Abstract
Tamm-Horsfall protein (THP) is a glycoprotein expressed exclusively in thick ascending limbs (TAL) of the kidney. We recently described a novel protective role of THP against acute kidney injury (AKI) via downregulation of inflammation in the outer medulla. Our current study investigates the mechanistic relationships among the status of THP, inflammation, and tubular injury. Using an ischemia-reperfusion model in wild-type and THP−/− mice, we demonstrate that it is the S3 proximal segments but not the THP-deficient TAL that are the main targets of tubular injury during AKI. The injured S3 segments that are surrounded by neutrophils in THP−/− mice have marked overexpression of neutrophil chemoattractant MIP-2 compared with wild-type counterparts. Neutralizing macrophage inflammatory protein-2 (MIP-2) antibody rescues S3 segments from injury, decreases neutrophil infiltration, and improves kidney function in THP−/− mice. Furthermore, using immunofluorescence volumetric imaging of wild-type mouse kidneys, we show that ischemia alters the intracellular translocation of THP in the TAL cells by partially shifting it from its default apical surface domain to the basolateral domain, the latter being contiguous to the basolateral surface of S3 segments. Concomitant with this is the upregulation, in the basolateral surface of S3 segments, of the scavenger receptor SRB-1, a putative receptor for THP. We conclude that TAL affects the susceptibility of S3 segments to injury at least in part by regulating MIP-2 expression in a THP-dependent manner. Our findings raise the interesting possibility of a direct role of basolaterally released THP on regulating inflammation in S3 segments.
Keywords: CXCL2, ischemia-reperfusion, uromodulin, acute kidney injury
tamm-horsfall protein (THP) is a unique glycoprotein because it is exclusively expressed in the kidney, specifically in thick ascending limbs (TALs) and early distal tubules (4, 15, 29). Although this glycoprotein was discovered 60 years ago (37), its functions are still not fully understood (35). The association of THP with acute and chronic forms of renal disease, such as tubulointerstitial nephritis, familial juvenile hyperuricemic nephropathy, and more recently chronic kidney disease, argues for important regulatory functions of this glycoprotein in maintaining normal function of renal tubules (4, 5, 14, 16, 19, 20, 35, 41).
The role of THP in the setting of acute kidney injury (AKI) is even less well-understood. AKI remains a grave disease without a specific therapy and associated with increased risk of death (39). Experimental models of AKI such as ischemia-reperfusion injury (IRI) have been instrumental in understanding the complex pathogenesis of this disease. Indeed, AKI is a dynamic injury that involves epithelial and endothelial cell injury and alterations in various processes such vascular permeability, oxidative stress, and inflammation (8, 9, 26). The role of inflammation in the pathophysiology of AKI involves various cells of the innate and adaptive immune response (18). Among these, neutrophils are believed to play a detrimental role in the ischemic insult (3, 18). Neutrophil infiltration of the injured site is mediated in part by the release of specific chemokines that function as powerful attractants. Macrophage inflammatory protein-2 (MIP-2) is part of the CXC chemokine family (CXCL-2) and is a major neutrophil chemoattractant (2, 34). This chemokine is thought to play an important role in neutrophil recruitment to the site of injury during AKI (22, 23, 40). Although MIP-2 expression is induced after ischemia-reperfusion (36), its specific tubular localization has not been well-defined in the context of AKI.
We recently presented the first lines of evidence demonstrating that, contrary to the traditional belief, THP is actually protective of renal tubules during AKI (12). Kidney injury, especially necrosis and neutrophil infiltration, is significantly aggravated in THP knockout mice compared with wild-type. We also showed that THP is not requisite for cast formation in the setting of AKI. The current studies were designed to address the mechanisms whereby THP protects the renal tubules in the outer medulla. We aimed to determine, using a THP knockout mouse model, the effect of THP deficiency on the specific type of tubule(s) involved in injury (TAL vs. S3) and the relationship to the neutrophil chemoattractant MIP-2.
We therefore tested the hypothesis that THP-deficient TAL is more prone to injury by an augmented expression of MIP-2 that attracts neutrophils to the outer medulla. However, our results show that, instead of suspected THP-deficient TAL of the outer stripe (OS), necrosis predominantly affects S3 proximal segments. In addition, lack of THP led to an upregulation of MIP-2 in these S3 segments during AKI, and neutralizing MIP-2 rescued these tubules from injury. Our results indicate that a normal THP protects the kidney from acute injury by inhibiting a relay of inflammatory signaling between TAL and S3 segments. Our findings that ischemia increases ectopic THP expression on the basolateral surface and that putative THP receptors are present on S3 segments also suggest a direct THP-S3 link, a suggestion that warrants further validation.
MATERIALS AND METHODS
Animal surgery.
