were predictive of unfavorable outcomes (60, 65). Taken together, the clinical data confirmed that recurrent SDs do occur spontaneously in the cortex of TBI patients, and specific patterns of these events are independently associated with worse clinical outcome.
An inadequate SD-associated CBF response can delay repolarization by depriving the tissue at risk of essential nutrients required to restore ionic balance across neuronal cell membranes (12, 32). This CBF response and the duration of depolarization are critical determinants of the harmful effects of SD, since prolonged depolarization in metabolically compromised tissue results in either infarction or selective neuronal necrosis (62). To evaluate the CBF response to SD in patients with aneurysmal subarachnoid hemorrhage, ECoG electrode strips were also equipped with laser Doppler probes (34). With this technique, spreading ischemia was commonly observed in the human brain, often reducing CBF to ischemic levels. Thereafter, thermal diffusion probes were used alongside ECoG strips for CBF monitoring in TBI patients, and inverse neurovascular coupling was similarly observed (68). As in rodent models of focal ischemia (127), inverse neurovascular coupling in TBI patients was observed in the vicinity of evolving lesions, occasionally during ongoing ischemic episodes (Fig. 6A), and progressively reduced CBF in a stepwise fashion with each successive SD (68).
Fig. 6.
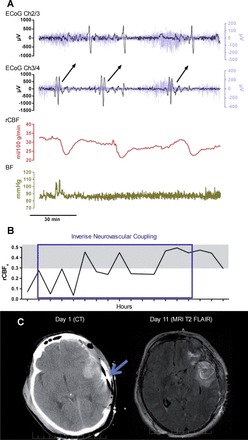
Inverse neurovascular coupling with SD in a representative postoperative case of TBI. A: bipolar electrocortigraphic (ECoG) recordings: black traces depict SD (0.01 Hz high-pass filtering); blue traces in the background represent spreading depression of activity (0.5–50 Hz bandpass filtering). Synchronous changes of regional cerebral blood flow (rCBF) are shown in the red trace. Note that rCBF drops with each subsequent SD and returns to lower CBF after the passage of each event. Blood pressure (BP) variations are represented by the gold trace. B: gradual onset of impaired autoregulation during the period of inverse coupling, as demonstrated by the increasing correlation between CBF and BP. Gray shaded area indicates the range of correlation considered to be significant, indicative of impaired autoregulation. C: brain images showing placement of electrode (day 1 CT, blue arrow) directly adjacent to an intracerebral hematoma where cortical lesions developed (day 11 MRI). Reproduced from Hinzman et al. (68) with the permission of Oxford University Press (license no. 3816350088958).
Inverse coupling was additionally confirmed by the assessment of the partial pressure of oxygen in the brain tissue in one patient. The data revealed that the change in local partial pressure of oxygen with SD transformed from physiological transient hyperoxic response to inverse transient hypoxic response over time (68). In addition, in this study, the inverse CBF response with SD coincided with the loss of autoregulation as assessed by correlations between CBF and mean arterial pressure (Fig. 6B) (68). Importantly, the loss of autoregulation after TBI, as mentioned above, is considered a risk factor for ischemic damage following TBI. Speculatively, the ischemic damage could result from transformation of hyperemic CBF response to SD to spreading ischemia. The evolving characteristics of SDs and impairment of autoregulation suggest that continuous monitoring of these variables is important for personalized diagnosis and treatment of secondary injury (Fig. 7).
Fig. 7.
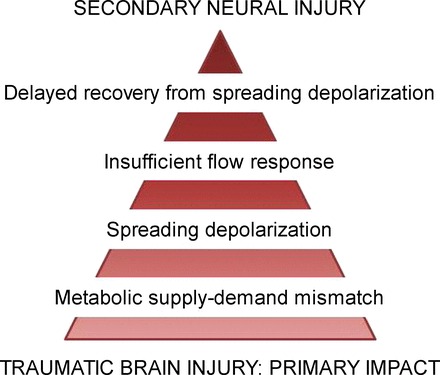
A subpopulation of SD events that evolve in TBI is attributed high injurious potential. Recurrent SD waves occur in 55–60% of TBI patients requiring surgical alleviation of their symptoms. The insufficiency of the CBF response, which may manifest as spreading ischemia, hampers repolarization, thereby prolonging SD. Because longer SD duration has been associated with larger injury, this chain of events is thought to contribute to the expansion of secondary damage in TBI.
