Abstract
Angiopoietin-2 (Ang-2) is a key mediator of pulmonary vascular permeability. This study tested the association between plasma Ang-2 and mortality in pediatric acute respiratory distress syndrome (ARDS), with stratification for prior hematopoietic cellular transplantation (HCT), given the severe, yet poorly understood, ARDS phenotype of this subgroup. We enrolled 259 children <18 years of age with ARDS; 25 had prior HCT. Plasma Ang-2, von Willebrand Factor antigen (vWF), and vascular endothelial growth factor (VEGF) were measured on ARDS days 1 and 3 and correlated with patient outcomes. Day 1 and day 3 Ang-2 levels were associated with mortality independent of age, sex, race, and P/F ratio [odds ratio (OR) 3.7, 95% CI 1.1–11.5, P = 0.027; and OR 10.2, 95% confidence interval (CI) 2.2–46.5, P = 0.003, for each log10 increase in Ang-2]. vWF was associated with mortality (P = 0.027), but VEGF was not. The association between day 1 Ang-2 and mortality was independent of levels of both vWF and VEGF (OR 3.6, 95% CI 1.1–12.1, P = 0.039, for each log10 increase in Ang-2). 45% of the cohort had a rising Ang-2 between ARDS day 1 and 3 (adjusted mortality OR 3.3, 95% CI 1.2–9.2, P = 0.026). HCT patients with a rising Ang-2 had 70% mortality compared with 13% mortality for those without (OR 16.3, 95% CI 1.3–197.8, P = 0.028). Elevated plasma levels of Ang-2 were associated with mortality independent of vWF and VEGF. A rising Ang-2 between days 1 and 3 was strongly associated with mortality, particularly in pediatric HCT patients, suggesting vulnerability to ongoing endothelial damage.
Keywords: acute respiratory distress syndrome, angiopoietin, endothelial injury, hematopoietic stem cell transplant, pediatrics
pediatric acute respiratory distress syndrome (ARDS) is characterized by pathologic permeability of the pulmonary endothelium and epithelium leading to intra-alveolar edema rich in leukocytes and inflammatory mediators (16, 25, 28). Clinically, children manifest severe oxygenation failure, and although mortality is lower than in adults, they may progress to death in ∼20–40% of cases (26, 27, 43).
As such, there is considerable interest in identifying molecular regulators of endothelial permeability. The Tie-2 receptor was discovered in 1993 and became the second identified endothelium-specific receptor, preceded by the discovery of vascular endothelial growth factor-receptor (VEGF-R) in 1989 (9, 10, 18, 34, 37, 38). Tie-2 is activated by the agonist angiopoietin-1 (Ang-1) and is regulated by the partial agonist angiopoietin-2 (Ang-2) (3, 23). Endothelial stress causes exocytosis of Weibel-Palade bodies, where Ang-2 is stored (12, 24, 32). Ang-2 then saturates the Tie-2 receptor and increases endothelial cell permeability by internalizing vascular endothelial (VE)-cadherin adherens junction proteins (3, 14). Ang-2 also upregulates the NF-κB pathway and promotes leukocyte firm adhesion and transmigration across the endothelial barrier (17, 41).
The clinical significance of elevated Ang-2 has been demonstrated in both animals and humans. Ang-2 overexpression in mice is uniformly lethal due to disrupted angiogenesis (23, 40). In contrast, Ang-2 knockout mice have a protective phenotype in response to a variety of endothelial insults; they consistently fail to upregulate tissue factor and leukocyte adhesion molecules, resulting in maintained endothelial barriers and improved survival (4, 6, 11, 39, 45). In humans, elevated plasma Ang-2 has been associated with mortality in both adult and pediatric septic shock (15, 33, 49). Elevated Ang-2 has also been correlated with mortality and pulmonary leak in adult ARDS, but this association has not been tested in children (1, 7).
Therefore, a principal aim of this study was to test the association of Ang-2 with mortality in pediatric ARDS patients. To account for possible confounding effects, we also measured von Willebrand Factor (vWF), which is coreleased from Weibel-Palade bodies, and VEGF, which mediates downstream pathways of Ang-2 signaling.
A secondary aim of this study was to test the association between Ang-2 and mortality in pediatric hematopoietic cellular transplant patients (HCT), whom we have previously found have significantly higher ARDS mortality than other children (52, 53). Endothelial damage is a hallmark of posttransplant complications, yet the molecular pathogenesis of ARDS in HCT patients is poorly understood. Given the ability to modulate Ang-2 signaling with Ang-2 neutralizing antibodies, soluble Tie-2, and exogenous Ang-1, identifying Ang-2 dysregulation in clinically high-risk subgroups has the potential for direct therapeutic translation (29, 30, 39, 48, 51).
METHODS
Patients.