Animal experiments and protocols were approved by the St. Louis VA Animal Care and Use Committee and conducted in conformity with the “Guiding Principles for Research Involving Animals and Human Beings.” THP knockout animals (129/SvEv THP−/−) and wild-type background strain have been characterized in detail in previous publications (12, 24, 25). Animals were 8–10 wk old at the time of surgery. Ischemia-reperfusion surgery was as previously described (12, 17). In brief, mice were anesthetized with isoflurane and placed on a homeothermic table to maintain core body temperature at 37°C. Both renal pedicles were exposed via a midline incision and occluded with micro aneurysm clamps for 22 min followed by 6- or 24-h reperfusion depending on the time point studied. This clamp time was chosen because of our previous work showing that the corresponding injury was significant enough without causing diffuse necrosis/or infarction of the outer medulla (12). This is especially relevant to our studies investigating the effect of THP on the type of tubule injured. Sham surgery consisted of an identical procedure without application of the micro aneurysm clamps. All animals received subcutaneous analgesic (0.1 mg/kg bruprenorphine) at the end of surgery.
Serum creatinine was measured using capillary electrophoresis (28), performed at the UT Southwestern O'Brien Center of Excellence. Serum was obtained from maxillary vein bleeds before surgery (baseline) and 24 h after surgery (sham and IRI; n = 6 per strain per experimental group).
Tissue harvesting and staining.
Six or 24 hours after surgery, kidneys were perfusion-fixed in situ with 30 ml 4% paraformaldehyde in PBS, pH 7.4; 100-μm vibratome sections for THP, MIP-2, and SR-B1 staining were obtained 24 h later. Alternatively, kidneys were dehydrated with increasing concentrations of ethanol and paraffin-embedded for histological studies using light microscopy as previously described (10, 12).
We used the following primary antibodies for immunostaining: a sheep polyclonal IgG for THP (AbD Serotec, cat. no. 8595-0054), a goat polyclonal IgG for MIP-2 (Santa Cruz Biotechnology, cat. no. Sc-26537), and a SR-B1 rabbit polyclonal antibody for SR-B1(Abcam, cat. no. Ab396-100). The following conjugated secondary antibodies were used: Alexa Fluor 555 donkey anti-sheep IgG and Alexa Fluor 647 chicken anti-goat IgG (both from Molecular Probes, cat. no. A21436 and A21469, respectively). Brush borders were stained with Oregon green-488 phalloidin (Molecular Probes, cat. no. O7466) and nuclei were stained with DAPI (Molecular Probes, cat. no. D21490). Negative controls were obtained by incubating tissues from sham and ischemia animals with secondary antibodies in the absence of primary antibodies.
Light microscopy and operational definitions for identification of tubular segments.
Paraffin-embedded kidneys were sectioned at 4 μm and stained using periodic acid Schiff (PAS). This stain can differentiate proximal tubules in the outer medulla (predominantly S3 segments, identified by the pink brush border) from nonproximal tubules (predominantly TAL, lacking any apical staining and appearing thinner). Nonnecrotic S3 tubules could still be identified after injury by the pink brush-border remnant rim present at the apical area (see Fig. 2B). Since we previously showed that necrosis, rather than apoptosis, is the predominant injury phenotype in THP knockout mice (12), we quantitated necrosis as an indicator of injury by tubule type. This was accomplished by counting tubules in the OS of the medulla (5 ×20 fields/kidney section) and grouping them into three types: S3, non-S3, and necrotic tubules. No necrotic tubules are expected in sham. After injury, necrotic tubules are expected to appear, alongside a decrease in the number of identifiable S3 or non-S3 tubules. Comparing the changes in the tubule type percentages between sham and IRI in THP+/+ and THP−/− mice will identify the tubule type affected by necrosis. Six animals per strain for each experimental group (sham THP+/+, sham THP−/−, IRI THP+/+, IRI THP−/−) were used. Experiments were performed on coded sections, enabling blinded scoring.
Fig. 2.
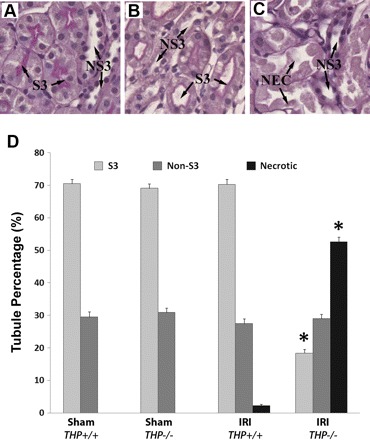
Quantitation of necrosis in the kidney outer stripe of THP−/− and THP+/+ mice according to tubular type using PAS stain. A–C: representative enlarged fields of outer stripe kidney sections stained with PAS after sham (A: THP+/+) and IRI surgery (B: THP+/+ and C: THP−/−). These images show examples of the type of tubules in the outer stripe: S3 segments [characterized by their pink brush staining, seen in sham (A) and after IRI (B)], non-S3 segments (NS3; they lack the brush-border stain and are typically thinner than S3, comprised primarily of thick limbs), and necrotic tubules (NEC; after IRI seen as frequently detached tubular fragments without nuclei). D: quantitation of tubular type in the outer stripe of THP+/+ and THP−/− kidneys after sham and IRI surgery (n = 6 animals/group). Bars are tubule percentages ± SE. *Statistical significance (P < 0.05) compared with the corresponding tubule type in THP+/+. After IRI, S3 tubules in THP−/− sections are less frequently identified; this is concomitant with a significant increase in the percentage of necrotic tubules. No change is seen in the percentage of non-S3 tubules. Therefore, necrotic tubules in THP−/− are predominantly S3 segments.