Evidence for the Occurrence of SD in Experimental Models of TBI
SD waves were first demonstrated as a sequel of TBI in a rat model of contusional injury induced by fluid percussion (135). Although the genesis of SD was not universal (i.e., SDs were shown in 6 of the 10 animals), the events were reliably detected as recurrent transient negative shifts of the DC potential with the concomitant depression of the ECoG. As expected for SD waves, the negative DC shifts were also associated with transient increases of the interstitial K+ concentration (79). The first SD occurred ∼1 h after impact, and subsequent SDs occurred as often as 9–10 events/h (135). The experiments were later extended by the assessment of regional CBF, using [14C]iodoan tipyrine-based autoradiography (112). The analysis of the autoradiograms indicated local transient hyperemic CBF response to SDs, superimposed on a baseline CBF considerably reduced by the contusion itself. The response was blunted, however, since CBF barely exceeded baseline flow in the hemisphere contralateral to the injury (112). The above observations established without doubt that SD occurred spontaneously in the cortex after experimental TBI, with features similar to SD events under cerebral ischemia. The conditions leading to SD elicitation were not defined explicitly, but low CBF in association with SD occurrence and high interstitial K+ level related to the trauma and SDs were reported (108, 112). It was not explicitly tested whether the SDs augmented injury, although inverse CBF responses to SDs were occasionally observed. Furthermore, the frequency and duration of SDs recurrence were associated with the severity of brain injury (121). Finally, high-frequency SD recurrence in combination with ICP >20 mmHg was concluded to demonstrate advancing secondary injury and poor outcome after TBI (121).
Although contusions are common with impact injuries, other pathologies such as various types of intracranial hematoma also prevail in severe TBI. Subdural hematoma, for instance, is associated with high morbidity and mortality and causes injury by raising intracranial pressure, compressing the brain, and triggering a continuum of initial and subsequent SDs, as in focal ischemia (64). SDs were also shown to emerge following intracerebral hemorrhage in a swine model (105). Finally, hemorrhage in the subarachnoid space, particularly following hemolysis, can induce neural damage by provoking SDs and scavenging NO, thereby promoting vasoconstrictive spreading ischemia (33). These findings together suggest that hemorrhagic lesions in combination with contusions may account for the high incidence of SD in patients with severe TBI (60, 64).
THERAPEUTIC POSSIBILITIES
At present there are no targeted interventions available to restore autoregulatory function or prevent SDs and concomitant CBF changes in humans with TBI. Current therapeutic efforts focus on normalization of systemic hemodynamic parameters known to affect CBF regulation (blood pressure, plasma osmotic pressure, plasma and erythrocyte volumes, and PaO2 and PaCO2 using the so-called Lund protocol) to maintain adequate perfusion of the injured areas (53).
On the basis of results from recent studies, a fairly novel therapeutic approach emerged, namely maintaining the CPP at a level where autoregulatory function is most preserved and therefore optimal for the individual patient (20, 21, 25, 26, 86). When the pressure reactivity index is monitored over a certain period, it exhibits a U-shaped curve when plotted as a function of CPP. The optimal CPP for the patient is then determined as the value corresponding to the lowest PRx value of the curve. This value can then be targeted in subsequent patient management as a more personalized approach than simply maintaining CPP within the broad range of 60–140 mmHg considered to be normal for an adult population. The optimal CPP can in theory be targeted more narrowly by ICP control and adjusting blood pressure of the patient, thereby optimizing autoregulatory function. One study provided evidence for the efficacy of this approach: smaller differences between optimal CPP and actual CPP values maintained in patients throughout neurointensive monitoring were associated with more favorable outcomes (20). Actual CPPs that were well below optimal CPPs were associated with higher mortality, and those substantially above optimal CPPs were associated with increased disability (5, 132).
In animal models, 1 h posttraumatic hypothermia was shown to improve TBI-related autoregulatory dysfunction in response to sudden hypotension (48), but these promising results could not be translated to clinical settings (4). As mentioned above, in newborn pigs, administration of norepinephrine prevented CBF reductions in response to decreasing blood pressure via the inhibition of MAPKs (and probably via increasing perfusion pressure) (10). Also, in piglets (9), glucagon treatment and inhibition of tPA fully restored hypotension-induced pial arterial dilation after FPI. Another interesting study demonstrated that carbon-based antioxidant nanovectors (by annihilation of ROS) targeted to P-selectin on injured cultured cerebral endothelial cells decreased generation of superoxide (89). Further studies should examine the possibility whether in vivo treatment with these antioxidant clusters can restore autoregulation of CBF. Also, further studies should examine whether targeting the involved cellular pathways (Figs. 3 and 4) is capable of preventing and/or treating dilatory dysfunction of cerebral vessels during decreases in perfusion pressure. To restore autoregulatory responses to increases in perfusion pressure, further studies are needed to determine mechanisms of impaired myogenic constriction of cerebral vessels.
