INTRODUCTION
Leukocyte cell‐derived chemotaxin‐2 (LECT2) is a highly conserved protein with roles in cell development, sepsis, liver disease, metabolic syndrome, arthritis, cancer, and amyloidosis, among others. We will review its genetics, structure, role in physiology, and its future as a biomarker and therapeutic target across many diseases. Studies involving LECT2 were identified using PubMed and compiled into a single review paper. Research on LECT2 suggests a future as a biomarker of disease and potential therapeutic target.
BACKGROUND
The discovery of leukocyte cell‐derived chemotaxin‐2 (LECT2) was the result of a drive to find novel biomarkers of inflammatory diseases.1 It was first isolated in humans by Yamagoe et al.1 from phytohemagglutinin‐stimulated T‐cell leukemia SKW‐3 cells and identified as a chemotaxin of neutrophils. Chemotaxins are substances released by cells that stimulate the movement of white blood cells and LECT2 derived its name from this in vitro chemotactic ability at its initial discovery.1 However, it is now known to have several other functions in the human body and it is no longer limited to chemotaxis. There has been a rapid expansion of knowledge regarding its function with major developments in liver regeneration,2, 3, 4, 5, 6, 7, 8 immune modulation,1, 9, 10, 11, 12, 13, 14, 15 bone growth,9, 16, 17, 18, 19, 20 neuronal development,21 glucose metabolism and metabolic syndrome,10, 22, 23, 24, 25, 26, 27 cancer,9, 28, 29, 30, 31, 32, 33 and amyloidosis,34, 35, 36, 37, 38, 39 among others. It is often found secreted in the bloodstream and expression of this protein has been identified in liver cells, neurons, various epithelial cells, parathyroid cells, and white blood cells, although it is felt to be most often secreted by the liver.40 However, despite the multifunctional role of LECT2 in several different organ systems, there has yet to be a comprehensive review that summarizes all of the current knowledge and information. We will attempt to synthesize current reported literature on this increasingly important protein and explore its potential future in modern clinical medicine in relation to disease pathophysiology (Table 1)6, 13, 17, 18, 22, 28, 29, 33, 41, 42 and as a biomarker (Table 2).2, 3, 6, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 22, 23, 24, 28, 29, 30, 31, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42
Table 1.
Association of leukocyte cell‐derived chemotaxin‐2 with pathophysiology and disease in different organ systems
| Organ system | Findings | Ref. | Study description (subjects, sample size, study design) |
|---|---|---|---|
| Inflammation and fibrosis | LECT2 expression changes with signs of inflammation or fibrosis | 40 | In vivo human study using immunohistochemistry to study 28 different organs in the human body (n = 68) for LECT2 expression in normal and diseased states |
| Immune system | Activated immune response and prosurvival role with infection and sepsis | 15 | In vivo and in vitro nonhuman study of healthy fish (n = 4) demonstrating increased cytokine expression, induced chemotaxis, and monocyte / macrophage activation by LECT2 mediated by C‐type lectin receptor (PaCLR) that is inhibited by anti‐PaCLR antibodies |
| Activated immune response and prosurvival role with infection and sepsis | 10 | In vivo nonhuman study in mice using models of bacterial infection and cecal ligation to show that LECT2 had a protective effect in mice through CD209a receptor interaction to activate macrophages | |
| Activated immune response and prosurvival role with infection and sepsis | 12 | In vitro nonhuman activity of fish macrophages and their response to recombinant LECT2 using qPCR, chemotactic activity assays, and polyacrylamide gel electrophoresis | |
| Activated immune response and prosurvival role with infection and sepsis | 13 | In vivo human retrospective study of healthy controls (n = 31) and patients admitted to the intensive care unit with confirmed sepsis (n = 23) comparing serum LECT2 to outcomes, vital signs, routine bloodwork, and cytokines | |
| Neutrophil chemotaxis | 9 | In vitro human study of neutrophil chemotaxis compared with fMLP | |
| Bone | Involved in the pathogenesis of osteoarthritis and rheumatoid arthritis | 16 | In vivo nonhuman study in WT and LECT2‐deficient mice after induction of arthritis by anti‐type II collagen antibodies and lipopolysaccharide (n = 7 in each group) as assessed by hind paw thickness, histology, and cytokine expression. Confirmed by hydrodynamic gene transfer of LECT2 into deficient mice to monitor improvement (n = 10 in each group) |
