Abstract
Interstitial cells of mesenchymal origin form gap junctions with smooth muscle cells in visceral smooth muscles and provide important regulatory functions. In gastrointestinal (GI) muscles, there are two distinct classes of interstitial cells, c-Kit+ interstitial cells of Cajal and PDGFRα+ cells, that regulate motility patterns. Loss of these cells may contribute to symptoms in GI motility disorders.
Endothelial cells have long been recognized for their critical role in regulating vascular tone, but similar recognition of the important regulatory functions provided by various classes of interstitial cells in visceral smooth muscles has been slower to be accepted. Several classes of interstitial cells, referred to by a variety of terms, including interstitial cells of Cajal (ICC), ICC-like (ICC-LI) cells, PDGFRα+ cells, fibroblast-like cells, and teleocytes, have been described morphologically and provide various regulatory functions. Interstitial cells can contribute to the excitability of smooth muscle tissues because some classes of these cells form gap junctions with smooth muscle cells (SMCs). Interstitial cells express a variety of receptors for neurotransmitters, hormones, paracrine substances, and inflammatory mediators, and these cells also express second-messenger pathways and ion channels that allow them to mediate postjunctional responses to neurotransmission and other biological regulatory substances. Because of extensive morphological studies and some naturally occurring mutant animals with defects in ICC, interstitial cells in the gastrointestinal (GI) tract have been studied in greatest detail, but it should be noted that all smooth muscles display some types of interstitial cell populations. In many cases, the physiological functions and roles in pathophysiology of interstitial cells are still unknown. This short review describes various aspects of physiological regulation that have been associated with interstitial cells of GI muscles and how they enhance the motor behaviors of visceral smooth muscles.
Morphology of Interstitial Cells and Relation to Smooth Muscle Cells
Interstitial cells are distinctly different than SMCs, often displaying multiple processes and typically few thick filaments. Ultrastructural features of ICC include an abundance of mitochondria, moderately well developed Golgi, thin and intermediate filaments, and rough and smooth endoplasmic reticulum (ER) (23, 24, 66, 90). Some ICC display caveolae and a basal lamina. Mitochondria and cisternae of ER are often prevalent in the perinuclear region, and regions of close apposition between the ER and plasma membrane are common. Pacemaker functions and neural responses of ICC appear to depend on Ca2+ release mechanisms from internal stores (3, 39, 108, 125), and the close appositions between ER and the plasma membrane suggest that much of this signaling occurs in microdomains. There is an abundance of rough ER in PDGFRα+ cells, giving them a fibroblast-like appearance, and these cells also lack caveolae and a basal lamina. PDGFRα+ cells were referred to as fibroblast-like cells for many years, but now distinctive chemical coding (such as specific labeling with antibodies for PDGFRα) has provided a more precise means of referring to these cells.
ICC and PDGFRα+ cells form gap junctions with SMCs (41, 67). SMCs, ICC, and PDGFRα+ cells express numerous gap-junction genes and proteins (15, 35, 98). Electrical coupling causes the interstitial cells and SMCs to function as a multicellular syncytium we have called the SIP syncytium (94). This structure serves as the pacemaker in GI muscles and transducer of neural and other regulatory inputs. Electrical coupling allows conductance changes in one type of cell to affect the excitability of the other types of cells in the SIP syncytium.
Intramuscular ICC (ICC-IM) and ICC clustered within the region of the deep muscular plexus in the small intestine (ICC-DMP) are very closely associated with varicosities of motoneurons in the tunica muscularis. The close associations with neural processes were common observations in many classical ultrastructural studies of ICC (17, 21, 51, 90). Structured junctions between ICC and nerve varicosities can be found with spacing of ∼20 nm, and pre- and postjunctional synaptic proteins are present (10). Connectivity of this sort can also be found between varicosities and SMCs (28, 79); however, the only morphometric study comparing connectivity of neurons with ICC and SMCs found far more frequent junctional connections between ICC and nerve varicosities than between neurons and SMCs (16). If the regions of close spacing are sites of neurotransmitter release, then very high concentrations of transmitters might be achieved at postjunctional receptors, and metabolism or uptake of transmitters might also be accelerated. FIGURE 1 shows representative images of ICC and PDGFRα+ cells using immunohistochemical techniques, and their relationships with each other and the processes of enteric motoneurons.
FIGURE 1.
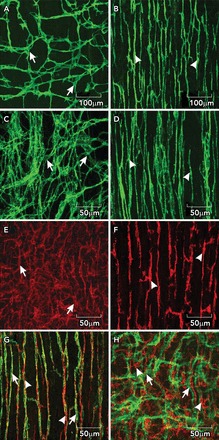
ICC and PDGFRα+ cells in the small intestine
A and B: whole mounts of c-Kit+ ICC-MY (arrows) and ICC-DMP (arrowheads) in the murine small intestine. Note the well defined network structure of ICC-MY, and the more distributed and parallel orientation of ICC-DMP that run parallel to the circular muscle and are closely aligned with nerve processes of the deep muscular plexus. C and D: images from the small intestine of Macaca fascicularis (cynomolgus monkey) showing relatively the same distributions of ICC-MY (arrows) and ICC-DMP (arrowheads) in the primate intestine. E and F: distributions of PDGFRα+ cells at the level of the myenteric plexus (E; PDGFRα+-MY; arrows) and PDGFRα+ cells in the deep muscular plexus (F; PDGFRα+-DMP; arrowheads) in the murine intestine. Intramuscular ICC and PDGFRα+ are closely associated with enteric motor nerves. G: the relationship between ICC-DMP (green; arrows) in very close contact with inhibitory motor neurons highlighted by an antibody for nNOS (red; arrowheads). ICC and PDGFRα+ cells are distinct populations of interstitial cells. H: ICC-MY (green; arrows) and PDGFRα+ cells (red; arrowheads) in the same tissue. Scale bars are shown for each panel.