Patients were prospectively enrolled from five pediatric intensive care units in California and Wisconsin between 2008 and 2014. Inclusion criteria were age between 30 days and 18 years; respiratory support of either high-flow nasal cannula ≥5 l/min, noninvasive CPAP/BiPAP, or invasive mechanical ventilation; and meeting the American European Consensus Conference diagnostic criteria for ARDS (5). The Berlin Definition of ARDS was published in 2012 and, therefore, not used in enrollment criteria (2). Patients were excluded if they were younger than 36 wk-corrected gestational age, had been enrolled previously, or had a limitation of care in place at the time of screening. Patients were further excluded when an insufficient blood sample was available for Ang-2 measurement. This study was approved by the University of California–San Francisco Institutional Review Board with consent obtained from each patient or surrogate.
Baseline characteristics.
Demographic information included age, sex, race, and ethnicity. The etiology of lung injury was defined as direct (pneumonia or aspiration) or indirect (nonpulmonary sepsis, trauma, transfusions, or other), given recent findings highlighting Ang-2 release in systemic inflammatory states, such as sepsis (8). Illness severity was assessed with Pediatric Risk of Mortality III (PRISM-3) scores based on variables present in the first 24 h of admission (20, 35). Oxygenation failure was described using each patient's worst PaO2/FiO2 ratio (P/F ratio) within the first 24 h of enrollment.
Molecular measurements.
Plasma Ang-2 was measured on ARDS days 1 and 3 (0–24 h and 48–72 h after meeting ARDS criteria, respectively) using a solid-phase sandwich ELISA immunoassay (R&D Systems, Minneapolis, MN) with reported least detectable dose (LDD) of 8.29 pg/ml. Assays were performed in duplicate with <15% variability between readings, and the final recorded value was the average of the two. Patients whose Ang-2 levels rose from day 1 to day 3 were described to have a positive change in Ang-2, hereafter referred to as rising Ang-2. vWF and VEGF were measured with a Luminex multiplex ELISA immunoassay (Myriad RBM, Austin, TX), with LDD of 7.8 μg/ml and 62 pg/ml, respectively. Coefficients of variation were <15%, and there was no reported cross-reactivity for any of these assays.
Outcome.
The primary outcome was ICU death while on the ventilator or within 24 h of extubation. This outcome was chosen to exclude deaths occurring distant to extubation, which might represent non-ARDS-related mortality or complications from underlying comorbidities. ARDS patients who died while intubated likely had ARDS or respiratory failure as the primary cause of death. We included patients who died within 24 h of extubation in this group to capture patients who had redirection of their goals of care and were compassionately extubated with the expectation that they were in the terminal phase of dying and would not survive extubation. All-cause ICU and all-cause hospital death were also analyzed. The secondary outcome was the highest pediatric logistic organ dysfunction (PELOD) score for PICU survivors. The PELOD scores comprise clinical and laboratory derangements and is a validated surrogate for PICU morbidity (20). The PELOD was derived from the worst organ failure score measured on study days 1–7, 14, 21, and 28.
Statistics.
Differences between categorical variables were tested using the χ2-test or the Fisher exact statistic, as appropriate. Differences between nonnormally distributed continuous variables were tested using Wilcoxon rank sum, and associations between nonnormally distributed continuous variables were tested using Spearman's rank correlation coefficient. Nonnormally distributed continuous variables with significant Spearman's associations were then log10-transformed and tested for association using linear regression. Nonnormally distributed continuous variables were divided into quartiles and tested for association with mortality by the nonparametric test of trend. Mortality odds were assessed using the logistic regression following log10-transformation for nonnormally distributed continuous variables. All analyses used 95% confidence intervals and two-tailed P values based on robust standard error estimates with a nominal significance level of α = 0.05. Regression models were compared using likelihood ratios. Analyses were performed using STATA software, version 13.1 (StataCorp, College Station, TX).
RESULTS
Cohort description.
There were 299 patients enrolled during the study period; 40 were subsequently excluded from analysis due to lack of plasma available for analysis, leaving 259 patients (Table 1). Baseline characteristics were not different between study patients and those excluded due to lack of Ang-2 measurement (Table 2). The study population was 55% male, 64% Caucasian, and had a median age of 5.2 years [interquartile range (IQR) 1.1–13.2]. A history of HCT was present in 10%, and 62% of patients had ARDS from direct lung injury. The median P/F ratio was 137 (IQR 90–222), and the median PRISM-3 was 12.5 (IQR 7.5–20.5). The overall mortality was 15% (38/259); the first patient death occurred on ARDS day 4, and the last death occurred 257 days after extubation.
Table 1.
