Abstract
Despite considerable progress in the understanding of endothelial barrier regulation and the identification of approaches that have the potential to improve endothelial barrier function, no drug- or stem cell-based therapy is presently available to reverse the widespread vascular leak that is observed in acute respiratory distress syndrome (ARDS) and sepsis. The translational gap suggests a need to develop experimental approaches and tools that better mimic the complex environment of the microcirculation in which the vascular leak develops. Recent studies have identified several elements of this microenvironment. Among these are composition and stiffness of the extracellular matrix, fluid shear stress, interaction of endothelial cells (ECs) with pericytes, oxygen tension, and the combination of toxic and mechanic injurious stimuli. Development of novel cell culture techniques that integrate these elements would allow in-depth analysis of EC biology that closely approaches the (patho)physiological conditions in situ. In parallel, techniques to isolate organ-specific ECs, to define EC heterogeneity in its full complexity, and to culture patient-derived ECs from inducible pluripotent stem cells or endothelial progenitor cells are likely to advance the understanding of ARDS and lead to development of therapeutics. This review 1) summarizes the advantages and pitfalls of EC cultures to study vascular leak in ARDS, 2) provides an overview of elements of the microvascular environment that can directly affect endothelial barrier function, and 3) discusses alternative methods to bridge the gap between basic research and clinical application with the intent of improving the translational value of present EC culture approaches.
Keywords: endothelial barrier, acute respiratory distress syndrome, cell culture
the endothelium forms a thin layer of single cells that separates the vascular lumen from the surrounding tissues. This monolayer of tightly adhering endothelial cells regulates, in addition to a spectrum of other processes, the barrier properties of the vascular wall. In the healthy state, the endothelium regulates the passage of fluid and nutrients to and from the tissue. The rate of exchange depends on several factors, including the organ-specific nature of endothelial cells and the mode of transport across the endothelium (via transcellular vs. paracellular routes). In pathophysiological conditions, endothelial barrier function may be severely impaired, resulting in acute vascular leak as seen in severe sepsis and anaphylactic shock or chronic vascular leak as seen in diabetic retinopathy or atherosclerosis. The resulting excess of interstitial fluid (tissue edema) may result in severe organ damage and carries with it high morbidity and mortality. There is no direct therapy targeting vascular leak despite our understanding of endothelial barrier function at cellular and molecular levels. This review focuses on the question whether the present approaches to study endothelial barrier function in vitro can be improved to advance our understanding of endothelial permeability and to yield new therapies.
The lungs are particularly vulnerable to vascular leak and fluid accumulation, which cause interstitial and ultimately alveolar edema and thereby compromise gas exchange. Although the pulmonary lymphatics have an enormous draining capacity (138), pulmonary capillary leak associated with failure of the lymphatic system often results in flooding of the alveolar space with direct impairment of gas exchange and respiratory failure, the underlying characteristics of acute respiratory distress syndrome (ARDS) (10). ARDS may be precipitated by factors as diverse as sepsis, pneumonia, pancreatitis, drug toxicity, and multiple blood transfusions. Besides pathophysiological events like inflammation and microthrombosis, disruption of the pulmonary endothelial barrier is at the center of ARDS pathophysiology (95). The disruption of the pulmonary endothelial barrier results in widespread pulmonary edema and the presence of proteinaceous fluid in the alveolar space observed in the acute phase of ARDS (0–72 h). Mechanisms underlying disruption of the pulmonary endothelial barrier include defective cell-cell contacts at the level of adherens junctions (AJs), contraction of endothelial cells by increased actomyosin contractility, and dissociation or denuding of cells from the extracellular matrix (ECM). In parallel, other structural components of the barrier like the glycocalyx and ECM are degraded. Together these processes result in the formation of large intercellular gaps and, if the insults persist, cell detachment, apoptosis, and pyroptosis (Fig. 1). For an overview of the factors underlying endothelial barrier disruption and strategies to reverse endothelial barrier disruption, we refer the reader to recent reviews on this topic (50, 98, 99).
Fig. 1.
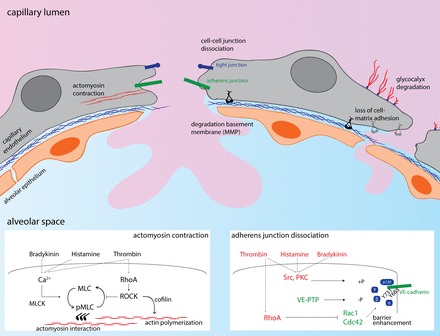
Mechanisms of endothelial barrier disruption at the level of alveolo-capillary barriers. The alveolo-capillary barrier exists at the pulmonary microvascular endothelium and a layer of type I alveolar epithelial cells (which cover 95% of the alveolar epithelial surface area). The basolateral side of both cell layers faces a thin sheet of extracellular matrix. The most important and extensively studied mechanisms for barrier disruption are depicted here, with the inserts showing major signaling events during actomyosin contraction and adherens junction dissociation, with barrier-disruptive signaling shown in red and barrier-protective signaling shown in green. MLC, myosin light chain; MLCK, myosin light chain kinase; MMP, matrix metalloprotease; RhoA, Ras homolog family member A; ROCK, Rho kinase; PKC, protein kinase C; VE-PTP, vascular endothelial protein tyrosine phosphatase.
Reviewing the scientific progress on endothelial barrier dysregulation in ARDS suggests two conclusions. The first is that studies from the last 30 years have identified several factors in barrier regulation, such as the composition and regulation of cell-cell junctions, the actomyosin contractile apparatus in endothelial cells, and the activation of signaling proteins like RhoGTPases, kinases, and phosphatases. The second is that, even with better understanding of endothelial permeability, there are as yet no clinical strategies to protect the endothelial barrier and reverse the course of ARDS. Recent ARDS trials still show unacceptably high mortality rates, ranging between 35–41% (43, 162). The reasons for the shortcoming are unclear. One explanation may be that the vast majority of experimental therapies proposed were directed at modulating inflammation and the immune response (44, 60, 74) as opposed to the leaky endothelial barrier. However, even therapies that focused on protection of the endothelium have failed to reduce ARDS mortality, as evidenced by late trials on activated protein C (119), statins (97, 108), and β2-adrenergic agonists (46). An alternative explanation may be the translational gap between basic science and clinical research. Factors such as culture conditions used for endothelial monolayer studies or even in vivo studies may not have mimicked the complexity of clinical conditions. Recent reviews have addressed the question why animal models have failed to provide adequate treatment (94), and other reviews have provided useful guidelines for animal models more suitable for studies of ARDS (96).
Here we will discuss the advantages and disadvantages of endothelial cell cultures as models of ARDS with the intent of improving the models. The second part of the review provides an overview of the microvascular environment that affects endothelial barrier function and is necessary to consider. The last part will address novel culturing approaches that aim to incorporate elements of the microvascular environment to better mimic (patho)physiological conditions. In this part, we will also discuss the attempts to optimize the relevance of the cell-based studies by using patient-derived cells. Optimizing endothelial cell approaches may narrow the translational gap.
Advantages and Pitfalls of Endothelial Cell Culture Approaches
Regulation of endothelial barrier function was well studied before endothelial cell culture was feasible (92, 12) (for review see Ref. 65). The development of endothelial cell isolation methods in 1973 (70) and the availability of primary endothelial cells were key conditions that spurred the field and have contributed to understanding of ARDS pathophysiology.
Advantages and value of endothelial cell study.
For overview on the present understanding of endothelial barrier regulation, we refer to comprehensive reviews on endothelial barrier regulation in general (5, 98, 99) and in the pulmonary microvessels (17, 34). It is clear that in vitro studies have importantly contributed to our understanding of endothelial barrier regulation, stressing the importance and relevance of endothelial cell culture studies.