Role of Interstitial Cells in Pacemaker Activity
The idea that ICC might provide pacemaker activity originated from the morphological evidence showing that ICC were electrically coupled to SMCs, and this idea was later supported by dissection experiments in the 1980s that showed that pacemaker activity was focused in regions of tissue that contained networks of ICC (6, 11, 36, 100). Removal of ICC networks rendered muscles void of pacemaker activity and inhibited active slow-wave propagation (96). Subsequent studies using mutant animals in which ICC failed to develop (44, 113) or in mice treated with neutralizing c-Kit antibodies (107) confirmed the obligatory role of ICC in pacemaking. Patch-clamp studies of GI SMCs failed to display spontaneous pacemaker activity or conductances compatible with the generation or regeneration of slow waves. Freshly isolated ICC display autorhythmicity, with ion conductances and pharmacologies consistent with the slow waves recorded in intact muscles (39, 45, 73, 124).
Selective impalement of ICC in intact muscles showed (FIGURE 2, A AND B) 1) omnipresent slow-wave activity, as also observed in smooth muscle cells (19, 59, 60); 2) faster rates of depolarization (upstroke phase) compared with slow waves recorded from SMCs in the same muscles (59, 61); 3) upstrokes of slow waves in ICC were phase advanced vs. the onset of depolarization in nearby SMCs (40). Elegant experiments in which dual impalements of ICC and SMC were accomplished clearly demonstrated electrical coupling between these cells (15, 40). Taken together, these findings supported the concept that ICC generate electrical slow waves and actively propagate these events through networks of ICC that are electrically coupled to the circular and longitudinal muscle layers. Slow waves conduct to SMCs passively, and no mechanism that is capable of regenerating slow waves has been identified in SMCs. Propagation of slow waves in ICC networks is critical for organ-level motility patterns such as gastric peristalsis and intestinal segmentation.
FIGURE 2.
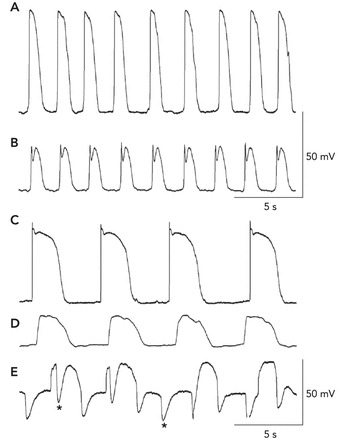
Recordings of electrical activity from the different cell types of the SIP syncytium
A and B: slow waves recorded by impalements of an ICC-MY and a circular SMC in the murine small intestine (as in Refs. 60, 61), respectively. The resting potentials in these cells were −70 mV in the ICC-MY and −68 mV in the SMC. The slow-wave events in ICC are much larger in amplitude, and the upstroke depolarization is ∼1 V/s. Slow waves conduct to SMCs, and the smaller amplitude is a result of the passive nature of this communication (i.e., decremental conduction). C–E: recordings from an ICC-MY, a SMC, and a fibroblast-like cell of the rabbit small intestine (as in Refs. 58, 59), respectively. In these recordings, the resting potentials were −68 mV for the ICC-MY, −64 mV for the SMC, and −43 mV for the fibroblast-like cell. Note again that the amplitude and rise time of the slow waves recorded from ICC-MY are much greater than the events recorded in SMCs. Small oscillations at the frequency of slow waves were also observed in fibroblast-like cells, but a prominent activity in these cells was spontaneous transient hyperpolarizations (STHs; asterisks denote examples). These events appeared to be due to SK channels because they were blocked by apamin. STHs occurred during and between slow waves. Cells were identified by filling them with fluorescent dyes during impalements.
ICC are a minor component of the total cells in smooth muscle tissues, representing <10% of cells (81). Thus it was hard to recognize these cells in enzymatic dispersions of the muscle layers. This problem was solved by generation of a reporter strain of mice in which a bright green fluorescent protein was expressed in ICC (88). Isolation of ICC from these mice allowed more detailed experiments into the mechanism of slow-wave activity. Studies on intact muscles by the groups of David Hirst, Hikaru Suzuki, and Dirk van Helden had suggested that slow waves were due to a Ca2+-activated Cl− conductance (CaCC) (39, 57, 60, 108), but other studies, performed on cultured ICC, suggested a nonselective cation conductance was the inward current likely to be responsible for slow waves (65). When it became possible to study freshly isolated ICC, long-duration “autonomous” currents were observed upon step depolarizations of these cells (31). These large-amplitude, unique currents displayed by ICC are due to a CaCC with the properties of Ano1 (aka Tmem16a) (124). Studies on intact muscles showed that genetic deactivation of Ano1 completely abolished slow-wave activity (FIGURE 3) (45). Ano1 expression was rapidly lost in cell culture, and the autorhytmicity retained by ICC was altered vs. the pacemaker activity of cells in situ.
FIGURE 3.
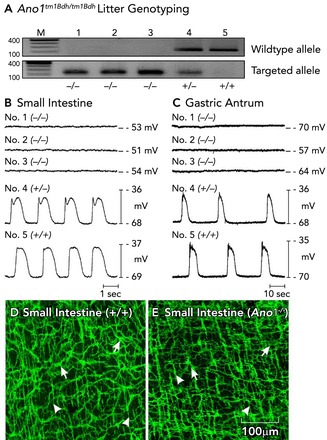
Loss of pacemaker activity in Ano1−/− mice
A: genotyping of Ano1tm1Bdh/tm1bdh mice. The wild-type allele was absent in animals 1–3 in this representative litter, proving that these mice were Ano1−/−. Animals 4 and 5 were a heterozygote and a wild-type homozygote, respectively. B and C: intracellular electrical recordings from jejunal and antral circular muscles of each of the five animals. Slow waves were absent in the muscles of Ano1−/− mice. Slow waves with a normal amplitude and frequency were recorded from animals that were heterozygous or homozygous for Ano1 (animals 4 and 5, respectively). In contrast to what has been claimed by others (102), we observed no significant differences in the development or density of ICC-MY (pacemaker class of ICC in these organs; see Table 1). D and E: ICC [ICC-MY (arrows) and ICC-DMP (arrowheads)] have a normal distribution and density in the small intestines of Ano1−/− mice, suggesting that Ano1 is not critical for the development of ICC. Figure is redrawn from Ref. 45 with permission. Scale bar for D and E is shown in E.
