Abstract
Exercise effectively prevents the development of obesity and obesity-related diseases such as type 2 diabetes. Capsinoids (CSNs) are capsaicin analogs found in a nonpungent pepper that increase whole body energy expenditure. Although both exercise and CSNs have antiobesity functions, the effectiveness of exercise with CSN supplementation has not yet been investigated. Here, we examined whether the beneficial effects of exercise could be further enhanced by CSN supplementation in mice. Mice were randomly assigned to four groups: 1) high-fat diet (HFD, Control), 2) HFD containing 0.3% CSNs, 3) HFD with voluntary running wheel exercise (Exercise), and 4) HFD containing 0.3% CSNs with voluntary running wheel exercise (Exercise + CSN). After 8 wk of ingestion, blood and tissues were collected and analyzed. Although CSNs significantly suppressed body weight gain under the HFD, CSN supplementation with exercise additively decreased body weight gain and fat accumulation and increased whole body energy expenditure compared with exercise alone. Exercise together with CSN supplementation robustly improved metabolic profiles, including the plasma cholesterol level. Furthermore, this combination significantly prevented diet-induced liver steatosis and decreased the size of adipocyte cells in white adipose tissue. Exercise and CSNs significantly increased cAMP levels and PKA activity in brown adipose tissue (BAT), indicating an increase of lipolysis. Moreover, they significantly activated both the oxidative phosphorylation gene program and fatty acid oxidation in skeletal muscle. These results indicate that CSNs efficiently promote the antiobesity effect of exercise, in part by increasing energy expenditure via the activation of fat oxidation in skeletal muscle and lipolysis in BAT.
Keywords: exercise, capsinoids, obesity, energy expenditure, fat oxidation
obesity is rapidly becoming a major global health problem (4) and is a major risk factor for several common diseases, including type 2 diabetes, cardiovascular diseases, and cancer (32). Obesity develops when energy intake chronically exceeds total energy expenditure (51). Conventional antiobesity strategies have focused on repressing energy intake by suppressing appetite or inhibiting intestinal fat absorption; however, increasing energy expenditure by activating the metabolic function of skeletal muscle or brown fat could serve as an alternative and effective antiobesity intervention, thereby avoiding the potential adverse effects associated with conventional antiobesity therapies such as depression, oily bowel movements, and steatorrhea.
Exercise is one of the most efficient ways to prevent obesity and type 2 diabetes through an increase in energy expenditure (25, 27, 52). However, people in the developed world are becoming less physically active as a result of lifestyle changes and the nature of their work. Moreover, constraints such as lack of time, limited access. and injuries frequently become barriers to exercise (48). For example, a recent epidemiological study estimated that in a 1-yr period, 39% of women adopted some type of physical activity, but the attrition rate exceeded 30% within only months (46).
Capsaicin is the pungent component in chili pepper, and it is known to have an antiobesity effect (18, 20). Capsinoids (CSNs) are capsaicin analogs found in a nonpungent type of chili pepper, “CH-19 Sweet” (23, 24). CSNs differ from capsaicin in their chemical structure only at the center linkage of an ester bond, resulting in reduced (<0.1%) pungency, which facilitates daily intake, while maintaining the metabolic effect. Several studies have shown that CSNs increase energy expenditure and suppress body fat accumulation in mice (31). A single administration of capsiate, a CSN, was found to increase oxygen consumption, and chronic administration for 2 wk diminished fat accumulation in mice (31, 39). Moreover, chronic treatment with CSNs via their inclusion in a high-fat diet (HFD) for 12 wk was shown to dramatically suppress body weight gain and fat accumulation (Inoue N, Nogusa Y, Hara-Kimura Y, Okabe-Nogusa Y, Ohyama K, Tsukamoto-Yasui M, and Ono K, unpublished observations). In adult humans, a 4-wk treatment with CSNs was found to increase oxygen consumption, and a 12-wk treatment decreased the abdominal fat mass in subjects with body mass indexes (BMIs) of 25–35 (15, 50). Mechanistically, oral CSN supplements have been reported to activate transient receptor potential vanilloid subtype 1 (TRPV1) in the gut (14, 47), resulting in increased sympathetic efferent activity and thermogenesis (41). Activation of TRPV1 signaling has been reported to positively regulate exercise endurance and energy expenditure in mice (30).
We propose that the combination of dietary supplements with exercise may be effective and realistic for obesity control. Although there have been many investigations into the individual beneficial effects of exercise and dietary supplements, little is known about their combined effects. Thus we investigated the effects of daily intake of CSNs in combination with exercise on the development of obesity in C57BL/6J mice.
MATERIALS AND METHODS
Animals and diets.