Modified esterase staining specific for neutrophil detection using the naphthol AS-D chloroacetate esterase kit (Sigma, cat. no. 91C) was performed according to the manufacturer's specifications. Neutrophils were counted per intermediate power field (×20 magnification) and a general average was computed for each animal group (n = 6 per strain for each experimental group).
Fluorescence microscopy.
Vibratome sections (100-μm thickness) were stained for THP, MIP-2, or SR-B1 as previously described (11) and viewed under an Olympus Fluoview laser-scanning confocal microscope available at St. Louis University. Images were collected under ×20 or ×60 magnification. Previously described fluorescence-based operational definitions of kidney layers and structures were used to guide localizing proteins of interest within each kidney layer (11).
Three-dimensional reconstruction and volume rendering of THP expression.
Stacks of z-planes were collected at 0.4-μm intervals. Three-dimensional (3D) volume rendering was done using Voxx, a voxel-based 3D imaging software available from the Indiana Center for Biological Microscopy. Videos were produced in addition to snapshot images representing views from different angles as previously described (11).
MIP-2 neutralization in THP−/− mice.
THP−/− mice were subjected to IRI surgery. Following the release of the renal clamp, they received an intraperitoneal injection of 100 μg of MIP-2 neutralizing antibody (n = 5; monoclonal rat anti-mouse MAB452, clone 40605, R&D Systems) or 100 μg of isotype IgG control (n = 5; also from R&D Systems). Mice were killed at 24 h following surgery. Serum creatinine at baseline and 24 h after surgery was analyzed as above. Kidneys were processed as described above to quantitate injury histologically by tubular type and assess neutrophil infiltration as described above.
Statistical analysis.
Values of each experimental group are reported as means ± SE. A chi square test was used to compare the difference in proportions and a two-tailed t-test was used to examine the difference in means for continuous data. Statistical significance was determined at the 0.05 significance level.
RESULTS
Renal IRI in THP−/− mice causes significant necrosis of S3 segment and not TAL in the outer medulla.
We studied the effect of renal IRI on THP+/+ and THP−/− mice 24 h after IRI surgery. We focused on the OS of the outer medulla because it is the main site of injury. As shown in Fig. 1, significant necrosis and cast formation were seen in THP−/− OS compared with THP+/+. This is consistent with our previous findings described recently (12).
Fig. 1.
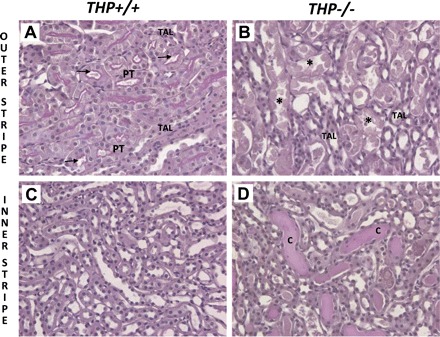
Periodic acid Schiff (PAS)-stained sections of the outer medulla from wild-type and Tamm-Horsfall protein (THP)-deficient kidneys 24 h after ischemia-reperfusion injury (IRI). Panels are ×20 fields of representative kidney sections after IRI from the outer medulla [outer stripe (A and B) and inner stripe (C and D)] of wild-type (A and C) and THP−/− mice (B and D). Injury in THP+/+ outer medulla is very mild and consisted primarily of proximal tubular dilation in the outer stripe (arrows in A). However, in THP−/− sections, significant tubular necrosis is seen in the outer stripe (* in B) and cast formation in the inner stripe (labeled C in D). PT, proximal tubule; TAL, thick ascending limb.
