Abstract
Proanthocyanidins (PACs) are plant-derived, multifunctional compounds that possess high interactivity with extracellular matrix (ECM) components. The documented affinity of PACs for type-I collagen is directly correlated with their structural features and degree of polymerization. In this investigation, centrifugal partition chromatography (CPC) was used to sequentially deplete less active monomeric and polymeric PACs from a crude Pinus massoniana bark extract to create refined mixtures enriched in oligomeric PACs. The ability of these oligomeric PACs to modify the mechanical properties of the dentin collagen matrix and their biocompatibility with dental pulp cells (DPCs) was evaluated in an innovative biomimetic environment. The refined mixtures displayed high interactivity with dentin collagen as demonstrated by a significant increase (> 5-fold) in the modulus of elasticity of the dentin matrix. In a simplified model of the dentin-DPC complex, DPCs embedded within their native ECM in the presence of PAC-treated dentin exhibited increased proliferation. Quantitative gene expression analyses indicated that exposure to PAC-treated dentin increased the expression of key biomineralization and odontogenic differentiation regulators, including RUNX2, BMP2, OCN, and DSPP. LC-MS/MS analysis revealed that PACs two to four units long (dimers, trimers, and tetramers) were being released from dentin into media, influencing cell behavior. Overall, the results suggested that PAC dimers, trimers, and tetramers are not only biocompatible, but enhance the differentiation of DPCs towards a phenotype that favors biomineralization. PAC-enriched refined mixtures can influence the field of biomaterials and regeneration by serving as renewable, non-cytotoxic agents that can increase the mechanical properties of biomaterials.
Keywords: ECM, dentin, collagen cross-linking, odontoblast differentiation, DPCs, proanthocyanidins
Graphical Abstract
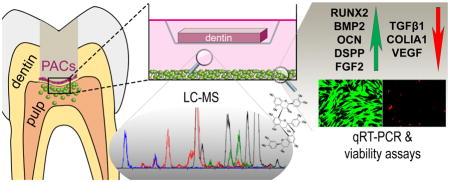
Pine bark extract is a renewable source of structurally diverse proanthocyanidins (PACs), multifunctional compounds whose interaction with collagen can be tailored to specific purposes by enrichment of selected PACs from the complex mixture. Oligomeric PACs were enriched from the extract and were shown here to sustain desired tissue modification and were thus assessed for cellular response in a model of the dentin-pulp interface. This model was developed to mimic leaching of potentially reactive compounds into pulp tissue. Dental pulp cells exposed to PAC-treated dentin showed increased proliferation and expression of genes necessary for extracellular matrix deposition and biomineralization, processes crucial for forming new dentin. Thus, collagen-interactive PACs may also enhance tissue regeneration and have broad impact in tissue engineering.
1. Introduction
The mechanical properties of collagen-based tissues are largely due to post-translational modification induced by enzymatic cross-links in the C- and N-terminal telopeptides of type-I collagen [1]. The native strength of type-I collagen can be enhanced by use of natural or synthetic chemicals participating in non-enzymatic cross-links [2–4]. Plant-derived proanthocyanidins (PACs) are particularly active collagen cross-linkers [5] with potential effects across many other biological systems, as has been reviewed [6–8].
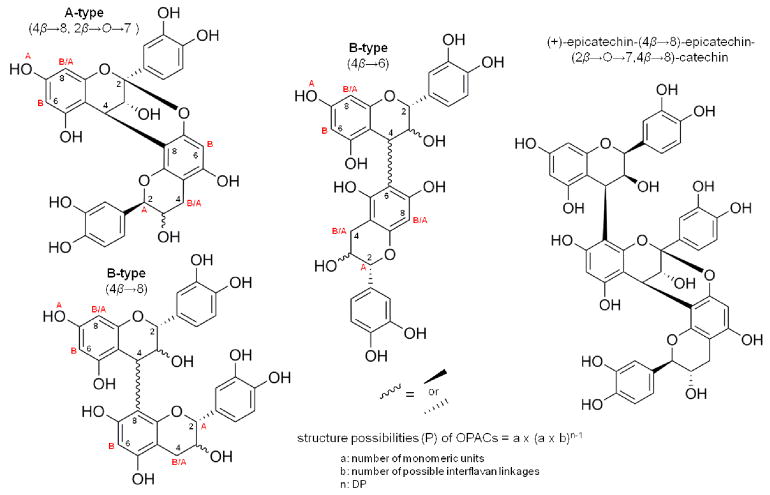
Pinus massoniana bark extract is a rich source of structurally unique PACs [9] that have been shown to mechanically reinforce the dentin matrix and reduce biodegradation rates [10]. These features are of clinical relevance when repairing lost dental tissue substance. PACs comprise a complex group of structurally related but highly diverse compounds composed of condensed flavan-3-ol monomer units. PACs can differ in linkage type, constituent monomers, and degree of polymerization (DP) (Fig. 1), thus just one plant can produce thousands of unique, but structurally related, PACs. In P. massoniana bark, four different monomeric units ((+)-catechin, (−)-catechin, (+)-epicatechin, (−)-epicatechin) have been observed, with seven possible linkage types, leading to over 3,000 theoretical PAC structures for trimers (DP = 3) alone. These structural parameters are critical as they determine PAC binding to type-I collagen in a dentin model [9,11] and explain the specific collagen targeting abilities of certain PAC subtypes.
Figure 1.
Interflavan linkages in oligomeric PACs from Pinus massoniana bark. Monomer units (+/− catechin and +/− epicatechin) are linked to sequential monomer units by an A-type (4β→8 and 2β→O→7) or B-type (4β →8 or 4β→6) bond. In pine bark, all OPACs have at least one A-type connection, with multiple A- or B-type bonds added. A represents bond site for additional A-type linkage, B represents bond site for additional B-type linkage. (+)-epicatechin-(4β→8)-epicatechin-(2β→O→7,4β→8)-catechin found in pine bark is a trimer with an A- and B-type interflavan linkage.