Patient characteristics
| Demographics | All (n = 259) | Nonsurvivors (n = 38) | Survivors (n = 221) | |
|---|---|---|---|---|
| Age, (median years, IQR) | 5.2 (1.1–13.2) | 7.0 (2.3–13.9) | 5.0 (1–13.1) | P = 0.353 |
| Male (n, %) | 143 (55.4) | 27 (71.1) | 116 (52.7) | P = 0.036 |
| Race (n, %) | ||||
| Caucasian | 166 (64.3) | 23 (60.5) | 143 (65.0) | P = 0.714 |
| African American | 19 (7.4) | 4 (11.1) | 15 (6.8) | |
| Asian/Pacific Islander | 17 (6.6) | 1 (2.8) | 16 (7.2) | |
| Multiple | 20 (7.8) | 3 (7.9) | 17 (7.7) | |
| American Indian/Alaskan Native | 3 (1.2) | 1 (2.8) | 2 (0.9) | |
| Unknown | 33 (12.8) | 6 (16.7) | 27 (12.2) | |
| Hispanic/Latino Ethnicity (n, %) | 95 (36.8) | 14 (38.9) | 81 (36.5) | P = 0.760 |
| Comorbidities (n, %) | ||||
| HCT | 25 (9.6) | 12 (31.6) | 13 (5.9) | P < 0.001 |
| ARDS Etiology (n, %) | ||||
| Sepsis | 56 (21.9) | 12 (31.6) | 44 (20.1) | P = 0.557 |
| Trauma | 13 (5.1) | 2 (5.3) | 11 (5.1) | |
| Transfusion | 7 (2.7) | 1 (2.6) | 6 (2.8) | |
| Other | 21 (8.2) | 2 (5.3) | 19 (8.7) | |
| Pneumonia | 151 (59.0) | 21 (55.3) | 130 (59.6) | |
| Aspiration | 8 (3.1) | 0 (0) | 8 (3.7) | |
| Severity of Illness (median, IQR) | ||||
| P/F Ratio (n = 221) | 110 (77.3–168.6) | 106.5 (73.3–128.3) | 111.3 (79.3–186.9) | P = 0.080 |
| PRISM-3 (n = 236) | 12.5 (7.5–20.5) | 20 (12–24) | 12 (7–19) | P < 0.001 |
IQR, interquartile range; HCT, hematopoietic cellular transplantation; ARDS, acute respiratory distress syndrome; PRISM-3, Pediatric Risk of Mortality III score.
Table 2.
Comparison of included vs. excluded patients
| Ang-2 Not Measured (n = 40) | Ang-2 Measured (n = 259) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age (median years, IQR) | 4.4 (1.2–10.1) | 5.2 (1.1–13.2) | P = 0.533 |
| Male (n, %) | 22 (55.0) | 143 (55.4) | P = 1.000 |
| Race (n, %) | P = 0.286 | ||
| Caucasian | 22 (55.0) | 166 (64.3) | |
| African American | 4 (10.0) | 19 (7.4) | |
| Asian/Pacific Islander | 4 (10.0) | 17 (6.6) | |
| Multiple | 7 (17.5) | 20 (7.8) | |
| American Indian/Alaskan Native | 0 (0) | 3 (1.2) | |
| Unknown | 3 (7.5) | 33 (12.8) | |
| Hispanic/Latino Ethnicity (n, %) | 15 (37.5) | 95 (36.8) | P = 0.879 |
| Comorbidities (n, %) | |||
| HCT | 1 (2.5) | 25 (9.6) | P = 0.223 |
| ARDS Etiology (n, %) | |||
| Sepsis | 6 (15.0) | 56 (21.9) | P = 0.649 |
| Trauma | 2 (5.0) | 13 (5.1) | |
| Transfusion | 0 (0) | 7 (2.7) | |
| Other | 6 (15.0) | 21 (8.2) | |
| Pneumonia | 25 (62.5) | 151 (59.0) | |
| Aspiration | 1 (2.5) | 8 (3.1) | |
| Severity of Illness (median, IQR) | |||
| P/F Ratio | 115.6 (79.9–160) | 110 (77.3–168.6) | P = 0.762 |
| PRISM-3 | 11 (7–14) | 12.5 (7.5–20.5) | P = 0.074 |
| Outcome | |||
| Mortality (n, %) | 2 (5.0) | 38 (14.7) | P = 0.094 |
| Survivor peak PELOD (median, IQR) | 12 (10–20) | 20 (11–23) | P = 0.060 |
PELOD, pediatric logistic organ dysfunction.
Plasma Ang-2 levels in pediatric ARDS.
Median plasma Ang-2 levels on day 1 and day 3 were 7.2 ng/ml (IQR 4.2–15.5) and 7.7 ng/ml (IQR 4.3–14.7), respectively. Patients with higher PRISM-3 scores had higher plasma Ang-2 on both day 1 (Spearman coefficient 0.322, P < 0.001) and day 3 (Spearman coefficient 0.337, P < 0.001). Patients with indirect lung injury had higher plasma Ang-2 on day 1 (P < 0.001) but not on day 3 (P = 0.491). Compared with non-HCT patients, HCT patients had higher plasma Ang-2 on both day 1 (14.7 ng/ml, IQR 6.8–25.2 vs. 6.9 ng/ml, IQR 4.1–13.2, P = 0.010) and day 3 (16.1 ng/ml, IQR 6.8–25.4 vs. 7.7 ng/ml, IQR 4.3–13.8, P = 0.006).
Plasma Ang-2 is associated with morbidity and mortality in pediatric ARDS.