| Polymorphisms of LECT2 associated with more severe forms of RA | 17 | In vivo human cross‐sectional study using DNA sequencing of adults with RA (n = 204) and controls (n = 197) found that the LECT2 Val58Ile polymorphism was associated with increased severity of disease in RA | |
| Polymorphisms of LECT2 associated with more severe forms of RA | 19 | In vivo human cross‐sectional study using DNA sequencing of adults with RA (n = 105) and controls (n = 101) found that the LECT2 Val58Ile polymorphism was associated with increased severity of disease in RA | |
| Expression of LECT2 associated with severity of disease in OA | 18 | In vivo human cross‐sectional study using isobaric tags for relative and absolute quantitation and Western blot in patients with OA (n = 17) and controls (n = 6) to identify LECT2 as a biomarker for severity of disease | |
| Intestines | Loss of LECT2 function in mice can lead to deregulated Wnt‐signaling in mice and possibly contribute to adenoma formation | 31 | In vivo nonhuman study of mice and small intestine adenoma formation involving the APC and Wnt signaling pathways. WT (induced AhCre+ Apc+/+ Mbd2+/+) mice were compared with Mbd2‐deficient (induced AhCre+ Apc+/+ Mbd2−/−) and Apcfl/fl (induced AhCre+ Apcfl/fl Mbd2+/+)) and Apcfl/fl Mbd2−/− (induced AhCre+ Apcfl/fl Mbd2−/−) mice to assess for changes in gene expression, adenoma formation, and changes in Wnt‐pathway signaling |
| Liver | Involvement in hepatic inflammation, injury, and recovery | 2 | In vivo nonhuman study of mice (n = 6) to assess for changes in LECT2 expression, serum transaminase levels, and cytokine release following Con A induced hepatic injury |
| Assessment of the effects of LECT2 on SEA‐induced hepatitis | 14 | In vivo nonhuman study that compared B6 mice (n = 10) to LECT2‐deficient mice (n = 10) when exposed to SEA. Also assessed B6 mice response when treated with SEA as well as LECT2 (n = 15) and not treated with LECT2 (n = 15) | |
| Changes after liver transplantation | 3 | In vivo human study of living related donor liver transplant donors (n = 5) and recipients (n = 5) where serum LECT2 and serum transaminases were trended over several days following the procedure | |
| Associated with acute liver failure survival | 6 | In vivo human study (n = 6) following adult patients with acute liver failure and compared serum LECT2 levels with serum transaminases and outcomes | |
| Upregulated and downregulated in certain human liver cancers | 9 | In vitro human study from samples of hepatoblastoma (n = 14) and hepatocellular carcinoma (n = 15) using qPCR to assess for up and down regulation | |
| LECT2 expression increased in mice with liver tumors | 9 | In vivo study of mice given an oncogenic form of β‐catenin with liver tumors (n = 15) showing increased LECT2 expression via Western blot, Northern blot, and immunohistochemistry | |
| Deficiency of LECT2 increases hepatic NKT‐cells in mice worsening hepatitis | 11 | In vivo mouse study (n = 6 at each time point) comparing WT and LECT2 deficient mice after Con A induced hepatitis to assess for cytokine production, histology of the liver, and presence of immune cells | |
| Tumor suppressor in certain HCCs | 28 | Multipart study using in vitro HCC cell lines and well as in vivo human and nonhuman subjects. Human patients with HCC (n = 73) were assessed by immunoblotting found that increased LECT2 suppresses MET phosphorylation and is associated with decreased vascular invasion and improved survival. Mice were studied via orthotopic liver injection experiments to assess the effects of loss of function LECT2 mutations on HCC. Cell lines were used to identify the HxGxD as the binding motif for LECT2 | |
| Expression pattern of LECT2 changes with staging of HCC | 42 | In vivo human subject study of liver biopsies using immunohistochemistry to compare the expression patterns of LECT2 in low‐grade malignant HCC (n = 9), advanced HCC (n = 5), and atypical hyperplasia (n = 19) | |
| Potential biomarkers in HCC | 29 | Multipart study using in vitro human cell lines and well as in vivo human and nonhuman subjects. Human patients with HCC (n = 54) were compared with healthy controls (n = 11) and patients with cirrhosis but no HCC (n = 16) by ELISA for LECT2, PCR to sequence β‐catenin, and qPCR to assess expression. Microarray was used to assess for LECT2 regulation in WT and β‐catenin knockout mice. Human cell lines were then assessed for β‐catenin regulation of LECT2 using qRT‐PCR, β‐catenin knockdown, and ChIP analysis. A mouse model of HCC (n = 9) was assessed for β‐catenin mutations via direct sequencing and then correlated with LECT2 expression via qRT‐PCR. | |
