Abstract
Background
Individuals infected with Mycobacterium tuberculosis (Mtb) may develop symptoms and signs of disease (tuberculosis disease) or may have no clinical evidence of disease (latent tuberculosis infection [LTBI]). Tuberculosis disease is a leading cause of infectious disease morbidity and mortality worldwide, yet many questions related to its diagnosis remain.
Methods
A task force supported by the American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America searched, selected, and synthesized relevant evidence. The evidence was then used as the basis for recommendations about the diagnosis of tuberculosis disease and LTBI in adults and children. The recommendations were formulated, written, and graded using the Grading, Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Results
Twenty-three evidence-based recommendations about diagnostic testing for latent tuberculosis infection, pulmonary tuberculosis, and extrapulmonary tuberculosis are provided. Six of the recommendations are strong, whereas the remaining 17 are conditional.
Conclusions
These guidelines are not intended to impose a standard of care. They provide the basis for rational decisions in the diagnosis of tuberculosis in the context of the existing evidence. No guidelines can take into account all of the often compelling unique individual clinical circumstances.
EXECUTIVE SUMMARY
Individuals infected with Mycobacterium tuberculosis (Mtb) may develop symptoms and signs of disease (TB disease) or may have no clinical evidence of disease (latent tuberculosis infection [LTBI]). TB disease is a leading cause of infectious disease morbidity and mortality worldwide, with many diagnostic uncertainties. A task force supported by the supported by the American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America appraised the evidence and derived the following recommendations using the Grading, Recommendations, Assessment, Development, and Evaluation (GRADE) approach (Table 1):
Table 1.
Interpretation of Strong and Weak (Conditional) Recommendations
| Strong Recommendation | Weak (Conditional) Recommendation | |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policy makers | The recommendation can be adopted as policy in most situations. | Policymaking will require substantial debate and involvement of various stakeholders. |
Testing for LTBI
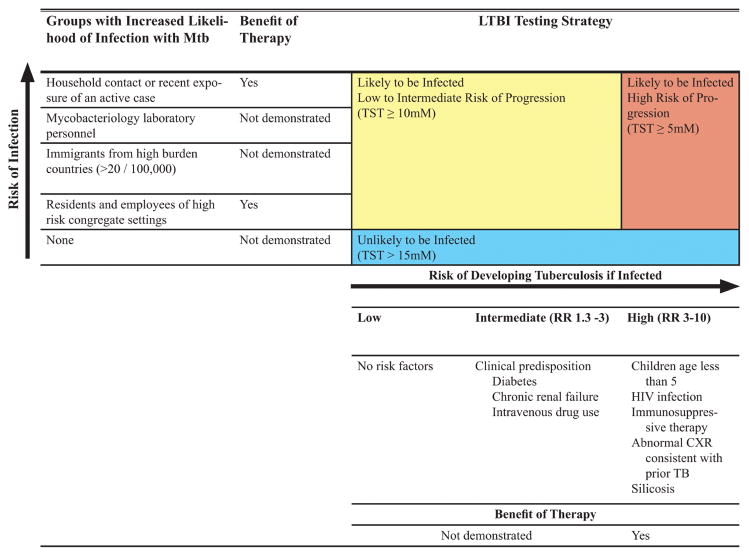
Our recommendations for diagnostic testing for LTBI are based upon the likelihood of infection with Mtb and the likelihood of progression to TB disease if infected, as illustrated in Figure 1.
Figure 1.
Paradigm for evaluation of those with latent tuberculosis infection (LTBI) based on risk of infection, risk of progression to tuberculosis, and benefit of therapy. In developing a diagnostic approach for the evaluation of those with suspected LTBI, we recommend the clinician weigh the likelihood of infection, the likelihood of progression to tuberculosis if infected, and the benefit of therapy (Horsburgh and Rubin, Clinical practice: latent tuberculosis infection in the United States. N Engl J Med 2011; 364:1441–8). Recommendations were formulated for each of the 3 groups illustrated above. These groups are concordant with current recommendations for the interpretation of the tuberculin skin test (American Thoracic Society, Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep 2000; 49:1–51). Abbreviations: CXR, chest radiograph; HIV, human immunodeficiency virus; LTBI, latent tuberculosis infection; Mtb, Mycobacterium tuberculosis; RR, relative risk; TB, tuberculosis; TST, tuberculin skin test.