Multiple electrode and imaging studies have shown that slow waves propagate actively in GI muscles and organs at rates exceeding 1 mm/s (6, 85). In the axis of the circular muscle layer, the propagation velocity can exceed 10 mm/s. This rate is too fast to be due to simple intracellular diffusion of Ca2+ or a second messenger causing sequential Ca2+ release from stores. A voltage-dependent mechanism for propagation is required. Some investigators suggested that changes in membrane potential are linked to enhanced production of IP3 (39, 108) or perhaps voltage-dependent sensitization of IP3 receptors that are also required for slow-wave generation and regeneration (105). Our own experiments suggested that the voltage-dependent mechanism is due to voltage-dependent Ca2+ entry, and the likely mechanism is activation of T-type Ca2+ channels. The controversy regarding the voltage-dependent mechanism is not fully resolved, but voltage-dependent IP3 production has not yet been demonstrated in ICC, whereas these cells express Cacna1H and display T-like Ca2+ currents (7, 85, 123). In this concept, Ca2+ entry is thought to produce Ca2+-induced Ca2+ release (CICR) from ryanodine and/or IP3 receptors. CICR appears to be the signal coupled to activation of CaCC (125). It is possible that Ca2+ entry could be the signal that “sensitizes” IP3 receptors, since these channels are sensitive to cytoplasmic Ca2+ levels (48).
Still unresolved is the question of how the plateau phase of slow waves is maintained for long durations (one to many seconds). Openings of CaCC depend on maintenance of Ca2+ at the cytoplasmic surface of the channels, and Ca2+ release events are typically transient in nature (86, 126). The mechanism for maintenance of CaCC activation to create the plateau phase of slow waves therefore requires a sustained means of Ca2+ delivery. More experiments will be required to understand the full mechanism of slow-wave generation and propagation, but this is an important question. The depolarization of SMCs caused by conduction of slow waves yields activation of voltage-dependent (L-type) Ca2+ channels in SMCs, and this is the primary signal that initiates contraction in GI muscles (84). Ca2+ entry is regulated in part by the amplitude and duration of slow waves, so the mechanism regulating slow-wave duration (i.e., plateau phase) is of critical importance to the regulation of contractile force in GI muscles.
Role of Interstitial Cells in Neurotransmission
ICC and Neurotransmission
The close proximity of interstitial cells (ICC-IM and ICC-DMP; see Table 1) and apparent specialized junctions with enteric motoneurons suggested that ICC are innervated (17, 21, 51). Neurotransmitter signals transduced by these cells can be conveyed to SMCs via gap junctions. Expression of receptors and effectors by interstitial cells and SMCs suggests that postjunctional responses to motor neurotransmission in GI muscles are likely to represent integrated responses of the SIP syncytium (FIGURE 4). More detailed reviews of this topic have been provided previously (92, 97).
Table 1.
Localization and proposed functional contributions of cellular components of the SIP syncytium in GI muscles
| Type of Cell and Anatomical Localization | Common Name(s) | Proposed Function(s) | Representative References |
|---|---|---|---|
| Myenteric ICC (in region between circular and longitudinal muscle layers) | ICC-MY; ICC-MP | 1) Pacemaker cells that generate electrical slow waves; 2) form networks that allow active (regenerative) propagation of slow waves; 3) voltage-dependent excitability due to expression of voltage-dependent Ca2+ channels; 4) role in setting resting membrane potential and basal excitability of SIP syncytium | 7, 19, 44, 83, 99, 107, 113, 123 |
| Intramuscular ICC (within bundles of smooth muscle cells and lying in close proximity to nerve varicosities) | ICC-IM; ICC-DMP in region of the deep muscular plexus of the small intestine | 1) Transduction of neurotransmitter signals from enteric motor neurons; 2) mechano-sensitivity; 3) lack of voltage-dependent excitability due to generally low expression of voltage-dependent Ca2+ currents; 4) contribution to active propagation in gastric muscles; 5) mediation of inflammatory input via protease-activated receptors | 8, 10, 14, 18, 33, 76, 104, 106, 112, 114 |
| ICC within septa between bundles of muscle | ICC-SEP | 1) Active propagation of slow waves into the depth of thicker muscles (as in human GI tract); 2) interactions with enteric neurons | 42, 74, 115 |
| ICC along the submucosal surface of the circular muscle layer in colon and to small extent in stomach | ICC-SM | 1) Pacemaker activity in the colon | 11, 100 |
| ICC at the serosal surface of the longitudinal muscle layer | ICC-SS | Unknown | 2, 13 |
| Fibroblast-like cells found in all regions of the GI tract; generally common localization with ICC; also in close apposition with varicosities of enteric neurons | *PDGFRα+ cells | 1) Mediation of purinergic inhibition (purinergic inhibitory junction potential); 2) mediation of inflammatory input via protease-activated receptors | 5, 47, 69–71, 104 |
| Smooth muscle cells | Circular SMCs, longitudinal SMCs, sphincteric SMCs, sling SMCs in stomach | 1) Generation of forces in motility; 2) maintenance of sphincter tone; 3) contributions to setting resting excitability of SIP syncytium; 4) transduction of neural and hormonal responses; 5) mediation of Ca2+ sensitization mechanisms | 32, 54, 75, 84, 91, 101, 103, 117 |
PDGFRα+ cells also have distinct localization and cellular morphologies when found within muscle bundles or in the myenteric region. They are often intertwined with ICC in these localizations. At present, no differences in cells within muscle bundles and cells in the myenteric region have been distinguished; thus, for the purposes of this overview description, these cells are not broken down into
PDGFRα+-MY and
PDGFRα+-IM classes.