Oligomeric PACs, which are by definition between two to nine condensed monomer units in size (DP = 2–9), interact most effectively with collagen-rich tissue and induce greater mechanical enhancement than monomers [9,12]. Pinus massoniana bark was evaluated in this report because it contains the single most potent dentin modifying compound purified to date, (+)-epicatechin-(4β→8)-epicatechin-(2β→O→7,4β→8)-catechin (Fig. 1) [9].
Aimed at standardizing complex mixtures and optimizing the bioactivity of PACs, a DESIGNER (Deplete and Enrich Select Ingredients to Generate Normalized Extract Resources) concept [13] was employed here for the enrichment of selected PAC subtypes. The approach utilizes phytochemical separation techniques, including centrifugal partition chromatography (CPC), to enrich highly bioactive oligomeric PACs from a crude extract of Pinus massoniana bark. This ultimately leads to a standardized intervention biomaterial of chemically-controlled PAC composition.
Biomaterials can trigger local host tissue responses [14], which must be evaluated to ensure safety and determine potential side effects. A dental model provides a unique micro-environment to determine localized tissue responses as biomaterials introduced in this environment generally elicit cell response within the biological barriers of the tooth [15]. Therefore, a simplified model of the dentin-pulp complex was developed where dental pulp cells (DPCs) growing within their native extracellular matrix (ECM) were co-cultured with dentin that has been treated with refined PAC mixtures. DPCs are a population of cells within pulp tissue that secrete collagen and induce biomineralization, and, thus, have potential in regenerative therapies. The aim of the present study was to determine the localized responses of DPCs towards oligomeric PACs and evaluate their safety and potential to enhance tissue regeneration.
2. Materials and Methods
2.1 Production of PAC-enriched refined mixtures
Powdered Pinus massoniana Lamb. inner bark extract was purchased from Xi’an Chukang Biotechnology Co. Ltd. (Shaanxi, China), Lot No. PB120212, and validated for identity using normal phase HPLC-PDA analysis. The crude extract was suspended in water and subjected to liquid-liquid partitioning with ethyl acetate (EtOAc) to deplete polymers. The upper phase (EtOAc layer) was separated and dried, and is designated here as the polymer knockout (pKO) refined mixture. The pKO was then separated using centrifugal partition chromatography (Armen CPC-E) with hexanes/EtOAc/methyl acetate/water (1:8:2:8) in ascending mode. This allowed for the depletion of monomers from pKO, affording a monomer/polymer knockout refined mixture, designated mpKO.
For direct treatment experiments, pKO and mpKO were dissolved in DPC media to the desired concentrations (10, 1.0, 0.1 ug/mL). For experiments using the dentin-pulp complex model, pKO and mpKO were dissolved in HEPES buffer (0.02M, pH = 7.4) to a concentration of 15% w/v.
2.2 HPLC-PDA analysis of extracts
Normal phase HPLC profiles were acquired using a Develosil Diol column (5 μm, 100 Å, 250 × 4.6 mm) interfaced to a Waters 600 HPLC with a 2996 PDA detector. Samples were prepared to 20 mg/mL in 98:2 methanol-acetic acid. Acetonitrile-acetic acid (98:2) was solvent A, and methanol-water-acetic acid (95:3:2) was solvent B. Separation was carried out according to a previously reported method [16] and PDA chromatograms were extracted at 280 nm for analysis.
2.3 Mechanical testing of biological tissue
A well-established dental model to determine PAC interactivity with type-I collagen was employed using extracted sound human molars following approval by the Institutional Review Board Committee of the University of Illinois at Chicago (protocol No. 2011-0312). Dentin beams (1.7 × 0.5 × 6.0 mm) were prepared as previously described [17]. The apparent modulus of elasticity was evaluated using dentin beams decalcified in 10% phosphoric acid for five hours. Specimens were tested using a customized three-point bending device (3 mm span) coupled to an EZ Graph test machine (Shimadzu, Kyoto, Japan), with a maximum deformation of 3%. The apparent modulus of elasticity was obtained before (baseline) and 24 hrs after 60 min treatment with pKO or mpKO prepared to 0.65% w/v in HEPES buffer (pH 7.0–7.2). Vehicle control consisted of 60 min treatment with HEPES buffer only. Statistical analysis was performed using two-way ANOVA and Tukey’s multiple comparisons tests.
2.4 Dental pulp cell culture
Human dental pulp-derived mesenchymal stem cells were kindly provided by Dr. Songtao Shi, (University of Pennsylvania). These cells have been previously characterized for multi-lineage differentiation and self-renewal capacity [18,19] and we have previously worked with them in regenerative studies [20]. Cells were cultured in αMEM (GIBCO) supplemented with 20% FBS, 1% L-glutamine and 1% antibiotic/antimycotic solution. DPCs were seeded onto ECM-coated or uncoated plates (15,000 cells/well for 24-well plates; 5,000 cell/well for 96-well plates). Treatment began 24 hrs after seeding. Direct treatments were conducted on 96-well plates and the dentin-pulp complex experiments were conducted on 24-well plates.
2.5 Generation of extracellular matrix
24- and 96-well plates were coated with ECM from DPCs using previously published protocols [21]. Briefly, after DPCs (100,000 cells/well for 24-well plates, 50,000 cells/well for 96-well plates) were cultured for three days, cells were lysed, followed by DNA digestion, resulting in an ECM-coated tissue culture plate.