We recommend performing an interferon-γ release assay (IGRA) rather than a tuberculin skin test (TST) in individuals 5 years or older who meet the following criteria: (1) are likely to be infected with Mtb, (2) have a low or intermediate risk of disease progression, (3) it has been decided that testing for LTBI is warranted, and (4) either have a history of BCG vaccination or are unlikely to return to have their TST read (strong recommendation, moderate-quality evidence). Remarks: A TST is an acceptable alternative, especially in situations where an IGRA is not available, too costly, or too burdensome.
We suggest performing an IGRA rather than a TST in all other individuals 5 years or older who are likely to be infected with Mtb, who have a low or intermediate risk of disease progression, and in whom it has been decided that testing for LTBI is warranted (conditional recommendation, moderate-quality evidence). Remarks: A TST is an acceptable alternative, especially in situations where an IGRA is not available, too costly, or too burdensome.
There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to disease, and in whom it has been determined that diagnostic testing for LTBI is warranted.
-
Guidelines recommend that persons at low risk for Mtb infection and disease progression NOT be tested for Mtb infection. We concur with this recommendation. However, we also recognize that such testing may be obliged by law or credentialing bodies. If diagnostic testing for LTBI is performed in individuals who are unlikely to be infected with Mtb despite guidelines to the contrary:
We suggest performing an IGRA instead of a TST in indivduals 5 years or older (conditional recommendation, low-quality evidence). Remarks: A TST is an acceptable alternative in settings where an IGRA is unavailable, too costly, or too burdensome.
We suggest a second diagnostic test if the initial test is positive in individuals 5 years or older (conditional recommendation, very low-quality evidence). Remarks: The confirmatory test may be either an IGRA or a TST. When such testing is performed, the person is considered infected only if both tests are positive.
We suggest performing a TST rather than an IGRA in healthy children <5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted (conditional recommendation, very low-quality evidence). Remarks: In situations in which an IGRA is deemed the preferred diagnostic test, some experts are willing to use IGRAs in children over 3 years of age.
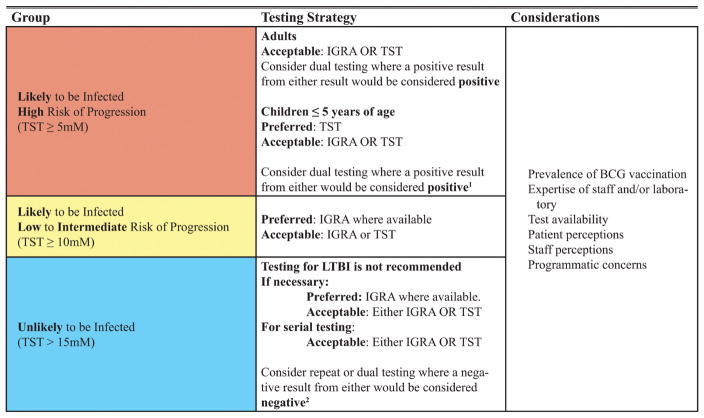
The preceding recommendations are summarized in Figure 2. While both IGRA and TST testing provide evidence for infection with Mtb, they cannot distinguish active from latent TB. Therefore, the diagnosis of active TB must be excluded prior to embarking on treatment for LTBI. This is typically done by determining whether or not symptoms suggestive of TB disease are present, performing a chest radiograph and, if radiographic signs of active TB (eg, airspace opacities, pleural effusions, cavities, or changes on serial radiographs) are seen, then sampling is performed and the patient managed accordingly.
Figure 2.
Summary of recommendations for testing for latent tuberculosis infection (LTBI). 1Performing a second diagnostic test when the initial test is negative is a strategy to increase sensitivity. This may reduce specificity, but the panel decided that this is an acceptable trade-off in situations in which the consequences of missing LTBI (ie, not treating individuals who may benefit from therapy) exceed the consequences of inappropriate therapy (ie, hepatotoxicity). 2Performing a confirmatory test following an initial positive result is based upon both the evidence that false-positive results are common among individuals who are unlikely to be infected with Mycobacterium tuberculosis and the committee’s presumption that performing a second test on those patients whose initial test was positive will help identify initial false-positive results. Abbreviations: IGRA, interferon-γ release assay; LTBI, latent tuberculosis infection; TST, tuberculin skin test.
