Abstract
Cells in the body are exposed to irregular mechanical stimuli. Here, we review the so-called fluctuation-driven mechanotransduction in which stresses stretching cells vary on a cycle-by-cycle basis. We argue that such mechanotransduction is an emergent network phenomenon and offer several potential mechanisms of how it regulates cell function. Several examples from the vasculature, the lung, and tissue engineering are discussed. We conclude with a list of important open questions.
Cells in an organism are continuously exposed to a variety of mechanical factors including osmotic stress, external pressure, shear stress, and tensile stress. For example, primary cilia in kidney (89), bone (50), cartilage (101), and eye (67) cells are sensitive to pressure. During body movement, muscle cells contract and exert stresses on all cells in the muscle, including themselves (72) as well as nerve cells (115). With every heartbeat, the propagating pressure waves in the arteries produce shear stresses along endothelial cells (ECs) (30) as well as circumferential stresses acting on vascular smooth muscle cells (VSMCs) (83). During breathing, the rhythmic contraction of the respiratory muscles generates cyclic stretch of the lung that is transmitted along the extracellular matrix (ECM), including mostly collagen and elastin (108), to all resident cells of the lung (126).
Nearly all cell types have machinery to sense and respond to mechanical stimuli. The sensing is achieved via mechanosensitive channels (133) and various transmembrane receptors such as integrins at focal adhesions (2) or cadherins at cell-cell junctions (15, 52). The most common immediate cellular response at the molecular scale is protein conformational change (71) at focal adhesions (64) or cell-cell contacts (132), which can lead to reorganization of focal adhesions (102) and the cytoskeleton (116) followed by biochemical signaling cascades (118, 122), turning genes on or off, and eventually expressing various intra- (81) and extracellular proteins (18, 22, 131) and enzymes (123). These processes involving sensing, transmission, and signaling are collectively called mechanotransduction (42, 47, 48, 82, 118).
Physiological rhythms acting within the body also exhibit fluctuations that may influence the process of mechanotransduction (5, 7, 120). For example, blood pressure is known to exhibit significant beat-to-beat variability that increases in diseases such as hypertension (70, 97). The depth of breathing also fluctuates from breath to breath (21). It has been proposed that, beside the magnitudes of mechanical stresses, the time period as well as the frequency of the stimuli determine the actual signaling response (39). Nevertheless, in laboratory conditions, the details of the mechanotransduction pathway are invariably studied using static or cyclic but monotonous stretch (MS). In a recent study, we presented evidence that fluctuations in cycle-by-cycle strain, called variable stretch (VS), applied to VSMCs fundamentally alter cellular bioenergetics, cytoskeletal organization, and signaling (7).
The main purpose of this review is to describe how fluctuations in mechanical stimuli regulate mechanotransduction. We first briefly review current concepts in conventional mechanotransduction including integrins, focal adhesions, cadherins, as well as their signaling. Next, we discuss why mechanotransduction should be considered as an emergent network phenomenon. Comparison of static stretch, transient stretch, and MS has recently been summarized (39). Here, the main focus will be on the so-called fluctuation-driven mechanotransduction (FDM) during VS in which peak strains vary from cycle to cycle while maintaining the same mean strain as MS. Examples will mostly be from the vasculature and the lung. Following some speculation on the possible roles of fluctuations in diseases and aging, we conclude with a list of important open questions.
Conventional Mechanotransduction
Biology of Cell Adhesion-Related Signaling
Mechanotransduction has been intensively studied and is known to play fundamental roles in the genesis and maintenance of normal tissue structure and function as well as regulate many cell functions such as migration and contraction (2, 16, 34, 39, 47, 69, 82, 100, 118). Mechanotransduction is a complex process that acts at several levels. The ECM interacts with cells through adhesion receptors such as integrins, which in turn structurally and functionally regulate cell membrane proteins and their linkages to the cytoskeleton, including actin microfilaments, microtubules, and intermediate filaments. These events involve multi-molecular conformational changes, biochemical reactions, as well as whole organelle responses such as the reorganization of the cytoskeleton, organelle biogenesis, or changes in organelle structure and function. Below, we provide a brief review of the main pathways in conventional mechanotransduction (see FIGURE 1), which are also involved in or are modulated by FDM.
FIGURE 1.
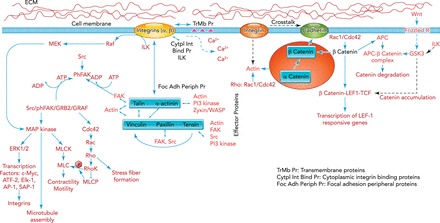
Schematics of cell adhesion-related signaling
The external domains of integrins interact with certain transmembrane proteins with major impact on signaling influencing cell motility as well as intracellular calcium levels. The intracellular domains of integrins also interact with several cytoplasmic proteins, including integrin-linked kinase (ILK), which regulate integrin outward signaling. At focal adhesions (FAs), the FA peripheral proteins, talin and α-actinin, bind directly to intracellular domains of integrins, connecting them to vinculin, paxillin, and tensin, as well as to Zyxin/WASP, filamin, or FA kinase (FAK), Src PI3 kinases. Once the integrin-mediated cell adhesion occurs, FAK is rapidly autophosphorylated, which recruits Src to further phosphorylate FAK at several tyrosine residues. This process regulates FA assembly and disassembly, while additional proteins such as GRB2 and GRAF may be recruited and further signaling occurs to Cdc42, Rho, and mitogen-activated protein kinase (MAPK). These signaling events regulate cell motility, cell cycle and proliferation, and apoptosis among others. MAPK can also be activated directly by integrins through Raf/MEK independent of FAK. MAPK-ERK1/2, after integrin activation, translocates to the nucleus and regulates various transcription factors including c-Myc, AP-1, ATF-2, Elk-1, and SAP-1 with fast expression of vinculin, actin, α-actinin, and β1 integrin. MAPK can phosphorylate and activate myosin light chain kinase (MLCK) directly and hence regulate cell contractility and motility. Furthermore, MAPK can physically associate with microtubules and enzymatically alter their structure. The other major signaling family, the Rho family of GTPases Cdc42/Rac/Rho, regulates actin assembly, stress fiber formation, and anchorage for integrin signaling. Rho kinase (RhoK) can also directly phosphorylate MLC as well as modulate it by the inhibition of MLC phosphatase (MLCP), and may act synergistically with the integrin-activated MAPK/MLCK/MLC contractility. Cell-cell adhesion sites at adhesion junctions link cadherins with each other and the actin cytoskeleton. The cadherin intracellular domain binds to β-catenin, which binds to α-catenin, and the complex binds directly to actin and α-actinin, establishing the link between cadherins and integrins. Cadherins are modulated by the Wnt signaling through the level of β-catenin availability. Wnt binds to ECM and activates the Frizzled R/GSK3/APC-β-catenin complex axis. The cross talk of integrin/ILK and GSK3 modulates the β-catenin/LEF1/TCF complex and hence the LEF1 responsive transcription. Similarly to integrins, there is both inside-out and outside-in signaling between cadherins and the Rho GTPases. Both positive and negative regulations are achieved; Rac1/Cdc42 strengthens the cadherin complex through β-catenin availability, but Rho negatively regulates cadherins through the actin cytoskeleton assembly. On the other hand, at cadherin-cadherin contacts, Rac1/Cdc42 becomes activated.