Both day 1 and day 3 Ang-2 were associated with morbidity and mortality. Compared with survivors, nonsurvivors had higher plasma Ang-2 on day 1 (11.6 ng/ml, IQR 6.9–25.2 vs. 6.6 ng/ml, IQR 4.1–13.8, P = 0.021) (Fig. 1). Higher Ang-2 quartile was associated with mortality (P = 0.049) and on univariate logistic analysis, the odds ratio for mortality was 2.8 for each log10 increase in Ang-2 (95% CI 1.1–7.1, P = 0.036). In those who survived, day 1 Ang-2 was associated with peak PELOD score (Spearman coefficient 0.259, P < 0.001).
Fig. 1.
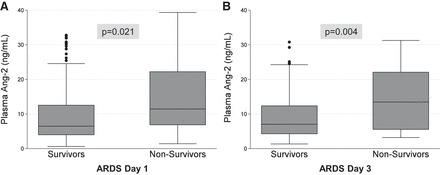
Plasma angiopoietin-2 (Ang-2) levels in pediatric acute respiratory distress syndrome (ARDS), stratified by mortality. Boxes represent median with IQR, whiskers extend to upper and lower adjacent values, and dots represent outliers.
Similarly, nonsurvivors had higher plasma Ang-2 on day 3 (13.5 ng/ml, IQR 5.6–22.1 vs. 7.5 ng/ml, IQR 4.3–13.4, P = 0.004). Higher Ang-2 quartile on day 3 was associated with mortality (P = 0.022) and on univariate logistic analysis, the OR for mortality was 5.2 for each log10 increase in day 3 Ang-2 (95% CI 1.7–16.2, P = 0.004). In those who survived, day 3 Ang-2 was associated with peak PELOD score (Spearman coefficient 0.386, P < 0.001).
On multivariable logistic regression, the associations between day 1 or day 3 Ang-2 and mortality were independent of age, sex, race, and P/F ratio (Table 3). These findings persisted on analysis of all-cause PICU mortality and all-cause hospital mortality (Table 4). In addition, there was an interaction between day 1 Ang-2 and HCT status (P = 0.077). Given this interaction effect and the baseline difference in Ang-2 levels between HCT and non-HCT patients, we further examined mortality according to HCT status. For non-HCT patients, the association between Ang-2 and mortality was independent of age, sex, race, and P/F ratio on both day 1 (adjusted OR 5.1, 95% CI 1.2–20.7, P = 0.023 for each log10 increase in Ang-2) and day 3 (adjusted OR 13.1, 95% CI 1.8–94.5, P = 0.011 for each log10 increase in Ang-2). For HCT patients, the association between day 1 or day 3 Ang-2 and mortality was not statistically significant (P = 0.372 and P = 0.882, respectively).
Table 3.
Odds of mortality based on Ang-2 measurements
| Predictor | Day 1 Ang-2* | Day 3 Ang-2* | Rising Ang-2 |
|---|---|---|---|
| All Patients | |||
| Univariate | 2.8 (1.1–7.1, P = 0.036) | 5.2 (1.7–16.2, P = 0.004) | 2.1 (0.9–4.9, P = 0.073) |
| Multivariable | 3.7 (1.2–11.5, P = 0.027) | 10.2 (2.2–46.5, P = 0.003) | 3.3 (1.2–9.2, P = 0.026) |
| No HCT | |||
| Univariate | 3.4 (1.1–10.7, P = 0.032) | 5.4 (1.4–21.2, P = 0.015) | 1.3 (0.5–3.4, P = 0.555) |
| Multivariable | 5.1 (1.2–20.7, P = 0.023) | 16.4 (2.4–109, P = 0.004) | 2.3 (0.7–7.4, P = 0.169) |
| HCT | |||
| Univariate | 0.4 (0.04–3.4, P = 0.372) | 0.8 (0.08–9.0, P = 0.882) | 16.3 (1.3–197.8, P = 0.028) |
| Multivariable | ** | ** | ** |
Mortality odds were calculated with logistic regression using log10 transformed distributions and are displayed as odds ratios per log10 increase in Ang-2, with 95% confidence intervals. Multivariable regression adjusted for age, sex, race, and P/F ratio.
These models could not be accurately completed due to limited sample size.
Table 4.
All-cause PICU and all-cause hospital mortality by Ang-2 level
| Predictor | Nonsurvivors | Survivors | Univariate Odds | Multivariable Odds |
|---|---|---|---|---|
| All-Cause PICU Mortality by Ang-2 Level | ||||
| Ang-2, day 1 | 11.6 (6.6–25.3) | 6.8 (4.1–13.5) | 3.2 (1.3–8.2, P = 0.014) | 4.0 (1.3–11.6, P = 0.012) |
| Ang-2, day 3 | 14.0 (6.4–23.6) | 7.3 (4.3–13.4) | 6.8 (2.1–21.8, P = 0.001) | 13.0 (2.8–60.9, P = 0.001) |
| All-Cause Hospital Mortality by Ang-2 Level | ||||
| Ang-2, day 1 | 9.8 (6.5–22.2) | 6.8 (4.0–14.6) | 2.5 (1.04–5.8, P = 0.040) | 3.0 (1.1–8.1, P = 0.027) |
| Ang-2, day 3 | 12.3 (5.0–21.4) | 7.6 (4.3–13.4) | 3.6 (1.3–10.3, P = 0.016) | 10.9 (2.5–47.5, P = 0.001) |
Mortality odds were calculated with logistic regression using log10 transformed distributions and are displayed as odds ratios per log10 increase in Ang-2 with 95% confidence intervals. Multivariable regression adjusted for age, sex, race, and P/F ratio. PICU, pediatric intensive care unit.