2.6 Dentin-DPC co-culture model
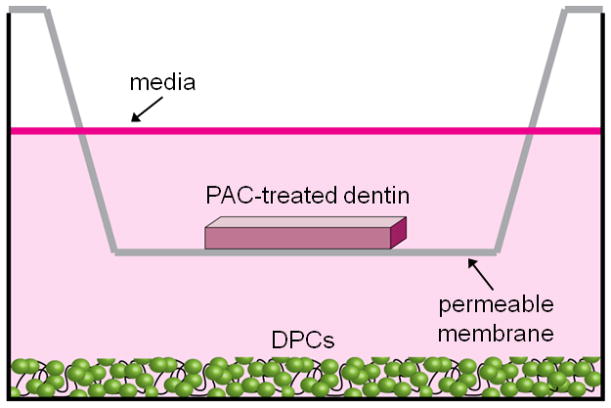
Dentin beams were superficially etched, using 32% H3PO4 (3M ESPE, Scotchbond Universal Etchant) for 15 s on each side, rinsed thoroughly and placed in 80 μL of pKO or mpKO (15% w/v), or HEPES buffer only (vehicle control). The known cytotoxic cross-linker, glutaraldehyde [22], was used as a negative control at 15% w/v). After 1 min of treatment beams were removed and rinsed ten times in distilled, sterile H2O. Treated dentin specimens wre immediately placed on a permeable polycarbonate membrane insert (Corning, 6.5 mm diameter, 8.0 μm pore size) above DPCs cultured on ECM-coated or non-coated 24 well plates and covered with 800 μL DPC media, as below (Fig. 2). This comprised the dentin-DPC co-culture model.
Figure 2.
Dentin-DPC co-culture model. DPCs were cultured on the bottom of an ECM-coated 24-well plate. Dentin was treated with PAC-enriched refined mixtures and placed above DPCs on a permeable membrane. Media (800 uL) was added to submerge dentin and DPCs.
2.7 Cell proliferation assay
Cell proliferation was determined with the MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega), using protocol guidelines specified by the manufacturer. Absorbance was measured using a Synergy 2 plate reader (Biotek) at 490 nm two hours after addition of MTS reagent. Experiments were conducted one, three, and five days after treatment. Statistical analysis was performed using a two-way ANOVA followed by Tukey’s multiple comparisons tests.
2.8 Live/dead staining
A cell viability imaging assay (Invitrogen LIVE/DEAD assay) was used to differentially stain live cells with calcein-AM and dead cells with ethidium homodimer, using protocol guidelines specified by the manufacturer. Cells were imaged using a fluorescent cell imager with 20× magnification (ZOE, Bio-Rad).
2.9 RNA extraction and qRT-PCR
RNA from quadruplicate culture experiments was isolated using the Aurum Total RNA extraction kit (Bio-Rad) per manufacturer’s protocol. cDNA synthesis was performed using Maxima First Strand kit (Thermo) per manufacturer’s protocol. Quantitative RT-PCR was performed for selected genes (Table S1) in quadruplicate as per published protocols [23]. Gene expression was normalized to GAPDH and β2 macroglobulin (B2M) (housekeeping genes) and the δδCt method was used to obtain fold change with respect to controls. Amplification and quantification was conducted using a CFX96 Touch Real-Time detection system (BioRad). Statistical analysis was performed using a one-way ANOVA followed by Tukey’s multiple comparisons tests.
2.10 LC-MS/MS Analysis of PACs in the co-culture model
A 200 μL aliquot of media was collected from three wells of each treatment group in the co-culture model. Ethyl acetate (600 μL) was added to the media, followed by vortex shaking. After settling, the upper phase (500 μL) was drawn off. Ethyl acetate extraction of each media aliquot was repeated for a total of three times before evaporation to dryness in a Speedvac (miVac QUATTRO, Genevac). Dried extracts from each of three replicate treatment wells were resuspended in MeOH, pooled, and dried again. The pooled, dried samples were weighed and resuspended to a 1 mg/mL solution with MeOH.
LCMS was performed on a Shimadzu Prominence HPLC system equipped with a Waters Xterra 2.1 × 150 mm reversed-phase column coupled to an AB Sciex 4000 QTRAP system with Parker Source 5000 LCMS gas generator. The mobile phase consisted of solvent A: 0.1% formic acid in water, and solvent B: 0.1% formic acid in acetonitrile. Solvent B in the mobile phase (expressed as % v/v) was linearly increased from 10% to 37% over the first 19 minutes, followed by a second linear increase from 37% to 95% from 19 to 20 minutes. This mobile phase composition was held constant for 5 minutes, and then returned to initial conditions in 1 minute. The mobile phase was held at initial conditions for 7 minutes to ensure equilibration. Samples were analyzed with an injection volume of 10 μL and a constant flow rate of 200 μL/min.
MS experiments were carried out in multiple reaction monitoring (MRM) scan mode, selecting for precursor ions in the first quadrupole (Q1), and product ions were generated by collision-induced dissociation (CID) in the linear accelerator collision cell (second quadrupole, Q2). The product ions were filtered, trapped, and scanned in the third quadrupole (Q3), which operated as a linear ion trap. Declustering potential, entrance potential and collision cell exit potential were −115 V, −10 V and −16 V, respectively, for all compounds. The collision energy was set to −20 V, −30 V, −40 V and −50 V for monomers, dimers, trimers, and tetramers, respectively. These parameters, as well as product ions, were determined by syringe infusion experiments using pure isolated compounds. Collision gas was set to medium, curtain gas was 20 psi, nebulizer and turbo gases were 40 psi. Ion spray voltage was −4000 V, and source temperature was 450 °C. The instrument was operated in negative mode.
Statistical analysis was performed by t-test, followed by Bonferroni-Dunn correction for multiple comparisons.