A key element in mechanotransduction is the coupling of the cell to the ECM by specialized multimolecular complexes, the focal adhesions (FAs). The FAs serve as dynamic biochemical signaling hubs that regulate numerous signaling proteins and alter adhesion receptor clustering and binding to the ECM or receptors on other cells (15, 33, 52, 93). The most studied adhesion events are the bidirectional integrin-mediated processes (43). Signals can arrive from the ECM to the cells to reorganize FAs, but FA proteins are also tightly regulated by biochemical events from inside the cell. Virtually all cells in the body express integrins with cell type specificity that is often regulated by changes in the cell's mechanical environment. Integrins form heterodimers, which consist of α and β subunits. Ligands, such as the RGD (Arg-Gly-Asp) sequence along collagen, fibronectin, or laminin, bind to integrin receptors depending on the subunit combinations (2, 44, 96). Following ligand binding, integrin subunits form domains and clusters (66) as well as undergo conformational changes from a low- to a high-affinity state (41). G-protein-coupled receptors and Ras-related small GTPases and their effectors modulate the affinity of integrin bindings (41, 104). The external (99) and internal (4) integrin domains interact with other proteins, which regulate the bidirectional signaling events. At FAs, talin and α-actinin bind directly to the intracellular domains, connecting them to vinculin, paxillin, and tensin, as well as to FA kinase (FAK), which, through mostly tyrosine phosphorylation, regulate actin filament formation (14). Thus cytoskeletal assembly and integrin signaling are tightly coupled. The most accepted view is that, upon integrin-mediated cell adhesion, FAK is rapidly autophosphorylated to recruit Src and further autophosphorylate FAK, which regulate FA assembly/disassembly with additional proteins such as Rho and mitogen-activated protein kinase (MAPK). However, MAPK can also be activated directly by integrins independent of FAK. After integrin activation, MAPK translocates to the nucleus and regulates various transcription factors (49), with a fast expression of vinculin, actin, α-actinin, and β1-integrin (134). MAPK can also phosphorylate and activate myosin light chain kinase (MLCK) and govern cell contractility and motility (56). Furthermore, MAPK can physically associate with microtubules and alter their structure (78). The other major signaling pathway is through the Rho family of GTPases, which regulates actin assembly, stress fiber formation, and anchorage for integrin signaling (19). Similar to MAPK, the Rho kinase can also phosphorylate MLC with an effect on cell contractility (3).
Cell-cell adhesion occurs via linking cadherins with each other and the actin cytoskeleton (15, 52). There are cell type-specific cadherins (110), which are members of the calcium-dependent homotypic cell-cell adhesion molecules and play fundamental roles in tissue development and homeostasis (121). The intracellular domain of cadherin binds to β-catenin, which in turn binds to α-catenin, and the full complex binds directly to actin and α-actinin (20), establishing a force transmission pathway between cadherins and integrins (127). Cadherins are modulated by the Wnt signaling through availability of β-catenin (37). Similarly to integrins, there is bidirectional signal transduction between cadherins and Rho GTPases. Additionally, there is an interplay between cadherins, activation of Rho, and the dynamics of microtubule, actin, and intermediate filaments (13, 130), implying a regulatory force transmission across cell-cell junctions (80).
Dynamic Network Phenomena in Mechanotransduction
There is evidence that cell adhesion and the related signaling discussed above may not simply be localized to FAs; rather, they involve larger-scale interactions originating within the network of actin filaments as a whole (28, 75, 85, 117). To understand the nature of network phenomena, we point out two key features. First, the cytoskeleton consists of active and passive cross links governed by their binding kinetics that are force dependent (57). Second, network formation is an active process since the myosin II motors that attach to and walk along actin filaments draw energy from ATP hydrolysis (40, 124). The active walk of the motors reorganizes the actin network and generates a prestress in the cytoskeleton. The prestress influences local processes such as FA assembly and hence is an essential component of adherent cells' ability to sense their environment (26). Indeed, even the stability of FA complexes depends on the contractile force in the actin cytoskeleton (38, 87). This was further corroborated by the fact that the FA proteome was enhanced by myosin II-induced contractility, suggesting a common myosin-related FA regulation (61, 98). While this mechanism may contribute little to force generation by skeletal muscle cells, myosin II motors still play a role in the development and cell-cell interactions of myoblasts (109).
The response of adherent cells to mechanical stimuli is also time-scale dependent (106). The development of tension in the cytoskeleton due to myosin activity is a fast process and reaches equilibrium in the order of 100 s (75). Within this time frame, all adherent cells exhibit common features in the way they respond to changes in substrate stiffness (75) and stretch (58, 116). As substrate stiffness increases, cells not only develop higher traction forces, but they also adjust the rate of force generation such that the time to equilibrium is the same independent of substrate stiffness (75). A similar phenomenon is observed in adherent cells subject to a transient equi-biaxial stretch pulse (58, 116). Following stretch, cell stiffness immediately drops, followed by a slow recovery phase. Again, cells adjust their rate of recovery in such a way that the time to reach equilibrium remains more or less the same regardless of stretch amplitude. A computational study showed that cross-linking proteins and active molecular motors in an elastic actin network were able to replicate the response of cells to changes in substrate stiffness (10). A more recent study also showed that an elastic network consisting of only actin and myosin can explain and unify three apparently unrelated phenomena (85): the effect of changes in substrate stiffness on cellular traction and stiffness, the dependence of the rate of traction force development on the stiffness of the substrate (75), and the physical response to transient (58, 116) and cyclic stretch (25, 31). These studies provide evidence that a dynamic network phenomenon may be responsible for the sensitive detection of environmental cues by the cell. Other studies have also demonstrated that complex self-organized structures naturally emerge in an active network as the myosin motors reorganize the actin (59). Such a network exhibits both active contractility and viscoelastic properties consistent with those observed in cells (55, 125). Also, in vitro models of actin-myosin gels (6, 105) successfully reproduced structural motifs found in the actin cytoskeleton (27, 46).
How can a simple mechanical model of the actin-myosin network reproduce the response to both stretch and substrate stiffness while producing self-organized structures as observed in living cells? Within a prestressed elastic network, the local force on a myosin cross link is a function of both the total average prestress in the network as well as the local structure of the network in the neighborhood of the myosin. This allows interactions to occur at two different length scales. At the microscale, the myosin motors are able to elastically interact with each other as they move along actin filaments and generate prestress in the network. At the larger scale of the entire network, the average prestress feeds back to the individual myosin motors to regulate their walk along the actin. Thus the strong link between internal prestress and adaptation to external stretch indicates that mechanotransduction emerges as a multi-scale network phenomenon that results from microscopic molecular processes attempting to maintain local force balance within a large and prestressed elastic network in response to an external stimulus. Furthermore, the polar nature of the actin necessarily leads to heterogeneities, and the directional motion of the motors driven away from equilibrium by ATP consumption acts as a dissipative nonlinear network that can generate structure and form (90). As discussed below, such a complex behavior also manifests in the response of cells to fluctuations in their mechanical environment.