Plasma vWF levels in pediatric ARDS.
The median plasma vWF level on day 1 was 233 μg/ml (IQR 178–306). Higher PRISM-3 score did not correlate with higher vWF levels (Spearman P = 0.685), and HCT patients had vWF levels similar to non-HCT patients (250 μg/ml, IQR 202–328 vs. 230 μg/ml, IQR 172–306, P = 0.189). Indirect lung injury patients had similar vWF levels compared with direct lung injury patients (P = 0.244). There was a positive correlation between Ang-2 and vWF levels on day 1 (Spearman correlation 0.199, P = 0.007) and log10 transformed Ang-2 and vWF displayed a linear association (regression coefficient 0.296, P = 0.023).
Plasma vWF is associated with mortality in pediatric ARDS.
Compared with survivors, nonsurvivors had higher plasma vWF on day 1 (275 μg/ml, IQR 219–328 vs. 219.5 μg/ml, IQR 169.5–300.5, P = 0.027) (Fig. 2). Higher vWF quartile was associated with mortality (P = 0.018), although the univariate logistic relationship between log10-transformed vWF and mortality was not significant (P = 0.224) due to the presence of three outliers with the highest vWF levels, all of whom survived. vWF was not associated with peak PELOD in survivors (Spearman P = 0.942). On multivariable logistic regression, vWF quartile did not influence the association between day 1 Ang-2 and mortality (interaction P = 0.771). The multivariable adjusted association between day 1 Ang-2 and mortality was independent of vWF quartile (P = 0.036), and the addition of vWF quartile to this model did not improve the model fit for mortality (likelihood ratio P = 0.164).
Fig. 2.
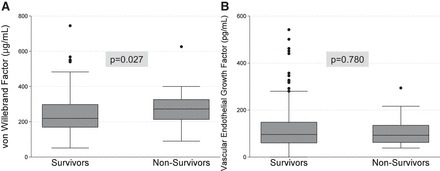
Plasma vWF and VEGF levels in pediatric ARDS, stratified by mortality. Boxes represent median with IQR; whiskers extend to upper and lower adjacent values, and dots represent outliers.
Plasma VEGF levels in pediatric ARDS.
The median plasma VEGF level on day 1 was 97 pg/ml (IQR 62–148). A higher PRISM-3 score did not correlate with higher VEGF levels (P = 0.630), and HCT patients had similar VEGF levels to non-HCT patients (96 pg/ml, IQR 61–173 vs. 97 pg/ml, IQR 62–146, P = 0.891). Indirect lung injury patients had similar VEGF levels compared with direct lung injury patients (P = 0.085). There was a weak correlation between Ang-2 and VEGF levels on day 1 (Spearman coefficient −0.144, P = 0.045), but there was no association between log10-transformed Ang-2 and VEGF (linear regression P = 0.182).
Plasma VEGF is not associated with mortality in pediatric ARDS.
Compared with survivors, nonsurvivors had similar plasma VEGF on day 1 (92.5 pg/ml, IQR 65.5–133 vs. 97 pg/ml, IQR 61–154, P = 0.780). Higher VEGF quartile was not associated with mortality (P = 0.652), and VEGF was not associated with peak PELOD in survivors (Spearman P = 0.473). On multivariable logistic regression, levels of VEGF did not influence the association between Ang-2 and mortality (interaction P = 0.404). The association between day 1 Ang-2 and mortality was independent of VEGF (P = 0.035), and the addition of VEGF to this model did not improve the model fit for mortality (likelihood ratio P = 0.472). In a multivariable model, including log-transformed Ang-2, vWF, and VEGF and adjusting for age, sex, race, and P/F ratio, Ang-2 was the only molecule that was associated with mortality (OR 3.6, 95% CI 1.1–12.1, P = 0.039).
Distribution of rising Ang-2 levels in pediatric ARDS.
Plasma from both day 1 and day 3 was available for Ang-2 measurement for 173 of the 259 patients; the remaining patients either did not have an indwelling line to draw blood on both days or did not consent to successive blood draws. No patients died between days 1 and 3. Of the 173 patients with plasma from both day 1 and day 3, a rising Ang-2 between ARDS day 1 and day 3 occurred in 45% of the cohort (78/173). PRISM-3 scores were not different among those with and without a rising Ang-2 (P = 0.238). Similar proportions of HCT patients and non-HCT patients had rising Ang-2 (10/18 vs. 68/155, P = 0.454).