Since THP is expressed in the TAL, we investigated further the effect of THP deficiency on the type of tubule affected by injury (S3 vs. TAL). Therefore, we surveyed the tubule type in the OS of kidney sections harvested 24 h after surgery from sham and ischemic mice of both strains using PAS stain. We classified the tubules into three groups: S3 (based on the appearance of a purple brush border in PAS), non-S3, and necrotic tubules. Non-S3 tubules in the OS consist predominantly of TAL and few collecting ducts. Examples of tubules are shown in Fig. 2, A–C. As detailed in the bar graph in Fig. 2D, THP knockout and wild-type shams had similar distribution of S3 and non-S3 in the OS (69.1 and 31.9%, respectively in THP−/−; 70.5 and 29.5% in THP+/+). Twenty-four hours after IRI, there was minimal necrosis in wild-type (2.2%), but the distribution of intact S3 and non-S3 remains the same. In THP−/−, IRI caused a significant increase in necrotic tubules (52.6%). This coincides with a statistically significant decrease in the number of intact S3 tubules (18.4%). However, the number of non-S3 tubules remained the same (29.0%; P = 0.47 vs. THP+/+ and P = 0.52 vs. sham). Therefore, these findings allow us to conclude that the necrotic tubules in THP−/− outer medulla are predominantly S3 segments.
To examine the functional relevance of the histological changes described above, we measured serum creatinine (Cr) in wild-type and THP−/− mice at baseline and after sham or IRI surgery. This was performed using capillary electrophoresis at the UT Southwestern O'Brien Center (Dallas, TX). Figure 3 shows that there was no difference in serum Cr at baseline or after sham surgery in mice from both strains. After IRI, THP−/− mice had a higher Cr compared with wild-type (0.96 ± 0.3 vs. 0.09 ± 0.01 mg/dl, respectively; P < 0.05). This is consistent with the injury seen in histology.
Fig. 3.
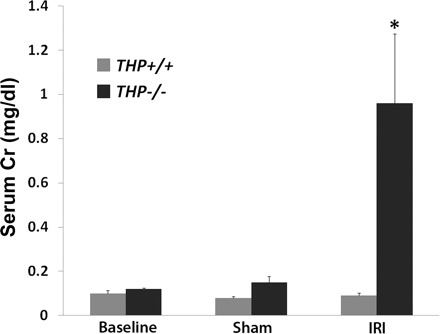
Serum creatinine (Cr) measurements using capillary electrophoresis. Values are means ± SE; n = 6 animals for each group. There was no difference in mean Cr at baseline or after sham surgery between wild-type and THP−/−. After IRI, there was no change in serum Cr in wild-type mice, but a significant increase in THP−/− mice. *Statistical significance between wild-type and THP−/− (P < 0.05).
Neutrophil infiltration after IRI is significantly increased in THP−/− OS and localizes around injured S3 segments.
We assessed a time course of neutrophil infiltration in the OS using a neutrophil-specific esterase stain at two time points after IRI surgery: 6 and 24 h (Fig. 4). There was minimal neutrophil presence in sham conditions of THP+/+ (0.1 ± 0.1/field) and THP−/− (0.2 ± 0.1/field) OS. After IRI, there was a small but significant increase of neutrophils in wild-type OS at 6 h (1.1 ± 0.3/field; P < 0.05 vs. sham). This trend remained at 24 h after IRI (2.6 ± 0.9/field; P < 0.05 vs. sham, but P = 0.1 vs. 6-h IRI). However, in THP−/− sections, neutrophil infiltration was markedly increased 6 h after IRI (18.6 ± 2.6/field; P < 0.05 vs. sham and IRI wild-type) and further increased at 24 h (51.6 ± 8.4/field; P < 0.05 vs. 6-h IRI THP−/− and IRI wild-type). Neutrophils localized around injured S3 segments, as shown in Fig. 4B.
Fig. 4.
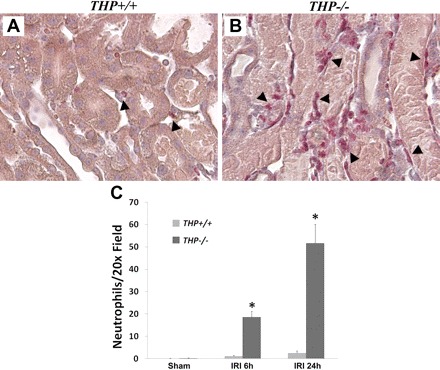
THP and neutrophil infiltration in the outer stripe. A and B: ×20 representative images of outer stripe kidney sections from THP+/+ and THP−/− kidneys after IRI stained with neutrophil-specific esterase stain. Arrowheads identify neutrophils in red color. Note the increase in neutrophil infiltration in THP−/− outer stripe around injured S3 segments compared with THP+/+. C: bar graphs represent quantitation of neutrophils per ×20 fields 6 h (IRI 6 h) and 24 h (IRI 24 h) after IRI. *Statistical significance between mouse stains (P < 0.05).
THP deficiency upregulates MIP-2 expression in the S3 segments of the OS during AKI.