GRANTS
This work was supported by grants from the American Heart Association (to P. Toth and Z. Ungvari), the Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00634/15 to P. Toth and E. Farkas), the Hungarian Brain Research Program (Grant No. KTIA_13_NAP-A-I/13 to E. Farkas and Grant No. KTIA_13_NAP-A-II/8 to P. Toth, E. Czeiter, and A. Buki), National Center for Complementary and Alternative Medicine (R01-AT006526 to Z. Ungvari), the Marie Slodowska-Curie Actions SMARTER 7th Framework Program of the European Union 606998 (to N. Szarka and A. Koller), and the Hungarian Scientific Research Fund (K111923 to E. Farkas and K108444 to A. Koller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.T., N.S., E.F., J.A.H., and A.K. prepared figures; P.T., N.S., E.F., E.E., E.C., Z.I.U., J.A.H., A.B., and A.K. drafted manuscript; P.T., N.S., E.F., E.E., E.C., K.A., Z.I.U., J.A.H., and A.K. edited and revised manuscript; P.T., N.S., E.F., E.E., E.C., K.A., Z.I.U., J.A.H., A.B., and A.K. approved final version of manuscript.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Muneer PM, Pfister BJ, Haorah J, Chandra N. Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Mol Neurobiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alluri H, Wiggins-Dohlvik K, Davis ML, Huang JH, Tharakan B. Blood-brain barrier dysfunction following traumatic brain injury. Metab Brain Dis 30: 1093–1104, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, Murray GD, Eurotherm3253 Trial Collaborators. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med 373: 2403–2412, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JD, Smielewski P. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 40: 2456–2463, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Armstead WM. Age dependent endothelin contribution to NOC/oFQ induced impairment of NMDA cerebrovasodilation after brain injury. Peptides 22: 39–46, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Armstead WM. Brain injury impairs prostaglandin cerebrovasodilation. J Neurotrauma 15: 721–729, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Armstead WM, Bohman LE, Riley J, Yarovoi S, Higazi AA, Cines DB. tPA-S(481)A prevents impairment of cerebrovascular autoregulation by endogenous tPA after traumatic brain injury by upregulating p38 MAPK and inhibiting ET-1. J Neurotrauma 30: 1898–1907, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstead WM, Riley J, Cines DB, Higazi AA. Combination therapy with glucagon and a novel plasminogen activator inhibitor-1-derived peptide enhances protection against impaired cerebrovasodilation during hypotension after traumatic brain injury through inhibition of ERK and JNK MAPK. Neurol Res 34: 530–537, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstead WMPD, Riley J, Vavilala MS. Norepinephrine protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury via block of ERK MAPK and IL-6 in juvenile pigs. J Neurotrauma In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Aukes AM, Vitullo L, Zeeman GG, Cipolla MJ. Pregnancy prevents hypertensive remodeling and decreases myogenic reactivity in posterior cerebral arteries from Dahl salt-sensitive rats: A role in eclampsia? Am J Physiol Heart Circ Physiol 292: H1071–H1076, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev 95: 953–993, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey DM, Jones DW, Sinnott A, Brugniaux JV, New KJ, Hodson D, Marley CJ, Smirl JD, Ogoh S, Ainslie PN. Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci Lond 124: 177–189, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, Ashwal S. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J Neurotrauma 31: 1497–1506, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Bedell EA, DeWitt DS, Uchida T, Prough DS. Cerebral pressure autoregulation is intact and is not influenced by hypothermia after traumatic brain injury in rats. J Neurotrauma 21: 1212–1222, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Bohlen HG, Harper SL. Evidence of myogenic vascular control in the rat cerebral cortex. Circ Res 55: 554–559, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol 35: 1116–1120, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 278: H1883–H1890, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Budohoski KP, Czosnyka M, de Riva N, Smielewski P, Pickard JD, Menon DK, Kirkpatrick PJ, Lavinio A. The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery 71: 652–660, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Budohoski KP, Reinhard M, Aries MJ, Czosnyka Z, Smielewski P, Pickard JD, Kirkpatrick PJ, Czosnyka M. Monitoring cerebral autoregulation after head injury. Which component of transcranial Doppler flow velocity is optimal? Neurocrit Care 17: 211–218, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Cipolla MJ, Sweet JG, Chan SL, Tavares MJ, Gokina NI, Brayden JE. Increased pressure-induced tone in rat parenchymal arterioles vs. middle cerebral arteries: Role of ion channels and calcium sensitivity. J Appl Physiol (1985) 117: 53–59, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cold GE, Jensen FT. Cerebral autoregulation in unconscious patients with brain injury. Acta Anaesthesiol Scand 22: 270–280, 1978. [DOI] [PubMed] [Google Scholar]