3. Results
3.1 HPLC analysis of crude pine extract and refined PAC mixtures
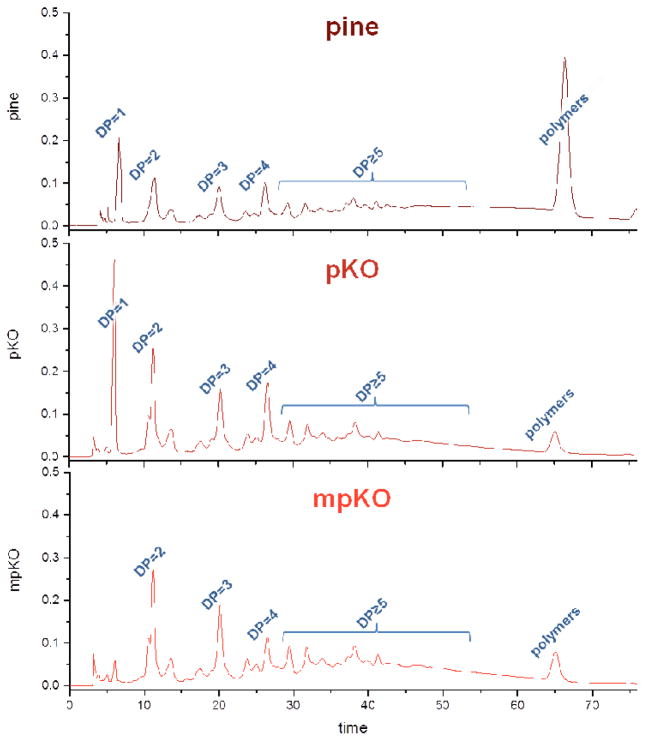
HPLC-UV analysis was conducted to validate the polymer and monomer knockout procedures. Annotated chromatograms of crude pine extract and refined mixtures are shown in Figure 3. In comparison to the crude pine extract, the subsequent liquid-liquid chromatography steps first reduced the concentration of polymers (pKO), making a PAC-enriched refined mixture, and then monomers (mpKO), making an oligomeric PAC-enriched refined mixture.
Figure 3.
HPLC-UV chromatograms (280 nm) of pKO and mpKO refined mixtures. DP = 1 (monomers), 2 (dimers), 3 (trimers), 4 (tetramers), ≥ 5 (pentamers and above). DP for polymers > 9.
3.2 Bulk dentin matrix modulus of elasticity
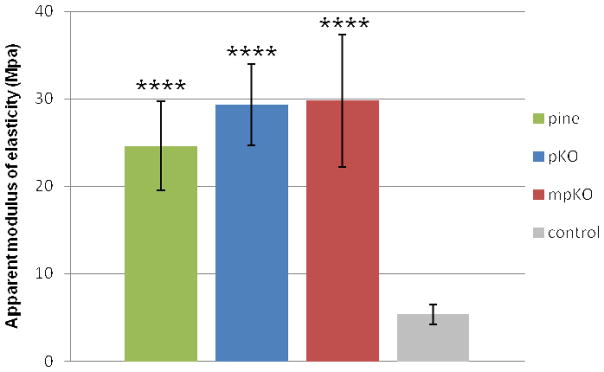
To evaluate cross-linking activity of the refined PAC mixtures, a modulus of elasticity assay was performed on treated dentin matrices. A statistically significant higher modulus of elasticity was observed for dentin treated with pine, pKO, and mpKO (p<0.0001) compared to the untreated control group (Fig. 4). Dentin matrices treated with the refined mixtures were statistically similar to the crude pine extract, thus, the DESIGNER strategy effectively refined the extracts, enriching oligomeric PACs, without apparent loss of tissue interactivity.
Figure 4.
Modulus of elasticity of dentin treated with pine crude extract (pine), pKO or mpKO refined mixtures. Assay performed 24 hrs after a 60 min treatment with indicated extract. Negative control group treated with vehicle only. ****, p<0.0001 compared to control by Tukey’s test after one-way ANOVA.
3.3 Tissue culture substrate selection
A simplified proliferation assay was first performed with DPCs seeded on uncoated and ECM-coated plates, generating results comparable to conventional, commonly reported toxicity assays. The use of ECM-coated plates allowed the determination of influence of native ECM on cellular behavior.
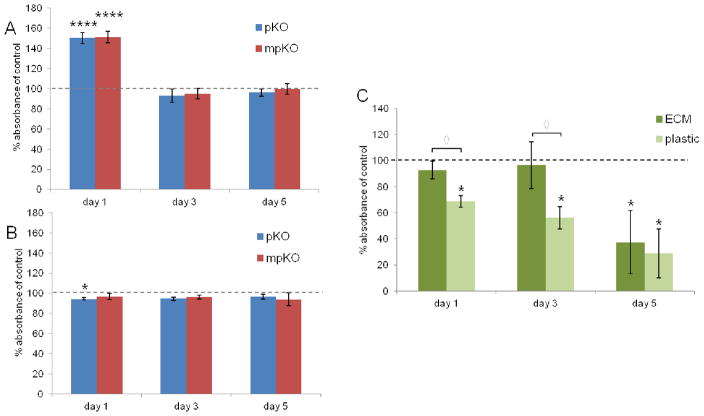
DPCs on uncoated culture plates showed an initial spike in proliferation in response to pKO and mpKO refined mixtures (Fig. 5A), and after day three and five, cell numbers were similar to control. DPCs seeded onto ECM-coated plates (Fig. 5B) did not show an increase in proliferation over control at any time point. In fact, pKO-treated cells had slightly reduced proliferation after one day of treatment, which recovered to control levels by day three. Live/dead staining (Fig. 6) shows that DPCs on ECM-coated plates exhibited healthy morphology and a negligible amount of cell death after treatment with refined extracts. The increase in proliferation was only observed when DPCs were seeded on uncoated plates, thus, cells on an uncoated plastic substrate were more sensitive to the proliferative effects of refined treatments than those on ECM-coated plates.
Figure 5.