Fluctuation-Driven Mechanotransduction
Fluctuations in Mechanical Stimuli
To appreciate fluctuation-driven mechanotransduction (FDM), we first review the type and amount of mechanical fluctuations cells in the body experience. These fluctuations depend on the location of the cell within the organ, the organ itself, as well as the species. In the lung, fluctuations are due to breath-to-breath variability of tidal volume (21) as well as regional variations in the mechanical properties of the ECM. Tidal stretches are superimposed on a preexisting stress corresponding to functional residual capacity. Furthermore, gravity generates a gradient in both the preexisting stress and the regional tidal volume so that the mean static stretch is larger and the dynamic tidal stretch is smaller in the upper regions of the lung. Although the strain variability is not known along the gravitational axis, the coefficient of variation (CV) of regional tidal volume for the pulmonary rib cage was higher than that of the abdominal compartment (0.39 vs. 0.28; P < 0.05) (21). The strain magnitudes also depend on the cell type. A detailed analysis of the mechanics of and strains in the alveoli are given in Ref. 92. Epithelial cells in the alveoli receive approximately two-dimensional strains and undergo 10–15% cyclic area strain during quiet breathing, with a maximum of ∼37% corresponding to deep inspirations (119). Because the lung is porous with thin membranous structures, fibroblasts might experience similar strains in the alveolar septal walls. On the other hand, airway smooth muscle cells are stretched up to only ∼5% strains during breathing fairly independently of location, whereas a deep inspiration could result in ∼25% strain in the larger airways and up to 40% strain in the smaller airways (63). The breath-to-breath fluctuations in tidal volume in normal adults have CV values between 20 and 50% (21), which may generate similar peak strain variations, although the actual strain distribution acting locally on cells is likely different from the global tidal volume distribution.
In the vasculature, both heart rate and stroke volume exhibit significant beat-to-beat variabilities (65) and, consequently, so does blood pressure. The amount of fluctuations in circumferential strain can be estimated from blood pressure variability and the stress-strain curve of the vessel wall. This calculation for VSMCs in the rat aorta resulted in a static strain of 70%, with the peak strain amplitudes uniformly distributed between 77.5 and 92.5%, while frequencies ranged from 2 to 6 Hz for high and low amplitudes, respectively (7). In this study, the design was specifically chosen so as to maintain a constant strain rate; in reality, however, strain rate also changes due to variability in heart rate, which varies in a long-range correlated manner according to a non-Gaussian distribution (88) and is highly sensitive to vascular disease (51). Shear stresses on the endothelium vary largely along the arterial tree (17, 54, 91), and it is estimated that peak shear stress is between ∼7 and 18 dyn/cm2 during a 12-h period (120). While blood pressures are similar in magnitude across mammals, the same cell type in different species can see very different frequencies and hence strain rates. The heart rate f is known to scale with body mass M as f∼M−0.25 (128), which provides a 20–25 times faster rate in the mouse compared with the elephant. How mechanotransduction is affected by these highly different rates is not understood.
General Considerations for FDM
The mechanisms of FDM have not been fully elucidated. However, our recent study suggests sweeping effects of VS on many cellular processes compared with MS in VSMCs (7). It is therefore possible that VS interferes with most molecular interactions in FIGURE 1. In the following, we will consider only a few possible mechanisms that could lead to different signaling during MS and VS. Since cells adhere to ECM molecules such as collagen or fibronectin through integrins, the stretching of the fibers will first be sensed by the integrins that are linked to talin and, through vinculin, to the cytoskeleton. Forces of different magnitudes may be sensed by multimodular proteins along a linked chain of mechanotransduction triggered by conformational changes and unfolding motifs (122). In this picture, mechanotransduction is governed by switch-like mechanisms so that stronger and weaker forces can be detected by unfolding different protein domains. During MS, domain folding might undergo cyclic variations, eventually leading to a nonequilibrium steady state. However, the occasionally larger than average force during VS can unfold different protein domains. If this happens a sufficient number of times, VS will induce phosphorylation and binding of a different set of signaling molecules than MS. Furthermore, while VS also maintains nonequilibrium conditions, cells and the stretch-sensitive subcellular structures may never reach a steady state. The average time for a motif to be in the unfolded state can also be different between MS and VS, allowing slower biochemical signaling pathways to be selectively triggered by the dynamics of stretch pattern.
There are several additional mechanisms not based on switch-like conformational changes that can be influenced by stretch pattern. For example, since bond dissociation depends on the force on a bond (29), the binding and unbinding rates will be affected not only by the mean force during MS but by the amount of fluctuations in VS. The mechanism here is likely related to the force-rate sensitivity of bond dissociation that should allow different sets of catch and slip bonds to be activated by MS and VS. Similarly, given sufficient time, the reinforcement of FAs by mechanical forces will be different during VS than MS. Most importantly perhaps, within the cytoskeleton, the network-related mechanosensing via myosin motors will certainly be affected by stretch pattern. For example, during the loading phase of cyclic stretch, myosin motors detach from actin while, during the unloading phase, they reattach and ratchet up tension (85). The repeated application of the same stretch in MS leads to a star-shape formation with a gradual decline of traction force. However, it is conceivable that the larger than average forces during VS would allow the cytoskeleton to break out of this stable configuration, which would lead to a different organization of the actin cytoskeleton. The continuous competition between detachments and ratcheting up the prestress, while in a steady state during MS, is repeatedly kicked out of the steady state during VS. This unsteady state of the cytoskeleton should consume more ATP and maintain a different dynamic network structure than during MS. Indeed, the fractal dimension of actin was higher following 4 h of VS than MS in VSMCs with substantial differences in subsequent signaling (7), suggesting that FDM triggers a transition between various structural states of the cytoskeleton (27).