A first advantage of cell culture studies is the ready accessibility of cultured endothelial cells for cellular imaging with high spatiotemporal resolution. The visualization of subcellular processes like activation/inactivation of monomeric GTPases (13, 141, 147), trafficking of caveolae in endothelial cells (101, 169), internalization of membrane proteins (47), phosphorylation of AJ proteins (84), and turnover of focal adhesions (164) is largely limited to cultured endothelial cell studies. In vivo imaging of fast processes like calcium dynamics in mouse lungs is feasible (83, 125) but is still limited in application because of poor resolution. The specificity and accessibility of cultured endothelial cells are essential for biochemical analyses of signaling proteins like kinases, phosphatases, and small GTPases (71). Although these studies can be performed on whole organ tissue, the presence of other cell types can result in a false positive signal when the measured activity is increased in nonendothelial cells or a false negative signal when the signal of the activity does not exceed the background noise. Thus cell-based studies have been invaluable in identifying molecular mechanisms but require confirmation in in vivo experiments to establish the relevance in humans.
Second, as endothelial monolayers can be cultured on a large scale and in a short period, the cells are an ideal platform for high-throughput screenings. Several studies have established this as an informative approach for identifying the barrier-modulating properties of drugs (52, 85). Endothelial barrier parameters suitable for high-throughput screening include transendothelial electrical resistance measurements (137), macromolecule passage, or automatized morphometric analyses. A growing number of pharmaceutical companies allow research institutions free access to their compound libraries (81, 123). Such screenings may provide an effective first step in developing therapies for vascular leak. In parallel with improving in vivo techniques, endothelial cell cultures combined with novel approaches such as microfluidics are expected to become the mainstay of endothelial barrier research in the coming years.
In addition to the methodological advances, the value of endothelial cell studies is evident by a number of clinical studies emerging from cell-based studies. A recent early clinical study demonstrated the protective effect of interferon-β-1a on pulmonary vascular leak in patients with ARDS (15) after its protective effect was discovered in endothelial studies (80). We recently demonstrated that imatinib protects the endothelial barrier under inflammatory conditions (6, 7) after two clinical cases reported fast resolution of pulmonary edema upon initiation of imatinib therapy (27, 113). In vitro studies provided the mechanistic underpinnings for clinical observations, paving the way for clinical translation. In a similar fashion, in vitro studies can provide mechanistic insights into the failure of clinical therapies in ARDS trials, such as the recent trials on statins and β2-adrenergic agonists (46, 97, 108). Statins were suggested to benefit both inflammation and vascular injury (144), but endothelial cell studies showed that the protective effects of statins on the endothelial barrier were only attained at very high concentrations and after prolonged treatment (69, 151b). In another endothelial cell study, in vitro findings that endothelial β2-adrenergic receptors are quickly downregulated upon endothelial stimulation with β-adrenergic agonists (73) provided insights into the lack of efficacy of β2-adrenergic agonists in ARDS (46). These studies illustrate the translational value of properly designed endothelial cell studies. Thus, in addition to advantages at the methodological level, the use of cultured endothelial cells offers unique opportunities for drug development and interpretation of clinical observations.
Pitfalls of relying solely on endothelial cell culture studies.
The main drawback of endothelial cell studies is that the cells, when extracted, lose important in situ characteristics. The differences in endothelial cell characteristics between extracted cells and their in vivo counterparts and the consequences of these differences for barrier regulation have been recently reviewed by Uhlig et al. (151). The translational value of in vitro endothelial studies may be limited by the following factors: 1) the use of endothelial cells from different species or different organs than the lung, 2) the use of agents to induce endothelial barrier disruption that have limited relevance in in vivo conditions or clinical vascular leak seen in ARDS, and 3) the shift in balance between signaling pathways involved in endothelial barrier regulation that is provoked by cell environmental and epigenetic changes.
The latter concern poses the greatest challenge to using endothelial cell cultures. In situ, endothelial cells are part of a microvascular environment, and as such they are subjected to 1) influences of the ECM and the glycocalyx; 2) the presence of other cell types that directly or indirectly interact with endothelial cells (pericytes, alveolar epithelial cells, fibroblasts, macrophages); 3) chemical stimuli, such as varying oxygen levels, paracrine signaling, and plasma constituents; and 4) mechanical forces like shear stress from the blood flow and cyclic stress from ventilation (Fig. 2). As the majority of these factors are usually absent in in vitro endothelial studies, the cells undergo major changes at the level of epigenetic profile (72), gene expression (132), and protein expression (36), which can affect barrier function in unknown ways.
Fig. 2.
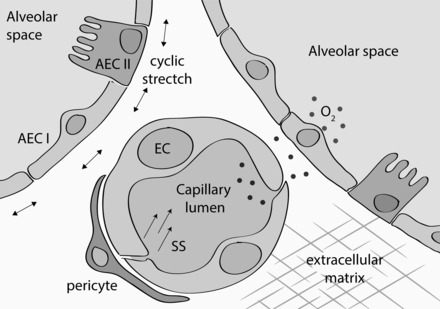
The microenvironment of lung microvascular endothelial cells. Depiction of the various elements that directly influence endothelial barrier function. AEC I, type I alveolar epithelial cell; AEC II, type II alveolar epithelial cell; EC, endothelial cell; SS, shear stress.
In the following sections, we discuss how elements of the microvascular environment influence endothelial barrier function and how these may be incorporated in novel endothelial cell culture strategies.
The Role of Microvascular Environment in Endothelial Barrier Regulation
Upon isolation, endothelial cells, which are typically “plastic” or highly changeable, undergo major changes in the extracellular environment and lose interaction with other cells. The effect of these changes on endothelial barrier function and regulation is still poorly understood but should be taken into account.
Influence of flow on endothelial cell barrier function.
Because of the presence of blood flow, mechanical forces exerted on endothelial cells in situ markedly differ from endothelial cells cultured under static conditions. At the interface of the flow with the endothelial cells, flow exerts a dragging force on the endothelium called fluid shear stress (31). Endothelial cells possess multiple mechanosensitive complexes such as Piezo channels that translate the shear stress into regulatory intracellular signals (150). The effects of shear stress on barrier function are largely determined by flow characteristics (87). Reviewing data on the effects of shear stress on endothelial barrier function, one should bear in mind that part of the in vitro studies were performed with aortic endothelial cells in the context of atherosclerosis. This context differs substantially from endothelial barrier regulation in the pulmonary microcirculation in which flow patterns (blood velocity and pulsatility are low) and endothelial phenotype are different compared with the aortic or peripheral microvascular cells.
The effect of shear stress on endothelial barrier function depends on both the velocity of the flow and nature of the flow (laminar vs. turbulent flow). Physiological shear stress attributable to laminar flow results in a slight enhancement of barrier function in cultured nonpulmonary (24, 26, 76) and pulmonary endothelial cells (53). The enhancement in the barrier may be preceded by a fast, temporary decrease in barrier function (100, 154), which is paralleled by alignment of the endothelial cells in the direction of the flow. Schwartz and others (30, 150) showed that intracellular signaling downstream from the vascular endothelial (VE)-cadherin/PECAM-1/VEGFR2 mechanosensory complex reduced the actomyosin contractile force at the level of the AJs, leading to stabilization of junctions. This is paralleled by activation of Rap1 (131) and stabilization of VE-cadherin at the cell junctions (2, 30, 41, 100). Alternatively, the presence of glycocalyx may explain the flow-induced improvement in barrier function. Glycocalyx is a meshwork of proteoglycans on the luminal exterior of the endothelial cell that determines endothelial barrier properties (127). The glycocalyx is involved in endothelial mechanosensing (142) and the endothelial inflammatory response (105). Formation and maintenance of the glycocalyx depend on the presence of flow (56, 165). This flow dependency may explain why flow enhances barrier function and why glycocalyx is often absent in cultured endothelial cells (117). Finally, shear stress may improve endothelial barrier function via phosphatidylinositol-3-kinase-nitric oxide-cGMP signaling (154), reduced intracellular stress (139), and upregulation of Sox18 (53), all of which enhance the endothelial barrier by driving the expression of the tight junction protein claudin-5 (45). In contrast to physiological shear stress, barrier disruption was observed under nonphysiological shear stress attributable to turbulent flow (2, 41, 100) or supraphysiological shear stress (167). These findings together demonstrate that flow affects various endothelial structures and pathways involved in barrier regulation. The presence of physiological flow may directly enhance the barrier properties of cultured endothelial cells compared with absent or disturbed flow.
ECM composition as regulator of endothelial barrier function.