| LECT2 is able to effect β‐catenin induced inflammation | 30 | In vivo nonhuman study of mice using inactivated APC type mice (to model tumor initiation) and Lpk‐myc+ mice (to model tumor progression) to assess the inflammatory setting of tumor development. These models were then cross bred with LECT2‐deficient mice to assess the effects of LECT2 on the development of hepatic inflammation and tumor development. | |
| Abnormal folding associated with amyloidosis | 35 | In vivo human study of subjects with hepatic amyloidosis (n = 130) using histology and microdissection / mass spectrometry to identify the prevalence of abnormally folding LECT2 | |
| Abnormal folding associated with amyloidosis | 38 | In vivo human study of retrospective samples from adult patients with hepatic amyloidosis (n = 70) to determine prevalence of abnormal LECT2 using immunohistochemistry and microdissection / mass spectrometry as well as a second phase to assess histologic morphology of abnormal LECT2 amyloidosis (n = 24) | |
| Endocrine and metabolic systems | Increased levels of LECT2 are associated with increased insulin resistance | 22 | Study of both in vivo human and nonhuman subjects. Human subjects involved DNA chip analysis of liver biopsies (n = 10 with type 2 diabetes and n = 7 healthy controls) to correlate LECT2 mRNA and obesity. Then also used LECT2 enzyme linked immunosorbent assays to correlate serum LECT2 with various markers of metabolic syndrome (n = 200). Afterward, mice were used to demonstrate LECT2 increasing insulin resistance in skeletal muscle via phosphorylation of Jun NH2‐terminal kinase and that LECT2 deletion increased insulin sensitization and other markers of metabolic syndrome. |
| LECT2 as a therapeutic target in metabolic syndrome | 23 | In vitro study of human HCC cell lines and in vivo study of mice of the effects of LECT2 and a dipeptidyl peptidase‐IV inhibitor (gemigliptin). HCC cell lines were assessed for molecular markers of fatty liver disease after LECT2 administration as well changes after gemigliptin administration via Western blot and histology. Mice fed a HFD without treatment (n = 7), with treatment (n = 7), and lean controls (n = 7) were assessed for body weight, glucose, and insulin tolerance tests, and Western blot for molecular markers of insulin signaling. | |
| LECT2 may have utility as a serum marker for obesity and NAFLD | 41 | In vivo cross‐sectional human study of Japanese adults (n = 231) using ELISA to measure LECT2 and compare levels to anthropometric and clinical variables to assess utility as serum biomarkers | |
| Oncology | Prognostic indicator in breast cancer | 33 | In vivo human study of breast cancer tissue samples using microarray (n = 247) and real time PCR (n = 98) to assess for gene expression and clinical data to develop gene expression profiles that could predict clinical outcome |
| Renal | Abnormal folding associated with amyloidosis | 34 | In vivo human study of a single patient with renal amyloidosis using biochemical analysis and immunohistochemistry to identify the abnormal folding of LECT2 as a novel cause of renal amyloidosis |
| Abnormal folding associated with amyloidosis | 36 | Case series using human subjects with renal amyloidosis to compare demographic, clinical, histologic features, and electron microscopy (n = 40), as well as genetic sequencing of the LECT2 gene (n = 10) | |
| Multiorgan system disease | Abnormal folding associated with amyloidosis | 37 | Human autopsy study with two phases to identify prevalence of abnormal LECT2 in amyloidosis by immunohistochemistry. Initial phase was a review of autopsies between 2010 and 2012 of those who died over the age of 45 (n = 524) with a second more focused phase of Hispanic (n = 376) and Native American Indians (n = 101). |
| Pulmonary | Abnormal folding associated with amyloidosis | 39 | In vivo human case report using immunohistochemistry to demonstrate abnormal LECT2 amyloidosis presenting as pulmonary‐renal syndrome with lung deposits in addition to already described renal deposits |
| Vascular | Associated with atherosclerotic signaling | 24 | In vitro study of human umbilical vein endothelial cells and THP‐1 cells treated with varying doses of LECT2 to assess for function and signaling pathways through Western blot and qPCR |
ChIP, chromatin immunoprecipitation; Con A, concanavalin A; ELISA, enzyme‐linked immunosorbent assay; fMLP, N‐formylmethionine‐leucyl‐phenylalanine; HCC, hepatocellular carcinoma; HFD, high‐fat diet; LECT2, leukocyte cell derived chemotaxin 2; Mbd2, methyl binding domain protein 2; NAFLD, nonalcoholic fatty liver disease; NKT, natural killer T cells; OA, osteoarthritis; PaCLR, Plecoglossus altivelis C‐type lectin receptor; PCR, polymerase chain reaction; qPCR, real time polymerase chain reaction; qRT‐PCR, quantitative reverse transcriptase‐polymerase chain reaction; RA, rheumatoid arthritis; SEA, staphylococcal enterotoxin A; WT, wild type.