- 24.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD, Centers for Disease Control and Prevention. Surveillance for traumatic brain injury-related deaths–United States, 1997–2007 morbidity and mortality weekly report. Surveill Summ 60: 1–32, 2011. [PubMed] [Google Scholar]
- 25.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke 27: 1829–1834, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. J Neurosurg 95: 756–763, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Dabertrand F, Hannah RM, Pearson JM, Hill-Eubanks DC, Brayden JE, Nelson MT. Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J Cereb Blood Flow Metab 33: 479–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis MJ. Perspective: physiological role(s) of the vascular myogenic response. Microcirculation 19: 99–114, 2012. [DOI] [PubMed] [Google Scholar]
- 29.DeWitt DS, Mathew BP, Chaisson JM, Prough DS. Peroxynitrite reduces vasodilatory responses to reduced intravascular pressure, calcitonin gene-related peptide, and cromakalim in isolated middle cerebral arteries. J Cereb Blood Flow Metab 21: 253–261, 2001. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt DS, Prough DS, Taylor CL, Whitley JM, Deal DD, Vines SM. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am J Physiol Heart Circ Physiol 263: H1276–H1284, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Dijkhuizen RM, Beekwilder JP, van der Worp HB, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res 840: 194–205, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17: 439–447, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab 18: 978–990, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ, COSBID Study Group. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 132: 1866–1881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 129: 3224–3237, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Drenckhahn C, Winkler MK, Major S, Scheel M, Kang EJ, Pinczolits A, Grozea C, Hartings JA, Woitzik J, Dreier JP, and COSBID Study Group. Correlates of spreading depolarization in human scalp electroencephalography. Brain 135: 853–868, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott SJ, Lacey DJ, Chilian WM, Brzezinska AK. Peroxynitrite is a contractile agonist of cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 275: H1585–H1591, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Enevoldsen EM, Jensen FT. Autoregulation and CO2 responses of cerebral blood flow in patients with acute severe head injury. J Neurosurg 48: 689–703, 1978. [DOI] [PubMed] [Google Scholar]
- 39.Enevoldsen EM, Jensen FT. “False” autoregulation of cerebral blood flow in patients with acute severe head injury. Acta Neurol Scand Suppl 64: 514–515, 1977. [PubMed] [Google Scholar]
- 40.Engelborghs K, Haseldonckx M, Van Reempts J, Van Rossem K, Wouters L, Borgers M, Verlooy J. Impaired autoregulation of cerebral blood flow in an experimental model of traumatic brain injury. J Neurotrauma 17: 667–677, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 129: 778–790, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Faraci FM, Baumbach GL, Heistad DD. Myogenic mechanisms in the cerebral circulation. J Hypertens Suppl 7: S61–S65, 1989. [PubMed] [Google Scholar]
- 43.Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab 36: 241–252, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feuerstein D, Backes H, Gramer M, Takagaki M, Gabel P, Kumagai T, Graf R. Regulation of cerebral metabolism during cortical spreading depression. J Cereb Blood Flow Metab In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fog M. The relationship between the blood pressure and the tonic regulation of the pial arteries. J Neurol Psychiatry 1: 187–197, 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foley N, Marshall S, Pikul J, Salter K, Teasell R. Hypermetabolism following moderate to severe traumatic acute brain injury: a systematic review. J Neurotrauma 25: 1415–1431, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Forbes HS. Regulation of the cerebral vessels; new aspects. AMA Arch Neurol Psychiatry 80: 689–695, 1958. [DOI] [PubMed] [Google Scholar]