Testing for TB Disease
We recommend that acid-fast bacilli (AFB) smear microscopy be performed, rather than no AFB smear microscopy, in all patients suspected of having pulmonary TB (strong recommendation, moderate-quality evidence). Remarks: False-negative results are sufficiently common that a negative AFB smear result does not exclude pulmonary TB. Similarly, false-positive results are sufficiently common that a positive AFB smear result does not confirm pulmonary TB. Testing of 3 specimens is considered the normative practice in the United States and is strongly recommended by the Centers for Disease Control and Prevention and the National Tuberculosis Controllers Association in order to improve sensitivity given the pervasive issue of poor sample quality. Providers should request a sputum volume of at least 3 mL, but the optimal volume is 5–10 mL. Concentrated respiratory specimens and fluorescence microscopy are preferred.
We suggest that both liquid and solid mycobacterial cultures be performed, rather than either culture method alone, for every specimen obtained from an individual with suspected TB disease (conditional recommendation, low-quality evidence). Remarks: The conditional qualifier applies to performance of both liquid and solid culture methods on all specimens. At least liquid culture should be done on all specimens as culture is the gold standard microbiologic test for the diagnosis of TB disease. The isolate recovered should be identified according to the Clinical and Laboratory Standards Institute guidelines and the American Society for Microbiology Manual of Clinical Microbiology.
We suggest performing a diagnostic nucleic acid amplification test (NAAT), rather than not performing a NAAT, on the initial respiratory specimen from patients suspected of having pulmonary TB (conditional recommendation, low-quality evidence). Remarks: In AFB smear-positive patients, a negative NAAT makes TB disease unlikely. In AFB smear-negative patients with an intermediate to high level of suspicion for disease, a positive NAAT can be used as presumptive evidence of TB disease, but a negative NAAT cannot be used to exclude pulmonary TB. Appropriate NAAT include the Hologic Amplified Mycobacteria Tuberculosis Direct (MTD) test (San Diego, California) and the Cepheid Xpert MTB/Rif test (Sunnyvale, California).
We recommend performing rapid molecular drug susceptibility testing for rifampin with or without isoniazid using the respiratory specimens of persons who are either AFB smear positive or Hologic Amplified MTD positive and who meet one of the following criteria: (1) have been treated for tuberculosis in the past, (2) were born in or have lived for at least 1 year in a foreign country with at least a moderate tuberculosis incidence (≥20 per 100 000) or a high primary multidrug-resistant tuberculosis prevalence (≥2%), (3) are contacts of patients with multidrug-resistant tuberculosis, or (4) are HIV infected (strong recommendation, moderate-quality evidence). Remarks: This recommendation specifically addresses patients who are Hologic Amplified MTD positive because the Hologic Amplified MTD NAAT only detects TB and not drug resistance; it is not applicable to patients who are positive for types of NAAT that detect drug resistance, including many line probe assays and Cepheid Xpert MTB/RIF.
We suggest mycobacterial culture of respiratory specimens for all children suspected of having pulmonary TB (conditional recommendation, moderate-quality evidence). Remarks: In a low incidence setting like the United States, it is unlikely that a child identified during a recent contract investigation of a close adult/adolescent contact with contagious TB was, in fact, infected by a different individual with a strain with a different susceptibility pattern. Therefore, under some circumstances, microbiological confirmation may not be necessary for children with uncomplicated pulmonary TB identified through a recent contact investigation if the source case has drug-susceptible TB.
We suggest sputum induction rather than flexible bronchoscopic sampling as the initial respiratory sampling method for adults with suspected pulmonary TB who are either unable to expectorate sputum or whose expectorated sputum is AFB smear microscopy negative (conditional recommendation, low-quality evidence).
We suggest flexible bronchoscopic sampling, rather than no bronchoscopic sampling, in adults with suspected pulmonary TB from whom a respiratory sample cannot be obtained via induced sputum (conditional recommendation, very low-quality evidence). Remarks: In the committee members’ clinical practices, bronchoalveolar lavage (BAL) plus brushings alone are performed for most patients; however, for patients in whom a rapid diagnosis is essential, transbronchial biopsy is also performed.
We suggest that postbronchoscopy sputum specimens be collected from all adults with suspected pulmonary TB who undergo bronchoscopy (conditional recommendation, low-quality evidence). Remarks: Postbronchoscopy sputum specimens are used to perform AFB smear microscopy and mycobacterial cultures.