A rise in Ang-2 levels between day 1 and day 3 is associated with mortality in pediatric ARDS.
A rising Ang-2 was observed in 61% of nonsurvivors (17/28) compared with 42% of survivors (61/145, P = 0.069). With adjustment for age, sex, race, and P/F ratio, the multivariable odds of mortality for those with a rising Ang-2 were 3.3 (95% CI 1.2–9.2, P = 0.026). However, on multivariable regression, there was an interaction between a rising Ang-2 and HCT status (P = 0.065). Therefore, we further examined mortality according to HCT status. For non-HCT patients, mortality was similar for those with a rising Ang-2 (15%; 10/68) compared with those without (12%; 10/87, P = 0.554) (Fig. 3). However, for HCT patients, mortality was 70% for those with a rising Ang-2 (7/10) compared with 13% for those without (1/8, P = 0.025) with univariate odds of mortality of 16.3 (95% CI 1.3–197.8, P = 0.028) (Fig. 4).
Fig. 3.
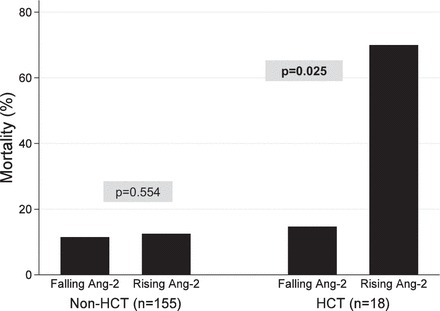
Pediatric ARDS mortality stratified by HCT status and rising or falling Ang-2.
Fig. 4.
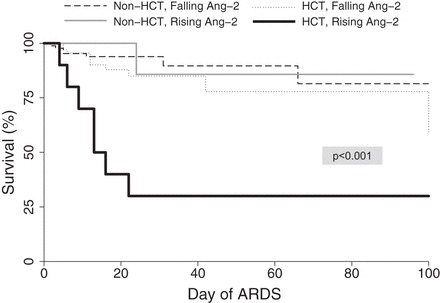
Survival estimates for pediatric ARDS patients based on HCT history and Ang-2. Dropout was recorded for ARDS-related mortality, defined as death while intubated or within 24 h of extubation. P < 0.001 for overall difference among four subgroups. P = 0.0164 for difference among only HCT patients with and without rising Ang-2.
DISCUSSION
The primary results of this study can be summarized as follows. First, both elevated plasma levels of Ang-2 on day 1 and day 3 of ARDS and rising levels of Ang-2 between day 1 and day 3 of ARDS were strongly associated with mortality in our pediatric study population. Second, for those patients who survived ARDS, elevated Ang-2 also predicted morbidity as measured by peak PELOD score. Third, levels of vWF and not VEGF were associated with mortality; however, the association of elevated Ang-2 plasma levels with mortality was independent of both vWF and VEGF. Fourth, the association between a rising Ang-2 and mortality was most prominent in HCT patients, who had a significantly higher mortality associated with rising Ang-2.
The association between elevated Ang-2 and mortality is consistent with recent literature on adult ARDS, as well as a variety of other illnesses, including sepsis and trauma. The overall raw Ang-2 distributions in survivors and nonsurvivors (median 6.6 ng/ml and 11.6 ng/ml) are similar to adult ARDS (median 7.9 ng/ml and 10.6 ng/ml in one reference) (42), suggesting that Ang-2 distributions are roughly similar between adult and pediatric ARDS patients. The portion of patients with rising Ang-2 was roughly similar to other reports and likely reflects the case mix of patients with direct and indirect lung injury (7, 47). The magnitude of the relationship between Ang-2 and mortality in this study is difficult to compare to adult data since the cohort characteristics are inherently different, and mortality was measured at different time points (7, 49). Nonetheless, these results provide further evidence for the importance of Ang-2 in endothelial dysregulation and for the importance of endothelial damage in illness severity and outcome. Future clinical trials of targeted endothelial stabilization via Ang-2 modulation may be warranted for pediatric ARDS and may benefit from the use of Ang-2 levels as part of enrollment criteria, which could be done as a point-of-care measurement. These results also suggest that resolving elevation of Ang-2 may be a surrogate biochemical marker for illness recovery; future studies should evaluate Ang-2 in concert with biomarkers of pulmonary epithelial injury for a potentially more comprehensive representation of lung damage.
Our findings that vWF was associated with mortality complements earlier ARDS studies in adults and children (7, 13, 42). However, although the association was present on nonparametric test of trend, it was not robust to univariate or multivariable logistic regression. Further, the addition of vWF to the logistic model of Ang-2 and mortality did not affect the relationship between Ang-2 and mortality, nor did it improve the model fit, again suggesting that the primary association between Ang-2 and mortality is not due to a confounding effect of vWF collinearity with Ang-2. These findings may also suggest that the primary effect of endothelial injury is not simply Weibel-Palade body exocytosis, but rather an upregulation of Ang-2 synthesis relative to other molecules. Also, Ang-2 itself may be pathogenic and not just a biomarker of endothelial injury. Identifying differential regulatory mechanisms for Ang-2 synthesis in response to endothelial signals should be a future goal of investigation.