Adhesion to the ECM is critical for endothelial cell survival and has potentially important consequences for the stability of the endothelial barrier. The ECM consists of a fine meshwork of filamentous proteins including laminin, collagen IV, perlecan, fibronectin, fibrin, and vitronectin (32, 75). The endothelial cytoskeleton is linked to the ECM via integrins, dimeric proteins consisting of α- and a β-subunits (8, 66, 133). The phenotype of transgenic mice lacking or defective in one or more of these ECM proteins is vascular instability and hemorrhage (116), stressing the relevance of the ECM for vessel formation. However, most of these studies were carried out in the context of embryonic development in which the regulation of the endothelial barrier is substantially different from the adult barrier.
Besides the effects of the composition and stiffness of ECM on vascular stability, there is growing evidence that specific characteristics of the ECM also affect endothelial barrier properties directly. Stiffening of the ECM occurs under various conditions, including atherosclerosis and calcification of the vascular wall, increased smooth muscle cell content, and changed composition of the ECM attributable to aging. In cultured endothelial cells, the use of “hard” culture plastics or matrices containing high concentrations of collagen leads to high ECM stiffness (for review see Ref. 63). Endothelial cells grown on these stiff substrates show enhanced RhoA activity (64, 82) and RhoA-myosin light chain (MLC)-driven actin polymerization, resulting in increased cell contractility and cellular stiffness (64). The increase in intracellular forces in turn leads to enhanced tension at cell-cell junctions (88), marked disruption of junctions, and enhanced permeability in response to barrier-disruptive agents such as thrombin (82). This in turn may lead to increased tension inside endothelial cells, setting up an amplification loop. The influence of ECM is not limited to endothelial barrier function alone but also involves related processes such as transendothelial migration of inflammatory cells (63).
Much more work is needed in this area. Studies need to define the role of ECM on the endothelial barrier in general. Although stiffness of lung tissue normally is only at an intermediate level compared with other organs (140), pathophysiological mechanisms such as fibrosis enhance ECM stiffness. Decreased lung tissue compliance observed during ARDS (10) underscores the need to study the effects of ECM stiffness on barrier function. Ingber and colleagues (93) recently demonstrated increased ECM rigidity in mice lung upon exposure to LPS caused by lysyl oxidase-mediated collagen cross linking. In this model, vascular leak could be reversed by lysyl oxidase inhibition (93), supporting the notion that ECM stiffness is a critical determinant of endothelial barrier function.
The effect of cyclic stretch on endothelial barrier function.
Endothelial cells in blood vessels are subjected to continuous cyclic stretch because of either the pulsatile movement of the arterial wall (at the level of arterial endothelial cells) or the repetitive expansion and collapse of the alveoli during normal breathing (at the level of lung microvascular endothelial cells). In conjunction with the stiffness of the ECM discussed above, these cyclic movements also alter the mechanical properties of the endothelial microenvironment.
In lung endothelial cells, the response to cyclic stretch from alveolar breathing motions depends on the level of stretch. Compared with static conditions, physiological stretch (5% increase) induces Rac1 activation and cortical actin ring formation (20, 131) and attenuates thrombin-induced barrier disruption (19, 131). Pathophysiological stretch (18% increase) resulted in enhanced RhoA expression, RhoA activity, and MLC phosphorylation (21, 23) and increased the magnitude of barrier disruption induced by thrombin (19) and interleukin-6 (23). Paxillin and p190RhoGAP were shown to mediate stretch-induced changes in RhoGTPases (22, 48).
To our knowledge, the combined influence of enhanced ECM stiffness and cyclic stretch has not been determined. However, as both ECM stiffness and cyclic stretch “signal” via RhoGTPases, it is expected that these factors will interact. One possibility is that physiological cyclic stretch may lead to pathophysiological stretch in the presence of a stiffer ECM. This concept may explain the clinical observation that stiff lungs are at a higher risk for ventilator-induced endothelial injury and loss of endothelial barrier.
The effect of oxygen tension on endothelial cell barrier function.
The effect of oxygen tension on endothelial cells in culture has been predominantly studied in the context of hypoxia and angiogenesis. The majority of endothelial cell studies do not take into account the effect of oxygen tension and are typically carried out in ambient air. Although there is only a small drop in oxygen tension from ambient air (21 kPa) to oxygen tension in the alveolar space (20 kPa), oxygen tension is significantly lower in the circulation (5–13 kPa) and even lower at the level of peripheral tissue (0.5–2.5 kPa) (135). Thus, even for endothelial cells obtained from the pulmonary microcirculation in which the estimated oxygen tension is 13 kPa (135), culturing endothelial cells in ambient air with an oxygen tension of 21 kPa induces a state of relative hyperoxia.
Oxygen tension may affect endothelial barrier function in several ways. Early studies have already indicated that hypoxia impairs the endothelial barrier both in cultured cells (67, 111) and in isolated microvessels (118, 149). This effect was mediated by production of oxygen radicals (12, 67) and by decreased cellular production of cAMP (111). The finding that ischemia-induced enhanced permeability occurs even in pulmonary venules at close proximity to alveoli with normal oxygen tension (118) indicates that the lung microvascular endothelium critically depends on blood-derived oxygen. It should be taken into account that the insult of ischemia, in which a decrease in oxygen tension is combined with nutrient deprivation (118), differs from hypoxia, in which there is only a decrease in oxygen tension (67, 111, 149). Hyperoxia may also have detrimental effects as shown by a study in which exposure of pulmonary artery endothelial cells to increasing levels of oxygen tension resulted in enhanced endothelial permeability (115), albeit at extremely high oxygen levels (up to 95%). The hyperoxic effects on the endothelial barrier were mediated by reactive oxygen species (115). The role of oxidative stress and intracellular signaling upon EC exposure to reactive oxygen species is reviewed elsewhere (25, 102). In particular, oxygen tension was shown to be an important determinant in the activational state of the RhoGTPases RhoA, RhoB, and Rac1 (157, 158).
In contrast to earlier studies, a recent study has demonstrated that hypoxia-inducible factor 2α (HIF-2α), a transcription factor that is stabilized during hypoxia, protects the endothelial barrier by preserving AJs in a vascular endothelial protein tyrosine phosphatase (VE-PTP)-dependent fashion (51). Mice lacking HIF-2α had an exaggerated response to lung inflammation, as measured by transvascular albumin flux and leukocyte extravasation. These effects could be recapitulated by inhibition of prolyl hydroxylase domain 2 (PHD2) (51), the PHD responsible for HIF-2α breakdown under normoxia. These recent data show that hypoxia may also have a preserving effect on the endothelial barrier.
In summary, both hypoxia and hyperoxia affect endothelial barrier function, mainly by oxygen radical formation and altering the activation state of RhoGTPases. Because oxygen tension importantly determines barrier function and because even lung endothelial cells critically depend on blood-derived oxygen, the oxygen level is an important determinant of endothelial barrier function.
Interaction with other cell types.
Neighboring cells play an important (but not fully appreciated) role in endothelial barrier regulation. As part of the microvascular environment, proximal cells such as fibroblasts and pericytes form cellular support for vascular stability. Vascular smooth muscle cells also interact with endothelial cells with hemidesmosomes and gap junctions (126). Epithelial cells interact with endothelial cells to regulate passage of solutes to and from the organ tissue (e.g., renal podocytes, alveolar epithelial cells, and hepatic stellate cells). These paracrine interactions may importantly influence endothelial barrier regulation.
The contribution of pericytes to endothelial barrier regulation remains controversial, as coculture studies have yielded conflicting results. In systemic vessels, pericytes were shown to stabilize the vessels (9) and protect against endothelial barrier disruption via upregulation of the tight junction protein zona occludens 1 (152). Recent unpublished studies by Kruse et al. showed that the N-cadherin interaction between pericytes and endothelial cells increased the adhesion of VE-cadherin junctions. Other studies showed that pericytes disrupted the brain endothelial barrier (78, 90) via the production of matrix metalloproteinase 9 and VEGF (91, 145, 170). Both barrier protection and barrier disruption were attributed to paracrine signaling (152, 170), whereas ECM production by pericytes enhanced endothelial barrier function (54). In the pulmonary vasculature, pericytes were shown to stabilize microvessels grown from lung microvascular cells (18), suggesting a supportive role of pericytes in lung microvessels. Conversely, pericytes may contribute to formation of aberrant vessels in pulmonary arterial hypertension (121) and mediate inflammation by producing cytokines during sepsis (37, 77). In a coculture model, pericytes failed to stabilize endothelial tubes, resulting in rarefaction (163). The dual effects of pericytes may be explained by differences in pericyte differentiation (146) and by the possibility that pericytes may themselves be activated and thus may modify endothelial function (37). The limited work on pericytes thus far precludes a definitive conclusion on the contribution of pericytes in regulating endothelial barrier and stresses the need for future research on pericyte interaction with the endothelium.