Proliferation of DPCs after exposure to biomaterials. (A) Direct treatment of DPCs seeded on uncoated culture plates with 10 ug/mL refined mixtures. ****, p<0.0001 compared to control by Tukey’s test. (B) Direct treatment of DPCs seeded on ECM-coated culture plates with 10 ug/mL refined mixtures. *, p<0.05 compared to control by Tukey’s test. (C) DPCs cultured on ECM-coated and uncoated culture plates and exposed to glutaraldehyde-treated (15% w/v) dentin in the dentin-DPC model. Results reported as fold change normalized to untreated control for each substrate (dashed line = 100%). n = 4 replicates per culture condition. *, p<0.05 compared to control; ◇, p<0.05 when comparing substrates by Mann-Whitney U test.
Figure 6.
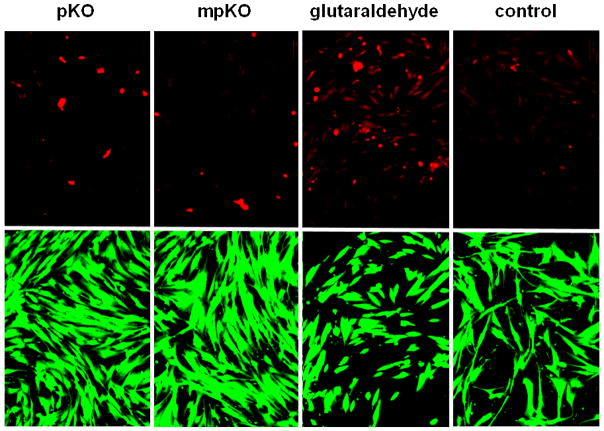
Cell viability of DPCs exposed to biomaterials at 10 μg/mL. Dead cells stained red by ethidium homodimer. Live cells stained green by calcein-AM. Shown at 20× magnification.
Next, the influence of ECM on DPC sensitivity was further explored by exposing cells to a known toxic compound. The dentin-DPC co-culture model (Fig. 2) was used, in which dentin was treated with the toxic collagen cross-linking agent, glutaraldehyde, and incubated with DPCs (Fig. 5C). Cells seeded on uncoated tissue culture plates had impaired proliferation after one, three, and five days. When DPCs were seeded on ECM-coated plates their proliferation was not impaired until five days after glutaraldehyde exposure. Collectively, these results demonstrate that DPCs cultured on their native ECM are more tolerant to biomaterials. Based on these findings, DPCs were seeded on ECM-coated plates for further biocompatibility studies.
3.4 Biocompatibility of refined PAC mixtures
The dentin-DPC co-culture model was used to determine proliferation and gene expression of DPCs in response to PACs that are released from dentin into the underlying pulp tissue. We anticipated certain compounds will bind more strongly to type-I collagen, changing the ratio of PACs within the leached materials compared to the original, freshly-prepared treatment. Thus, direct treatment of DPCs with the extracts (Fig. 5A and B) may not accurately represent the specific biomaterial composition that pulp tissue will be exposed to as a result of the in vivo release of PACs from dentin.
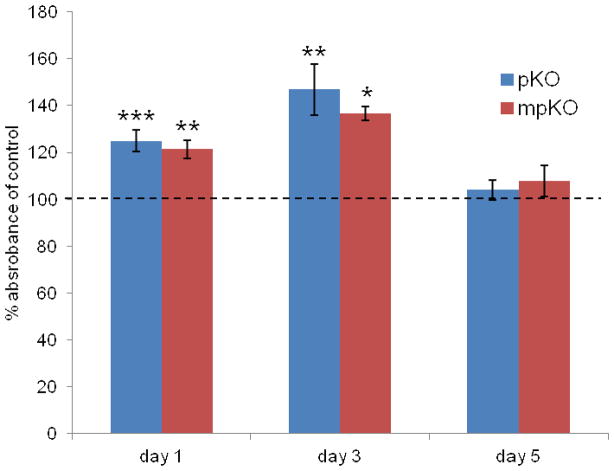
In the dentin-DPC co-culture model, DPC viability was not harmed after exposure to dentin treated with refined mixtures. In fact, proliferation of exposed groups was greater than control groups after one and three days (Fig. 7). After five days, proliferation was similar to control. pKO- and mpKO caused similar effects on proliferation at every time point. This pattern of proliferation differed when cells were exposed to refined mixtures directly (Fig. 5A and B).
Figure 7.
Proliferation of DPCs exposed to dentin treated with pKO- and mpKO-refined mixtures (15% w/v) in dentin-DPC co-culture model. Data normalized to absorbance of untreated control (dashed line = 100%). n = 4 culture replicates per treatment. *, p<0.05; **, p<0.01; ***, p<0.001 compared to control, by Tukey’s test after one-way ANOVA.
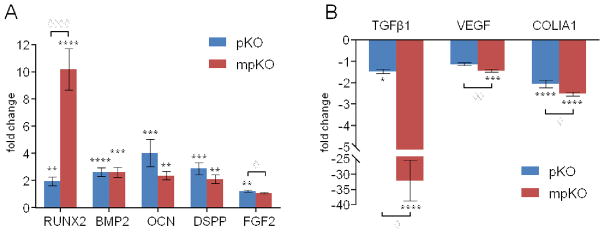
qRT-PCR revealed that odontoblast-associated marker genes were significantly affected by PACs released from treated dentin (Fig. 8). DPCs exposed to pKO- and mpKO-treated dentin expressed significantly more Runt-related transcription factor 2 (RUNX2), bone morphogenetic protein 2 (BMP2), fibroblast growth factor 2 (FGF2), osteocalcin (OCN), and dentin sialophosphoprotein (DSPP), but significantly less transforming growth factor-β1 (TGFβ1), vascular endothelial growth factor (VEGF), and collagen, type I, alpha 1 (COLIA1) when compared to untreated control. The magnitude of fold change of each gene differed between pKO- and mpKO-exposed groups.
Figure 8.