We can conceptually demonstrate why FDM is expected to utilize different signaling pathways than conventional mechanotransduction. Consider a linearly elastic element characterized by a spring constant k as a simplified representation of cell stiffness. The energy stored in the spring by stretching it to an extension x is E = kx2/2. Over N identical such cycles, the total energy delivered by MS is simply the sum of the energies in each cycle
| (1) |
Since the term inside the summation is independent of i, this can be written as EMS = NE. Consider now the case of VS in which the displacement is given as yi = x + ei, where ei is a zero mean random variable with variance σ2, which takes a different value in each cycle i. The energy stored in a given cycle i depends on ei, and the total energy is the sum of the energies communicated to the system in each cycle:
| (2) |
By substituting yi = x + ei and expanding the square, we obtain the following:
| (3) |
The first term in the parenthesis reduces to EMS, the second term is zero because e is zero mean, and the third term is by definition σ2. Hence, EVS simplifies to
| (4) |
Equation 4 demonstrates that the total energy depends on the variance of the cycle-by-cycle fluctuations added to peak strain. Several limitations apply here. First, we assumed that the system is linear and elastic. Nonlinearity would not alter the main conclusion from the above analysis, but the expression for the energy would be more complicated. Cells are also viscoelastic, and allowing for dissipation would require the knowledge of the relative energy dissipation during MS and VS, which is not known. However, a consequence of viscoelasticity is that stresses and forces are also a function of strain rate. Higher rates at the same amplitude will generate higher forces and consequently could trigger different mechanotransduction responses. Second, we assumed that k is constant. However, in response to MS and VS, cell stiffness will likely change. Indeed, during MS, cell stiffness declines (25). Unfortunately, it is not known how cell stiffness changes following VS, although the organization of the actin cytoskeleton, the most important contributor to cell stiffness, exhibits a stronger network structure during VS (7), which might result in higher stiffness. One could also argue that by elevating the extension x in Eq. 1 so that EMS = EVS, the difference between the two stretching modes should disappear. However, this is not necessarily the case. Consider a protein that unfolds at an extension x′ > x, where x is the mean value of y in Eq. 2. It is easy to see that VS can occasionally unfold the protein if the fluctuations are big enough, but MS will not be able to achieve that. Gradually increasing x in MS to above x′ so that EMS = EVS, MS can also be made to unfold the protein. However, MS will unfold the protein in every cycle, whereas VS unfold the protein only occasionally despite the total energy delivered by the two stretching modes being the same. Thus, during VS, the fraction of time the protein is in the phosphorylated state may be tuned for specific tasks. We can conclude that, due to either the elastic surplus energy or the different phosphorylation state of the cell, VS should result in different cytoskeletal organization, signaling, and gene and protein expressions compared with MS.
FDM Enhances Bioenergetics in VSMCs
An important consequence of the energy surplus is that cellular bioenergetics may respond differently to VS than to MS. We recently reported that ATP production rate in VSMCs in culture stretched equi-biaxially was higher by a factor of ∼2 following 4 h of VS compared with MS (7). In this study, ATP production was quantified by the intensity of the tetramethylrhodamine methyl ester dye, which is related to the inner mitochondrial membrane potential (mMP) (24) and hence ATP production (53). The fluctuation-enhanced increase in mMP thus implies higher ATP production, which was accompanied by a general increase in tyrosine phosphorylation, upregulation of key components of the electron transport chain including the ATP synthase, as well as various structural changes such as increased organization of the actin, mitcrotubule, and mitochondrial networks characterized by their fractal dimension and coefficient of variation (7).
Using a set of inhibitors, part of the mechanism related to ATP production was found to be as follows (7). When actin polymerization, microtubule depolymerization, the ATP synthase, FAK, or calcium availability were inhibited individually, ATP production and mitochondrial cluster size were altered, but VS maintained a higher mMP and hence ATP production than MS. Two cytoskeletal structures, microtubules and vimentin, however, play a key role in FDM since blocking their polymerization eliminated the difference between MS and VS cells. Inhibiting non-muscle myosin II, the dynamin-related protein 1, which regulates mitochondrial fission (84), or paprotrain, an inhibitor of mitotic kinesin-like protein 2, all reduced ATP production in VS cells to the levels found in MS cells. Interestingly, inhibiting Rho kinase also eliminated the differences between MS and VS cells, but via elevating mMP in MS cells. A conceptual diagram summarizing the differences in signaling and bioenergetics during MS and VS is shown in FIGURE 2. The main functional consequence of the enhanced ATP production by FDM was an increase in myosin light chain phosphorylation, which maintained VSMC contractility in vascular wall segments stretched using VS but declined it during MS. Thus VSMCs appear to utilize the mechanical energy surplus during VS and convert it to chemical energy stored in ATP, which, together with the reorganized cytoskeletal structure, may play crucial roles in many essential cell functions.
FIGURE 2.
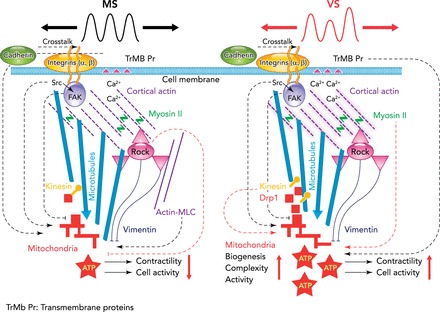
Schematic representation of the signaling to the mitochondria involved in monotonous stretch and variable stretch
Schematic representation of the signaling to the mitochondria involved in monotonous stretch (MS) (left) and variable stretch (VS) (right) of VSMCs. The effect of stretch enters the cell through integrins and transmembrane proteins (TRMb Pr) such as stretch-sensitive calcium channels. Fluctuations in stretch amplitude spread through focal adhesions, subcortical actin, and microtubules to eventually reach mitochondria. Mitochondrial activity characterized by the inner mitochondrial membrane potential (mMP) is significantly affected by both structural elements (solid and dashed lines) such as the microtubule network and signaling molecules (dotted lines) such as Src. The dotted lines do not necessarily indicate a direct effect of the corresponding molecule on mMP. Left: the black dotted lines represent mechanisms that make mMP higher during MS than the unstretched (US) condition. The red dotted lines (e.g., from myosin II to mitochondria) denote mechanisms that differentiate between MS and VS since inhibition of the corresponding molecule eliminates the difference between them. An arrow/perpendicular line at the end of a line means that the activation of the corresponding signaling molecule or the intactness of the corresponding structure serves to maintain a high/low level of mMP. Accordingly, since inhibition of microtubule and vimentin polymerization eliminates the difference between VS, MS, and US cells, these two cytoskeletal structures are fundamentally necessary for any mechanotransduction to the mitochondria. Src, which is closely associated with focal adhesions, plays a role in both MS and VS signaling since it eliminates the difference between MS and US but maintains the difference between MS and VS. While FAK is necessary for normal mMP for both MS and VS, it does not affect the difference between MS and VS. During MS, inhibiting the Rho kinase (red dotted line in left schematic) elevates mMP to the level of VS, suggesting that Rho kinase plays a silencing role in mMP during conventional mechanotransduction. Additionally, during MS, mechano-sensitive channels, ATP-dependent K+ channels, or MLC phosphorylation play a role in increasing mMP above that in the US condition. On the other hand, inhibition of motor molecules (non-muscle myosin II, kinesin motors, Drp1) reduces mMP during VS to that observed during MS, demonstrating the involvement of higher mechanical tension in the cortical actin (shaded purple in right schematic) and more active trafficking of mitochondria. Thus VS activates different signaling pathways during fluctuation-driven mechanotransduction, which increases biogenesis, the complexity of the mitochondrial network, ATP production, general cellular activity, and cell contractility. Vertical red arrows represent an increase or a decrease in activity. Figure was reproduced and adapted from Ref. 7 with permission from Nature Materials.