C57BL/6J male mice were purchased from Charles River (Kanagawa, Japan) at 7 wk of age and were housed in a controlled-lighting environment (lights on from 1600 to 0400) at 25 ± 1°C. They were fed CRF-1 (Charles River, Kanagawa, Japan) for 2 wk to stabilize their metabolic condition. The mice were divided into two groups: one group was given free access to a running wheel (Ex group; wheel diameter, 14 cm: Melquest, Toyama, Japan) connected to a counter, and the other group could not access the wheel. After habituation to wheel running for 1 wk, the mice were divided into two groups by body weight, food intake, and wheel running counts. The mice were allowed ad libitum access to water and either the HFD or HFD supplemented with 0.3% (wt/wt) CSNs (Yoyu-Lab, Gunma, Japan), depending on the group. The compositions of the diets are listed in Table 1. Thus we prepared four groups: 1) sedentary-HFD [Control: Ex(−)/CSN(−)], 2) sedentary-CSNs [CSN: Ex(−)/CSN(+)], 3) voluntary running-HFD [Exercise: Ex(+)/CSN(−)], and 4) voluntary running-CSNs [Exercise + CSN: Ex(+)/CSN(+)]. The mice were maintained on these diets for 8 wk. Body weight, food intake and running distance were monitored twice a week. All animal protocols were approved by the Animal Committee of Ajinomoto.
Table 1.
Composition of the experimental diets
| HFD, % | HFD + CSNs (0.3%) | |
|---|---|---|
| Casein | 20 | 20 |
| Sucrose | 10 | 10 |
| Corn starch | 22.48 | 22.48 |
| α-Corn starch | 7.47 | 7.47 |
| l-Cysteine | 0.3 | 0.3 |
| Cellulose | 5 | 5 |
| Lard | 30 | 30 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1 | 1 |
| Choline bitartrate | 0.25 | 0.25 |
| CSNs | — | 0.3 |
HFD, high-fat diet; CSNs, capsinoids.
Sampling procedures.
At the end of the experiment, the mice were anesthetized with isoflurane, and blood samples were collected by aortic puncture after 3 h of fasting. The blood samples were centrifuged at 3,000 rpm for 20 min at 4°C and stored at −20°C until analyzed. The organs [liver, mesenteric white adipose tissue (WAT), perirenal WAT, epididymal WAT, subcutaneous WAT, brown adipose tissue (BAT), gastrocnemius muscle, and soleus muscle] were excised and weighed. The organs were then immediately frozen in liquid nitrogen and stored at −80°C.
Blood analysis.
The plasma levels of glucose and glutamic pyruvic transaminase (GPT) were determined using a Fuji-drychem device (Fujifilm, Tokyo, Japan). The plasma insulin levels and leptin levels were determined by an enzyme-linked immunosorbent assay (ELISA; Morinaga, Kanagawa, Japan). The plasma levels of triglycerides (TG) and cholesterol were determined using the Wako TG-test kit (Wako, Osaka, Japan) and Cholesterol-E test kit (Wako), respectively.
Liver lipid analysis.
Lipids in the liver and gastrocnemius muscle were extracted using the Folch partition method (10) with slight modifications as follows: a portion of the tissue was homogenized in 2.5 ml of methanol, and 5 ml of chloroform were added. The mixture was horizontally shaken for 10 min and extracted at 4°C overnight. The extracted samples were filtered using a Kiriyama-rohto device (Kiriyama, Tokyo, Japan), and the volume was increased to 8 ml with methanol:chloroform (2:1, vol/vol). Then, 1.6 ml of saline were added. The mixture was horizontally shaken for 10 min and centrifuged at 2,000 rpm for 5 min at 4°C. The supernatant was removed, and the lower chloroform phase was filled to 6 ml with chloroform. Next, 2.5 ml of the lipid fraction in this phase were dried and weighed. The dried lipids were dissolved in 1 ml of 10% Triton/isopropanol, and the TG and nonesterified fatty acid (NEFA) levels were determined using a Wako TG-test kit (Wako) and NEFA-C test kit (Wako), respectively.
Quantitative real-time RT-PCR.
Total RNA was isolated from the gastrocnemius muscle and subcutaneous WAT using Ribozol (AMRESCO). cDNA was synthesized from 1 μg of RNA using an iScrip cDNA Synthesis Kit (Bio-Rad). After cDNA synthesis, quantitative real-time PCR was performed in 10 μl of iTaq Fast SYBR Green Supermix (Bio-Rad) using a fluorometric thermal cycler (ViiA 7 System; Life Technologies). The reaction mixtures were incubated for an initial denaturation at 95°C for 10 s, followed by 45 cycles of 95°C for 5 s and 60°C for 20 s. The sequences of the sense and antisense primers used in the amplification are shown in Table 2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or TATA box binding protein (TBP) was used as an internal control.
Table 2.
Primers used for real-time PCR analysis
| Gene | Sense | Antisense | Entrez Gene ID |
|---|---|---|---|
| Gapdh | CTGAGGACCAGGTTGCTCC | ACCACCCTGTGCTGTAGCC | 14433 |
| Tbp | ACCCTTCACCAATGACTCCTATG | TGACTGCAGCAAATCGCTTGG | 21374 |
| Aco | CATTGGCATGTGAGAACAG | AGCAAATCTGATGGCTTTGA | 11430 |
| Cox-1 | CACTAATAATCGGAGCCCCA | TTCATCCTGTTCCTGTCCT | 17708 |
| Lpl | CCAGCAACATTATCCAGTGCTAG | CAGTTGATGAATCTGTCCT | 16956 |
| Mcad | GCTCGTGAGCACATTGAAAA | CATTGTCCAAAAGCCAAACC | 11364 |
| Pgc-1α | CACTACAGACACCGCACACA | AGGCTTCATAGCTGTCGTACC | 19017 |
| Ucp-1 | CACCTTCCCCCTGGACACT | CCCTAGGACACCTTTATACCT | 22227 |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT | 12683 |
| Cox8B | GAACCATGAAGCCAACGACT | GCGAAGTTCACAGTGGTTCC | 12869 |
Histology.