Little is known about the effects of epithelial cells on endothelial barrier function and in particular the role of the alveolar epithelium, which is nearly opposed to endothelial cells in lung microvessels. Several studies have described methods for coculturing endothelial cells with alveolar epithelial cells (57, 58), but the focus in these studies has been on the effect of endothelial cell-derived mediators on the alveolar epithelial barrier or on combined effects of alveolar epithelial cells and endothelial cells on alveolo-capillary barriers (29, 109). One study has directly investigated the effect of cocultured epithelial cells on endothelial barrier function. Wang and colleagues (153) demonstrated that endothelial barrier disruption in response to sepsis is attenuated by coculturing endothelial cells with epithelial cells (A549 cell line). These effects could be recapitulated by exposure of endothelial cells to epithelial cell conditioned medium (153), indicating that the effect is driven by soluble mediators.
Several groups showed that epithelial cells form an important chain in inflammatory signaling to the pulmonary microvascular endothelium. Exposure of alveolar epithelial cells to carbon or metal particles induced upregulation of proinflammatory cytokines in the underlying endothelium (16, 136). Exposure of the alveolar epithelium to TNF-α (83) or hydrochloric acid (156) induces endothelial activation in a gap junction- and calcium-dependent manner (56, 83). Leukocyte transmigration elicits interaction between epithelial and endothelial cells by release of interleukin-6 and interleukin-8, respectively, which promote leukocyte transmigration (104). Another study showed that leukocyte transmigration was reduced when the proximity of the epithelial and endothelial layer was reduced (155), indicating that formation of a barrier in series enhances the function of the barrier that comprises both cells. In the context of ARDS, the distinct contribution of the alveolar epithelial and the endothelial barrier in disruption of alveolo-capillary barriers remains an important question. Thus the presence of nonendothelial cells in culture, such as pericytes and underlying epithelial cells, may have a profound influence on endothelial barrier regulation.
Novel Methods to Optimize Culture Conditions
To optimize the microenvironment of endothelial cells in culture, it is important to enhance the endothelial monolayer conditions used to study the barrier. Although specific elements such as flow, stretch, and matrix stiffness have to some extent been used, recent efforts in tissue engineering, microfabrication, and biomaterial sciences (124) have contributed to the development of sophisticated culturing systems (134) that integrate multiple elements.
Microfluidics and cultured microvessels.
“Microfluidics” is a technique used to manipulate fluids at a microscopic scale. On the basis of the development of blood vessels at a microscopic scale, assays and devices that have high control of the cell microenvironment can be developed, thereby allowing detailed monitoring of spatiotemporal regulation of endothelial cell dynamics. Additional advantages are the small volume of reagents and the small number of cells required as well as the ability to run multiple experiments in parallel. The latter advantage may provide the basis for screening barrier-modifying agents and drugs (124). Although Sackmann and colleagues (124) conclude that microfluidics is still in its early stages as a tool for addressing important biological questions, its value in addressing questions in endothelial cell biology has become evident by a number of recent publications.
One value of this approach is described in a study by Rexius-Hall et al. (120). Here they developed a microfluidic platform to generate an oxygen landscape to study the effects of oxygen tension variations on endothelial cells and described its utility in the real-time analysis of cell responses and cell-cell interactions within physiologically relevant oxygen landscapes. Although various methods for constructing microdevices have been described, in all these devices endothelial cells are grown in a biomaterial scaffold that supports adhesion of endothelial cells in a precast lumen or a set of lumina (Fig. 3). Microvessels are formed when endothelial cells cover the whole lumen (168) or via the formation of sprouting angiogenesis (86). The three-dimensional construction of these devices allows for application of continuous and controlled perfusion, changes in oxygen levels, variations in extracellular stiffness, and coculture with other cell types (168). Thus conditions can be adjusted to mimic organ environment and disease conditions as shown for the blood-brain barrier (161), atherosclerosis (41), and the tumor vasculature (166). Direct comparison with in vivo blood vessels shows that endothelial cells grown in a microfluidic system recapitulate well endothelial characteristics in vivo (1).
Fig. 3.
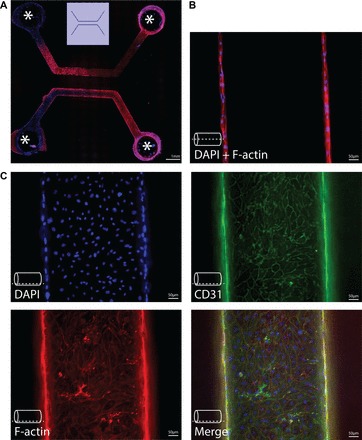
Endothelialized microvessels. Immunofluorescence imaging of endothelialized microvessels in vitro. Endothelial cells were grown in collagen casts forming microvessels with a diameter of 500 μm, as described (101). A: example of a cast for 2 parallel-running, linear microvessels, covered with human skin microvascular endothelial cells. Openings in the cast (indicated by asterisk) allow for perfusion of these microvessels. B: immunofluorescence imaging at middle z-height of a microvessel, showing coverage of opposing vessel walls with endothelial cells. The dashed line indicates the z-height at which images were obtained. C: immunofluorescence imaging of cell markers at the bottom of a microvessel. DAPI was for nuclear staining, F-actin for staining of the actin cytoskeleton, CD31 staining as an endothelial marker. The dashed line indicates the z-height at which images were obtained.
Endothelial barrier function can be measured in these set-ups by determining extravasation of tracer molecules (86, 130) or by cell-cell junctional integrity with immunofluorescence imaging (1). The application of barrier-disruptive agents [such as histamine (130) or TNF-α (86)] or barrier-protective agents [like cAMP (159) or resveratrol (1)] demonstrates that microfluidic devices provide a good platform to study endothelial barrier dynamics (159). The coculture of endothelial cells with alveolar epithelial cells (106) and the creation of a liquid-air interface (107) also create an environment that more realistically mimics alveolo-capillary barriers (128), rendering these devices of particular interest for the study of endothelial barrier regulation of the epithelial barrier or vice versa. Importantly, the use of microfluidic systems may not be limited to endothelial barrier regulation and vascular leak alone. Work of Tsa and colleagues and Zheng and colleagues (148, 168) demonstrated that microvascular occlusion and thrombosis can be induced in microfluidic systems, indicating that microfluidic culture systems provide a study platform for various pathophysiological processes in ARDS. A recent publication described a detailed protocol on the formation of microvascular networks in vitro (103).
Lung-on-a-chip.
Another important advancement is the development of organs on a chip. These functional organ units allow for drug testing on a large scale (“medium-level throughput”) and can be enriched with human cells and serum derived from patients (40), allowing a personalized medicine approach. Functional units at the chip level have been developed for a wide variety of organs (129), giving rise to the idea that connecting these functional units in series may yield a human on a chip (39). An elegant example of drug screening based on patient-derived microorgans is the recently developed assay that predicts treatment response in patients with cystic fibrosis (33).
For pulmonary edema and lung injury studies, such an integrative approach has been pioneered by Ingber and colleagues (61, 62), the so-called lung-on-a-chip model. They designed a microsystem that recapitulated alveolar characteristics, including surfactant synthesis, ventilatory stretch, pulmonary circulation, and alveolo-capillary air-liquid interface (61). The core of this system is a microporous, polydimethylsiloxane membrane on which alveolar epithelial cells are grown on the one side and endothelial cells are grown on the other side, with air- and fluid-filled channels passing over the sides (Fig. 4). Vacuum side chambers induce cyclic stretch, mimicking respiratory movement of the alveolus. The formation of intact cell monolayers as evidenced by continuous occludin and VE-cadherin staining at cell junctions as well as the intact leukocyte transmigration over this barrier indicated that the microsystem simulates the functional characteristics of the alveolo-capillary barriers (61).