FDM in Other Systems
Surfactant in the lung is secreted by type II epithelial cells and a key component of alveolar surface film stability. One of the strongest stimulants of surfactant production and secretion is a single large stretch (129). A period of 30-min cyclic stretch of ∼50% area strain applied to epithelial cells in culture showed first a decline in surfactant secretion after an additional incubation time of 30 min but resulted in increasing levels of secretion after further incubations for 60 and 210 min (23). Another study found an increase in secretion at 60 min when type II cells were stretched for 15 min but a decrease when these cells were stretched for 30 or 60 min (5). The delayed responses are consistent with the notion that stretch increases surfactant transport from the interior to the exterior through a surface layer with a limited number of secretory pores, which temporarily hinder secretion due to an interaction among the surfactant containing lamellar bodies, effectively creating a jam (68). When the stretch stops, the jam takes additional time to clear, implying that surfactant secretion is a fundamentally nonlinear process, with memory representing collective behavior at the level of a single cell. The jam maybe due to the organization of the cytoskeletal network around fusion pores since depolymerizing actin generally enhances surfactant secretion (94). Additionally, surfactant secretion is tightly regulated by membrane-bound target SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins called SNAP-23 and syntaxin-2 (1). Syntaxins in the plasma membrane also self-organize into clusters due to weak intermolecular attractive interactions with cluster sizes limited by crowding-induced repulsion (103). The available syntaxins in the membrane may group into a finite number of docking sites, allowing jamming to occur when secretion is stimulated. Interestingly, when 5% variability was added to stretch, surfactant secretion doubled (5), suggesting that VS reduces jamming. While the precise mechanism is not known, several possibilities arise. Stretch pattern can influence the cluster distribution of docking proteins to alter fusion pore-opening dynamics. The opening of calcium channels required for secretion (129) is a nonlinear stretch-dependent process (79). Since fluctuations can enhance signal transduction even in the simplest ion channel (8), VS might increase general calcium levels too. Finally, if ATP production is upregulated by VS in type II cells similarly to VSMCs (7), the excess ATP can depolymerize actin and act as a strong surfactant secretagauge. Elucidating the specific mechanisms will require more experiments.
ECs in the vasculature provide a selective barrier to circulating blood cells and macromolecules in the presence of shear stresses due to blood flow. Many studies have demonstrated that shear stress regulates various genes, which in turn control the production of vasoactive materials such as nitric oxide (NO), which relaxes VSMCs, and endothelin-1, which contracts VSMCs. While this knowledge comes from countless experiments using steady shear flow, only one study examined how physiological variability in shear flow based on observed in vivo flow pattern might influence vascular signaling (120). Both steady and variable shear flows upregulated superoxide dismutase that protects against oxidative stress; however, variable shear flow also upregulated NO synthase activity and NO as well as lowered TNF-α-induced leukocyte adhesion, implying more quiescent ECs. Little is known about the corresponding FDM mechanism. It is likely that the glycocalix, comprised of proteoglycans and glycoproteins on the surface of ECs, is a key player in mediating mechanotransduction (111). For example, varying shear stresses over long time scales may modulate glycocalix synthesis and degradation (36). The amount and clustering of glycocalix in turn modulate how instantaneous shear stress activates G-protein signaling, which is known to regulate the burst-like production and release of NO (60). Uncovering the exact mechanisms, however, awaits further studies.
Several studies also attempted to incorporate variability into tissue engineering approaches. Using a novel stretcher, Gelfoam samples were seeded with neonatal rat lung fibroblasts and uniaxially stretched with different levels of variability (0%, 25%, 50%, and 75%) superimposed on 20% mean strain (45). While the data showed that VS increased the mRNA expression of collagen-1α and lysyl oxidase, the mRNA expression of syndecan-4 was maximized at 25% variability. Further studies are required to assess any functional consequences of the altered expression profile. In another study using a novel bioreactor, VS (25% variability) was used in cardiac tissue engineering with a small but significant increase in twitch force compared with static stretch (76). There is only one study that tested the effects of varying the frequency alone (77). Cardiomyocyte constructs from neonatal rat in fibrin hydrogels were stimulated with constant, Gaussian, or uniform distribution of frequencies for 2 wk with 10% and 20% variations around the mean. The observed differences in protein expressions suggest that variable-frequency stimulation affected cell-cell coupling and growth pathway activation. Although these studies are descriptive, they present new opportunities to selectively tune the composition of tissue constructs.
Fluctuation-Driven Mechanotransduction in Diseases
While changes in stretch pattern are likely common to many diseases, here we only consider those that manifest in the respiratory and cardiovascular systems. Cycle-by-cycle variability in respiratory parameters has been measured in both healthy and diseased subjects (21, 114). Variability of tidal volume, as measured by the CV, was ∼26% in normal subjects, increased to 43% in obstructive lung disease, and decreased to 17% in restrictive lung disease (62). Considering that tidal volume is the driving signal for strain delivered to lung cells, mechanical stimuli can significantly be altered in diseases; however, the actual strains cells experience need to be experimentally determined since the local strain is further complicated by changes in local stiffness with lung disease. Separating variability into random and non-random components, healthy subjects under elastic loading decreased the random component of variability (12). Furthermore, variability in breathing pattern induced dyspnea in patients with restricted lung disease, indicating that such patients adopted a strategy of decreasing variability as a means of avoiding dyspnea (11). Regardless of whether breathing patterns are inherent to the disease or adopted to alleviate breathing problems, changes in variability will result in abnormal strains delivered to cells, which can trigger pathological signaling and contribute to the progression of the disease. This may affect remodeling of lung ECM by fibroblast in both fibrosis and emphysema, especially at the microscopic level where the regional heterogeneity of stretch is large. An extreme example of changing variability is taking it away altogether, which occurs in monotonous mechanical ventilation of patients. It has been reported that monotonous stretch during mechanical ventilation impairs surfactant metabolism and promotes cytokine release, whereas adding variability to tidal volume can be used as a potential treatment, which has shown positive results in induced lung injury models (9, 112, 113).
Blood pressure fluctuations arise in the cardiovascular system as a result of numerous feedback systems interacting in a noisy environment (35). These fluctuations are an intrinsic characteristic of the system in healthy subjects but are known to increase in hypertensive patients and animals (70, 107). Furthermore, blood pressure variability (BPV) occurs and has been characterized on different time scales ranging from beat-to-beat, to 24 h, to periodic doctor visits (86, 95). While mean blood pressure and in particular systolic blood pressure are considered primary diagnostic measurements for cardiovascular disease, higher BPV on the time scale of periodic doctor visits was identified as an important and independent predictor of stroke (95). Likewise, lower 24-h BPV correlated with lesser prevalence of target organ damage in patients with hypertension (86). Increases in BPV should certainly induce higher maximum strains acting on ECs as well as VSMCs, both of which are sensitive to variable mechanical stimuli (7, 120). While the general trend is that mean blood pressure and BPV go together, inducing increased BPV in rats via sinoaortic denervation results in aortic and left ventricular hypertrophy without an increase in mean blood pressure (73). This is quite important since cells in the vessel wall are likely able to accommodate an increase in static mean stretch associated with increased mean blood pressure but may react in unexpected ways to pathological increases in BPV. Increased BPV in humans is also associated with arterial wall remodeling and arterial wall stiffness (97), a strong and independent predictor of cardiovascular risk (74). Thus increased BPV is perhaps not just a symptom of hypertension but also an important mechanical signal involved in the progression of cardiovascular diseases.