Subcutaneous WAT was fixed in 4% paraformaldehyde for 24 h at 4°C. The samples were then dehydrated, embedded in paraffin, sliced into 7-μm-thick sections with a Leica RM2255 (Leica Microsystems; Vienna, Austria), deparaffinized, and rehydrated. The sections were then stained with hematoxylin and eosin (H&E).
Respiratory gas analysis and measurement of activity.
With the use of a respiratory gas analysis system consisting of an acrylic metabolic chamber, gas analyzers, and a switching system (ARCO2000-RAT/ANI System; Arco, Chiba, Japan), the O2 and CO2 concentrations of gas sampled from each metabolic chamber were measured as described previously (39). Briefly, room air was constantly pumped through the chamber, and expired air was dried in a thin cotton column and then introduced into the gas analyzer. The respiratory quotient (RQ: V̇co2/V̇o2) was calculated according to Weir (56). Fat oxidation was calculated based on the V̇o2 and carbon dioxide production. The spontaneous activity of the mice was simultaneously measured using an activity sensor (NS-AS01; Neuroscience, Tokyo, Japan). All measurements were performed in 4.5-min intervals.
cAMP levels and PKA activity in BAT.
cAMP levels and PKA activity were determined using the cAMP Direct Immunoassay Kit (Abcam, Cambridge, UK) and PKA Kinase Activity Assay Kit (Abcam), respectively.
Fatty acid oxidation in muscle.
The muscle fatty acid oxidation rate was determined in fresh muscle homogenate using a modification of the method of Kim et al. (21a). The oxidation rate of palmitate was measured by collecting and counting the 14CO2 produced during incubation. Twenty microliters of muscle homogenate were incubated with 380 μl of reaction mixture (pH 8.0) for 1 h. The final concentrations of the reaction mixture were as follows in millimoles per liter: 100 sucrose, 10 Tris·HCl, 5 potassium phosphate, 80 potassium chloride, 1 magnesium chloride, 2 l-carnitine, 0.1 malate, 2 ATP, 0.05 coenzyme A (CoA), 1 dithiothreitol, 0.2 EDTA, 5 nicotinamide, 0.001 trichostatin A, and 0.3% bovine serum albumin The substrate used was [1-14C]palmitate (0.4 μCi) with 2 mM BSA. After 60 min of incubation at 37°C, 200 μl of 1 M perchloric acid were injected to stop the reaction. The CO2 produced during the incubation was trapped by Whatman filter paper with 15 μl of hyamine hydroxide. Then, the filter paper was transferred to a glass scintillation vial that contained 4 ml of Emulsifier-Safe. The average counts per minute were measured over 3 min.
Statistical analysis.
The statistical analysis was performed using JMP version 9.0 (SAS Institute). The main and interaction effects of the CSNs and Ex on the metabolic parameters were determined by two-way ANOVA. Repeated-measures ANOVA with t-tests was used to compare the time courses. Other statistical comparisons utilized a two-tailed Student's t-test. The results are given as the means ± SE unless otherwise stated. P < 0.05 was considered significant throughout the study.
RESULTS
Effects of exercise and CSN supplementation on body weight gain and adiposity.
The metabolic effects of exercise and CSNs were evaluated in an 8-wk cohort of diet-induced obese mice. The mice given CSNs gained significantly less weight in both the normal and exercised groups under a HFD (Fig. 1A). The combination of exercise and CSNs dramatically (17%) suppressed weight gain at 56 days compared with that in the control [Ex(−)/CSN(−)] group. Body weight gain was significantly different between the Ex(−) and Ex(+) groups and between the CSN(−) and CSN(+) groups (Fig. 1B). The main effects of CSNs and exercise on final body weight gain were significant as determined by two-way ANOVA, whereas their interaction was not significant (P = 0.915). The reduction of body mass was accounted for by a decrease in fat as reflected in the weights of various white fat deposits, including the epididymal, mesenteric, and perirenal WAT (Fig. 1C). However, heart weight was not significantly affected by either exercise or the CSN treatment. Consistent with these results, the combination of exercise and CSNs significantly improved metabolic profiles. The blood levels of glucose, insulin, leptin, and cholesterol were all significantly decreased by the combination of exercise and CSNs (Table 3).
Fig. 1.