Gene expression after DPSC exposure to pKO- and mpKO-treated dentin for 5 days. Displayed as relative gene expression compared to negative control. (A) Genes upregulated by leaching of PACs include RUNX2 (Runt-related transcription factor 2), BMP2 (bone morphogenetic protein 2), OCN (osteocalcin), DSPP (dentin sialophosphoprotein), and FGF2 (fibroblast growth factor 2). (B) Genes downregulated by leaching of PACs include TGFβ1 (transforming growth factor beta-1), VEGF (vascular endothelial growth factor) and COL1A1 (collagen, type-I, alpha 1). *, p<0.05; **, p<0.01; ***, p<0.001 compared to control, by Tukey’s test. ◇, p<0.05; ◇◇, p<0.01; ◇◇◇◇, p<0.0001 between treatments, by Tukey’s test after one-way ANOVA.
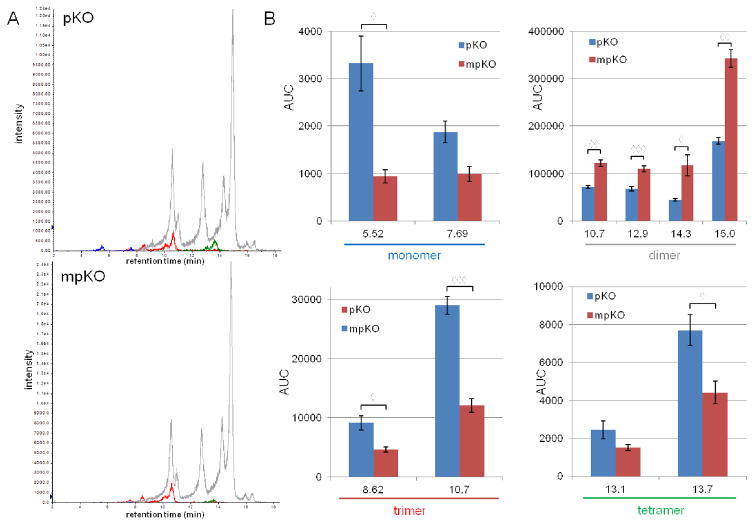
3.5 LC-MS detection of leached PACs
LC-MS analysis revealed that various PAC monomers, dimers, trimers, and tetramers are present in media exposed to pKO- and mpKO-treated dentin at levels that are readily detectable by MS. This confirms that PACs are released from dentin. Chromatograms composed of overlaid traces of mass-mass transitions corresponding to each PAC are shown in Figure 9A. To better visualize chromatographic data, figure 9B graphs the area under the curve (AUC) of each detected peak. Media exposed to pKO-treated dentin contained a higher concentration of monomers and tetramers, while media exposed to mpKO-treated dentin contained a higher concentration of dimers and trimers.
Figure 9.
HPLC-MS analysis of PACs leaching into media from treated dentin after 24 hrs. (A) Chromatogram of media exposed to pKO- and mpKO-treated dentin composed of overlaid traces of mass-mass transitions corresponding to monomers (blue, 289→137), dimers (gray, 575→449), trimers (red, 863→693), and tetramers (green, 1149→979). (B) Graphical representation of the chromatogram. Bars represent the AUC of each detected peak. Peaks are described by retention time. Average values and standard deviation of three analytical replicates are displayed. ◇, p<0.05; ◇◇, p<0.01; ◇◇◇, p<0.001 between treatments, by t-test (Bonferroni-Dunn correction).
4. Discussion
The pKO and mpKO refined mixtures were the result of enriching PACs from the more complex crude pine extract. Oligomeric PACs are renewable plant-derived compounds exhibiting a high level of interaction with type-I collagen rich dentin matrices [9,12]. The mechanical bioassay confirms the high interactivity of PACs with type-I collagen in dentin (representing 90% of the tissue). The remarkable increase (5.5 fold) in the modulus of elasticity of the native dentin matrix (Fig. 3) indicates that enriched fractions contain the bioactive PACs. These results demonstrate that CPC is a suitable technique for enriching bioactive PACs, as the process did not cause the already high modulus of elasticity induced by crude pine extract to be diminished.
While we have established previously that oligomeric PACs are effective for enhancing the mechanical properties of collagen [9,10,12], their biocompatibility with pulp tissue and potential regenerative effect on the dentin-pulp complex is unknown. To address this, the bioactivity of refined PAC mixtures was assessed in models of increasing complexity. First, direct treatment of DPCs on plastic culture plates resulted in increased proliferation. Next, to better mimic the pulp environment, DPCs were seeded on their native ECM and treated with refined mixtures; however, on this substrate refined PAC mixtures did not cause increased proliferation. Finally, a model representing the dentin-pulp complex was used, in which cells were seeded on native ECM and leaching of biomaterials was replicated by co-culturing the DPCs with dentin treated with refined mixtures (Fig. 2). In this model, PACs leached from dentin, increased proliferation and upregulated markers of odontogenic differentiation and mineralization. Collectively, these results demonstrate that not only are these refined mixtures, and the oligomeric PACs they contain, safe, but that these materials actually have beneficial effects on pulp cells.
Interestingly, the effects on proliferation changed as the model better mimicked the in vivo pulp tissue environment. Unlike the response of DPCs on uncoated tissue culture plates, when DPCs were seeded on their native ECM, direct treatment with refined mixtures did not cause a spike in proliferation. Therefore, ECM-seeded cells appeared to be more tolerant to PAC treatment. ECM-seeded DPCs were also more tolerant to glutaraldehyde, a toxic biomaterial [24], than cells on plastic culture plates (Fig. 5C). The ECM is a dynamic, multifunctional environment that can maintain cells in a more favorable state by supporting cellular attachment and migration [21,25,26]. Our results indicate that these conditions make cells more tolerant to foreign compounds. Additionally, it is reasonable to infer that the large quantity of collagen in the ECM may bind biomaterials, effecting delivery and prolonging or attenuating their ability to influence cells. Predictably, this phenomenon can also occur in native in vivo ECM. Presently, it is difficult to confirm this hypothesis using LC-MS techniques, but we would like to address the interaction of PACs and ECM in the future, perhaps using MALDI or solid-state NMR techniques.