The precise mechanism by which BPV and arterial stiffness interact and progress into diseased states remains to be elucidated. However, our recent findings that FDM governs VSMC signaling to maintain normal contractility coupled with observed high BPV in hypertensive patients offer a hypothesis for how these factors might play a role in hypertension. VSMC contractility is an important contributor to vascular stiffness (32). FIGURE 3 shows potential relationships between contractility and variability in strain, with red and green curves representing hypothetical diseased states and the black curve representing normal aortic function. We have measured the effects of strain variability in normotensive rat aorta at normal (black cross) and laboratory (blue cross) levels of variability (7) as well as in several samples from spontaneously hypertensive rats (red cross) exposed to normal variability (Imsirovic J, Suki B, unpublished data). The red arrow is the observed pathological state found in hypertension with increased contractility and strain variability. Thus increased variability in strain likely induces a more contractile VSMC phenotype. If experiments will find the red curve, then the increased BPV would represent a protective mechanism by which contractility is reduced compared with the peak of the red curve. In this case, BPV would have to be reduced close to the level of normal BPV to make sure that contractility is also lowered. Alternatively, if the green curve is found, then drugs that reduce BPV should also reduce mean blood pressure since contractility will decrease. Indeed, in hypertensive patients undergoing 3 mo of antihypertensive treatment, the reduction in BPV by amlodipine, a calcium channel blocker, was significantly associated with the reduction in mean blood pressure (135).
FIGURE 3.
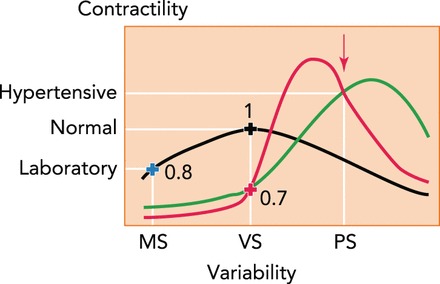
Possible relationships between FDM and VSMC contractility
MS (monotonous stretch) represents laboratory condition with no variability and low contractility. VS (variable stretch) is the homeostatic variability in normal arteries. PS (pathological stretch) is pathological variability in hypertensive patients with high contractility. The black curve represents the normal VSMC, and the red and green curves are two possible scenarios occurring in hypertension. The red arrow is based on actual observations in hypertensive subjects. The blue (n = 10) and black (n = 9) crosses and the numbers are contractility values normalized to VS from our measurements in Ref. 7. The red cross (n = 3) is our recent measurements (Imsirovic J, Suki B, unpublished observations).
Conclusions
Most cell types are highly sensitive to their physical environment, and physiological forces acting on them play a dominant role in many regulatory cell functions. Such mechanotransduction is invariably studied using monotonous mechanical stimuli; however, cells in the body are exposed to irregularly varying stimuli. Cells sense external mechanical forces via adhesion molecules and the cytoskeleton, and most if not all basic cell functions should also be sensitive to the fluctuations in mechanical stimuli. Evolutionary forces should favor structures that can adapt to and take advantage of existing fluctuations. This aspect of mechanotransduction has been overlooked in cell and tissue culture studies. The two main conclusions from this summary of known mechanisms involved in FDM are that 1) cells are capable of exploiting mechanical fluctuations to increase their ATP production rate and 2) FDM-induced stimulation of bioenergetics leads structural reorganization of the cytoskeleton and selective activation of signal transduction pathways.
Many challenges and open questions remain, however. At the most general level, we argued that FDM is a dynamic nonequilibrium state of the cell that never reaches a steady state. The thermodynamics of such driven systems is still under development. We also did not consider several important factors. Among others is the fact that fluctuations are often nonrandom because heart rate and breathing patterns show long-range correlations. It is not known whether correlations in fluctuating mechanical stimuli affect FDM. While strain rate also fluctuates and bond dissociations strongly depend on strain rate, their influence on conventional mechanotransduction and FDM is less well understood. At the level of many cells, FDM probably alters cell-cell interactions. The specific effects of FDM on single cells are likely cell-type dependent. However, the general organization and dynamics of the cytoskeleton, an active network under nonequilibrium conditions, may respond to FDM similarly in all cell types, which should be further studied. Nothing is known about the nuclear responses to FDM, and these also need to be elucidated. At the molecular level, the specific focal adhesion and other proteins that undergo conformational changes have not been studied. If the increase in the availability of ATP during FDM as seen in VSMCs is also confirmed in other cells and organs, FDM may turn out to be a very general mechanism, suggesting that hierarchical active networks may be capable of harnessing energy from fluctuations in their environment. This latter issue may also have evolutionary implications. Finally, we briefly addressed the possibility that alterations in the fluctuations coming from one organ and affecting FDM in other organs may contribute to disease pathogenesis and progression. The specific direction and magnitude of the changes in fluctuations will need to be experimentally mapped for different organs, organisms, and diseases. In summary, uncovering how cells deal with physiological variabilities may help us understand how cells work in real living tissues, with possible impact for several major diseases including atherosclerosis, neuro-degenerative diseases, metabolic disorders, aging, or cancer.
Footnotes
This study was supported by National Heart, Lung, and Blood Institute Grants HL-098976, HL-111745, and HL-122513.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: B.S., H.P., J.I., and E.B.-S. conception and design of research; B.S. and E.B.-S. prepared figures; B.S., H.P., J.I., and E.B.-S. drafted manuscript; B.S. approved final version of manuscript; H.P., J.I., and E.B.-S. analyzed data.