Fig. 4.
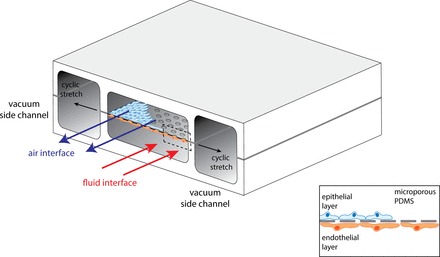
The lung-on-a-chip model. The model contains 4 parallel-running compartments, with 2 vacuum channels on the outer side of the model and 2 channels at the inner side of the model. The 2 inner channels are separated by a microporous permeability thickness dependence of polydimethylsiloxane (PDMS) membrane on which alveolar epithelial cells and endothelial cells can be cultured, mimicking the alveolocapillary barrier. By applying flow of air (upper channel) and fluid (lower channel), one exposes alveolo-capillary barriers to ventilation and circulation. With application of cyclic vacuum to the side channels, cyclic stretch is exerted on the alveolo-capillary barriers. [Adapted from Huh et al. (61, 62).]
Exposing the alveolo-capillary membranes to a combination of mechanic (cyclic stretch) and toxic (IL-2) stimuli demonstrated that this microsystem is useful for the study of ARDS, as several ARDS characteristics can be faithfully reproduced, e.g., endothelial barrier breakdown, alveolar flooding, formation of microthrombi. and oxygen desaturation (62). This system provided important information concerning the interplay between mechanical and toxic injury, which are by themselves insufficient to induce barrier disruption. Finally, drug testing was shown to be feasible in this model, and the effects could be reproduced in mouse models of lung vascular injury and pulmonary edema.
These culture techniques demonstrate that considerable progress has been made in 1) recapitulating many of the physiological conditions in culture, 2) improving pathophysiological relevance of disease models, and 3) improving the translational value by using patient-derived cells for a personalized medicine approach.
Evaluation of novel methods.
Compared with static cell culture methods, the techniques described above have made major steps in recapitulating in situ conditions, as they have incorporated physiological variables like flow, presence of other interacting cells, and adjustable matrix stiffness. As such, the microfluidic and the lung-on-a-chip approaches offer important advantages in that they provide integration of multiple physiological variables that are absent in the static culture. This results in, not only better understanding of complex (patho)physiological mechanisms, but also better conservation of the in situ endothelial characteristics. Validation experiments showed that experimental ARDS can in many ways be recapitulated in these culture approaches (62). When using human or even patient-derived cells, we anticipate that these models may even better reflect disease conditions than animal models do. As an additional advantage, the novel methods allow rapid accessibility to different cell types (via different compartments) for biochemical analyses and permit immunofluorescence analysis over time, providing longitudinal data.
The novel culture techniques described above are still in the development stage and need further validation to establish whether they better reflect in vivo conditions than present methods. Several issues need to be addressed, such as whether the endothelial monolayers grown in these models develop a glycocalyx, which is a strong indicator of physiological flow and the health of the endothelium. Another issue is whether the microfluidic models adequately simulate the complex branching patterns and flow variations of the pulmonary microcirculation and whether they allow assessment of the role of matrix stiffness and flow profiles in mediating endothelial injury. We expect that the answers to these questions, together with the incorporation of patient-derived cells, will importantly determine the place of these culture techniques in future translational ARDS research.
Use of Patient-Derived Cells
Besides innovation of cell culture techniques, the translational value of cell studies can be enhanced by using appropriate cell types. This includes among others the use of endothelial cells isolated from relevant organs and patient-derived cells. The concept of endothelial heterogeneity is well established in that the vascular tree harbors a great variety in morphology, histology, and function, which is paralleled by a highly differentiated pattern of gene expression (28). It is clear that endothelial heterogeneity importantly regulates endothelial barrier function in different tissue (3), not only between organs, but also within organs, as shown for lungs (79) (reviewed in Ref. 4). In lungs, the most important differences have been observed between macrovascular endothelial cells (derived from conduit vessels) and microvascular cells (derived from small arteries, capillaries, and venules). Postinjury at the alveolo-capillary interface, microvascular endothelial cells are considered most relevant for the study of ARDS.
Lung microvascular endothelial cells were shown to differ from macrovascular endothelial cells with respect to chemokine production (14), nitric oxide production (49, 89), and leukocyte adhesion (112). Also for endothelial barrier function, marked differences were reported. Human lung microvascular endothelial cells in culture form a significantly more restrictive barrier as measured by transendothelial electrical resistance (41.3 ± 3.0 Ohm/cm2) compared with human umbilical vein endothelial cells (27.6 ± 3.8 Ohm/cm2) (151a). Similar differences were observed in rat lung microvascular endothelial cells as measured by hydraulic conductance determinations (114). Both differences in inflammatory response and endothelial barrier regulation suggest that lung microvascular cells are the most appropriate endothelial cell type for defining the underlying basis of ARDS. Unfortunately, not all available microvascular endothelial cells recapitulate these characteristics (M. van der Heijden, V. W. M. van Hinsbergh, G. P. van Nieuw Amerongen, unpublished data), likely attributable to the high plasticity of endothelial cells when removed from their environment. Otherwise, fresh isolation of cells may not always be possible, as ethical considerations preclude use of lung tissue from patients with ARDS for microvascular endothelial cell isolation. These cells if available would have excellent translational value.
For these reasons, alternative methods have been investigated to obtain patient-derived cells. Endothelial progenitor cells (EPCs) can be obtained from the blood of patients with relative ease and expanded in culture (11). Different subtypes of EPCs exist, including early outgrowth EPCs (160) and endothelial colony-forming cells (ECFCs) (143), which both were shown to possess characteristics of mature endothelial cells (double positive in VE-cadherin and Flk1 or CD31 and vessel-forming capacity). Also differences in nature and origin exist between early-outgrowth EPCs and ECFCs (59), which need clarification especially from an epigenetic perspective. Isolation protocols and phenotype of progenitor cells have been reviewed (42, 59). An apparent advantage of EPCs is that they have a high proliferation capacity (68) and retain disease phenotype in culture (35). Although the isolation and expansion of EPCs may take too long to provide patient-tailored therapy, these cells may provide a rich source of patient material that yields a high translational value in in vitro endothelial studies. Joint efforts are directed toward collecting patient-derived cells to provide an open source for preclinical translational studies (38).
Conclusions and Recommendations
Despite a number of inherent shortcomings, cultured endothelial cells are fundamental for ARDS research and for developing targeted therapies. Studies employing endothelial cells, not only form the basis of a bottom-up approach for drug development strategies, but also contribute to the understanding of the pathophysiology of the disease. These studies would be most fruitful when embedded in a translational setting with input from both the clinical side (bedside to bench) and from basic endothelial biology (bench to bedside). The main drawback of endothelial studies is that culture conditions do not always fully recapitulate the microvascular environment of endothelial cells found in situ and that models of barrier disruption do not fully reflect the complex pathophysiology of ARDS. In this review, we have provided an overview of the main differences between endothelial cells in vitro and in situ and how these differences may affect endothelial barrier function.
The use of cell culturing techniques and the development of novel methods to assess endothelial barrier function will improve the translational value of endothelial culture studies. This may involve the use of devices that closely mimic the in situ condition, incorporating physiological factors such as flow, stretch, and an optimal ECM environment. Defining endothelial heterogeneity and using cells isolated from specific vascular beds and studying them before change in their phenotype will be needed to address specific research questions. In addition, the use of primary and freshly isolated cells from patients for personalized medicine approaches is potentially important if hurdles and means of obtaining these cells can be overcome.
GRANTS
This work was funded by Dutch Heart Foundation grant number 2014T064 (J. Aman) and 2011T072 (G. P. van Nieuw Amerongen) and the Netherlands Institute for Regenerative Medicine (E. M. Weijers). In addition, this work was funded by grant numbers P01 HL60678, P01 HL077806, and R01 HL45638 (A. B. Malik).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A. and E.M.W. prepared figures; J.A. and G.P.v.N.A. drafted manuscript; J.A., E.M.W., G.P.v.N.A., A.B.M., and V.W.v.H. edited and revised manuscript; J.A., E.M.W., G.P.v.N.A., A.B.M., and V.W.v.H. approved final version of manuscript.