Further, these analyses indicated that the association between plasma Ang-2 and mortality was independent of plasma levels of VEGF. Importantly, VEGF was not associated with ARDS-related mortality in our study. This is consistent with prior studies that have not associated VEGF levels with ARDS mortality; our cohort's plasma VEGF levels are also similar to those of adult ARDS cohorts and higher than those of adult healthy controls (44, 50). The lack of association between VEGF and mortality may be related to varying levels of other cytokines that interact with VEGF to facilitate vascular permeability, the need to measure VEGF locally in alveolar epithelial lining fluid, and the degree of VEGF receptor expression, which may significantly influence the pathobiology of VEGF in acute lung injury patients.
We also found that plasma Ang-2 is higher in HCT patients and rises in HCT nonsurvivors. Elevated Ang-2 levels in the HCT cohort may be due to higher baseline Ang-2 levels prior to the onset of critical illness. For example, the process of stem cell transplantation itself induces a rise in Ang-2 in the first 4 wk posttransplant (19, 31, 36), and persistently elevated Ang-2 levels correlate with ongoing cellular damage related to graft vs. host disease, thrombotic microangiopathy, and hepatic sinusoidal obstruction syndrome (46). Alternatively, these higher levels in HCT patients may indicate a higher susceptibility to endothelial damage or a dysregulated response to ARDS triggers (11, 21, 22). Identifying modifiable triggers of endothelial damage in transplant conditioning regimens and graft manipulation strategies should be a key goal of future investigation.
Interestingly, HCT patients with rising Ang-2 had significantly higher mortality than HCT patients with a stable or falling Ang-2, a finding that was less prominent in non-HCT patients. This finding suggests that acute, ongoing endothelial damage may propagate unchecked in HCT patients, perhaps due to changes or damage to regulatory proteins that abrogate the effect of rising Ang-2 in the healthier host. Preclinical data indicate that elevated Ang-2 alone is not sufficient to induce endothelial injury, but rather requires a network of other cytokines and intracellular proteins (3, 4). Future studies could focus on identifying molecular and genetic mechanisms of Ang-2 synthesis and release.
There are several strengths to this study. First, we used a large pediatric multicenter cohort with demographic diversity. Second, we measured plasma Ang-2 at two time points, allowing for the analysis of a trend over time. Third, we described ARDS-related mortality in addition to all-cause PICU and all-cause hospital mortality, which is particularly relevant to our HCT cohort of patients, who may survive ARDS but die prior to discharge from primary disease relapse or other transplant complications. Finally, we contextualized our Ang-2 associations with measurements of two other biologically relevant markers of endothelial injury.
This study also has some limitations. First, measurements from patient plasma may not comprehensively reflect local processes in the lung. This may be particularly important for patients with vasculopathies, such as pulmonary hypertension or autoimmune vasculitides. Second, we have limited data on HCT-specific characteristics, such as type of malignancy, type, and date of transplant, and relevant complications, which may affect Ang-2 levels both at baseline and in the setting of critical illness. Also, the number of patients with HCT included in this cohort was modest, so future studies in larger numbers of HCT patients with pediatric ARDS will be needed. Finally, the effect of medicines such as corticosteroids and extracorporeal membrane therapies, such as continuous renal replacement (CRRT) on levels of Ang-2, is unknown and may confound our results. To address this, we excluded patients who used CRRT on day 1 (n = 15) and found that the association between Ang-2 and mortality persisted (no patients used extracorporeal membrane oxygenation on day 1).
Conclusions.
Elevated plasma Ang-2 is strongly correlated with mortality in pediatric ARDS, suggesting a strong mechanistic link between endothelial injury and mortality. HCT patients manifest a particularly severe ARDS phenotype with a strong association between rising plasma Ang-2 and mortality, which may suggest ongoing endothelial damage. Further research needs to be done to elucidate the molecular steps involved in Ang-2 regulation in response to endothelial injury.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S.Z. and A. Sapru conception and design of research; M.S.Z. and A. Sapru analyzed data; M.S.Z., A. Spicer, B.O.O., C.C.D., C.S.C., M.A.M., and A. Sapru interpreted results of experiments; M.S.Z. and A. Sapru prepared figures; M.S.Z. and A. Sapru drafted manuscript; M.S.Z., A. Spicer, B.O.O., C.C.D., C.S.C., M.A.M., and A. Sapru edited and revised manuscript; M.S.Z., A. Spicer, B.O.O., M.A., C.C.D., C.S.C., M.A.M., and A. Sapru approved final version of manuscript; M.A. and A. Sapru performed experiments.
ACKNOWLEDGMENTS
We thank Dr. John Neuhaus, Professor of Epidemiology and Biostatistics at the University of California San Francisco, for his statistical review.