FIGURE 4.
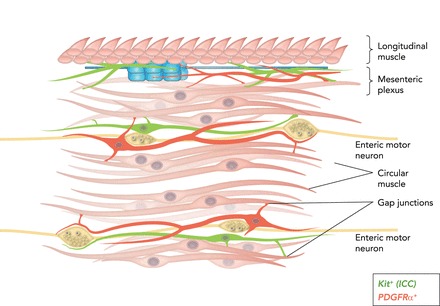
Cartoon showing SIP syncytium (94)
c-Kit+ ICC (green) and PDGFRα+ (fibroblast-like cells; red) are intermixed with smooth muscle fibers in bundles of cells within the circular muscle layer (and in some regions in the longitudinal muscle layer as well). Intramuscular ICC and PDGFRα+ cells are closely associated with the processes of enteric motoneurons and express receptors, second-messenger pathways, and ion channels facilitating responses to enteric motor neurotransmitters. ICC and PDGFRα+ cells are electrically coupled to SMCs, so responses elicited in the interstitial cells can conduct to SMCs and regulate the excitability of the musculature. ICC-MY and PDGFRα+ cells are also found in the region of the myenteric plexus. ICC-MY are pacemaker cells and generate electrical slow waves. PDGFRα+ cells are responsive to purines. Here again, the interstitial cells are electrically coupled to circular and longitudinal SMCs, and slow waves conduct from ICC-MY to SMCs to generate a phasic pattern of contractions in the musculature.
Early studies on mutants (W/WV and Sl/Sld mice) lacking most ICC-IM in the gastric fundus showed that postjunctional electrophysiological responses to nitrergic and cholinergic neurotransmission were decreased (9, 14, 112). Nitric oxide, which normally causes significant hyperpolarization in mouse fundus, had little effect on membrane potential in W/WV mutants, suggesting that a major transduction pathway for NO occurs in ICC-IM. The fast transient excitatory junction potentials (EJPs) elicited by cholinergic neurotransmission were also depressed in W/WV mutants.
More recent studies of rat and mouse mutants with reduced populations of ICC-IM have claimed that normal motor responses are observed in these mice and that ICC do not contribute to the postjunctional responses (1, 20, 43, 122). However, some of these studies have utilized regions of the GI tract in which there is an incomplete loss of ICC, making the results difficult to interpret (1, 122). Recent studies have also shown that the developmental defect in ICC-IM of W/WV is not consistent among the mutant animals, but, when the lesion was severe, nitrergic responses were depressed (95).
An interesting observation was made regarding cholinergic neurotransmission in that contractile responses to cholinergic neurotransmission can actually be elevated in W/WV muscles (12). In this study, Ca2+ sensitization responses to carbachol added to the solutions bathing muscles were compared with responses to cholinergic nerve stimulation. Carbachol caused phosphorylation of CPI-17 and MYPT, two components of Ca2+ sensitization pathways that are enhanced to inhibit myosin phosphatase (101), but cholinergic neurotransmission resulted in phosphorylation of only CPI-17. When ICC-IM were reduced in numbers in W/WV muscles or when metabolism of ACh was reduced by cholinesterase inhibitors, then MYPT phosphorylation was observed. These data suggest that cholinergic responsiveness was altered in W/WV muscles by recruitment of additional Ca2+ sensitization mechanisms, and, in these conditions, ACh released from nerve terminals reaches receptors not normally bound when ICC are present and when cholinesterases are active.
Experiments have also been performed recently utilizing the Cre/loxP technology to produce cell-specific lesions in nitric oxide receptors [soluble guanylate cyclase (sGC)] (32, 33, 75, 76). These studies have suggested that nitrergic regulation of contractile activity in GI organs results from integrated responsiveness from specific components of the SIP syncytium. For example, in the colon, arrhythmic contractions occurred in wild-type mice and in mice with specific knockdown of sGC in SMCs. Mice with global knockdown of sGC and mice with knockdown directed at ICC displayed rhythmic contractile activity. Blocking neural inputs switched the contractile pattern in wild-type and SMC knockdown mice to rhythmic contractions. These findings suggest that ICC are involved in tonic neural inhibition of the colon, a behavior observed in GI muscles of several species (116, 119). Responses to electrical field stimulation of intrinsic inhibitory neurons were, in contrast, mainly due to inhibitory responses generated by SMCs because knockdown of sGC in SMCs reduced these responses. In the esophagus, a different pattern of cell-specific regulation was noted (33). Basal tone of the lower esophageal sphincter was regulated equally by SMCs and ICC, but swallowing-induced relaxation responses appeared to be mainly mediated by ICC. These studies rely heavily on the ability of Cre recombinase to knock out expression of sGC in specific types of cells. As discussed recently, the degree of knockdown may be incomplete in studies in which inducible Cre is activated in adult animals (93).
PDGFRα+ Cells in Neurotransmission
There is another type of interstitial cell that was originally identified by electron microscopy that is also closely aligned with motor nerve terminals in GI muscles. These cells were called fibroblast-like cells for many years because of their morphological features. Fibroblast-like cells were shown to express small conductance Ca2+-activated K+ (SK3) channels (27, 62, 110), raising the possibility that they might have a role in responses to purine neurotransmitters in GI muscles. Recently, antibodies against PDGFRα were found to provide selective labeling of fibroblast-like cells throughout the GI tract (49, 50). A reporter strain of mice expressing a histone 2B-eGFP fusion protein driven by the endogenous Pdgfra promoter allowed identification of these cells for physiological and expression studies after enzymatic digestion of muscles.