To characterize the changes in expression and localization of MIP-2, we used immunofluorescence microscopy. We examined sections from all the kidney areas and focused on the outer medulla (outer and inner stripe, Fig. 5). MIP-2 was not significantly expressed in sham THP+/+ sections. There was faint staining in THP−/− kidneys localized to few S3 tubules. Six hours after IRI, MIP-2 staining was seen in few S3 segments in the OS of THP+/+ mice and occasional collecting ducts in the inner stripe of these mice. However, MIP-2 staining in THP−/− was markedly increased after IRI and localized predominantly to S3 segments of the outer medulla (Fig. 5D). Twenty-four hours after IRI, MIP-2 staining was comparable to the 6-h staining in wild-type and THP−/−, although some staining was lost from S3 segments that underwent necrosis (data not shown).
Fig. 5.
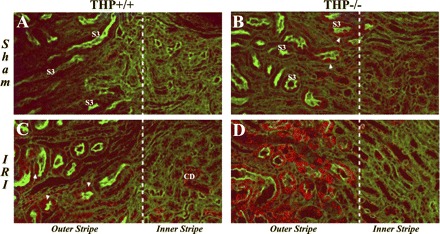
Effect of THP on macrophage inflammatory protein-2 (MIP-2) expression in IRI using immunofluorescence confocal microscopy. Panels are ×20 confocal images of outer medulla kidney sections from THP+/+ (A and C) and THP−/− mice (B and D) after sham and IRI surgery (6-h time point). MIP-2 stain is red. Brush border is stained green with Oregon Green-phalloidin, labeling S3 segments (S3). Dotted white line separates outer from inner stripe of the outer medulla. In sham THP−/−, a few S3 segments have weak MIP-2 staining (arrowhead in B) compared with no significant staining in THP+/+ (A). After IRI, MIP-2 expression becomes more noticeable in several S3 segments of THP+/+ outer stripe (arrowheads in C) and few collecting ducts (CD). However, in ischemic THP−/− outer medulla, MIP-2 expression is significantly increased and localized to S3 segments of the outer stripe (D, outer stripe).
MIP-2 neutralization after IRI rescues S3 segments, decreases neutrophil infiltration, and improves kidney function in THP−/− mice.
To investigate further the importance of MIP-2 upregulation in THP−/− mice, we neutralized MIP-2 following IRI surgery using a specific neutralizing antibody and harvested kidneys 24 h later. A nonspecific IgG isotype antibody was used as control. We used the same methods described above to measure neutrophil infiltration and to determine the type of tubules affected by necrotic injury. As shown in Fig. 6, A, C, and E, neutrophil infiltration was significantly reduced by MIP-2 neutralization compared with IgG control. Figure 6, B, D, and F, also shows that MIP-2 neutralization decreased the percentage of necrotic tubules from 52.5% (with nonspecific IgG) to 33.0%. A concomitant increase in the percentage of intact S3 segments was also observed in MIP-2 antibody-treated mice [35.4% compared with 15.3% in IgG-treated control mice (P < 0.05)]. No change was observed in non-S3 tubules. Therefore, we conclude that MIP-2 neutralization in THP−/− mice rescued S3 segments from injury. This was also reflected in measuring kidney function (Fig. 6G) where MIP-2 neutralization prevented a rise in serum Cr 24 h after IRI compared with control (0.17 ± 0.03 vs. 0.64 ± 0.17 mg/dl, respectively; P < 0.05).
Fig. 6.
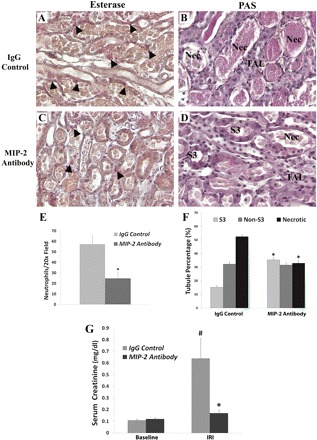
MIP-2 neutralization in THP−/− after IRI. A and C: ×20 representative fields of kidney sections stained with a neutrophil-specific esterase from THP−/− mice after IRI treated with nonspecific IgG or MIP-2-neutralizing antibody, respectively. Neutrophils (red color, arrowheads) are abundant next to necrotic S3 segments in control (A), whereas there are significantly fewer observed after MIP-2 neutralization (C). This is quantitated in the bar graphs (E), where * denotes statistical significance between the 2 groups. B and D: ×20 representative fields of kidney sections stained with PAS from control and MIP-2-neutralized THP−/− mice, respectively. NEC are commonly seen in B, affecting predominantly S3 segments and not TALs. However, neutralization of MIP-2 (D) rescued S3 segments, which can now be recognized by the characteristic brush border. F: bar graphs show the corresponding quantitation of tubular type in the outer stripe in the control and neutralization groups. *Statistical significance between the 2 groups (P < 0.05). G: bar graphs represent the average serum Cr of the 2 groups at baseline and 24 h after IRI. #Statistical significance vs. baseline. *Statistical significance between groups.
AKI increases basolateral expression of THP in the TAL of wild-type mice.