We suggest flexible bronchoscopic sampling, rather than no bronchoscopic sampling, in adults with suspected miliary TB and no alternative lesions that are accessible for sampling whose induced sputum is AFB smear microscopy negative or from whom a respiratory sample cannot be obtained via induced sputum (conditional recommendation, very low-quality evidence). Remarks: Bronchoscopic sampling in patients with suspected miliary TB should include bronchial brushings and/or transbronchial biopsy, as the yield from washings is substantially less and the yield from BAL unknown. For patients in whom it is important to provide a rapid presumptive diagnosis of tuberculosis (ie, those who are too sick to wait for culture results), transbronchial biopsies are both necessary and appropriate.
We suggest that cell counts and chemistries be performed on amenable fluid specimens collected from sites of suspected extrapulmonary TB (conditional recommendation, very low-quality evidence). Remarks: Specimens that are amenable to cell counts and chemistries include pleural, cerebrospinal, ascitic, and joint fluids.
We suggest that adenosine deaminase levels be measured, rather than not measured, on fluid collected from patients with suspected pleural TB, TB meningitis, peritoneal TB, or pericardial TB (conditional recommendation, low-quality evidence).
We suggest that free IFN-γ levels be measured, rather than not measured, on fluid collected from patients with suspected pleural TB or peritoneal TB (conditional recommendation, low-quality evidence).
We suggest that AFB smear microscopy be performed, rather than not performed, on specimens collected from sites of suspected extrapulmonary TB (conditional recommendation, very low-quality evidence). Remarks: A positive result can be used as evidence of extrapulmonary TB and guide decision making because false-positive results are unlikely. However, a negative result may not be used to exclude TB because false-negative results are exceedingly common.
We recommend that mycobacterial cultures be performed, rather than not performed, on specimens collected from sites of suspected extrapulmonary TB (strong recommendation, low-quality evidence). Remarks: A positive result can be used as evidence of extrapulmonary TB and guide decision making because false-positive results are unlikely. However, a negative result may not be used to exclude TB because false-negative results are exceedingly common.
We suggest that NAAT be performed, rather than not performed, on specimens collected from sites of suspected extrapulmonary TB (conditional recommendation, very low-quality evidence). Remarks: A positive NAAT result can be used as evidence of extrapulmonary TB and guide decision making because false-positive results are unlikely. However, a negative NAAT result may not be used to exclude TB because false-negative results are exceedingly common. At present, NAAT testing on specimens other than sputum is an off-label use of the test.
We suggest that histological examination be performed, rather than not performed, on specimens collected from sites of suspected extrapulmonary TB (conditional recommendation, very low-quality evidence). Remarks: Both positive and negative results should be interpreted in the context of the clinical scenario because neither false-positive nor false-negative results are rare.
We recommend one culture isolate from each mycobacterial culture-positive patient be submitted to a regional genotyping laboratory for genotyping (strong recommendation, very low-quality evidence).
GUIDELINE STATEMENT.
These guidelines are not intended to impose a standard of care. They provide the basis for rational decisions in the diagnostic evaluation of patients with possible latent tuberculosis or tuberculosis. Clinicians, patients, third-party payers, stakeholders, or the courts should never view the recommendations contained in these guidelines as dictates. Guidelines cannot take into account all of the often compelling unique individual clinical circumstances. Therefore, no one charged with evaluating clinicians’ actions should attempt to apply the recommendations from these guidelines by rote or in a blanket fashion. Qualifying remarks accompanying each recommendation are its integral parts and serve to facilitate more accurate interpretation. They should never be omitted when quoting or translating recommendations from these guidelines.
Acknowledgments
The writing committee thanks Drs Mike Iseman and Jeffrey Starke for their critical examination of the manuscript. The committee is particularly indebted to Kevin Wilson for his patience and his editing skills.
Footnotes
These guidelines were endorsed by the European Respiratory Society on 20 June 2016.
For permissions, journals.permissions@oup.com.
Potential conflicts of interest.
D. L. C. has received speaking fees from Qiagen. C. L. D. serves on the data and safety monitoring board (DSMB) for Otsuka America Pharmaceutical, Inc, served on a DSMB for Sanofi Pasteur Inc, received research support from Insmed, and received travel support from Qiagen. L. R. serves as a speaker and on an advisory committee for Boehringer Ingelheim and F. Hoffmann-La Roche, serves on an advisory committee and received research support from Biogen Inc, served on an advisory committee for AstraZeneca, GlaxoSmithKline, and Sanofi Pasteur, served as a consultant for Bayer HealthCare, served as a speaker for Cipla. T.M.S. is employed by the US Centers for Disease Control and Prevention (CDC). T. R. S. serves on a DSMB for Otsuka. P. A. L. is employed by the CDC. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.