References
- 1.Abonyo BO, Gou D, Wang P, Narasaraju T, Wang Z, Liu L. Syntaxin 2 and SNAP-23 are required for regulated surfactant secretion. Biochemistry 43: 3499–3506, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE 2002: pe6, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 50: 197–263, 1998. [PubMed] [Google Scholar]
- 5.Arold SP, Bartolak-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 296: L574–L581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backouche F, Haviv L, Groswasser D, Bernheim-Groswasser A. Active gels: dynamics of patterning and self-organization. Phys Biol 3: 264–273, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bartolak-Suki E, Imsirovic J, Parameswaran H, Wellman TJ, Martinez N, Allen PG, Frey U, Suki B. Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nat Mater 14: 1049–1057, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Bezrukov SM, Vodyanoy I. Noise-induced enhancement of signal transduction across voltage-dependent ion channels. Nature 378: 362–364, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Boker A, Graham MR, Walley KR, McManus BM, Girling LG, Walker E, Lefevre GR, Mutch WA. Improved arterial oxygenation with biologically variable or fractal ventilation using low tidal volumes in a porcine model of acute respiratory distress syndrome. Am J Respir Crit Care Med 165: 456–462, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Borau C, Kim T, Bidone T, Garcia-Aznar JM, Kamm RD. Dynamic mechanisms of cell rigidity sensing: insights from a computational model of actomyosin networks. PLos One 7: e49174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brack T, Jubran A, Tobin MJ. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am J Respir Crit Care Med 165: 1260–1264, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Brack T, Jubran A, Tobin MJ. Effect of elastic loading on variational activity of breathing. Am J Respir Crit Care Med 155: 1341–1348, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol 14: 546–556, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res 70: 422–433, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4: 118–132, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S, Fisher AB. Mechanotransduction: forces, sensors, and redox signaling. Antioxid Redox Signal 20: 868–871, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT, van Haperen R, Wentzel JJ, Gijsen F, van der Steen AF, de Crom R, Krams R. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195: 225–235, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol 18: 417–426, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol 142: 573–586, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol 8: 56–65, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Dellaca RL, Aliverti A, Lo Mauro A, Lutchen KR, Pedotti A, Suki B. Correlated variability in the breathing pattern and end-expiratory lung volumes in conscious humans. PLos One 10: e0116317, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Physiological cyclic stretch directs L-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. FASEB J 14: 1775–1783, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Edwards YS, Sutherland LM, Power JH, Nicholas TE, Murray AW. Cyclic stretch induces both apoptosis and secretion in rat alveolar type II cells. FEBS Lett 448: 127–130, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Ehrenberg B, Montana V, Wei MD, Wuskell JP, Loew LM. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J 53: 785–794, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldib M, Dean DA. Cyclic stretch of alveolar epithelial cells alters cytoskeletal micromechanics. Biotechnol Bioeng 108: 446–453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Entcheva E, Bien H. Mechanical and spatial determinants of cytoskeletal geodesic dome formation in cardiac fibroblasts. Integr Biol (Camb) 1: 212–219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etienne J, Fouchard J, Mitrossilis D, Bufi N, Durand-Smet P, Asnacios A. Cells as liquid motors: mechanosensitivity emerges from collective dynamics of actomyosin cortex. Proc Natl Acad Sci USA 112: 2740–2745, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science 316: 1148–1153, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Fisher AB, Al-Mehdi AB, Manevich Y. Shear stress and endothelial cell activation. Crit Care Med 30: S192–197, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Gao YZ, Saphirstein RJ, Yamin R, Suki B, Morgan KG. Aging impairs smooth muscle-mediated regulation of aortic stiffness: a defect in shock absorption function? Am J Physiol Heart Circ Physiol 307: H1252–H1261, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10: 21–33, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem 104: 1964–1987, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Glass L. Synchronization and rhythmic processes in physiology. Nature 410: 277–284, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med 259: 393–400, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2: a002915, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 14: 2508–2519, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475: 316–323, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu S, Chen J, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochem Biophys Res Commun 329: 423–428, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 88: 521–530, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Imsirovic J, Derricks K, Buczek-Thomas JA, Rich CB, Nugent MA, Suki B. A novel device to stretch multiple tissue samples with variable patterns: application for mRNA regulation in tissue-engineered constructs. Biomatter 3: e24650, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingber DE. The architecture of life. Sci Am 278: 48–57, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J 20: 811–827, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575–599, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun 328: 751–755, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng 12: 369–400, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Jelinek HF, Imam HM, Al-Aubaidy H, Khandoker AH. Association of cardiovascular risk using non-linear heart rate variability measures with the framingham risk score in a rural population. Front Physiol 4: 186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42: 283–323, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Kadenbach B, Ramzan R, Wen L, Vogt S. New extension of the Mitchell Theory for oxidative phosphorylation in mitochondria of living organisms. Biochim Biophys Acta 1800: 205–212, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Karau KL, Krenz GS, Dawson CA. Branching exponent heterogeneity and wall shear stress distribution in vascular trees. Am J Physiol Heart Circ Physiol 280: H1256–H1263, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Kim T, Hwang W, Lee H, Kamm RD. Computational analysis of viscoelastic properties of crosslinked actin networks. PLoS Comput Biol 5: e1000439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol 137: 481–492, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA 104: 9994–9999, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnan R, Park CY, Lin YC, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, Butler JP, Fredberg JJ. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLos One 4: e5486, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kruse K, Joanny JF, Julicher F, Prost J, Sekimoto K. Asters, vortices, and rotating spirals in active gels of polar filaments. Phys Rev Lett 92: 078101, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol Cell Physiol 267: C753–C758, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Kuo JC, Han X, Hsiao CT, Yates JR 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13: 383–393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuratomi Y, Okazaki N, Ishihara T, Arai T, Kira S. Variability of breath-by-breath tidal volume and its characteristics in normal and diseased subjects. Ventilatory monitoring with electrical impedance pneumography. Japan J Med 24: 141–149, 1985. [DOI] [PubMed] [Google Scholar]
- 63.LaPrad AS, Lutchen KR, Suki B. A mechanical design principle for tissue structure and function in the airway tree. PLoS Comput Biol 9: e1003083, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SE, Kamm RD, Mofrad MR. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J Biomech 40: 2096–2106, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Liu H, Yambe T, Sasada H, Nanka S, Tanaka A, Nagatomi R, Nitta S. Comparison of heart rate variability and stroke volume variability. Auton Neurosci 116: 69–75, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Loftus JC, Liddington RC. New insights into integrin-ligand interaction. J Clin Invest 100: 77–81, 1997. [PubMed] [Google Scholar]
- 67.Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, Joos KM, Iomini C, Obukhov AG, Sun Y. Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci USA 111: 12871–12876, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majumdar A, Arold SP, Bartolak-Suki E, Parameswaran H, Suki B. Jamming dynamics of stretch-induced surfactant release by alveolar type II cells. J Appl Physiol 112: 824–831, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mammoto T, Mammoto A, Ingber DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol 29: 27–61, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A, Investigators ELSA . Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 19: 1981–1989, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Marszalek PE, Lu H, Li H, Carrion-Vazquez M, Oberhauser AF, Schulten K, Fernandez JM. Mechanical unfolding intermediates in titin modules. Nature 402: 100–103, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol 91: 693–702, 2001. [DOI] [PubMed] [Google Scholar]