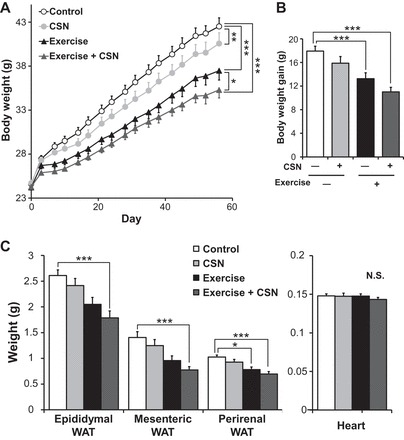
Capsinoid (CSN) supplementation with exercise additively suppressed body weight gain and adiposity in diet-induced obesity. A: body weight development. B: body weight gain. C: tissue weight of C57BL/6J mice fed an high-fat diet (HFD, Control), HFD supplemented with CSNs, HFD together with voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. WAT, white adipose tissue. The values represent the means ± SE (n = 7–8). *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3.
Effects of CSN supplementation plus exercise on blood parameters
| Control | CSN | Exercise | Exercise + CSN | P Value | |
|---|---|---|---|---|---|
| Blood glucose, mg/ml | 31.45 ± 2.27 | 25.28 ± 0.90* | 21.84 ± 0.80‡ | 23.00 ± 1.14† | 0.0003 |
| Insulin, ng/ml | 6.00 ± 1.08 | 3.89 ± 0.56 | 3.63 ± 0.53* | 3.12 ± 0.63† | 0.0481 |
| Leptin, ng/ml | 22.81 ± 1.45 | 21.47 ± 2.75 | 16.93 ± 3.61 | 12.03 ± 1.98† | 0.0262 |
| Cholesterol, mg/ml | 18.79 ± 0.98 | 15.13 ± 1.15* | 16.11 ± 0.40* | 15.15 ± 0.44† | 0.0120 |
| TG, mg/ml | 5.36 ± 0.63 | 5.51 ± 0.34 | 5.04 ± 0.47 | 4.70 ± 0.21 | 0.5755 |
Values represent the means ± SE (n = 7–8). The levels of blood glucose, insulin, leptin, cholesterol, and triglyceride (TG) were measured in C57BL/6J mice fed a HFD (Control), HFD supplemented with CSNs, HFD together with voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. P values were calculated by one-way ANOVA.
P < 0.05 and
P < 0.01, significant difference compared with the control group.
Effects of exercise and CSN supplementation on energy expenditure.
The critical parameter contributing to body weight control is the energy balance between caloric intake and energy expenditure (29). Food intake was not affected by CSNs in either the Ex(−) or Ex(+) groups (Fig. 2A). We then measured the energy expenditure, which comprises physical activity and thermogenesis. The physical activity (locomotive activity and running distance) of the mice during the treatment was not affected by the CSN supplementation (Fig. 2, B and C). However, the whole body oxygen consumption and fat oxidation were significantly increased by the combination of exercise and CSNs compared with the control group (Fig. 2, D and E).
Fig. 2.
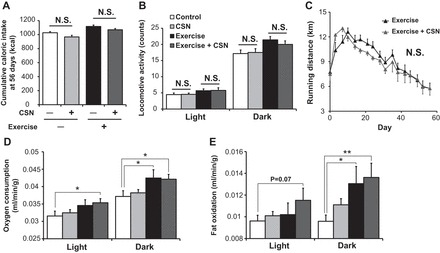
CSN supplementation with exercise additively increased energy expenditure. A: food intake. B: locomotive activity. C: running distance. D: oxygen consumption. E: fat oxidation of C57BL/6J mice fed a HFD (Control), HFD supplemented with CSNs, HFD in addition to voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. The values represent the means ± SE (n = 7–8). *P < 0.05, **P < 0.01.
CSN supplementation with exercise improved HFD-induced liver steatosis.
Next, we examined the metabolic consequences of the antiobesity effects of exercise and CSNs. It is well known that a HFD induces liver steatosis; thus lipids should accumulate in the liver. Therefore, we measured the lipid content in the liver, as assessed by the levels of total lipids, TG, and NEFA. Total lipid levels tended to be decreased by exercise or CSN treatment but not significantly. However, the combination of exercise and CSNs significantly reduced the total lipid content in the liver (Fig. 3A). Similar effects were observed for TG and NEFA levels (Fig. 3, B and C). Importantly, the combination of exercise and CSNs significantly reduced the total lipid, TG, and NEFA levels in the liver by 64, 46, and 78%, respectively (Fig. 3, A–C). The plasma levels of GPT, a sensitive parameter in diagnosing fatty liver in humans (21), were significantly reduced in the mice that exercised (Fig. 3D).
Fig. 3.
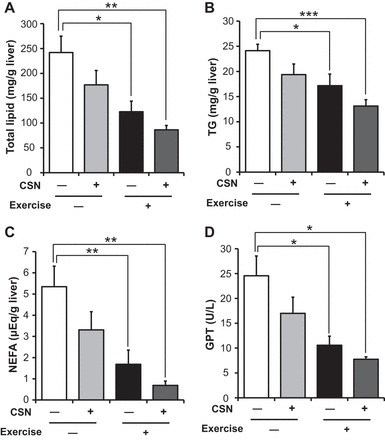
CSN supplementation with exercise improved HFD-induced liver steatosis. A: hematoxylin and eosin (H&E) liver staining. B–D: liver levels of total lipids (B), triglycerides (TGs; C); nonesterified fatty acid (NEFA; D). F: glutamic pyruvic transaminase (GPT) levels in the plasma of C57BL/6J mice. Mice were fed a HFD (control), HFD supplemented with CSNs, HFD together with voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. The values represent the means ± SE (n = 7–8). *P < 0.05, **P < 0.01, ***P < 0.001.