We used immunofluorescence confocal volumetric imaging and subsequent 3-D reconstruction to examine THP expression in sham and after ischemia in wild-type mice (see supplemental material for videos that were produced; the online version of this article contains supplemental data). Snapshots of the reconstructed animations are shown in Fig. 7. In sham mice, THP expression is predominantly apical with occasional basolateral THP staining. After IRI, THP staining is again present on the apical border, but it was more evidently detected in the basolateral/interstitial aspect of TAL, contiguous to the basolateral border of S3 segments (Fig. 7, E and F). These results suggest that ischemia alters the intracellular translocation of THP in the TAL cells by partially shifting it from its default apical surface domain to the basolateral domain.
Fig. 7.
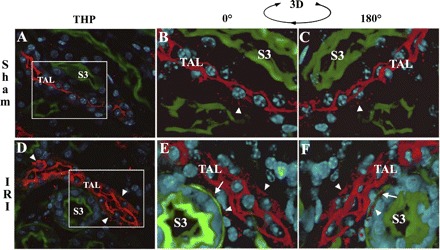
Changes of THP cellular distribution with ischemia using immunofluorescence with volumetric 3-dimentional (3D) reconstruction. THP stain is red. Brush-border stain is green with Oregon Green-phalloidin. Nuclei are stained blue with DAPI. A and D: ×60 single-plane images from sham (A) and IRI (D) of THP+/+ outer medulla. Volume stacks (15 μm) spanning the areas outlined by the white boxes in A and D were reconstructed in 3D using Voxx. Snapshots of the 3D animations (see supplemental materials, videos 1 and 2) are shown in B and C for sham, and E and F for IRI. C and F: 180° rotations compared with B and E, respectively. In sham, THP staining is predominantly apical (seen at the center of the TAL), with occasional basal/interstitial staining (arrowhead in B and C). After ischemia, THP staining is not only present at the apical side, but it can now be clearly detected at the basolateral/interstitial side of the TAL (arrowheads D, E, and F), in proximity to the basolateral aspect of S3 segments (arrows in E and F).
Ischemia shifts SR-B1, a THP receptor, to the basolateral side of S3 segments.
Pfistershammer et al. (30) showed recently that the scavenger receptor SR-B1 is a receptor for THP on macrophages. We studied the expression of SRB-1 after IRI in wild-type kidneys (Fig. 8). In sham outer medulla, SR-BI is expressed in the intracellular compartment of S3 segments (arrowhead, Fig. 8B). After IRI, SR-B1 staining localizes predominantly at the basolateral aspects of S3 segments (arrowheads in Fig. 8C), adjacent to TAL. In THP−/− outer medulla, the distribution of SR-BI remained cellular in S3 segments after ischemia (Fig. 8D).
Fig. 8.
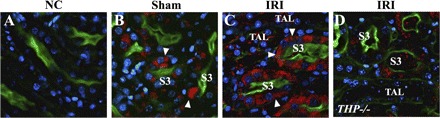
Effect of ischemia SR-B1 expression in the outer medulla. Panels are ×60 representative confocal images of outer stripe sections from THP+/+ (B and C) and THP−/− (D) stained for SR-B1 (red). Brush-border stain appears green with Oregon Green-phalloidin. Nuclei are stained blue with DAPI. A: negative control without primary antibody. In sham condition, SR-B1 stain predominantly localizes to the cellular compartment of S3 segments (arrowhead in B). After ischemia in THP+/+, SR-B1 redistributes the basolateral aspect in S3 segments (arrowheads in C). In contrast, SR-B1 staining after ischemia in THP−/− sections remains mostly cytoplasmic (D).
DISCUSSION
In this study, we investigated in a model of AKI the relationship of the protective role of THP, tubular injury, and MIP-2 expression. We showed using THP knockout mice that S3 proximal segments are the main targets of the THP-protective effect. We also demonstrated a differential effect of THP on the expression of the neutrophil chemoattractant MIP-2 also in the S3 segments, which could explain the predisposition of these tubules to injury and neutrophil infiltration. This was verified by neutralizing MIP-2 after IRI in THP−/− mice, which rescued S3 segments. The heightened inflammatory signaling in S3 of THP−/− kidneys is consistent with our previous findings that THP alters the expression of the inflammatory receptor Toll-like receptor 4 (TLR4) in these tubular segments. Indeed, TLR4 is upstream from MIP-2 in the inflammatory signaling axis in AKI (40). Therefore, THP derived from the TAL is affecting the response of S3 segments to injury. Our findings confirm the seminal proposition by Safirstein (32, 33) that the TAL is a key element in regulating molecular responses of neighboring tubular elements during kidney injury.