- 73.Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 20: 1865–1872, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 115: 2628–2636, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Mitrossilis D, Fouchard J, Guiroy A, Desprat N, Rodriguez N, Fabry B, Asnacios A. Single-cell response to stiffness exhibits muscle-like behavior. Proc Natl Acad Sci USA 106: 18243–18248, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan KY, Black DL. Creation of a bioreactor for the application of variable amplitude mechanical stimulation of fibrin gel-based engineered cardiac tissue. In: Cardiac Tissue Engineering: Methods and Protocols, edited by Radisic M, Black IIILD. New York: Springer Science + Business Media, 2014, p. 177–187. [DOI] [PubMed] [Google Scholar]
- 77.Morgan KY, Black LD 3rd.. Investigation into the effects of varying frequency of mechanical stimulation in a cycle-by-cycle manner on engineered cardiac construct function. J Tissue Eng Regen Med. In press. [DOI] [PubMed] [Google Scholar]
- 78.Morishima-Kawashima M, Kosik KS. The pool of map kinase associated with microtubules is small but constitutively active. Mol Biol Cell 7: 893–905, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naruse K, Sokabe M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol Cell Physiol 264: C1037–C1044, 1993. [DOI] [PubMed] [Google Scholar]
- 80.Ng MR, Besser A, Brugge JS, Danuser G. Mapping the dynamics of force transduction at cell-cell junctions of epithelial clusters. Elife 3: e03282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okada M, Matsumori A, Ono K, Furukawa Y, Shioi T, Iwasaki A, Matsushima K, Sasayama S. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol 18: 894–901, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell 10: 11–20, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res 32: 275–292, 1995. [DOI] [PubMed] [Google Scholar]
- 84.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta 1833: 1256–1268, 2013. [DOI] [PubMed] [Google Scholar]
- 85.Parameswaran H, Lutchen KR, Suki B. A computational model of the response of adherent cells to stretch and changes in substrate stiffness. J Appl Physiol 116: 825–834, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 5: 93–98, 1987. [DOI] [PubMed] [Google Scholar]
- 87.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng CK, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Phys Rev Lett 70: 1343–1346, 1993. [DOI] [PubMed] [Google Scholar]
- 89.Praetorius HA, Frokiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288: F133–F141, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Prigogine I. Time, structure, fluctuations. Science 201: 777–785, 1978. [DOI] [PubMed] [Google Scholar]
- 91.Reneman RS, Hoeks AP. Wall shear stress as measured in vivo: consequences for the design of the arterial system. Med Biol Eng Comput 46: 499–507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Lung Cell Mol Physiol 301: L625–L635, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romer LH, Birukov KG, Garcia JG. Focal adhesions: paradigm for a signaling nexus. Circ Res 98: 606–616, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Rose F, Kurth-Landwehr C, Sibelius U, Reuner KH, Aktories K, Seeger W, Grimminger F. Role of actin depolymerization in the surfactant secretory response of alveolar epithelial type II cells. Am J Respir Crit Care Med 159: 206–212, 1999. [DOI] [PubMed] [Google Scholar]
- 95.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375: 895–905, 2010. [DOI] [PubMed] [Google Scholar]
- 96.Ruoslahti E. Integrins. J Clin Invest 87: 1–5, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 60: 369–377, 2012. [DOI] [PubMed] [Google Scholar]
- 98.Schiller HB, Friedel CC, Boulegue C, Fassler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep 12: 259–266, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwartz MA. Integrin signaling revisited. Trends Cell Biol 11: 466–470, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol 20: 551–556, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shao YY, Wang L, Welter JF, Ballock RT. Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50: 79–84, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shemesh T, Geiger B, Bershadsky AD, Kozlov MM. Focal adhesions as mechanosensors: a physical mechanism. Proc Natl Acad Sci USA 102: 12383–12388, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, Lang T. Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317: 1072–1076, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Smyth SS, Joneckis CC, Parise LV. Regulation of vascular integrins. Blood 81: 2827–2843, 1993. [PubMed] [Google Scholar]
- 105.Soares E, Silva M, Depken M, Stuhrmann B, Korsten M, MacKintosh FC, Koenderink GH. Active multistage coarsening of actin networks driven by myosin motors. Proc Natl Acad Sci USA 108: 9408–9413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stamenovic D, Rosenblatt N, Montoya-Zavala M, Matthews BD, Hu S, Suki B, Wang N, Ingber DE. Rheological behavior of living cells is timescale-dependent. Biophys J 93: 39–41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su DF, Miao CY. Blood pressure variability and organ damage. Clin Exp Pharmacol Physiol 28: 709–715, 2001. [DOI] [PubMed] [Google Scholar]
- 108.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol 98: 1892–1899, 2005. [DOI] [PubMed] [Google Scholar]
- 109.Swailes NT, Colegrave M, Knight PJ, Peckham M. Non-muscle myosins 2A and 2B drive changes in cell morphology that occur as myoblasts align and fuse. J Cell Sci 119: 3561–3570, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7: 619–627, 1995. [DOI] [PubMed] [Google Scholar]
- 111.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng 16: 505–532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thammanomai A, Hamakawa H, Bartolak-Suki E, Suki B. Combined effects of ventilation mode and positive end-expiratory pressure on mechanics, gas exchange and the epithelium in mice with acute lung injury. PLos One 8: e53934, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thammanomai A, Hueser LE, Majumdar A, Bartolak-Suki E, Suki B. Design of a new variable-ventilation method optimized for lung recruitment in mice. J Appl Physiol 104: 1329–1340, 2008. [DOI] [PubMed] [Google Scholar]
- 114.Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA. Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol 65: 309–317, 1988. [DOI] [PubMed] [Google Scholar]
- 115.Tock Y, Ljubisavljevic M, Thunberg J, Windhorst U, Inbar GF, Johansson H. Information-theoretic analysis of de-efferented single muscle spindles. Biol Cybern 87: 241–248, 2002. [DOI] [PubMed] [Google Scholar]
- 116.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature 447: 592–595, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trichet L, Le Digabel J, Hawkins RJ, Vedula SR, Gupta M, Ribrault C, Hersen P, Voituriez R, Ladoux B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci USA 109: 6933–6938, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tschumperlin DJ. Mechanotransduction. Compr Physiol 1: 1057–1073, 2011. [DOI] [PubMed] [Google Scholar]
- 119.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 86: 2026–2033, 1999. [DOI] [PubMed] [Google Scholar]
- 120.Uzarski JS, Scott EW, McFetridge PS. Adaptation of endothelial cells to physiologically-modeled, variable shear stress. PLos One 8: e57004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 21: 211–220, 1999. [DOI] [PubMed] [Google Scholar]
- 122.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct 35: 459–488, 2006. [DOI] [PubMed] [Google Scholar]
- 123.Wang BW, Chang H, Lin S, Kuan P, Shyu KG. Induction of matrix metalloproteinases-14 and -2 by cyclical mechanical stretch is mediated by tumor necrosis factor-alpha in cultured human umbilical vein endothelial cells. Cardiovasc Res 59: 460–469, 2003. [DOI] [PubMed] [Google Scholar]
- 124.Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci USA 98: 7765–7770, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang S, Wolynes PG. Active contractility in actomyosin networks. Proc Natl Acad Sci USA 109: 6446–6451, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waters CM, Sporn PH, Liu M, Fredberg JJ. Cellular biomechanics in the lung. Am J Physiol Lung Cell Mol Physiol 283: L503–L509, 2002. [DOI] [PubMed] [Google Scholar]
- 127.Weber GF, Bjerke MA, DeSimone DW. Integrins and cadherins join forces to form adhesive networks. J Cell Sci 124: 1183–1193, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science 276: 122–126, 1997. [DOI] [PubMed] [Google Scholar]
- 129.Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990. [DOI] [PubMed] [Google Scholar]
- 130.Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol 160: 11–16, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yasuda T, Kondo S, Homma T, Harris RC. Regulation of extracellular matrix by mechanical stress in rat glomerular mesangial cells. J Clin Invest 98: 1991–2000, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. Alpha-catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 12: 533–542, 2010. [DOI] [PubMed] [Google Scholar]
- 133.Yoshimura K, Sokabe M. Mechanosensitivity of ion channels based on protein-lipid interactions. J R Soc Interface 7, Suppl 3: S307–S320, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12: 9–18, 2002. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X-CELLENT) study. Hypertension 58: 155–160, 2011. [DOI] [PubMed] [Google Scholar]