- 48.Fujita M, Wei EP, Povlishock JT. Effects of hypothermia on cerebral autoregulatory vascular responses in two rodent models of traumatic brain injury. J Neurotrauma 29: 1491–1498, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Golding EM. Sequelae following traumatic brain injury. The cerebrovascular perspective. Brain Res Rev 38: 377–388, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Golding EM, Contant CF Jr, Robertson CS, Bryan RM Jr. Temporal effect of severe controlled cortical impact injury in the rat on the myogenic response of the middle cerebral artery. J Neurotrauma 15: 973–984, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Grafstein B. Mechanism of spreading cortical depression. J Neurophysiol 19: 154–171, 1956. [DOI] [PubMed] [Google Scholar]
- 53.Grande PO. The “Lund Concept” for the treatment of severe head trauma–physiological principles and clinical application. Intensive Care Med 32: 1475–1484, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Grande PO, Asgeirsson B, Nordstrom CH. Volume-targeted therapy of increased intracranial pressure: the Lund concept unifies surgical and non-surgical treatments. Acta Anaesthesiol Scand 46: 929–941, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Guo ZN, Shao A, Tong LS, Sun W, Liu J, Yang Y. The role of nitric oxide and sympathetic control in cerebral autoregulation in the setting of subarachnoid hemorrhage and traumatic brain injury. Mol Neurobiol 53: 3606–3615, 2016. [DOI] [PubMed] [Google Scholar]
- 56.Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma 21: 9–20, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Halpern W, Osol G. Influence of transmural pressure of myogenic responses of isolated cerebral arteries of the rat. Ann Biomed Eng 13: 287–293, 1985. [DOI] [PubMed] [Google Scholar]
- 58.Hansen AJ, Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand 113: 437–445, 1981. [DOI] [PubMed] [Google Scholar]
- 59.Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol 300: H1557–H1565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartings JA, Bullock MR, Okonkwo DO, Murray LS, Murray GD, Fabricius M, Maas AI, Woitzik J, Sakowitz O, Mathern B, Roozenbeek B, Lingsma H, Dreier JP, Puccio AM, Shutter LA, Pahl C, Strong AJ, Co-Operative Study on Brain Injury Depolarizations. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol 10: 1058–1064, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Hartings JA, Rolli ML, Lu XC, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci 23: 11602–11610, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartings JASC, Kirov SA, Ayata C, Hinzman JM, Foreman B, Andrew RD, Boutelle MG, Brennan KC, Carlson AP, Dahlem MA, Drenckhahn C, Dohmen C, Fabricius M, Farkas E, Feuerstein D, Graf R, Helbok R, Lauritzen M, Major S, Oliveira-Ferreira A, Richter F, Rosenthal ES, Sakowitz OW, Sánchez-Porras R, Santos E, Schöll M, Strong AJ, Westover MB, Winkler MKL, Witte OW, Woitzik J, Dreier JP. The continuum of spreading depolarizations in acute cortical lesion development: re-examining Leão's legacy. J Cereb Blood Flow Metab In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, Mazzeo AT, Tortella FC, Bullock MR, Co-Operative Study of Brain Injury Depolarizations. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma 26: 1857–1866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartings JA, Vidgeon S, Strong AJ, Zacko C, Vagal A, Andaluz N, Ridder T, Stanger R, Fabricius M, Mathern B, Pahl C, Tolias CM, Bullock MR, Co-Operative Studies on Brain Injury Depolarizations. Surgical management of traumatic brain injury: a comparative-effectiveness study of 2 centers. J Neurosurg 120: 434–446, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, Dreier JP, Puccio A, Shutter LA, Pahl C, Strong AJ, Co-Operative Study on Brain Injury Depolarizations. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain 134: 1529–1540, 2011. [DOI] [PubMed] [Google Scholar]
- 66.Hartings JA, Wilson JA, Hinzman JM, Pollandt S, Dreier JP, DiNapoli V, Ficker DM, Shutter LA, Andaluz N. Spreading depression in continuous electroencephalography of brain trauma. Ann Neurol 76: 681–694, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, Boutelle MG. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leao's spreading depression. J Cereb Blood Flow Metab 29: 166–175, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Hinzman JM, Andaluz N, Shutter LA, Okonkwo DO, Pahl C, Strong AJ, Dreier JP, Hartings JA. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain 137: 2960–2972, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Hinzman JM, DiNapoli VA, Mahoney EJ, Gerhardt GA, Hartings JA. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp Neurol 267: 243–253, 2015. [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann U, Ayata C. Neurovascular coupling during spreading depolarizations. Acta Neurochir Suppl 115: 161–165, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Johnson U, Nilsson P, Ronne-Engstrom E, Howells T, Enblad P. Favorable outcome in traumatic brain injury patients with impaired cerebral pressure autoregulation when treated at low cerebral perfusion pressure levels. Neurosurgery 68: 714–721, 2011. [DOI] [PubMed] [Google Scholar]