How does THP in TAL regulate MIP-2 expression in neighboring S3 segments? The process is unlikely to be mediated through the urinary luminal space because THP cannot access S3 segments from that route. Since TAL and S3 segments run side-by-side in the OS, it is possible that a THP-dependent cross talk exists between TAL and S3 segments in the outer medulla. The concept of TAL-S3 cross talk in the medulla has been previously suggested by others (13, 27, 32). A few autocrine or paracrine factors have been proposed as potential mediators, such as prostaglandins (PGE2), nitric oxide, kinins, and adenosine (13). However, data in the setting of AKI are very sparse. Could THP itself be a major mediator of this trans-tubular signaling?
Although THP is predominantly an apically secreted protein, its basolateral and interstitial expression has been well-described previously. In a physiological state, Bachmann et al. (4) showed using immunogold electron microscopy that the apical-to-basolateral distribution of THP within the TALs lies approximately in a 2:1 ratio. More recently, Jennings et al. (16) also suggested a possible role for basolateral THP in the context of studying familial juvenile nephropathy. In injury models, previous studies suggest significant THP expression in the interstitium (14, 35, 41). We recently showed that ischemia-reperfusion promotes the interstitial presence of THP around S3 segments (12). In the current study, we further expand these findings by showing that ischemia shifts THP expression partially to the basolateral aspect of TAL, which could explain the presence of THP in the interstitium (through an enhanced basolateral release). The expression by S3 tubules of a putative receptor for THP (SRB-1) in an appropriate distribution (at the basolateral aspect of S3) could support a direct role for THP on S3 segments. Figure 9 depicts working models whereby a TAL-S3 tubular cross talk could be dependent on THP. Although our current and previous data (12) suggest a direct THP-S3 interaction (Fig. 9, model A), it is also important to consider, at this developing investigative stage, other alternative possibilities that do not necessarily involve a direct THP-S3 interaction; for example, the protective effects of THP on S3 segments could be relayed through a secondary message produced from TAL (Fig. 9, model B). Indeed, the current work underscores the importance of future investigations to further elucidate the role of THP in mediating TAL-S3 interactions in the outer medulla in maintaining homeostasis and during injury.
Fig. 9.
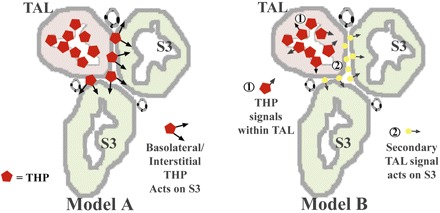
Possible mechanisms of THP-mediated TAL-S3 tubular cross talk. A and B: conceptual models illustrating possible mechanisms of THP-mediated cross talk between S3 segments and TAL. A: possibility where basolateral/interstitial THP released from TAL could act directly on S3 segments. B: alternative explanation where THP affects the TAL, which in turn releases a secondary mediator that acts on S3 tubules. Another possible “hybrid” mechanism (not depicted here) could involve THP in the interstitium without a direct effect on S3, but rather through an effect on interstitial or endothelial cells.
Inflammation is now believed to play a major role in the pathophysiology of AKI (8). In addition to involving various cells from the innate and adaptive immune system, neutrophil infiltration is a prominent feature of this response in murine AKI (7, 18). TLR signaling is at the core of the inflammatory response and is believed to be activated in ischemic kidney injury (22, 40). These proteins recognize specific molecular patterns of pathogens and select endogenous ligands and initiate among others the NF-κβ and MAPKK signaling cascades that produce a myriad of cytokines and chemokines (1). Tubular epithelial cells are believed to be involved in the ensuing inflammatory response after AKI in part by activating TLR signaling (TLR2 and TLR4) and the subsequent production of cytokines such as TNF-α and chemokines such as CXCL2 (MIP-2) and MCP-1 (21–23, 38, 40). We focused on MIP-2 in this manuscript because of the significant neutrophil infiltration around S3 segments seen after injury in THP−/− medulla and our previous findings of TLR4 upregulation specifically in these segments (12).
MIP-2 is part of the CXC chemokine family and is a major neutrophil chemoattractant (2, 34). MIP-2 is expressed in the kidney (31), and induced with injury (22, 36, 40), but its specific tubular localization has not been well-defined. The role of MIP-2 in the recruitment of neutrophils after kidney injury has been previously studied by Miura and colleagues (23), where neutralization of MIP-2 by specific antibodies in a mouse model of ischemia-reperfusion reduced neutrophil infiltration and attenuated kidney injury (23). Our current data further extend these studies, by showing that the renal epithelial MIP-2 expression after injury localizes primarily to S3 segments and is regulated by THP. Since we previously showed that THP alters the expression of TLR4 in these S3 segments, we speculate that THP regulates a TLR4-MIP-2 signaling axis in S3 segments, which could add up to be an important determinant of neutrophil infiltration and susceptibility to kidney injury. Future studies are needed to understand why TAL, independent of THP, is inherently more resistant to neutrophil-induced injury compared with S3 segments.