CSN supplementation with exercise diminished cell size in subcutaneous WAT.
The morphological and biochemical changes in WAT in response to exercise and CSNs were examined. Metabolic improvements are strongly associated with a reduction in adipose cell size. Histological examination (H&E staining) of subcutaneous WAT revealed that the adipocyte size significantly decreased in the WAT from the mice that received both exercise and CSNs (Fig. 4A). To quantitatively assess the difference, we calculated the average cell size in a 1-mm field of view of H&E-stained tissue and observed that exercise and CSNs indeed significantly diminished cell size (Fig. 4B). Because we observed a significant increase in energy expenditure that was associated with exercise and CSN supplementation (Fig. 2C), we hypothesized that nonshivering thermogenesis through brown adipocytes may be altered. Thus we measured the mRNA expression levels of thermogenic genes [uncoupling protein-1 (Ucp1) and peroxisome proliferator-activated receptor gamma coactivator 1α (Pgc-1α)] in WAT. As previously reported (5, 58), exercise significantly increased Ucp-1 and Pgc-1α expression (main effect P = 0.02), although the effects of CSNs did not reach significance, with P values of 0.64 (Ucp-1) and 0.87 (Pgc-1α) (Fig. 4C). However, the expression levels of lipoprotein lipase (Lpl), a key enzyme of lipolysis, and adiponectin (AdipQ), a differentiation marker, did not change among the groups (Fig. 4C).
Fig. 4.
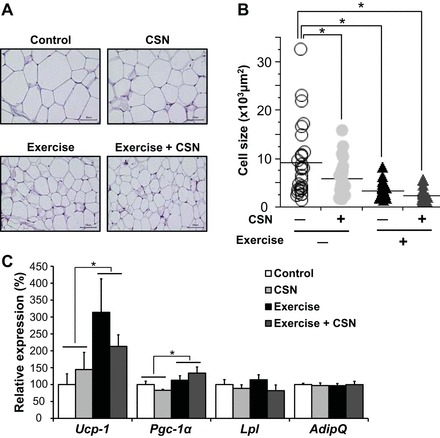
CSN supplementation with exercise decreased cell size in subcutaneous WAT. A: H&E staining of subcutaneous WAT. B: cell size of A (in × 103 μm2). The sizes of 100 randomly chosen cells in representative H&E-stained slides (n = 5) were quantified by ImageJ (1.48v). Each dot represents a cell, and the horizontal bar is the group mean. C: mRNA expression levels of uncoupling protein-1 (Ucp-1), peroxisome proliferator-activated receptor gamma coactivator-1α (Pgc-1a), lipoprotein lipase (Lpl), and adiponectin (AdipQ) in the subcutaneous WAT of C57BL/6J mice fed a HFD (Control), HFD supplemented with CSNs (CSN), HFD together with voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. The values represent the means ± SE (n = 7–8). *P < 0.05.
CSN supplementation combined with exercise increased lipolysis in BAT.
It is well known that the sympathetic nervous system (SNS) is activated during exercise (54). Additionally, it has been reported that CSNs increase BAT sympathetic nervous activity (41). We therefore hypothesized that CSN treatment with exercise would additively activate the SNS. To address this hypothesis, we determined cAMP levels in BAT. Exercise significantly increased cAMP levels, and the combination of exercise and CSNs tended to further increase cAMP levels (Ex: 54%; Ex + CSN: 87%) in BAT (Fig. 5A). We further determined the PKA activity in BAT, because increased intercellular cAMP activates PKA. Similar to the effect on cAMP levels, the combination of exercise and CSNs resulted in the highest PKA activity (Fig. 5B).
Fig. 5.
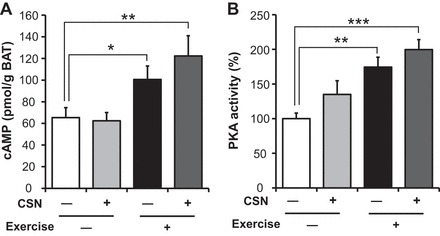
CSN supplementation with exercise increased cAMP levels and protein kinase A (PKA) activity in brown adipose tissue (BAT). A: level of cyclic AMP (cAMP). B: PKA activity in the BAT of C57BL/6J mice fed a HFD (Control), HFD supplemented with CSNs, HFD together with voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. The values represent the means ± SE (n = 7–8). *P < 0.05, **P < 0.01, ***P < 0.001.
CSN supplementation combined with exercise increased fatty acid oxidation in the gastrocnemius muscle.