- 72.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat Rev Neurosci 11: 361–370, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jullienne A, Obenaus A, Ichkova A, Savona-Baron C, Pearce WJ, Badaut J. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res 94: 609–622, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jullienne A, Roberts JM, Pop V, Paul Murphy M, Head E, Bix GJ, Badaut J. Juvenile traumatic brain injury induces long-term perivascular matrix changes alongside amyloid-beta accumulation. J Cereb Blood Flow Metab 34: 1637–1645, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Junger EC, Newell DW, Grant GA, Avellino AM, Ghatan S, Douville CM, Lam AM, Aaslid R, Winn HR. Cerebral autoregulation following minor head injury. J Neurosurg 86: 425–432, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, Diaz-Arrastia R. Cerebral vascular injury in traumatic brain injury. Exp Neurol 275: 353–366, 2016. [DOI] [PubMed] [Google Scholar]
- 77.Kim CD, Shin HK, Lee HS, Lee JH, Lee TH, Hong KW. Gene transfer of Cu/Zn SOD to cerebral vessels prevents FPI-induced CBF autoregulatory dysfunction. Am J Physiol Heart Circ Physiol 282: H1836–H1842, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Kontos HA. Regulation of the cerebral circulation. Annu Rev Physiol 43: 397–407, 1981. [DOI] [PubMed] [Google Scholar]
- 79.Kubota M, Nakamura T, Sunami K, Ozawa Y, Namba H, Yamaura A, Makino H. Changes of local cerebral glucose utilization, DC potential and extracellular potassium concentration in experimental head injury of varying severity. Neurosurg Rev 12, Suppl 1: 393–399, 1989. [DOI] [PubMed] [Google Scholar]
- 80.Lam JM, Hsiang JN, Poon WS. Monitoring of autoregulation using laser Doppler flowmetry in patients with head injury. J Neurosurg 86: 438–445, 1997. [DOI] [PubMed] [Google Scholar]
- 81.Langlois JA, Sattin RW. Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC). J Head Trauma Rehab 20: 187–188, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Lassen NA. Autoregulation of cerebral blood flow. Circ Res Suppl 15: 201–204, 1964. [PubMed] [Google Scholar]
- 83.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 39: 183–238, 1959. [DOI] [PubMed] [Google Scholar]
- 84.Lassen NA. Control of cerebral circulation in health and disease. Circ Res 34: 749–760, 1974. [DOI] [PubMed] [Google Scholar]
- 85.Lassen NA, Christensen MS. Physiology of cerebral blood flow. Br J Anaesth 48: 719–734, 1976. [DOI] [PubMed] [Google Scholar]
- 86.Lazaridis C, Smielewski P, Steiner LA, Brady KM, Hutchinson P, Pickard JD, Czosnyka M. Optimal cerebral perfusion pressure: are we ready for it? Neurol Res 35: 138–148, 2013. [DOI] [PubMed] [Google Scholar]
- 87.Leão A. Spreading depression of activity in the cerebral cortex. J Neurophysiol 7: 359–390, 1944. [DOI] [PubMed] [Google Scholar]
- 88.Lewelt W, Jenkins LW, Miller JD. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J Neurosurg 53: 500–511, 1980. [DOI] [PubMed] [Google Scholar]
- 89.Marcano DC, Bitner BR, Berlin JM, Jarjour J, Lee JM, Jacob A, Fabian RH, Kent TA, Tour JM. Design of poly(ethylene glycol)-functionalized hydrophilic carbon clusters for targeted therapy of cerebrovascular dysfunction in mild traumatic brain injury. J Neurotrauma 30: 789–796, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Marmarou A. Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir Suppl 86: 7–10, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Marmarou A, Fatouros PP, Barzo P, Portella G, Yoshihara M, Tsuji O, Yamamoto T, Laine F, Signoretti S, Ward JD, Bullock MR, Young HF. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J Neurosurg 93: 183–193, 2000. [DOI] [PubMed] [Google Scholar]
- 92.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg 104: 720–730, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Mathew BP, DeWitt DS, Bryan RM Jr, Bukoski RD, Prough DS. Traumatic brain injury reduces myogenic responses in pressurized rodent middle cerebral arteries. J Neurotrauma 16: 1177–1186, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Mayevsky A, Manor T, Meilin S, Doron A, Ouaknine GE. Real-time multiparametric monitoring of the injured human cerebral cortex–a new approach. Acta Neurochir Suppl 71: 78–81, 1998. [DOI] [PubMed] [Google Scholar]
- 95.McHedlishvili G. Physiological mechanisms controlling cerebral blood flow. Stroke 11: 240–248, 1980. [DOI] [PubMed] [Google Scholar]