Studies of intact murine and human GI muscles have clearly demonstrated that the purine neurotransmitter(s) binds to P2Y1 receptors to elicit the postjunctional effects of purinergic neurotransmission (i.e., the fast inhibitory junction potential that is linked to inhibition of contractions) (29, 30, 46). Purinergic effects are mediated, in part, by activation of apamin-sensitive, small-conductance Ca2+-activated K+ channels. PDGFRα+ cells display high expression of P2ry1 (P2Y1 receptors) and Kcnn3 (SK3 channels), and expression of P2ry1 and Kcnn1-3 genes is far lower in SMCs than in PDGFRα+ cells. Immunohistochemical analysis showed PDGFRα+ cells to be intertwined with ICC and the processes of excitatory (labeled with antibodies to vesicular acetylcholine transporter) and inhibitory (labeled with antibodies to nNOS) motoneurons. These data suggest that, when purines are released from inhibitory neurons, there is likelihood that receptors and effectors expressed by PDGFRα+ cells and appropriate for purinergic neurotransmission might be activated. Immunohistochemical and expression studies also demonstrated that PDGFRα+ cells are a population of cells distinct from ICC (49, 50, 70, 71). Similar interstitial cells with the same distributions and overlapping immunohistochemical properties were found in human colon (70) and stomach (34).
Voltage-clamp studies showed that PDGFRα+ cells express a Ca2+-dependent K+ conductance that was activated by purines and blocked by apamin (71). Some PDGFRα+ cells displayed spontaneous, periodic activation of K+ currents (spontaneous transient outward currents or STOCs), and these events were also blocked by apamin. Single channels with a conductance (∼10 pS) and sensitivity to intracellular Ca2+ (EC50 = 364 nM), consistent with expression of SK3 channels, were observed in excised membrane patches. Application of P2Y1 agonists caused activation of a conductance with the same properties, and apamin and a selective P2Y1 antagonist, MRS2500, inhibited these responses.
Studies to compare the relative responsiveness of SMC and PDGFRα+ cells to purines were performed on cells from the murine colon (58). Previous studies have shown that SMCs express P2Y receptor-driven, apamin-sensitive responses, but the current density resolved by activation of these channels was low, and depolarized potentials were needed to resolve the outward currents initiated by purines (64). Application of ATP caused significant hyperpolarization responses in PDGFRα+ cells, and these responses were partially blocked by MRS2500, a selective P2Y1 antagonist. Held at potentials equivalent to the resting potentials of cells in intact muscles, ATP caused depolarization of SMCs. Selective P2Y1 agonists also caused significant hyperpolarization of PDGFRα+ cells and little or no response in SMCs. Taken together, these data suggest that SMCs are unlikely to mediate the signature purinergic response in GI muscles: fast transient hyperpolarization. This response more likely results from purinergic activation of P2Y1 receptors and SK3 channels in PDGFRα+ cells.
The question of how P2Y1 receptors link to activation of SK3 channels in PDGFRα+ cells was addressed in imaging studies in which these cells were loaded with Fluo-4 and identified by a reporter molecule (eGFP) expressed in cell nuclei (4, 5). Purines were found to activate Ca2+ transients in PDGFRα+ cells from gastric fundus and colon. Responses were blocked in P2ry1−/− mice. The Ca2+ transients to P2Y1 selective agonists were blocked by MRS2500, but responses to ATP were not completely blocked by P2Y1 antagonists, nor were responses in muscles of P2ry1−/− mice. These data tend to confirm other studies suggesting that another more P2Y1-selective purine, and not ATP, is the purinergic neurotransmitter in GI muscles (47, 80).
Ca2+ imaging studies were also used to study the sequence of events in response to purinergic neurotransmission (5). With cholinergic and nitrergic components of neural inputs blocked, stimulation of intrinsic motoneurons evoked Ca2+ transients in PDGFRα+ and SMCs. The postjunctional responses were abolished by MRS2500 and in muscles of P2ry1−/− mice. The latency in the Ca2+ transients evoked in PDGFRα+ cells were equivalent to electrophysiological responses. The response evoked in SMCs during the stimulus was a slowly developing reduction in Ca2+. Gap-junction uncouplers inhibited responses of SMCs to nerve stimulation but did not affect the timing of Ca2+ transients evoked in PDGFRα+ cells. These data showed that PDGFRα+ cells are directly innervated by enteric inhibitory motoneurons and that these cells mediate responses to purinergic neurotransmitters by releasing Ca2+ from intracellular stores. Postjunctional hyperpolarization responses are conducted to SMCs via gap junctions.
Recently, cells identified morphologically as fibroblast-like cells were impaled and filled with dye to verify sites of recording in rabbit small intestine (58). In contrast to recordings from ICC and SMCs, fibroblast-like cells generated spontaneous transient hyperpolarizations (STHs). These events were often superimposed on low-amplitude oscillations at the frequency of slow waves (FIGURE 2, C-E). The STHs were reduced by MRS2500 and blocked by apamin, suggesting they were due, in part, to binding of P2Y1 receptors, possibly from tonic release of purine neurotransmitters, but some fraction of this activity may have been due to spontaneous activation of SK channels in these cells. These results are consistent with the integrative nature of the SIP syncytium and the cell-specific behavior of fibroblast-like cells. Slow waves appear to conduct to these cells with decrement, and the cells are capable of purinergic activation of SK-like currents. Spontaneous activation of SK channels also occurs via spontaneous Ca2+ release events that have been observed in this class of interstitial cells in the stomach and colon (4, 5). Ongoing STHs in electrically coupled cells within the SIP syncytium would tend to produce a hyperpolarizing influence (tonic inhibition) in intact GI muscles and decrease the input resistance of the SIP syncytium, reducing the amplitude of conducting slow waves and reducing the probability of reaching the threshold for generation of action potentials in SMCs.