REFERENCES
- 1.Abaci HE, Shen YI, Tan S, Gerecht S. Recapitulating physiological and pathological shear stress and oxygen to model vasculature in health and disease. Sci Rep 4: 4951, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Sarai RK, Altangerel A, Clark JF, Weinbaum S, Curry FE. Microvascular permeability to water is independent of shear stress, but dependent on flow direction. Am J Physiol Heart Circ Physiol 304: H1077–H1084, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res 100: 174–190, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Amado-Azevedo J, Valent ET, van Nieuw Amerongen GP. Regulation of the endothelial barrier function: A filum granum of cellular forces, Rho-GTPase signaling and microenvironment. Cell Tissue Res 355: 557–576, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, Groeneveld AB, Vonk Noordegraaf A, van Hinsbergh VW, van Nieuw Amerongen GP. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation 126: 2728–2738, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Aman J, Peters MJ, Weenink C, van Nieuw Amerongen GP, Vonk Noordegraaf A. Reversal of vascular leak with imatinib. Am J Respir Crit Care Med 188: 1171–1173, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Aman J, van Nieuw Amerongen GP, van Hinsbergh VWM. Cell-matrix adhesion proteins in the regulation of endothelial permeability. In: Endothelial Cytoskeleton, edited by Rosado JA. Boca Raton, FL: CRC, 2013, pp. 167–199. [Google Scholar]
- 9.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature 468: 557–561, 2010. [DOI] [PubMed] [Google Scholar]
- 10.ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin Definition. JAMA 307: 2526–2533, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 29: 1650–1655, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Barnard ML, Matalon S. Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J Appl Physiol 72: 1724–1729, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Baumer Y, Spindler V, Werthmann RC, Bünemann M, Waschke J. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol 220: 716–726, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Beck GC, Yard BA, Breedijk AJ, van Ackern K, van der Woude FJ. Release of CXC-chemokines by human lung microvascular endothelial cells (LMVEC) compared with macrovascular umbilical vein endothelial cells. Clin Exp Immunol 118: 298–303, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellingan G, Maksimow M, Howell DC, Stotz M, Beale R, Beatty M, Walsh T, Binning A, Davidson A, Kuper M, Shah S, Cooper J, Waris M, Yegutkin GG, Jalkanen J, Salmi M, Piippo I, Jalkanen M, Montgomery H, Jalkanen S. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. Lancet Respir Med 2: 98–107, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Bengalli R, Mantecca P, Camatini M, Gualtieri M. Effect of nanoparticles and environmental particles on a cocultures model of the air-blood barrier. Biomed Res Int 2013: 801214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Bichsel CA, Hall SR, Schmid RA, Guenat OT, Geiser T. Primary human lung pericytes support and stabilize in vitro perfusable microvessels. Tissue Eng Part A 21: 2166–2176, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 155: 44–54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birukova AA, Zebda N, Cokic I, Fu P, Wu T, Dubrovskyi O, Birukov KG. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res 317: 859–872, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birukova AA, Tian Y, Meliton A, Leff A, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 302: L965–L975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth R, Noh S, Kim H. A multiple-channel, multiple-assay platform for characterization of full-range shear stress effects on vascular endothelial cells. Lab Chip 14: 1880–1890, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res 77: 26–34, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan CF, Verbridge SS, Vlachos PP, Rylander MN. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adh Migr 8: 517–524, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnevale-Schianca F, Gallo S, Rota-Scalabrini D, Sangiolo D, Fizzotti M, Caravelli D, Capaldi A, Anselmetti G, Palesandro E, D'Ambrosio L, Coha V, Obert R, Aglietta M, Grignani G. Complete resolution of life-threatening bleomycin-induced pneumonitis after treatment with imatinib mesylate in a patient with Hodgkin's lymphoma: Hope for severe chemotherapy-induced toxicity? J Clin Oncol 29: e691–e693, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 100: 10623–10628, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury F, Howat WJ, Phillips GJ, Lackie PM. Interactions between endothelial cells and epithelial cells in a combined cell model of airway mucosa: Effects on tight junction permeability. Exp Lung Res 36: 1–11, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol 23: 1024–1030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis GE, Senger DR. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97: 1093–1107, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Duluc L, Wojciak-Stothard B. Rho GTPases in the regulation of pulmonary vascular barrier function. Cell Tissue Res 355: 675–685, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Duong HT, Comhair SA, Aldred MA, Mavrakis L, Savasky BM, Erzurum SC, Asosingh K. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ 1: 475–486, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 22: 985–992, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Edelman DA, Jiang Y, Tyburski JG, Wilson RF, Steffes CP. Cytokine production in lipopolysaccharide-exposed rat lung pericytes. J Trauma 62: 89–93, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Edwards AM, Arrowsmith CH, Bountra C, Bunnage ME, Feldmann M, Knight JC, Patel DD, Prinos P, Taylor MD, Sundström M; SGC Open Source Target-Discovery Partnership. Preclinical target validation using patient-derived cells. Nat Rev Drug Discov 14: 149–150, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Eisenstein M. Artificial organs: Honey, I shrunk the lungs. Nature 519: S16–S18, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14: 248–260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estrada R, Giridharan GA, Nguyen MD, Prabhu SD, Sethu P. Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics 5: 32006–3200611, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 368: 795–805, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Fink MP, Warren HS. Strategies to improve drug development for sepsis. Nat Rev Drug Discov 13: 741–758, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ. SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol Heart Circ Physiol 294: H891–H900, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, Khan Z, Lamb SE; BALTI2 Study Investigators. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): A multicentre, randomised controlled trial. Lancet 379: 229–235, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8: 1223–1234, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Gawlak G, Tian Y, O'Donnell JJ 3rd, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J 28: 3249–3260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geiger M, Stone A, Mason SN, Oldham KT, Guice KS. Differential nitric oxide production by microvascular and macrovascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 273: L275–L281, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: A new take on sepsis pathogenesis. Sci Transl Med 3: 88ps25, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, Zhao YY, Komarova YA, Vogel SM, Malik AB. HIF2α signaling inhibits adherens junctional disruption in acute lung injury. J Clin Invest 125: 652–664, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J Virol 85: 2296–2303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross CM, Aggarwal S, Kumar S, Tian J, Kasa A, Bogatcheva N, Datar SA, Verin AD, Fineman JR, Black SM. Sox18 preserves the pulmonary endothelial barrier under conditions of increased shear stress. J Cell Physiol 229: 1802–1816, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartmann C, Zozulya A, Wegener J, Galla HJ. The impact of glia-derived extracellular matrices on the barrier function of cerebral endothelial cells: An in vitro study. Exp Cell Res 313: 1318–1325, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Henderson-Toth CE, Jahnsen ED, Jamarani R, Al-Roubaie S, Jones EA. The glycocalyx is present as soon as blood flow is initiated and is required for normal vascular development. Dev Biol 369: 330–339, 2012. [DOI] [PubMed] [Google Scholar]
- 57.Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: Development of an alveolo-capillary barrier in vitro. Lab Invest 84: 736–752, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Hermanns MI, Fuchs S, Bock M, Wenzel K, Mayer E, Kehe K, Bittinger F, Kirkpatrick CJ. Primary human coculture model of alveolo-capillary unit to study mechanisms of injury to peripheral lung. Cell Tissue Res 336: 91–105, 2009. [DOI] [PubMed] [Google Scholar]