PACs released from dentin caused an increase in cell proliferation compared to control (Fig. 7), which was not observed with DPCs on ECM-coated plates after direct treatment (Fig. 5B). We hypothesize that, as highly interactive PACs cross-link collagen, they will be depleted from the original, freshly-prepared extract, while PACs with less dentin interaction will be released into media in greater concentrations. Therefore, selective binding of highly-interactive compounds to dentin collagen will alter the ratio of PACs that interact with DPCs compared to direct treatment with the freshly-prepared original extract, where there is no dentin matrix, and depletion does not occur. Differences in total PAC composition and ratio may explain the difference in activity seen between these two modes of PAC delivery. There is a dearth of information regarding the bioactivity of PACs larger than dimers on human cells, thus, additive effects, synergism, or antagonism may be involved in the total activity profile of the complex PAC mixtures evaluated here and will be explored in follow-up studies.
DPC gene expression in response to PACs was also evaluated using the dentin-DPC co-culture model. Marker genes of the odontogenic lineage, including BMP2, RUNX2, OCN, and DSPP were upregulated after exposure to treated dentin (Fig. 8). These genes are involved in mineralization and dentinogenesis, indicating that the functional ability and differentiation of DPCs was not undermined by PACs, but enhanced. On the other hand, the expression of TGFβ1, VEGF, and COLIA1 were reduced by treatment. TGFβ1 is expressed in early odontogenesis and has an inverse relationship to RUNX2 and DSPP [27]. High DSPP expression, indicative of induction of biomineralization, combined with the presence of type-I collagen in the ECM, may have triggered the observed decrease in COLIA1 expression [28]. PACs have been previously shown to inhibit angiogenesis in vitro [29,30], which may explain the slight decrease in VEGF expression. PACs may be directly involved in altering the expression of each of these genes individually, or the observed results may be downstream effects caused by PACs influencing the expression of master transcription factors such as RUNX2 or growth factors such as BMP2. Our future efforts will be concentrated on evaluating the pathway-specific effects of these enriched PAC mixtures and isolated oligomeric PACs.
LC-MS analysis of media from the dentin-DPC co-culture model confirms that PACs were released from treated dentin into cell culture media. MS is a very sensitive detection technique and, anticipating that PACs are present in the media at low concentrations, the vast majority of PACs stay bound to dentin. The chromatograms presented here are based on ion count, but the ionization sensitivity can vary widely based on analyte properties and instrument parameters. Follow-up studies involved pure compounds will be used a response calibrants to accurately quantify the amount of PACs leaching into media and affecting DPCs and also quantify PACs that stay bound to dentin.
Although released PACs were not quantified comparisons can be made between the media from both treatment groups. pKO-treated dentin released more monomers and tetramers into media than mpKO-treated dentin. Correspondingly, these PACs may contribute to the greater upregulation of DSPP and FGF2 seen in pKO-treated groups compared to mpKO-treated groups. As expected, mpKO-treated dentin released more dimers and trimers into the media. These PACs may have contributed to the observed greater upregulation of RUNX2 and greater downregulation of TGFβ1, VEGF, and COLIA1 seen in mpKO-treated groups compared to pKO-treated groups. Future biological experiments using pure compounds can confirm this hypothesis and reveal the influence of individual PACs on DPCs. Due to the complex mixture of PACs in media, we are unable to state exactly which PACs are responsible for the observed effects on proliferation and gene expression. Despite this, it is clear that PACs, especially oligomeric PACs in the dimer to tetramer range present in the media of the dentin-pulp cell co-culture, influenced DPC proliferation and differentiation.
Admittedly, the dentin-DPC co-culture model used here may exaggerate PAC release from the dentin matrix when compared to actual in vivo constraints because leaching is allowed to occur from all sides of the dentin specimen. In a tooth, biomaterials will reach the pulp tissue only by diffusing through dentin tubules, and there are models which more closely replicate this event [22,31]. Considering the safety and biocompatibility aims of this study, it was preferable to overestimate the release of PACs. With these compounds, even if excessive leaching occurs in vivo, our data shows that the effects will not be harmful, and may even benefit cells of the pulp tissue. A limitation of our simplified model is the lack of representation of other pulp cells as well as neurons and endothelial cells, as well as vasculature and innervation present in in vivo pulp tissue, which would influence the exposure and behavior of pulp tissue to PACs. These types of models will be considered for future study.
The biological responses of DPCs to biomaterials observed in the present study differed due to changes in tissue culture substrate and method of PAC delivery. Therefore, mimicking the in vivo environment as well as exposure method is essential to obtaining meaningful results from translational studies investigating biomaterials. The dentin-DPC co-culture model used here can be modified by replacing the dentin with other forms of collagen matrices or decellularized matrix scaffolds containing biomaterials, and by replacing the DPCs with other site appropriate cell types, to infer the biocompatibility or regenerative benefits of novel biomaterials.
5. Conclusion
Pinus massoniana Lamb. bark offers a potent, renewable source of selective collagen-interactive oligomeric PACs that can be enriched by efficient, scalable chromatographic procedures. We demonstrate that not only are oligomeric PACs biocompatible with DPCs, but they can also promote the native regenerative properties of this cell population, indicated by upregulation of key genes involved in differentiation, dentinogenesis, and biomineralization. The results are critical in the ongoing evaluation of oligomeric PACs as potent tissue reparative and regenerative biomaterials.