Role of Interstitial Cells in Pathophysiology
Research into the causes of GI motility disorders has largely focused on problems that might arise from lesions in enteric neurons or SMCs. Certainly, there are motility disorders that are due to developmental defects in enteric neurons, as in Hirschsprung's disease; neuropathies that can develop with age, as in diabetes; and remodeling of sensory pathways, as may be important in several functional bowel diseases. However, there appears to be a wide range of motility disorders that may be due to causes other than those directly related to cellular pathologies of enteric neurons and SMCs. The important regulatory functions provided by interstitial cells suggest that lesions in these cells might lead to defective electrical pacing, inappropriate coordination between regions of the SIP syncytium due to lack of connectivity between ICC and loss of active propagation of slow waves in the ICC networks, reduced or unbalanced neural regulation due to reduced or altered excitatory and/or inhibitory neural inputs, altered baseline excitability of the SIP syncytium, and altered responses to stretch (Table 1). Further suggestion that interstitial cells may be important factors in GI motility disorders comes from pathological reports showing reduced numbers of ICC or abnormal distributions of ICC networks that might lead to problems of connectivity in GI muscles of human patients with motor defects. It should be stressed that this type of information does not prove cause-and-effect, but studies of animal models with reduced populations of ICC have generally supported the idea that reductions in this cell population might be causative in certain types of motility disorders. One issue of importance is the quality of morphological evaluation that has been performed to compare populations of ICC in healthy and diseased muscles. A recent paper speaks to the procedures that should be followed in this type of clinical evaluation (63). Table 2 summarizes some of the motility disorders in which pathologists have reported reduced or defective ICC populations that might be linked to symptoms.
Table 2.
Motility disorders in which ICC loss or dysfunction has been reported
| Motility Disorder | Major Symptoms That Might be Related to Loss of Interstitial Cells | References |
|---|---|---|
| Achalasia | Reduction in swallowing-induced relaxation of lower esophageal sphincter | 22, 56, 68 |
| Diabetic gastropathy | Delayed gastric emptying; reduction in gastric accommodation and/or response to inhibitory neurotransmission | 26, 38, 53, 78, 82, 111 |
| Idiopathic gastroparesis | Weakened or dyscoordinated antral contractions leading to prolonged period of gastric emptying; loss of responsiveness to digestive neural control | 121 |
| Pyloric stenosis | Inability of the pyloric sphincter to relax to aid gastric emptying | 72, 109 |
| Intestinal pseudo-obstruction | Delayed or blocked intestinal transit | 25, 52, 55, 120 |
| Ulcerative colitis | Fecal stasis due to decreased motility in proximal colon; increased transit times in proximal colon; enhanced transit in rectosignmoid areas; reduced colonic motility following a meal | 89 |
| Slow transit constipation | Reduced force of colonic contractions; weakened propulsive contractions (mass movements); loss of coordination produced during peristaltic reflex; constipation | 37, 77, 118 |
| Internal anal sphincter achalasia | Reduction in internal anal relaxation during defecation reflex | 87 |
Little is known about how loss or defects in PDGFRα+ cells might impact motility, and, as yet, this group of interstitial cells appears to have been evaluated in a very limited number of human tissue samples (34). Here again, the importance of these cells in purinergic neurotransmission and some inflammatory responses might make them candidates for pathologists to examine once accurate immunohistochemical criteria, for their evaluation has been developed for the clinical lab.
Footnotes
The authors thank Peter Blair for the images in FIGURE 1, C AND D.
Work on ICC has been funded by Program Project Grants from the National Institute of Diabetes and Digestive and Kidney Diseases: P01 DK-41315 to K.M.S. and S.M.W., and R01 DK-57236 to S.M.W. and S.J.H. Work on PDGFRα+ cells has been funded by R01 DK-091336 to K.M.S. and S.M.W. Work on ICC and PDGFRα+ cells in situ has been funded by the 24th General Assembly of the Japanese Association of Medical Sciences to Y.K.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: K.M.S., Y.K., S.J.H., and S.M.W. conception and design of research; K.M.S., Y.K., S.J.H., and S.M.W. analyzed data; K.M.S., Y.K., S.J.H., and S.M.W. interpreted results of experiments; K.M.S., Y.K., S.J.H., and S.M.W. prepared figures; K.M.S. and S.M.W. drafted manuscript; K.M.S., Y.K., S.J.H., and S.M.W. edited and revised manuscript; K.M.S., Y.K., S.J.H., and S.M.W. approved final version of manuscript; Y.K., S.J.H., and S.M.W. performed experiments.