- 59.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1584–1595, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hotchkiss RS, Sherwood ER. Immunology. Getting sepsis therapy right. Science 347: 1201–1202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4: 159ra147, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: The relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res 116: 895–908, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3: 112ra122, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwa C, Aird WC. The history of the capillary wall: Doctors, discoveries, and debates. Am J Physiol Heart Circ Physiol 293: H2667–H2679, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Inauen W, Payne DK, Kvietys PR, Granger DN. Hypoxia/reoxygenation increases the permeability of endothelial cell monolayers: Role of oxygen radicals. Free Radic Biol Med 9: 219–223, 1990. [DOI] [PubMed] [Google Scholar]
- 68.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105: 2783–2786, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 288: L1026–L1032, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52: 2745–2756, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jennings RT, Knaus UG. Rho family and Rap GTPase activation assays. Methods Mol Biol 1124: 79–88, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Jiang YZ, Manduchi E, Jiménez JM, Davies PF. Endothelial epigenetics in biomechanical stress: Disturbed flow-mediated epigenomic plasticity in vivo and in vitro. Arterioscler Thromb Vasc Biol 35: 1317–1326, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med 158: S146–S153, 1998. [DOI] [PubMed] [Google Scholar]
- 74.Johnson ER, Matthay MA. Acute lung injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 23: 243–252, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalluri R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Kang H, Cancel LM, Tarbell JM. Effect of shear stress on water and LDL transport through cultured endothelial cell monolayers. Atherosclerosis 233: 682–690, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim CO, Huh AJ, Kim MS, Chin BS, Han SH, Choi SH, Jeong SJ, Choi HK, Choi JY, Song YG, Kim JM. LPS-induced vascular endothelial growth factor expression in rat lung pericytes. Shock 30: 92–97, 2008. [DOI] [PubMed] [Google Scholar]
- 78.King GL, Berman AB, Bonner-Weir S, Carson MP. Regulation of vascular permeability in cell culture. Diabetes 36: 1460–1467, 1987. [DOI] [PubMed] [Google Scholar]
- 79.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Kiss J, Yegutkin GG, Koskinen K, Savunen T, Jalkanen S, Salmi M. IFN-beta protects from vascular leakage via up-regulation of CD73. Eur J Immunol 37: 3334–3338, 2007. [DOI] [PubMed] [Google Scholar]
- 81.Kogej T, Blomberg N, Greasley PJ, Mundt S, Vainio MJ, Schamberger J, Schmidt G, Hüser J. Big pharma screening collections: More of the same or unique libraries? The AstraZeneca-Bayer Pharma AG case. Drug Discov Today 18: 1014–1024, 2013. [DOI] [PubMed] [Google Scholar]
- 82.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 300: C146–C154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuebler WM, Parthasarathi K, Wang PM, Bhattacharya J. A novel signaling mechanism between gas and blood compartments of the lung. J Clin Invest 105: 905–913, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Küppers V, Vockel M, Nottebaum AF, Vestweber D. Phosphatases and kinases as regulators of the endothelial barrier function. Cell Tissue Res 355: 577–586, 2014. [DOI] [PubMed] [Google Scholar]
- 85.Kurimoto N, Nan YS, Chen ZY, Feng GG, Komatsu T, Kandatsu N, Ko J, Kawai N, Ishikawa N. Effects of specific signal transduction inhibitors on increased permeability across rat endothelial monolayers induced by neuropeptide Y or VEGF. Am J Physiol Heart Circ Physiol 287: H100–H106, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Lee H, Kim S, Chung M, Kim JH, Jeon NL. A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res 91: 90–98, 2014. [DOI] [PubMed] [Google Scholar]
- 87.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA 107: 9944–9949, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lo HP, Ackland-Berglund CE, Pritchard KA Jr, Guice KS, Oldham KT. Attenuated expression of inducible nitric oxide synthase in lung microvascular endothelial cells is associated with an increase in ICAM-1 expression. J Pediatr Surg 36: 1136–1142, 2001. [DOI] [PubMed] [Google Scholar]
- 90.Lonigro AJ, McMurdo L, Stephenson AH, Sprague RS, Weintraub NL. Hypotheses regarding the role of pericytes in regulating movement of fluid, nutrients, and hormones across the microcirculatory endothelial barrier. Diabetes 45, Suppl 1: S38–S43, 1996. [DOI] [PubMed] [Google Scholar]
- 91.Machida T, Takata F, Matsumoto J, Takenoshita H, Kimura I, Yamauchi A, Dohgu S, Kataoka Y. Brain pericytes are the most thrombin-sensitive matrix metalloproteinase-9-releasing cell type constituting the blood-brain barrier in vitro. Neurosci Lett 599: 109–114, 2015. [DOI] [PubMed] [Google Scholar]
- 92.Majno G, Shea SM, Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol 42: 647–672, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mammoto A, Mammoto T, Kanapathipillai M, Wing Yung C, Jiang E, Jiang A, Lofgren K, Gee EP, Ingber DE. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nat Commun 4: 1759, 2013. [DOI] [PubMed] [Google Scholar]
- 94.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 20: 195–203, 2014. [DOI] [PubMed] [Google Scholar]
- 95.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM; Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725–738, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, Johnston P, Hopkins PA, Johnston AJ, McDowell C, McNally C; HARP2 Investigators; Irish Critical Care Trials Group. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 371: 1695–1703, 2014. [DOI] [PubMed] [Google Scholar]
- 98.Mehta D, Ravindran K, Kuebler WM. Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 307: L924–L935, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 100.Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: In vivo and in vitro investigations. J Vasc Res 42: 77–89, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol 150: 1057–1070, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morgan JP, Delnero PF, Zheng Y, Verbridge SS, Chen J, Craven M, Choi NW, Diaz-Santana A, Kermani P, Hempstead B, López JA, Corso TN, Fischbach C, Stroock AD. Formation of microvascular networks in vitro. Nat Protoc 8: 1820–1836, 2013. [DOI] [PubMed] [Google Scholar]
- 104.Mul FP, Zuurbier AE, Janssen H, Calafat J, van Wetering S, Hiemstra PS, Roos D, Hordijk PL. Sequential migration of neutrophils across monolayers of endothelial and epithelial cells. J Leukoc Biol 68: 529–537, 2000. [PubMed] [Google Scholar]
- 105.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol 286: H1672–H1680, 2004. [DOI] [PubMed] [Google Scholar]
- 106.Nalayanda DD, Puleo CM, Fulton WB, Wang TH, Abdullah F. Characterization of pulmonary cell growth parameters in a continuous perfusion microfluidic environment. Exp Lung Res 33: 321–335, 2007. [DOI] [PubMed] [Google Scholar]
- 107.Nalayanda DD, Wang Q, Fulton WB, Wang TH, Abdullah F. Engineering an artificial alveolar-capillary membrane: A novel continuously perfused model within microchannels. J Pediatr Surg 45: 45–51, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.National Heart, Lung, and Blood Institute; ARDS Clinical Trials Network; Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, Rock P, Douglas IS, deBoisblanc BP, Hough CL, Hite RD, Thompson BT. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 370: 2191–200, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neuhaus W, Samwer F, Kunzmann S, Muellenbach RM, Wirth M, Speer CP, Roewer N, Förster CY. Lung endothelial cells strengthen, but brain endothelial cells weaken barrier properties of a human alveolar epithelium cell culture model. Differentiation 84: 294–304, 2012. [DOI] [PubMed] [Google Scholar]
- 111.Ogawa S, Koga S, Kuwabara K, Brett J, Morrow B, Morris SA, Bilezikian JP, Silverstein SC, Stern D. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am J Physiol Cell Physiol 262: C546–C554, 1992. [DOI] [PubMed] [Google Scholar]
- 112.Otto M, Bittinger F, Kriegsmann J, Kirkpatrick CJ. Differential adhesion of polymorphous neutrophilic granulocytes to macro- and microvascular endothelial cells under flow conditions. Pathobiology 69: 159–171, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Overbeek MJ, van Nieuw Amerongen GP, Boonstra A, Smit EF, Vonk-Noordegraaf A. Possible role of imatinib in clinical pulmonary veno-occlusive disease. Eur Respir J 32: 232–235, 2008. [DOI] [PubMed] [Google Scholar]
- 114.Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 291: L30–L37, 2006. [DOI] [PubMed] [Google Scholar]
- 115.Payne DK, Owens MW, Grisham M. Early albumin leakage in pulmonary endothelial monolayers exposed to varying levels of hyperoxia. Free Radic Res 25: 229–238, 1996. [DOI] [PubMed] [Google Scholar]
- 116.Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131: 1619–1628, 2004. [DOI] [PubMed] [Google Scholar]
- 117.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res 102: 770–776, 2008. [DOI] [PubMed] [Google Scholar]
- 118.Qiao RL, Sadurski R, Bhattacharya J. Hydraulic conductivity of ischemic pulmonary venules. Am J Physiol Lung Cell Mol Physiol 264: L382–L386, 1993. [DOI] [PubMed] [Google Scholar]