Table 2.
Leukocyte cell‐derived chemotaxin‐2 as a biomarker of disease
| Disease | Findings | Ref. |
|---|---|---|
| Acute liver failure | Potential prognostic indicator | 6 |
| HCC | Potential prognostic indicator | 28, 29, 42 |
| RA | Potential marker for severity of disease | 17, 22 |
| OA | Potential marker of grade and severity of disease | 18 |
| Metabolic syndrome | Potential marker of severity of insulin resistance and obesity | 41 |
| Sepsis | Potential prognostic indicator | 13 |
| Breast cancer | Potential predictor of recurrence and mortality in female smokers | 33 |
HCC, hepatocellular carcinoma; OA, osteoarthritis; RA, rheumatoid arthritis.
METHODS
Research papers were identified using PubMed and Ovid Medline using the term “leukocyte cell‐derived chemotaxin 2.” Papers identified were reviewed by all authors for relevance to the subject matter and if they were published in peer‐reviewed journals. These studies were also used to identify further publications not found in the PubMed search. There were a total of 52 papers included in the study. Inclusion criteria included any publication that directly studied LECT2 in vitro either in human or nonhuman studies, and studies were excluded if they did not discuss LECT2 in the setting of either physiology or disease. Included studies were found in various searches from May to October 2016. References include publication years ranging from 1996 to 2016.
Protein
The protein was first purified and identified in humans from phytohemagglutinin‐stimulated SKW‐3 leukemic T‐cells as a 16 kDa secreted protein with 133 amino acids in humans.1, 43, 44 At the time of discovery, the human protein structure was found to be 48% similar to myb‐induced myeloid protein‐1 in chickens, 80% similar to mouse LECT2, and 86% similar to bovine LECT2 (also known as chondromodulin‐II).1, 45 It has since been isolated in various species from mammals to shellfish.12, 15 This suggests an important regulatory function because it is relatively conserved across a wide variety of species. It also suggests that a common ancestor of LECT2 was passed down several generations and modified. The typical mouse LECT2 protein is 151 amino acids, although there is also an atypical type that is smaller at 132 amino acids due to the early termination signal from exon 4.46 The initial studies by Yamagoe et al.1 revealed in vitro chemotactic properties of LECT2, which contributed to its name. Further studies have shown that LECT2 can form various oligomers in physiologic conditions, but the noncovalent binding of a zinc atom stabilizes the structure and prevents oligomer formation.47 There are three disulfide bonds between six evolutionarily conserved cysteine residues.47 The crystal structure of LECT2 was recently identified by Zheng et al.48 (Figure 1).
Figure 1.