References
- 1.Alberti E, Mikkelsen HB, Wang XY, Diaz M, Larsen JO, Huizinga JD, Jimenez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol 292: G1499–G1510, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Aranishi H, Kunisawa Y, Komuro T. Characterization of interstitial cells of Cajal in the subserosal layer of the guinea-pig colon. Cell Tiss Res 335: 323–329, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Baker SA, Drumm BT, Sauer D, Hennig GW, Ward SM, Sanders KM. Spontaneous Ca2+ transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol. In press. [DOI] [PMC free article] [PubMed]
- 4.Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca2+ signalling of fibroblast-like (PDGFR+) cells in the murine gastric fundus. J Physiol 591: 6193–6208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol 593: 1945–1963, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer AJ, Publicover NG, Sanders KM. Origin and spread of slow waves in canine gastric antral circular muscle. Am J Physiol Gastrointest Liver Physiol 249: G800–G806, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol 293: C1645–C1659, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Beckett EA, Bayguinov YR, Sanders KM, Ward SM, Hirst GD. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol 559: 259–269, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J Physiol 543: 871–887, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol 493: 193–206, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol 273: 42–51, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Bhetwal BP, Sanders KM, An C, Trappanese DM, Moreland RS, Perrino BA. Ca2+ sensitization pathways accessed by cholinergic neurotransmission in the murine gastric fundus. J Physiol 591: 2971–2986, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tiss Res 290: 11–20, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA 93: 12008–12013, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol 550: 829–844, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel EE, Posey-Daniel V. Effects of scorpion venom on structure and function of esophageal lower sphincter (LES) and body circular muscle (BCM) from opossum. Can J Physiol Pharmacol 62: 360–373, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 246: G305–G315, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Dickens EJ, Edwards FR, Hirst GD. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol 531: 827–833, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol 514: 515–531, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farre R, Wang XY, Vidal E, Domenech A, Pumarola M, Clave P, Huizinga JD, Jimenez M. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil 19: 484–496, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Faussone Pellegrini MS, Cortesini C, Romagnoli P. Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal's interstitial cells. Arch Ital Anat Embriol 82: 157–177, 1977. [PubMed] [Google Scholar]
- 22.Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicro Cytol 17: 673–685, 1985. [PubMed] [Google Scholar]
- 23.Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicro Cytol Pathol 21: 439–460, 1989. [PubMed] [Google Scholar]
- 24.Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Micro Res Tech 47: 248–266, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Feldstein AE, Miller SM, El-Youssef M, Rodeberg D, Lindor NM, Burgart LJ, Szurszewski JH, Farrugia G. Chronic intestinal pseudoobstruction associated with altered interstitial cells of Cajal networks. J Ped Gastroenterol Nutri 36: 492–497, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg 9: 102–108, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharm Sci 92: 35–42, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Gabella G. Cells of visceral smooth muscles. J Sm Mus Res 48: 65–95, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Gallego D, Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M. Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol 590: 1943–1956, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol 291: G584–G594, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol 559: 411–422, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groneberg D, Konig P, Lies B, Jager R, Seidler B, Klein S, Saur D, Friebe A. Cell-specific deletion of nitric oxide-sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterol 145: 188–196, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Groneberg D, Zizer E, Lies B, Seidler B, Saur D, Wagner M, Friebe A. Dominant role of interstitial cells of Cajal in nitrergic relaxation of murine lower oesophageal sphincter. J Physiol 593: 403–414, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grover M, Bernard CE, Pasricha PJ, Parkman HP, Abell TL, Nguyen LA, Snape W, Shen KR, Sarr M, Swain J, Kendrick M, Gibbons S, Ordog T, Farrugia G. Platelet-derived growth factor receptor alpha (PDGFRalpha)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil 24: 844–852, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanani M, Farrugia G, Komuro T. Intercellular coupling of interstitial cells of cajal in the digestive tract. Inter Rev Cytol 242: 249–282, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol 372: 501–520, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterol 118: 14–21, 2000. [DOI] [PubMed] [Google Scholar]
- 38.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterol 121: 427–434, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol 540: 907–919, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach: a stochastic process. J Physiol 535: 165–180, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/Wnu mouse small intestine. J Auton Nerv Syst 80: 142–147, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tis Res 311: 299–313, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Huizinga JD, Liu LW, Fitzpatrick A, White E, Gill S, Wang XY, Zarate N, Krebs L, Choi C, Starret T, Dixit D, Ye J. Deficiency of intramuscular ICC increases fundic muscle excitability but does not impede nitrergic innervation. Am J Physiol Gastrointest Liver Physiol 294: G589–G594, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol 590: 1957–1972, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterol 140: 608–617, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 131: 691–702, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol 72: 107–115, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Imaizumi M, Hama K. An electron microscopic study on the interstitial cells of the gizzard in the love-bird (Uroloncha domestica). Zeitschrift Zellforschung Mikroskopische Anatomie 97: 351–357, 1969. [DOI] [PubMed] [Google Scholar]
- 52.Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-Kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol 92: 332–334, 1997. [PubMed] [Google Scholar]
- 53.Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with Type 2 diabetes mellitus. J Gastroenterol 41: 1076–1087, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Kamm KE, Leachman SA, Michnoff CH, Nunnally MH, Persechini A, Richardson AL, Stull JT. Myosin light chain kinases and kinetics of myosin phosphorylation in smooth muscle cells. Prog Clin Biol Res 245: 183–193, 1987. [PubMed] [Google Scholar]
- 55.Kenny SE, Vanderwinden JM, Rintala RJ, Connell MG, Lloyd DA, Vanderhaegen JJ, De Laet MH. Delayed maturation of the interstitial cells of Cajal: a new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J Ped Surg 33: 94–98, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Khelif K, De Laet MH, Chaouachi B, Segers V, Vanderwinden JM. Achalasia of the cardia in Allgrove's (triple A) syndrome: histopathologic study of 10 cases. Am J Surg Pathol 27: 667–672, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflügers Arch 445: 202–217, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Kito Y, Kurahashi M, Mitsui R, Ward SM, Sanders KM. Spontaneous transient hyperpolarizations in the rabbit small intestine. J Physiol 592: 4733–4745, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kito Y, Mitsui R, Ward SM, Sanders KM. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 308: G378–G388, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol 553: 803–818, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol 288: C710–C720, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Klemm MF, Lang RJ. Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin Exper Pharm Physiol 29: 18–25, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Gershon MD, Hutson J, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol (Berl) 118: 271–301, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Koh SD, Dick GM, Sanders KM. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol Cell Physiol 273: C2010–C2021, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol 513: 203–213, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol 576: 653–658, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol 62: 295–316, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Kraichely RE, Farrugia G. Achalasia: physiology and etiopathogenesis. Dis Esophagus 19: 213–223, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor-alpha-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol 307: C561–C570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J Cell Mol Med 16: 1397–1404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589: 697–710, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langer JC, Berezin I, Daniel EE. Hypertrophic pyloric stenosis: ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal. J Ped Surg 30: 1535–1543, 1995. [DOI] [PubMed] [Google Scholar]