It is known that a HFD induces lipid accumulation not only in the liver but also in muscle. Exercise significantly decreased TG levels, and the combination of exercise and CSNs tended to further decrease TG levels (Ex: 37%; Ex + CSN: 42%) in the gastrocnemius muscle (Fig. 6A). Skeletal muscle is one of the most active organs in dissipating energy, and it plays a central role in systemic energy homeostasis. Exercise is well known to increase the expression of genes involved in fatty acid oxidation, such as acyl-CoA oxidase (Aco) and cytochrome c oxidase-1 (Cox-1), through the transcriptional pathway of Pgc-1α (28, 45, 59). Hence, we hypothesized that exercise and CSNs would additively activate the expression of fat oxidative genes in muscle, which could contribute to increased energy expenditure. As shown in Fig. 5B, the combination of exercise and CSNs significantly increased the mRNA levels of Lpl, Cox-1, Aco, and medium-chain acyl dehydrogenase (Mcad) compared with the expression levels in the control group. Additionally, CSNs significantly increased the mRNA levels of Cox-1 under the exercise conditions. By contrast, myosin heavy chain-1/β (Mhc-1/β) mRNA expression did not change (Fig. 6B), indicating that muscle fiber type changes did not occur in this setting. Furthermore, we directly determined the fatty acid oxidation rate in muscle using [14C]palmitate. The oxidation rate tended to be increased by the exercise or the CSN treatment but not significantly. However, the combination of exercise and CSNs significantly increased the oxidation rate in the gastrocnemius muscle (Fig. 6C).
Fig. 6.
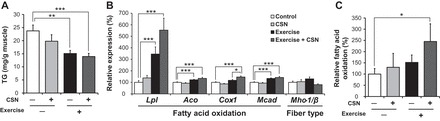
CSN supplementation with exercise increased fatty acid oxidation by activating the oxidative phosphorylation (OXPHOS) gene program in the gastrocnemius muscle. A: TG levels in the gastrocnemius muscle. B: mRNA expression levels of lipoprotein lipase (Lpl), acyl-CoA oxidase (Aco), cytochrome c oxidase-1 (Cox-1), medium-chain acyl dehydrogenase (Mcad), and myosin heavy chain-1/β (Mhc-1/β) in the gastrocnemius muscle. C: fatty acid oxidation rate in the gastrocnemius muscle of C57BL/6J mice fed a HFD (Control), HFD supplemented with CSNs (CSN), HFD together with voluntary exercise (Exercise), or HFD supplemented with CSNs in addition to voluntary exercise (Exercise + CSN) for 56 days. The values represent the means ± SE (n = 7–8). *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Exercise is important in treating obesity because of its capacity to increase energy expenditure (13, 17). Despite the well-documented health benefits of exercise, constraints such as lack of time, limited access, and injuries frequently become barriers to exercise (48). Hence, we propose that the combination of antiobesity food components with exercise may be effective in weight control by compensating for or adding to individual effects. Although there have been many investigations into the individual beneficial effects of exercise or food components, little is known about their combined effects on obesity development. This study demonstrated that the combination of exercise and CSNs additively reduced adiposity (Fig. 1C). Furthermore, CSN supplementation with exercise improved the status of peripheral markers such as blood glucose, insulin, and leptin levels, providing additional supporting evidence for the additive effect (Table 3). Although several groups, including ours, have reported independent effects of CSNs on obesity (Inoue N, Nogusa Y, Hara-Kimura Y, Okabe-Nogusa Y, Ohyama K, Tsukamoto-Yasui M, and Ono K, unpublished observations; Refs. 31, 39), this report is the first to show that CSNs have an antiobesity effect under exercise conditions. The combination of exercise and tea catechin was also reported to be more effective than the effects of the individual components in suppressing obesity (33). Catechins, such as epigallocatechin gallate (EGCG), have antiobesity effects in mice and humans (22, 34, 35, 57). These observations support our finding that the combination of exercise and antiobesity food components has increased efficacy for suppressing obesity and that CSNs are a promising candidate for combining with exercise.
The antiobesity mechanisms of CSNs include increased energy expenditure (15, 39, 40). Given the established effect of exercise on energy expenditure, we expected that CSNs would provide an additive increase in energy expenditure. Indeed, the combination of exercise and CSNs increased whole body fat oxidation, whereas food intake and exercise amounts were unchanged (Fig. 2). Thus the combination of exercise and CSNs may conceivably suppress obesity via an increase in basal energy expenditure through a significant increase in fat oxidation that involves an increase in the expression of fatty acid oxidative genes in muscle (Fig. 5, B and C). Exercise is well known to upregulate Pgc-1α gene expression, and downstream genes are transcriptionally regulated by Pgc-1α (28, 45, 59). For example, swimming exercise resulted in a twofold increase in Pgc-1α mRNA and protein levels in the skeletal muscle of rats (2), and exercise training induced a marked increase in Pgc-1α mRNA content in humans (44). Additionally, chronic administration of CSNs has been shown to increase the expression of Pgc-1α and its target genes in the gastrocnemius muscle (Inoue N, Nogusa Y, Hara-Kimura Y, Okabe-Nogusa Y, Ohyama K, Tsukamoto-Yasui M, and Ono K, unpublished observations). In the present study, the increase in Pgc-1α expression by only exercise or CSNs was smaller than that in previous reports (P = 0.15 and P = 0.07; data not shown), possibly because of differences in experimental conditions, such as follow-up duration or type of exercise (forced or voluntary). These results suggest that the increased fat oxidation in muscle induced by the combination of exercise and CSNs plays an important role in increasing the energy expenditure of the whole body.