- 96.McHedlishvili GI, Mitagvaria NP, Ormotsadze LG. Vascular mechanisms controlling a constant blood supply to the brain (“autoregulation”). Stroke 4: 742–750, 1973. [DOI] [PubMed] [Google Scholar]
- 97.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol 25: 350–364, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mellander S. Functional aspects of myogenic vascular control. J Hypertens Suppl 7: S21–S31, 1989. [PubMed] [Google Scholar]
- 99.Menon DK, Schwab K, Wright DW, Maas AI, Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehab 91: 1637–1640, 2010. [Google Scholar]
- 100.Menyhart A, Makra P, Szepes BE, Toth OM, Hertelendy P, Bari F, Farkas E. High incidence of adverse cerebral blood flow responses to spreading depolarization in the aged ischemic rat brain. Neurobiol Aging 36: 3269–3277, 2015. [DOI] [PubMed] [Google Scholar]
- 101.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 56: 1746–1748, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Mueller SM, Heistad DD, Marcus ML. Total and regional cerebral blood flow during hypotension, hypertension, and hypocapnia. Effect of sympathetic denervation in dogs. Circ Res 41: 350–356, 1977. [DOI] [PubMed] [Google Scholar]
- 103.Muizelaar JP, Lutz HA 3rd, Becker DP. Effect of mannitol on ICP and CBF and correlation with pressure autoregulation in severely head-injured patients. J Neurosurg 61: 700–706, 1984. [DOI] [PubMed] [Google Scholar]
- 104.Muizelaar JP, Ward JD, Marmarou A, Newlon PG, Wachi A. Cerebral blood flow and metabolism in severely head-injured children. Part 2. Autoregulation. J Neurosurg 71: 72–76, 1989. [DOI] [PubMed] [Google Scholar]
- 105.Mun-Bryce S, Wilkerson AC, Papuashvili N, Okada YC. Recurring episodes of spreading depression are spontaneously elicited by an intracerebral hemorrhage in the swine. Brain Res 888: 248–255, 2001. [DOI] [PubMed] [Google Scholar]
- 106.Nawashiro H, Shima K, Chigasaki H. Immediate cerebrovascular responses to closed head injury in the rat. J Neurotrauma 12: 189–197, 1995. [DOI] [PubMed] [Google Scholar]
- 107.Newell DW, Aaslid R, Stooss R, Seiler RW, Reulen HJ. Evaluation of hemodynamic responses in head injury patients with transcranial Doppler monitoring. Acta Neurochirurgica 139: 804–817, 1997. [DOI] [PubMed] [Google Scholar]
- 108.Nilsson P, Hillered L, Olsson Y, Sheardown MJ, Hansen AJ. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab 13: 183–192, 1993. [DOI] [PubMed] [Google Scholar]
- 109.Offenhauser N, Windmuller O, Strong AJ, Fuhr S, Dreier JP. The gamut of blood flow responses coupled to spreading depolarization in rat and human brain: from hyperemia to prolonged ischemia. Acta Neurochir Suppl 110: 119–124, 2011. [DOI] [PubMed] [Google Scholar]
- 110.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res 68: 359–367, 1991. [DOI] [PubMed] [Google Scholar]
- 111.Overgaard J, Tweed WA. Cerebral circulation after head injury. 1. Cerebral blood flow and its regulation after closed head injury with emphasis on clinical correlations. J Neurosurg 41: 531–541, 1974. [DOI] [PubMed] [Google Scholar]
- 112.Ozawa Y, Nakamura T, Sunami K, Kubota M, Ito C, Murai H, Yamaura A, Makino H. Study of regional cerebral blood flow in experimental head injury: changes following cerebral contusion and during spreading depression. Neurol Med-Chirurgica 31: 685–690, 1991. [DOI] [PubMed] [Google Scholar]
- 113.Panerai RB, White RP, Markus HS, Evans DH. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke 29: 2341–2346, 1998. [DOI] [PubMed] [Google Scholar]
- 114.Park L, Koizumi K, El Jamal S, Zhou P, Previti ML, Van Nostrand WE, Carlson G, Iadecola C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 45: 1815–1821, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 15: 379–393, 2014. [DOI] [PubMed] [Google Scholar]
- 116.Price RS, Kasner SE. Hypertension and hypertensive encephalopathy. Handb Clin Neurol 119: 161–167, 2014. [DOI] [PubMed] [Google Scholar]
- 117.Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus 25: E7, 2008. [DOI] [PubMed] [Google Scholar]
- 118.Reinert M, Khaldi A, Zauner A, Doppenberg E, Choi S, Bullock R. High extracellular potassium and its correlates after severe head injury: relationship to high intracranial pressure. Neurosurg Focus 8: e10, 2000. [DOI] [PubMed] [Google Scholar]
- 119.Risher WC, Ard D, Yuan J, Kirov SA. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J Neurosci 30: 9859–9868, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Risher WC, Croom D, Kirov SA. Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia 60: 1709–1720, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogatsky GG, Sonn J, Kamenir Y, Zarchin N, Mayevsky A. Relationship between intracranial pressure and cortical spreading depression following fluid percussion brain injury in rats. J Neurotrauma 20: 1315–1325, 2003. [DOI] [PubMed] [Google Scholar]