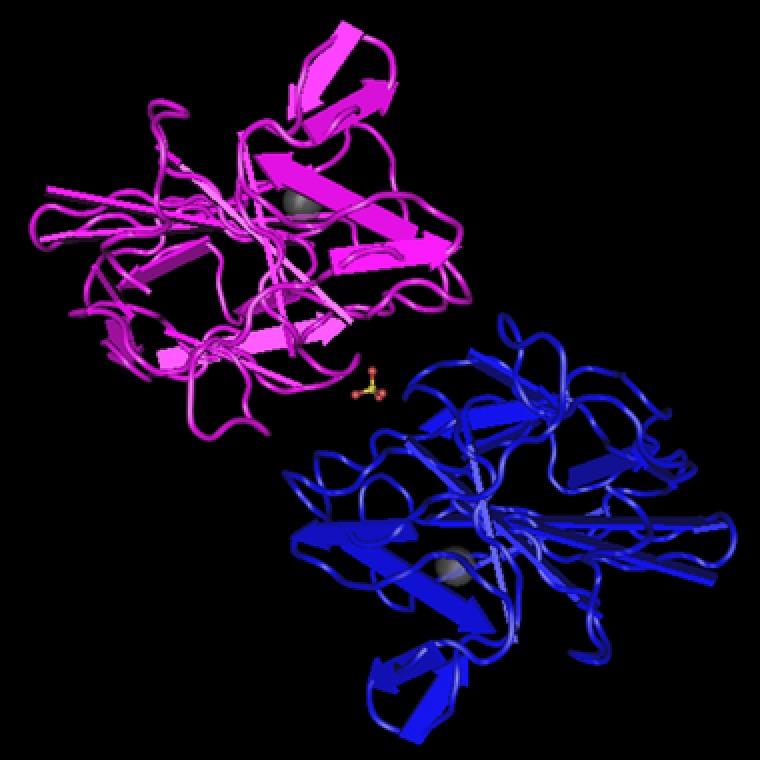
The crystal structure of human LECT2 identified by Zheng et al.
Although discovered in leukemic T‐cells, it was later identified in fetal livers, adult hepatic cell lines, and as a secreted protein in the blood.49 Subsequent studies have estimated its concentration in the bloodstream at ∼20 ng/mL in adult humans, but this can vary depending on disease state.13 Prior to secretion, it is generally found in the cytoplasm of cells. Using immunofluorescence, Yamagoe et al.1 demonstrated that the LECT2 protein is found in the cytoplasm of human hepatocytes and is also localized in some endothelial cells of hepatic arteries, portal veins, and central veins prior to secretion.49
In terms of function, the structure of LECT2 protein is similar to the M23 family of metalloendopeptidases, but it is unclear if they share any common functions. It has also been found to be similar to bovine chondromodulin‐II, which is involved in bone health. This has led researchers to investigate where else in the body LECT2 could be found.40, 43 Nagai et al.40 studied the LECT2 protein in various tissues of the body. They found that LECT2 is generally expressed in vascular, endothelial, and smooth muscle cells, adipocytes, cerebral nerve cells, apical squamous epithelia, parathyroid cells, sweat and sebaceous glandular epithelia, Hassall bodies, and some mononuclear cells in immunohematopoietic tissue. Additionally, in cells and tissues where LECT2 is normally found, decreased expression is observed during tissue inflammation, fibrosis, and pathology. This suggests that when LECT2 is a part of normal function, the degeneration of tissue homeostasis can downregulate its expression.40 Furthermore, tissues that generally did not have LECT2 expressed at baseline included osteoblasts, chondrocytes, cardiac tissue, smooth muscle cells in the gastrointestinal tract, and the epithelial cells of some tissues. In these tissues, LECT2 expression increased during disease conditions. It appears that in cells where LECT2 is not a normal part of cellular function, it is upregulated in disease settings and is likely to play a critical role in pathobiology.40
Interaction of LECT2 with other proteins and receptors is still under investigation, but some molecular pathways have been elucidated. For example, in hepatocellular carcinoma (HCC), LECT2 has the ability to bind and inhibit hepatocyte growth factor/MET receptor through protein tyrosine phosphatase 1B recruitment.28 It can also bind to CD209a receptors in mice macrophages to affect the immune system.22 LECT2 has also been noted to increase phosphorylation of Jun NH2‐terminal kinase (JNK) resulting in both insulin resistance and atherosclerotic disease.10, 24
However, what we know about LECT2 pathways is eclipsed by what we do not know. The M23 proteins are a group of the protease family and it would be easy to assume that LECT2 has a similar role in the body. Despite this, there has been no substrate discovered at this time for LECT2 protease activity.47 Either its substrate is very specific and has yet to be discovered or there is no substrate and the function of LECT2 is unrelated to its protease cousins. Using in vitro animal studies, LECT2 was found to target osteoblasts and promote bone growth, but its target that causes this is not known.50 The same applies to its in vitro chemotactic ability.1, 9 It is clear that LECT2 interacts with a wide range of proteins and cells in the human body, but before we can reliably target LECT2 as a potential biomarker or drug target, more work will need to be done mapping out its pathways.