- 119.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD; PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366: 2055–2064, 2012. [DOI] [PubMed] [Google Scholar]
- 120.Rexius-Hall ML, Mauleon G, Malik AB, Rehman J, Eddington DT. Microfluidic platform generates oxygen landscapes for localized hypoxic activation. Lab Chip 14: 4688–4695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmüller P, Humbert M, Guignabert C. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 129: 1586–1597, 2014. [DOI] [PubMed] [Google Scholar]
- 122.Rous P, Gilding HP, Smith F. The gradient of vascular permeability. J Exp Med 51: 807–830, 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roy A, McDonald PR, Sittampalam S, Chaguturu R. Open access high throughput drug discovery in the public domain: A Mount Everest in the making. Curr Pharm Biotechnol 11: 764–778, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature 507: 181–189, 2014. [DOI] [PubMed] [Google Scholar]
- 125.Samapati R, Yang Y, Yin J, Stoerger C, Arenz C, Dietrich A, Gudermann T, Adam D, Wu S, Freichel M, Flockerzi V, Uhlig S, Kuebler WM. Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am J Respir Crit Care Med 185: 160–170, 2012. [DOI] [PubMed] [Google Scholar]
- 126.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18: 1217–1223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sellgren KL, Butala EJ, Gilmour BP, Randell SH, Grego S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip 14: 3349–3358, 2014. [DOI] [PubMed] [Google Scholar]
- 129.Shamir ER, Ewald AJ. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 15: 647–664, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shao J, Wu L, Wu J, Zheng Y, Zhao H, Lou X, Jin Q, Zhao J. A microfluidic chip for permeability assays of endothelial monolayer. Biomed Microdevices 12: 81–88, 2010. [DOI] [PubMed] [Google Scholar]
- 131.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304: 40–49, 2005. [DOI] [PubMed] [Google Scholar]
- 132.Shima DT, Saunders KB, Gougos A, D'Amore PA. Alterations in gene expression associated with changes in the state of endothelial differentiation. Differentiation 58: 217–226, 1995. [DOI] [PubMed] [Google Scholar]
- 133.Silva R, D'Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: The keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol 28: 1703–1713, 2008. [DOI] [PubMed] [Google Scholar]
- 134.Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, Byzova T, Carmeliet P, Chilian W, Cooke JP, Davis GE, Eichmann A, Iruela-Arispe ML, Keshet E, Sinusas AJ, Ruhrberg C, Woo YJ, Dimmeler S; American Heart Association Council on Basic Cardiovascular Sciences, and Council on Cardiovascular Surgery and Anesthesia. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: A scientific statement from the American Heart Association. Circ Res 116: e99–e132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5: 712–721, 2005. [DOI] [PubMed] [Google Scholar]
- 136.Snyder-Talkington BN, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotubes induce human microvascular endothelial cellular effects in an alveolar-capillary co-culture with small airway epithelial cells. Part Fibre Toxicol 10: 35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20: 107–126, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Staub NC. Pulmonary edema. Physiol Rev 54: 678–811, 1974. [DOI] [PubMed] [Google Scholar]
- 139.Steward R Jr, Tambe D, Hardin CC, Krishnan R, Fredberg JJ. Fluid shear, intercellular stress, and endothelial cell alignment. Am J Physiol Cell Physiol 308: C657–C664, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Swift J, Discher DE. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci 127: 3005–3015, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szulcek R, Beckers CM, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJ, Minshall RD, van Hinsbergh VW, van Nieuw Amerongen GP. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc Res 99: 471–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 87: 320–330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tasev D, Konijnenberg LS, Amado-Azevedo J, van Wijhe MH, Koolwijk P, van Hinsbergh VW. CD34 expression modulates tube-forming capacity and barrier properties of peripheral blood-derived endothelial colony-forming cells (ECFCs). Angiogenesis 19: 325–338, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis 6: 242–248, 2006. [DOI] [PubMed] [Google Scholar]
- 145.Thanabalasundaram G, Pieper C, Lischper M, Galla HJ. Regulation of the blood-brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res 1347: 1–10, 2010. [DOI] [PubMed] [Google Scholar]
- 146.Thanabalasundaram G, Schneidewind J, Pieper C, Galla HJ. The impact of pericytes on the blood-brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol 43: 1284–1293, 2011. [DOI] [PubMed] [Google Scholar]
- 147.Tian X, Tian Y, Gawlak G, Meng F, Kawasaki Y, Akiyama T, Birukova AA. Asef controls vascular endothelial permeability and barrier recovery in the lung. Mol Biol Cell 26: 636–650, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest 122: 408–418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tucker VL, Huxley VH. O2 modulation of single-vessel hydraulic conductance. Am J Physiol Heart Circ Physiol 254: H317–H323, 1988. [DOI] [PubMed] [Google Scholar]
- 150.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Uhlig S, Yang Y, Waade J, Wittenberg C, Babendreyer A, Kuebler WM. Differential regulation of lung endothelial permeability in vitro and in situ. Cell Physiol Biochem 34: 1–19, 2014. [DOI] [PubMed] [Google Scholar]
- 151a.van der Heijden M, van Nieuw Amerongen GP, van Bezu J, Paul MA, Groeneveld AB, van Hinsbergh VW. Opposing effects of the angiopoietins on the thrombin-induced permeability of human pulmonary microvascular endothelial cells. PLoS One 6: e23448, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151b.van Nieuw Amerongen GP, Vermeer MA, Nègre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VW. Simvastatin improves disturbed endothelial barrier function. Circulation 102: 2803–2809, 2000. [DOI] [PubMed] [Google Scholar]
- 152.Wang YL, Hui YN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye 21: 1501–1510, 2007. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Taneja R, Wang W, Yao LJ, Veldhuizen RA, Gill SE, Fortin D, Inculet R, Malthaner R, Mehta S. Human alveolar epithelial cells attenuate pulmonary microvascular endothelial cell permeability under septic conditions. PLoS One 8: e55311, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Warboys CM, Eric Berson R, Mann GE, Pearson JD, Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol 298: H1850–H1856, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weppler A, Rowter D, Hermanns I, Kirkpatrick CJ, Issekutz AC. Modulation of endotoxin-induced neutrophil transendothelial migration by alveolar epithelium in a defined bilayer model. Exp Lung Res 32: 455–482, 2006. [DOI] [PubMed] [Google Scholar]
- 156.Westphalen K, Monma E, Islam MN, Bhattacharya J. Acid contact in the rodent pulmonary alveolus causes proinflammatory signaling by membrane pore formation. Am J Physiol Lung Cell Mol Physiol 303: L107–L116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005. [DOI] [PubMed] [Google Scholar]
- 158.Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, Prendergast GC, Wilkins MR. Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 110: 1423–1434, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wong KH, Truslow JG, Tien J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31: 4706–4714, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu L, Wary KK, Revskoy S, Gao X, Tsang K, Komarova YA, Rehman J, Malik AB. Histone demethylases KDM4A and KDM4C regulate differentiation of embryonic stem cells to endothelial cells. Stem Cell Reports 5: 10–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yeon JH, Na D, Choi K, Ryu SW, Choi C, Park JK. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices 14: 1141–1148, 2012. [DOI] [PubMed] [Google Scholar]
- 162.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH; Study Group OSCAR. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 368: 806–813, 2013. [DOI] [PubMed] [Google Scholar]
- 163.Yuan K, Orcholski ME, Panaroni C, Shuffle EM, Huang NF, Jiang X, Tian W, Vladar EK, Wang L, Nicolls MR, Wu JY, de Jesus Perez VA. Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am J Pathol 185: 69–84, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci 120: 137–148, 2007. [DOI] [PubMed] [Google Scholar]
- 165.Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One 9: e86249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA 109: 13515–13520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang J, Friedman MH. Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am J Physiol Heart Circ Physiol 302: H983–H991, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 109: 9342–9347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zloza A, Kim DW, Broucek J, Schenkel JM, Kaufman HL. High-dose IL-2 induces rapid albumin uptake by endothelial cells through Src-dependent caveolae-mediated endocytosis. J Interferon Cytokine Res 34: 915–919, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zozulya A, Weidenfeller C, Galla HJ. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res 1189: 1–11, 2008. [DOI] [PubMed] [Google Scholar]