- 122.Rogers ML, Feuerstein D, Leong CL, Takagaki M, Niu X, Graf R, Boutelle MG. Continuous online microdialysis using microfluidic sensors: dynamic neurometabolic changes during spreading depolarization. ACS Chem Neurosci 4: 799–807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 9: 231–236, 2013. [DOI] [PubMed] [Google Scholar]
- 124.Scheller D, Kolb J, Tegtmeier F. Lactate and pH change in close correlation in the extracellular space of the rat brain during cortical spreading depression. Neurosci Lett 135: 83–86, 1992. [DOI] [PubMed] [Google Scholar]
- 125.Schwarzmaier SM, Kim SW, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma 27: 121–130, 2010. [DOI] [PubMed] [Google Scholar]
- 126.Schwarzmaier SM, Terpolilli NA, Dienel A, Gallozzi M, Schinzel R, Tegtmeier F, Plesnila N. Endothelial nitric oxide synthase mediates arteriolar vasodilatation after traumatic brain injury in mice. J Neurotrauma 32: 731–738, 2015. [DOI] [PubMed] [Google Scholar]
- 127.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab 26: 1018–1030, 2006. [DOI] [PubMed] [Google Scholar]
- 128.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 81: 1065–1096, 2001. [DOI] [PubMed] [Google Scholar]
- 129.Sorond FA, Serrador JM, Jones RN, Shaffer ML, Lipsitz LA. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound Med Biol 35: 21–29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci 70: 1355–1359, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steffensen AB, Sword J, Croom D, Kirov SA, MacAulay N. Chloride Cotransporters as a Molecular Mechanism underlying Spreading Depolarization-Induced Dendritic Beading. J Neurosci 35: 12172–12187, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 30: 733–738, 2002. [DOI] [PubMed] [Google Scholar]
- 133.Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke 33: 2738–2743, 2002. [DOI] [PubMed] [Google Scholar]
- 134.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8: ra2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sunami K, Nakamura T, Kubota M, Ozawa Y, Namba H, Yamaura A, Makino H. Spreading depression following experimental head injury in the rat. Neurol Med-Chirurgica 29: 975–980, 1989. [DOI] [PubMed] [Google Scholar]
- 136.Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochirurgica 148: 255–268, 2006. [DOI] [PubMed] [Google Scholar]
- 137.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab 35: 1871–1881, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab 31: 2096–2105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–H308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34: 1887–1897, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ungvari Z, Koller A. Selected contribution: NO released to flow reduces myogenic tone of skeletal muscle arterioles by decreasing smooth muscle Ca2+ sensitivity. J Appl Physiol 91: 522–527, 2001. [DOI] [PubMed] [Google Scholar]
- 143.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab 28: 1071–1085, 2008. [DOI] [PubMed] [Google Scholar]
- 144.Vavilala MS, Tontisirin N, Udomphorn Y, Armstead W, Zimmerman JJ, Chesnut R, Lam AM. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care 9: 45–54, 2008. [DOI] [PubMed] [Google Scholar]
- 145.Villalba N, Sonkusare SK, Longden TA, Tran TL, Sackheim AM, Nelson MT, Wellman GC, Freeman K. Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function. J Am Heart Assoc 3: e001474, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vinall PE, Simeone FA. Cerebral autoregulation: an in vitro study. Stroke 12: 640–642, 1981. [DOI] [PubMed] [Google Scholar]
- 147.von Bornstadt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, Sandow N, Kura S, Eikermann-Haerter K, Endres M, Boas DA, Moskowitz MA, Lo EH, Dreier JP, Woitzik J, Sakadzic S, Ayata C. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron 85: 1117–1131, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Windmuller O, Lindauer U, Foddis M, Einhaupl KM, Dirnagl U, Heinemann U, Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain 128: 2042–2051, 2005. [DOI] [PubMed] [Google Scholar]
- 149.Woitzik J, Hecht N, Pinczolits A, Sandow N, Major S, Winkler MK, Weber-Carstens S, Dohmen C, Graf R, Strong AJ, Dreier JP, Vajkoczy P, COSBID Study Group. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology 80: 1095–1102, 2013. [DOI] [PubMed] [Google Scholar]
- 150.Yu GX, Mueller M, Hawkins BE, Mathew BP, Parsley MA, Vergara LA, Hellmich HL, Prough DS, Dewitt DS. Traumatic brain injury in vivo and in vitro contributes to cerebral vascular dysfunction through impaired gap junction communication between vascular smooth muscle cells. J Neurotrauma 31: 739–748, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998. [DOI] [PubMed] [Google Scholar]