Genetics
Yamagoe et al.,1 the group that initially isolated the protein, mapped the LECT2 gene to 5q31.1‐q32 in humans.43 This location is near several other immune‐modulating proteins, including interleukin (IL)‐3/4/5/9 and granulocyte macrophage‐colony stimulating factor.43 The LECT2 gene is ∼8 kb and contains four exons and three introns.43 The gene is translated as 1–1.3 kb mRNA with four different major transcription start sites and several minor start sites.43, 51 Polymorphisms were quickly identified. The first identified was at codon 58 in exon 3 that can code for either valine or isoleucine.43, 51 Subsequent studies have found that this polymorphism, Val58Ile, is associated with worsening joint destruction in rheumatoid arthritis (RA).17, 19 Additionally, there is a single nucleotide polymorphism at position 172 that is implicated in renal amyloidosis from LECT2.36 Other polymorphism and disease associations are still under investigation. General population data for frequency of polymorphisms have not been published, to the best of our knowledge. Definitive expression is still under investigation and although mRNA is largely identified in the liver, further areas of expression continue to be identified.49
Mouse LECT2 was first described by Yamagoe et al.46 and it is found on chromosome 13 and contains five potential exons with five major transcription start sites. However, the commonly expressed mRNA does not contain exon 4 and is closer to the human product. The gene occasionally includes exon 4 producing an atypical LECT2 protein through a frameshift and results in early termination.46 It is thought that this atypical mouse LECT2 has its own unique function in mouse physiology and does not have a human counterpart.46
Gene regulation
Given that LECT2 has multiple functions across many organ systems, there is no one clear molecular pathway that regulates LECT2 expression. LECT2 is associated with liver regeneration and there is evidence that the β‐catenin / Wnt‐pathway directly activates LECT2 expression.9 Wnt activation disrupts the normal function of cellular destruction pathways leading to an accumulation of β‐catenin in the cytoplasm.52 The β‐catenin then binds to the TCF/LEF family of transcription factors and binds to the identified promoter regions of both mice and human Lect2 genes resulting in increased expression.9 Additionally, methyl binding domain protein 2 (Mbd2) can also downregulate expression by binding to its promoter region and is also part of the Wnt‐pathway.31 Interestingly, LECT2 has negative feedback on the same pathway that induces its expression by inhibiting Wnt signaling.31 It accomplishes this by inhibiting all four activators of the Wnt/β‐catenin pathway, including ΔNLRP6, FlagAx2, ΔN β‐catenin, and TCF4‐VP16.31 It is felt that LECT2 must be working at the levels of the TCF4 transcription factor or below to be able to inhibit all four of these activators. Additionally, tumor necrosis factor alpha, interferon‐γ, and adenosine monophosphate‐activated protein kinase all seem to downregulate LECT2 expression in mice, but the exact mechanism remains unclear.2, 22, 23 We will discuss further molecular pathways in the sections below.
Current quantitation and analytical methods
Current studies have used several different methods of analyzing LECT2 in subjects. Clinical studies on most species were performed using enzyme‐linked immunosorbent assay (ELISA) plates.14, 53 ELISA plates using antibodies from across a wide spectrum of species have been developed and are readily obtained from a number of biomedical companies. Other studies have used Suppression Subtractive Hybridization, Northern Blot, Western Blot, immunohistochemistry, and real‐time reverse‐transcriptase polymerase chain reaction (PCR).9, 40 Although all of these methods are available for research purposes, quantification is not yet readily available in the clinical setting at this time and further studies are needed.