In addition to our studies in AKI, the use of THP knockout mice has been instrumental in uncovering the multifaceted role of THP as a defense agent against stone formation (24) and bladder infections (6, 25). Indeed, THP−/− mice show an inability to clear inoculated bladder infections. However, it is important to note that these mice do not develop spontaneous urinary tract infections (6, 25). Therefore, it is unlikely that superimposed kidney infections in our IRI model in THP−/− mice could explain the augmented inflammatory response and the increased susceptibility to kidney injury.
In summary, we showed data that the protective effect of THP in AKI involves primarily S3 segments of the outer medulla. THP has a differential effect on the expression of the neutrophil chemoattractant MIP-2 in these S3 segments, thereby affecting neutrophil infiltration. Our findings raise the possibility of a direct role of basolaterally released THP on downregulating inflammation in S3 segments, a hypothesis that warrants further investigation. We propose that THP is a mediator of tubular cross talk aimed at stabilizing the outer medulla in the face of injury.
GRANTS
This work was supported by a Carl Gottschalk Award from the American Society of Nephrology to T. M. El-Achkar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge J. Lucas from UT Southwestern O'Brien Center for assistance with capillary electrophoresis.
REFERENCES
- 1. Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol 4: 499–511, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int 63: 401–415, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985. [DOI] [PubMed] [Google Scholar]
- 5. Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65: 791–797, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Bolisetty S, Agarwal A. Neutrophils in acute kidney injury: not neutral any more. Kidney Int 75: 674–676, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006. [DOI] [PubMed] [Google Scholar]
- 10. El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290: F1034–F1043, 2006. [DOI] [PubMed] [Google Scholar]
- 11. El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol 293: F1187–F1196, 2007. [DOI] [PubMed] [Google Scholar]
- 12. El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol 39: 1551–1561, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Howie AJ, Brewer DB. Extratubular deposits of Tamm-Horsfall protein in renal allografts. J Pathol 139: 193–206, 1983. [PubMed] [Google Scholar]
- 15. Hoyer JR, Sisson SP, Vernier RL. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest 41: 168–173, 1979. [PubMed] [Google Scholar]
- 16. Jennings P, Aydin S, Kotanko P, Lechner J, Lhotta K, Williams S, Thakker RV, Pfaller W. Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J Am Soc Nephrol 18: 264–273, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Kelly KJ, Plotkin Z, Dagher PC. Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Ida Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Pare G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar S. Mechanism of injury in uromodulin-associated kidney disease. J Am Soc Nephrol 18: 10–12, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Lech M, Avila-Ferrufino A, Allam R, Segerer S, Khandoga A, Krombach F, Garlanda C, Mantovani A, Anders HJ. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol 183: 4109–4118, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66: 1159–1166, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 286: F795–F802, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Paroni R, Fermo I, Cighetti G, Ferrero CA, Carobene A, Ceriotti F. Creatinine determination in serum by capillary electrophoresis. Electrophoresis 25: 463–468, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Peach RJ, Day WA, Ellingsen PJ, McGiven AR. Ultrastructural localization of Tamm-Horsfall protein in human kidney using immunogold electron microscopy. Histochem J 20: 156–164, 1988. [DOI] [PubMed] [Google Scholar]
- 30. Pfistershammer K, Klauser C, Leitner J, Stockl J, Majdic O, Weichhart T, Sobanov Y, Bochkov V, Saemann M, Zlabinger G, Steinberger P. Identification of the scavenger receptors SREC-I, Cla-1 (SR-BI), and SR-AI as cellular receptors for Tamm-Horsfall protein. J Leukoc Biol 83: 131–138, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Roche JK, Keepers TR, Gross LK, Seaner RM, Obrig TG. CXCL1/KC and CXCL2/MIP-2 are critical effectors and potential targets for therapy of Escherichia coli O157:H7-associated renal inflammation. Am J Pathol 170: 526–537, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol 4: 1387–1395, 1994. [DOI] [PubMed] [Google Scholar]
- 33. Safirstein R, Megyesi J, Saggi SJ, Price PM, Poon M, Rollins BJ, Taubman MB. Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1095–F1101, 1991. [DOI] [PubMed] [Google Scholar]
- 34. Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 11: 152–176, 2000. [DOI] [PubMed] [Google Scholar]
- 35. Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003. [DOI] [PubMed] [Google Scholar]
- 36. Stroo I, Stokman G, Teske GJ, Raven A, Butter LM, Florquin S, Leemans JC. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int Immunol 22: 433–442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamm I, Horsfall FL., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med 74: 106–108, 1950. [PubMed] [Google Scholar]
- 38. Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, Nemenoff RA, Quigg RJ, Holers VM. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol 178: 1819–1828, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zager RA, Cotran RS, Hoyer JR. Pathologic localization of Tamm-Horsfall protein in interstitial deposits in renal disease. Lab Invest 38: 52–57, 1978. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