Both single and chronic CSN supplementation protocols have been reported to increase energy expenditure (11, 15, 31, 39, 40, 50). This CSNs-dependent increase in energy expenditure may be mediated by activation of the SNS. CSNs increase BAT sympathetic nervous activity and the release of norepinephrine (41). Because the SNS is activated and the release of catecholamines increases during exercise (55), the release of catecholamines may be synergistically regulated by the combination of exercise with CSNs. Catecholamines released from sympathetic nerve terminals promote lipid mobilization and thermogenesis via β3-adrenoreceptors (12, 36). In the present study the cAMP levels in BAT were additively increased by the combination of exercise and CSNs (Fig. 5A), which indicates that exercise and CSNs additively activated β3-adrenoreceptors and increased cAMP levels, resulting in activation of PKA (Fig. 5B). Activated PKA phosphorylates hormone-sensitive lipase (HSL) and induces lipolysis (1, 8). Because fatty acids are a UCP-1 substrate (9), the combination of exercise and CSNs could induce energy expenditure and thermogenesis by driving UCP-1 activity.
UCP-1 is a thermogenous protein that produces heat by uncoupling electron transport from ATP production (6) during adaptive nonshivering thermogenesis (36). UCP-1 has recently attracted attention as a target for suppressing obesity. CSNs and exercise have been reported to increase UCP-1 levels in interscapular BAT (31, 37, 38). Furthermore, exercise has been reported to induce browning in subcutaneous WAT (5). However, in this research, we did not observe an increase in Ucp-1 expression in interscapular BAT in response to the exercise and CSN treatment (data not shown). Moreover, we did not observe any differences in the mRNA levels in the subcutaneous WAT among the groups. These results suggest that the suppression of obesity by the combination of exercise and CSNs does not involve an increase in the expression of Ucp-1 in either interscapular BAT or subcutaneous WAT.
Recently, CSNs have been reported to activate the transient receptor potential cation channel, subfamily A, member 1 (TRPA1) and the TRPV1 channel (49). TRPA1 is sensitive to cold stimuli below 17°C (43) and to various reagents such as allyl isothiocyanate, cinnamaldehyde, and farnesyl thiosalicylic acid (3). However, the specific function of this protein has not yet been determined. Further studies are necessary to elucidate the role of the activation of TRPA1 by CSNs in the antiobesity effect.
Excessive accumulation of TG in the liver in response to a HFD has been reported to induce disorders such as fatty liver and to lead to liver cirrhosis and even hepatocellular cancer (54). In the present study, the combination of exercise and CSNs decreased the total lipid, TG, and NEFA levels in the liver (Fig. 3, A–C). Plasma cholesterol and GPT levels were also significantly reduced by the combination of exercise and CSNs (Table 3 and Fig. 3D). Previous studies have reported that exercise suppresses liver lipid accumulation (26), possibly via mechanisms involving increased fat oxidation and decreased lipogenesis in the liver (7, 42). Furthermore, capsiate was reported to reduce hepatic TG levels in rats fed a HFD (53), and CSNs were shown to reduce hepatic TG levels in mice fed a HFD (Inoue N, Nogusa Y, Hara-Kimura Y, Okabe-Nogusa Y, Ohyama K, Tsukamoto-Yasui M, and Ono K, unpublished observations). These data suggested that the combination of exercise and CSNs may suppress lipid accumulation by increasing fat oxidation in the liver. Hence, we measured the expression levels of genes involved in fatty acid oxidation in the liver but found that they were not changed in this cohort (data not shown). Therefore, the suppression of lipid accumulation is thought to be a secondary effect of increasing energy expenditure in muscle.
Some studies have reported that CSNs increase energy expenditure and suppress obesity in humans. Yoneshiro et al. (6) reported that CSNs acutely increase energy expenditure through the activation of BAT in humans and that chronic CSN treatment increases cold-induced thermogenesis in humans (61). Additionally, CSNs or CH-19 sweet pepper fruits containing CSNs were found to decrease visceral adiposity in subjects with BMIs of 25–35 kg/m2 (19, 50). CSN has already been filed with the Food and Drug Administration as a new dietary ingredient. Combining exercise with CSNs in the human diet could thus be a powerful tool for fighting obesity.
DISCLOSURES
K. Ohyama, Y. Nogusa, K. Suzuki, and M. Bannai are employees of Ajinomoto Co., Inc.
AUTHOR CONTRIBUTIONS
Author contributions: K.O., K. Suzuki, and M.B. conception and design of research; K.O. and Y.N. performed experiments; K.O. and K. Shinoda analyzed data; K.O. prepared figures; K.O. and M.B. drafted manuscript; K.O., S.K., and M.B. edited and revised manuscript; M.B. interpreted results of experiments; M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank A. Morita, K. Uchino, R. Iritani, and the staff of A-TEC Corporation for support in caring for the mice. We also appreciate the technical help provided by H. Ishizaki, N. Nishikawa, and M. Ogawa in many of the experiments.