REFERENCES
- 1.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med 187: 736–742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One 8: e70459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 12: 1286–1293, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA, NHLBI ARDS Network . Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 40: 1731–1737, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB, NHLBI ARDS Network . Distinct molecular phenotypes of direct versus indirect ARDS in single and multi-center studies. Chest 147: 1539–1548, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. Tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 7: 1471–1480, 1992. [PubMed] [Google Scholar]
- 10.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851–858, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med 12: 235–239, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Flori HR, Ware LB, Milet M, Matthay MA. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med 8: 96–101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14: 25–36, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano JS Jr Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS. Admission angiopoietin levels in children with septic shock. Shock 28: 650–654, 2007. [PMC free article] [PubMed] [Google Scholar]
- 16.Groeneveld AB, Raijmakers PG, Teule GJ, Thijs LG. The 67 gallium pulmonary leak index in assessing the severity and course of the adult respiratory distress syndrome. Crit Care Med 24: 1467–1472, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hughes DP, Marron MB, Brindle NP. The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-κB inhibitor ABIN-2. Circ Res 92: 630–636, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem Biophys Res Commun 195: 301–309, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Kumpers P, Koenecke C, Hecker H, Hellpap J, Horn R, Verhagen W, Buchholz S, Hertenstein B, Krauter J, Eder M, David S, Gohring G, Haller H, Ganser A. Angiopoietin-2 predicts disease-free survival after allogeneic stem cell transplantation in patients with high-risk myeloid malignancies. Blood 112: 2139–2148, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Leteurtre S, Martinot A, Duhamel A, Gauvin F, Grandbastien B, Nam TV, Proulx F, Lacroix J, Leclerc F. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making 19: 399–410, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99: 11,205–11,210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, Neumann F, Isermann B, Hegenbart U, Ho AD, Dreger P. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood 118: 1685–1692, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Mandriota SJ, Pyke C, Di Sanza C, Quinodoz P, Pittet B, Pepper MS. Hypoxia-inducible angiopoietin-2 expression is mimicked by iodonium compounds and occurs in the rat brain and skin in response to systemic hypoxia and tissue ischemia. Am J Pathol 156: 2077–2089, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 49: 119–133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med 189: 1301–1308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthay MA. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest 122 6 Suppl: 340S–343S, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 4: e269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss A. The angiopoietin:Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev 24: 579–592, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Nomura S, Ishii K, Inami N, Kimura Y, Uoshima N, Ishida H, Yoshihara T, Urase F, Maeda Y, Hayashi K. Evaluation of angiopoietins and cell-derived microparticles after stem cell transplantation. Biol Blood Marrow Transplant 14: 766–774, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 274: 15,732–15,739, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3: e46, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, Renkonen R, Knuutila S, Huebner K, Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol 12: 1698–1707, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 24: 743–752, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Porkholm M, Bono P, Saarinen-Pihkala UM, Kivivuori SM. Higher angiopoietin-2 and VEGF levels predict shorter EFS and increased non-relapse mortality after pediatric hematopoietic SCT. Bone Marrow Transplant 48: 50–55, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci USA 90: 9355–9358, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985, 1983. [DOI] [PubMed] [Google Scholar]
- 39.Stiehl T, Thamm K, Kaufmann J, Schaeper U, Kirsch T, Haller H, Santel A, Ghosh CC, Parikh SM, David S. Lung-targeted RNA interference against angiopoietin-2 ameliorates multiple organ dysfunction and death in sepsis. Crit Care Med 42: e654–62, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Tadros A, Hughes DP, Dunmore BJ, Brindle NP. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of angiopoietin-1. Blood 13: 4407–4409, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld AB. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 42: 691–700, 2014. [DOI] [PubMed] [Google Scholar]
- 43.The Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 16: 428–439, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med 164: 1601–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Ueda N, Chihara D, Kohno A, Tatekawa S, Ozeki K, Watamoto K, Morishita Y. Predictive value of circulating angiopoietin-2 for endothelial damage-related complications in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 20: 1335–1340, 2014. [DOI] [PubMed] [Google Scholar]
- 47.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, van Hinsbergh VW, Bouw MP, van der Hoeven JG, Groeneveld AB. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 35: 1567–1574, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Heijden M, van Nieuw Amerongen GP, Chedamni S, van Hinsbergh VW, Johan Groeneveld AB. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin Ther Targets 13: 39–53, 2009. [DOI] [PubMed] [Google Scholar]
- 49.van der Heijden M, van Nieuw Amerongen GP, van Hinsbergh VW, Groeneveld AB. The interaction of soluble Tie2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 33: 263–268, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Ware LB, Kaner RJ, Crystal RG, Schane R, Trivedi NN, McAuley D, Matthay MA. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. Eur Respir J 26: 101–105, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol 214: 472–481, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Zinter MS, DuBois SG, Spicer A, Matthay K, Sapru A. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med 40: 1536–1544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med 43: 1986–1994, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