- 73.Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA 86: 7280–7284, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterol 133: 907–917, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lies B, Beck K, Keppler J, Saur D, Groneberg D, Friebe A. Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol 593: 4589–4601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lies B, Groneberg D, Friebe A. Toward a better understanding of gastrointestinal nitrergic neuromuscular transmission. Neurogastro Motil 26: 901–12, 2014. [DOI] [PubMed] [Google Scholar]
- 77.Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 51: 496–501, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller SM, Narasimhan RA, Schmalz PF, Soffer EE, Walsh RM, Krishnamurthi V, Pasricha PJ, Szurszewski JH, Farrugia G. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastro Motil 20: 349–357, 2008. [DOI] [PubMed] [Google Scholar]
- 79.Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tis Res 309: 219–227, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA 104: 16359–16364, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ordog T, Redelman D, Horvath VJ, Miller LJ, Horowitz B, Sanders KM. Quantitative analysis by flow cytometry of interstitial cells of Cajal, pacemakers, and mediators of neurotransmission in the gastrointestinal tract. Cytometry 62: 139–149, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 49: 1731–1739, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Ordog T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol 518: 257–269, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol Cell Physiol 260: C917–C925, 1991. [DOI] [PubMed] [Google Scholar]
- 85.Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol 290: C1411–C1427, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Parker I, Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J Physiol 491: 663–668, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piotrowska AP, Solari V, Puri P. Distribution of interstitial cells of Cajal in the internal anal sphincter of patients with internal anal sphincter achalasia and Hirschsprung disease. Arch Pathol Lab Med 127: 1192–1195, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, Ward SM, Yan W, Sanders KM. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterol 138: 1068–1078, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterol 111: 1447–1455, 1996. [DOI] [PubMed] [Google Scholar]
- 90.Rumessen JJ, Mikkelsen HB, Thuneberg L. Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterol 102: 56–68, 1992. [DOI] [PubMed] [Google Scholar]
- 91.Sanders KM. Invited review: mechanisms of calcium handling in smooth muscles. J Appl Physiol 91: 1438–1449, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588: 4621–4639, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanders KM, Keef KD. Cellular mediators of nitrergic neurotransmission in GI smooth muscles: no easy answer. J Physiol 593: 4511–4512, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility-insights from smooth muscle biology. Nature Rev Gastro Hepat 9: 633–645, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanders KM, Salter AK, Hennig GW, Koh SD, Perrino BA, Ward SM, Baker SA. Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of cajal. J Neurogastro Motil 20: 171–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanders KM, Stevens R, Burke E, Ward SW. Slow waves actively propagate at submucosal surface of circular layer in canine colon. Am J Physiol Gastrointest Liver Physiol 259: G258–G263, 1990. [DOI] [PubMed] [Google Scholar]
- 97.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seki K, Komuro T. Immunocytochemical demonstration of the gap junction proteins connexin 43 and connexin 45 in the musculature of the rat small intestine. Cell Tis Res 306: 417–422, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Sha L, Farrugia G, Harmsen WS, Szurszewski JH. Membrane potential gradient is carbon monoxide-dependent in mouse and human small intestine. Am J Physiol Gastrointest Liver Physiol 293: G438–G445, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol Cell Physiol 252: C215–C224, 1987. [DOI] [PubMed] [Google Scholar]
- 101.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 102.Stanich JE, Gibbons SJ, Eisenman ST, Bardsley MR, Rock JR, Harfe BD, Ordog T, Farrugia G. Ano1 as a regulator of proliferation. Am J Physiol Gastrointest Liver Physiol 301: G1044–G1051, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stull JT, Kamm KE, Taylor DA. Calcium control of smooth muscle contractility. Am J Med Sci 296: 241–245, 1988. [DOI] [PubMed] [Google Scholar]
- 104.Sung TS, Kim HU, Kim JH, Lu H, Sanders KM, Koh SD. Protease-activated receptors modulate excitability of murine colonic smooth muscles by differential effects on interstitial cells. J Physiol 593: 1169–1181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol 525: 105–111, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol 546: 751–763, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-Kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tis Res 280: 97–111, 1995. [DOI] [PubMed] [Google Scholar]
- 108.van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol 524: 245–265, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterol 111: 279–288, 1996. [DOI] [PubMed] [Google Scholar]
- 110.Vanderwinden JM, Rumessen JJ, de Kerchove d'Exaerde A Jr, Gillard K, Panthier JJ, de Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tis Res 310: 349–358, 2002. [DOI] [PubMed] [Google Scholar]
- 111.Wang XY, Huizinga JD, Diamond J, Liu LW. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastro Motil 21: 1092–1095, 2009. [DOI] [PubMed] [Google Scholar]
- 112.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20: 1393–1403, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480: 91–97, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol 573: 147–159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. Am J Physiol Gastrointest Liver Physiol 259: G264–G273, 1990. [DOI] [PubMed] [Google Scholar]
- 116.Waterman SA, Costa M. The role of enteric inhibitory motoneurons in peristalsis in the isolated guinea-pig small intestine. J Physiol 477: 459–468, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ 27: 201–206, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterol 123: 1459–1467, 2002. [DOI] [PubMed] [Google Scholar]
- 119.Wood JD. Excitation of intestinal muscle by atropine, tetrodotoxin, and xylocaine. Am J Physiol 222: 118–125, 1972. [DOI] [PubMed] [Google Scholar]
- 120.Yamataka A, Ohshiro K, Kobayashi H, Lane GJ, Yamataka T, Fujiwara T, Sunagawa M, Miyano T. Abnormal distribution of intestinal pacemaker (c-Kit-positive) cells in an infant with chronic idiopathic intestinal pseudoobstruction. J Ped Surg 33: 859–862, 1998. [DOI] [PubMed] [Google Scholar]
- 121.Zarate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut 52: 966–970, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol 298: G14–G24, 2010. [DOI] [PubMed] [Google Scholar]
- 123.Zheng H, Park KS, Koh SD, Sanders KM. Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am J Physiol Cell Physiol 306: C705–C713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu MH, Sung TS, O'Driscoll K, Koh SD, Sanders KM. Intracellular Ca2+ release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Physiol Cell Physiol 308: C608–C620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.ZhuGe R, Sims SM, Tuft RA, Fogarty KE, Walsh JV Jr. Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J Physiol 513: 711–718, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