REFERENCES
- 1.Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273: 215–221, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Baraldi PG, Preti D, Materazzi S, Geppetti P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem 53: 5085–5107, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther 82: 509–524, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Carlson CL, Winder WW. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J Appl Physiol 86: 669–674, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Degerman E, Smith CJ, Tornqvist H, Vasta V, Belfrage P, Manganiello VC. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci USA 87: 533–537, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 11.Haramizu S, Mizunoya W, Masuda Y, Ohnuki K, Watanabe T, Yazawa S, Fushiki T. Capsiate, a nonpungent capsaicin analog, increases endurance swimming capacity of mice by stimulation of vanilloid receptors. Biosci Biotechnol Biochem 70: 774–781, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31: 1120–1124, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr 72: 558S–563S, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, Fushiki T, Yazawa S, Watanabe T, Tominaga M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 44: 958–967, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Inoue N, Matsunaga Y, Satoh H, Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci Biotechnol Biochem 71: 380–389, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Jeukendrup AE, Saris WH, Wagenmakers AJ. Fat metabolism during exercise: a review–part II.: regulation of metabolism and the effects of training. Int J Sports Med 19: 293–302, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Kang JH, Goto T, Han IS, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity (Silver Spring) 18: 780–787, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH19 sweet, a non-pungent cultivar of red pepper, in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem 70: 2824–2835, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr 116: 1272–1278, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Kawai N, Kawai T, Kawai K. [Ultrasonic and laboratory studies on fatty liver in white-collar workers]. Nihon Shokakibyo Gakkai Zasshi 92: 1058–1065, 1995. [PubMed] [Google Scholar]
- 21a.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 29: 615–623, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kobata K, Sutoh K, Todo T, Yazawa S, Iwai K, Watanabe T. Nordihydrocapsiate, a new capsinoid from the fruits of a nonpungent pepper, capsicum annuum. J Nat Prod 62: 335–336, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kobata K, Todo T, Yazawa S, Iwai K, Watanabe T. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, from the fruits of a nonpungent cultivar, CH-19 Sweet, of Pepper (Capsicum annuum L). J Agric Food Chem 46: 1695–1697, 1998. [Google Scholar]
- 25.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc 36: 1985–1996, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci 63: 1393–1409, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee IM. Physical activity, and cancer prevention–data from epidemiologic studies. Med Sci Sports Exerc 35: 1823–1827, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, Ma S, Chen X, Zhu T, Cao T, Liu D, Nilius B, Huang Y, Yan Z, Zhu Z. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res 22: 551–564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda Y, Haramizu S, Oki K, Ohnuki K, Watanabe T, Yazawa S, Kawada T, Hashizume S, Fushiki T. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol 95: 2408–2415, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett 580: 2917–2921, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I. Reduction of diet-induced obesity by a combination of tea-catechin intake and regular swimming. Int J Obes (Lond) 30: 561–568, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord 26: 1459–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr 81: 122–129, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504: 82–106, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Oh-ishi S, Kizaki T, Toshinai K, Haga S, Fukuda K, Nagata N, Ohno H. Swimming training improves brown-adipose-tissue activity in young and old mice. Mech Ageing Dev 89: 67–78, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Oh KS, Kim EY, Yoon M, Lee CM. Swim training improves leptin receptor deficiency-induced obesity and lipid disorder by activating uncoupling proteins. Exp Mol Med 39: 385–394, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Biochem 65: 2735–2740, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Ohnuki K, Niwa S, Maeda S, Inoue N, Yazawa S, Fushiki T. CH19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Biosci Biotechnol Biochem 65: 2033–206, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol 110: 789–798, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem 277: 32571–32577, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 4: 529–539, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Sallis JF, Haskell WL, Fortmann SP, Vranizan KM, Taylor CB, Solomon DS. Predictors of adoption and maintenance of physical activity in a community sample. Prev Med 15: 331–341, 1986. [DOI] [PubMed] [Google Scholar]
- 47.Sasahara I, Furuhata Y, Iwasaki Y, Inoue N, Sato H, Watanabe T, Takahashi M. Assessment of the biological similarity of three capsaicin analogs (capsinoids) found in non-pungent chili pepper (CH-19 Sweet) fruits. Biosci Biotechnol Biochem 74: 274–278, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annu Rev Nutr 20: 21–44, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, Yazawa S, Tominaga M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br J Pharmacol 165: 1476–1486, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr 89: 45–50, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 104: 531–543, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, Lindstrom J, Louheranta A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr 7: 147–165, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Tani Y, Fujioka T, Sumioka M, Furuichi Y, Hamada H, Watanabe T. Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J Nutr Sci Vitaminol (Tokyo) 50: 351–355, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta 1801: 299–310, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Urhausen A, Gabriel H, Kindermann W. Blood hormones as markers of training stress and overtraining. Sports Med 20: 251–276, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab 49: 54–63, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, Sun Q. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 300: R1115–R1125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Z, Okutsu M, Akhtar YN, Lira VA. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol 110: 264–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr 95: 845–850, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123: 3404–3408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