Supplementary Material
Acknowledgments
This research was funded by grants R01 DE021040, T32 DE018381, and R56 DE023806 from NIDCR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miguez P, Pereira P, Atsawasuwan P, Yamauchi M. Collagen Cross-linking and Ultimate Tensile Strength in Dentin. J Dent Res. 2004;83:807–810. doi: 10.1177/154405910408301014. [DOI] [PubMed] [Google Scholar]
- 2.Bedran-Russo AK, Pauli GF, Chen S-N, McAlpine J, Castellan CS, Phansalkar RS, Aguiar TR, Vidal CMP, Napotilano JG, Nam J-W, Leme Aa. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 2014;30:62–76. doi: 10.1016/j.dental.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65:118–124. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 4.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res. 2003;64A:427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 5.He L, Mu C, Shi J, Zhang Q, Shi B, Lin W. Modification of collagen with a natural cross-linker, procyanidin. Int J Biol Macromol. 2011;48:354–359. doi: 10.1016/j.ijbiomac.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Tang CF, Fang M, Liu RR, Dou Q, Chai ZG, Xiao YH, Chen JH. The role of grape seed extract in the remineralization of demineralized dentine: Micromorphological and physical analyses. Arch Oral Biol. 2013;58:1769–1776. doi: 10.1016/j.archoralbio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Cos P, De Bruyne T, Hermans N, Apers S, Vanden Berghe D, Vlietinck AJ. Proanthocyanidins in health care: current and new trends. Curr Med Chem. 2004;11:1345–59. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 8.Verstraeten SV, Fraga CG, Oteiza PI. Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance. Food Funct. 2015;6:32–40. doi: 10.1039/C4FO00647J. [DOI] [PubMed] [Google Scholar]
- 9.Nam JW, Phansalkar RS, Lankin DC, Bisson J, McAlpine JB, Leme AA, Vidal CMP, Ramirez B, Niemitz M, Bedran-Russo A, Chen SN, Pauli GF. Subtle Chemical Shifts Explain the NMR Fingerprints of Oligomeric Proanthocyanidins with High Dentin Biomodification Potency. J Org Chem. 2015;80:7495–7507. doi: 10.1021/acs.joc.5b01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguiar TR, Vidal CMP, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo aK. Dentin biomodification potential depends on polyphenol source. J Dent Res. 2014;93:417–22. doi: 10.1177/0022034514523783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal CMP, Leme Aa, Aguiar TR, Phansalkar R, Nam J-W, Bisson J, McAlpine JB, Chen S-N, Pauli GF, Bedran-Russo A. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir. 2014;30:14887–93. doi: 10.1021/la5034383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phansalkar RS, Nam JW, Chen SN, McAlpine JB, Napolitano JG, Leme A, Vidal CMP, Aguiar T, Bedran-Russo AK, Pauli GF. A galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential. Fitoterapia. 2015;101:169–178. doi: 10.1016/j.fitote.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarenga RR, Friesen JB, Nikolic D, Simmler C, Napolitano JG, van Breemen RB, Lankin DC, McAlpine JB, Pauli GF, Chen SN. K-Targeted Metabolomic Analysis Extends Chemical Subtraction to DESIGNER Extracts: Selective Depletion of Extracts of Hops (Humulus lupulus) J Nat Prod. 2014 doi: 10.1021/np500376g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333–355. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 16.Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH. HPLC separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J Agric Food Chem. 2006;54:1571. doi: 10.1021/jf0525941. [DOI] [PubMed] [Google Scholar]
- 17.Bedran-Russo AKB, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res. 2009;88:807–811. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem Cell Properties of Human Dental Pulp Stem Cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Walboomers XF, Shi S, Fan M, Jansen Ja. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 20.Ravindran S, Zhang Y, Huang CC, George A. Odontogenic induction of dental stem cells by extracellular matrix-inspired three-dimensional scaffold. Tissue Eng Part A. 2014;20:92–102. doi: 10.1089/ten.TEA.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravindran S, Gao Q, Kotecha M, Magin RL, Karol S, Bedran-Russo A, George A. Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue Eng Part A. 2012;18:295–309. doi: 10.1089/ten.TEA.2011.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheffel DLS, Bianchi L, Soares DG, Basso FG, Sabatini C, de Souza Costa CA, Pashley DH, Hebling J. Transdentinal Cytotoxicity of Carbodiimide (EDC) and Glutaraldehyde on Odontoblast-like Cells. Oper Dent. 2015;40:44–54. doi: 10.2341/13-338-L. [DOI] [PubMed] [Google Scholar]
- 23.Ravindran S, Huang CC, George A. Extracellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSCs. Front Physiol. 2014;4:395. doi: 10.3389/fphys.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang-Lee LLH, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res. 1990;24:1185–1201. doi: 10.1002/jbm.820240905. [DOI] [PubMed] [Google Scholar]
- 25.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A. 2010;16:431–40. doi: 10.1089/ten.TEA.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unterbrink A, O’Sullivan M, Chen S, MacDougall M. TGFβ-1 Downregulates DMP-1 and DSPP in Odontoblasts. Connect Tissue Res. 2002;43:354–358. doi: 10.1080/03008200290000565. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711–35. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Zhang K, Nam S, Anderson RA, Jove R, Wen W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis. 2010;31:481–488. doi: 10.1093/carcin/bgp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakanishi T, Mukai K, Hosokawa Y, Takegawa D, Matsuo T. Catechins inhibit vascular endothelial growth factor production and cyclooxygenase-2 expression in human dental pulp cells. Int Endod J. 2015;48:277–82. doi: 10.1111/iej.12312. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi L, Ribeiro APD, de Oliveira Carrilho MR, Pashley DH, de Souza Costa CA, Hebling J. Transdentinal cytotoxicity of experimental adhesive systems of different hydrophilicity applied to ethanol-saturated dentin. Dent Mater. 2013;29:980–90. doi: 10.1016/j.dental.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.