NORMAL FUNCTION AND CLINICAL SIGNIFICANCE
Cell cycle and development
Bovine chondromodulin‐II was identified before human LECT2 discovery and is structurally very similar, as discussed before.45 In vitro, exposure to chondromodulin‐II stimulated the growth of rabbit growth‐plate chondrocytes, mouse MC3T3‐E1 cells, and rat UMR‐106 osteoblastic cells.41, 47 This stimulation of bone growth hints at a role in inducing cell division, proliferation, and development, especially when considering that LECT2 is upregulated by the β‐catenin / WNT pathway. This pathway is involved in a wide range of functions, including cell fate specification, cell proliferation, and cell migration.9 In fact, LECT2 is being increasingly studied in the role of cellular development across the body. LECT knockout mice have been found to have decreased development of axons in neurons as well as altered expression of neutrophils that can affect neuronal development.21
Immune modulation and infection
Although there was evidence of neutrophil chemotaxis in vitro at the time of its discovery, the full function of LECT2 in the immune system is not known.1, 9 Several studies have attempted to clarify this but the results are nuanced and its function is not easily classified as either pro‐inflammatory or anti‐inflammatory.
In mice, LECT2 has been found to activate macrophages in times of sepsis via the CD209a receptor when placed in models of Escherichia coli sepsis, Pseudomonas aeruginosa sepsis, and cecal ligation and puncture.10 In fact, mice that died at 24 h in the Escherichia coli model had lower levels of circulating LECT2 (15.21 ± 1.03 ng/mL vs. 22.02 ± 1.22 ng/mL; P < 0.001) which may correlate with humans studies that we will discuss below.10 In that same study, recombinant LECT2 administration at a concentration of 30–135 ng/mg improved mouse survival from these models when compared with normal saline.10, 48 Dang et al.14 demonstrated an increased lethality from staphylococcal enterotoxin A in LECT2 knockout (LECT2‐KO) mice.14 More promisingly, exogenous LECT2 administration (5 μg/mouse at 0.5 h and 6 h) improved mouse survival from in those same mice from <30% to ∼50% (P < 0.05).14 LECT2 can also upregulate macrophage gene expression in certain species of fish, which again implies a conserved function across species.12, 15
The role of LECT2 in sepsis in humans is also being investigated. A Japanese study of 23 patients with sepsis admitted to the intensive care unit with confirmed infections and 31 healthy volunteer patients found that on admission to the intensive care unit, patients with sepsis initially had decreased circulating levels of LECT2 compared with the healthy controls (5.3 ± 4.1 ng/mL vs. 19.7 ± 3.4 ng/mL; P < 0.0001) that then increased by the time of discharge but was still less than the healthy controls.13 LECT2 was also found to be inversely and strongly correlated to C‐reactive protein, a commonly used inflammatory marker. Patients who recovered had an increase in LECT2 values during their admission, whereas the one patient who died in the intensive care unit had decreasing levels just prior to death. However, the study was not designed to comment on correlation vs. causation. It was unable to determine if the low levels of LECT2 contributed to disease severity or if it was simply downregulated in the initial setting of sepsis.
Bone health
In humans, LECT2 has been identified as a human biomarker for the severity of osteoarthritis (OA) by Ikeda et al.18 In that study, researchers obtained cartilage from six healthy controls and 17 patients with OA. These samples were analyzed using isobaric tags for relative and absolute quantitation and Western blot. LECT2 was found to be increased in patients with OA as well as in patients >65 years of age with P < 0.05. This is especially intriguing when taken in the context of a mouse model by Wu et al.20 in 2010. They found that mice with β‐catenin upregulation would convert normal articular chondrocytes into arthritic chondrocytes leading to an OA phenotype by 5 months of age, which is earlier compared with wild‐type (WT) mice.20 This study did not specifically analyze LECT2; however, it is known that β‐catenin increases LECT2 expression and that LECT2 in increased in human OA.9, 18
Given its effects on immune modulation and bone health, it is not surprising that LECT2 has a role in auto‐immune or RA. Okumura et al.16 assessed LECT2‐KO mice compared with WT mice with induction of arthritis by arthritogenic monoclonal antibody cocktails at 6–7 weeks of age and then assessed 5 days later. These mice were assessed via hind paw swelling, histology, ELISA, and RNA analysis. LECT2‐KO mice had worsening hind paw thickness and characteristics of articular inflammation on histology (P < 0.05). Certain chemokines and ILs (specifically IL‐1β and IL‐6) were found to be higher in the hind paws of LECT2‐KO mice by 1.8‐fold and 2.8‐fold, respectively. A set of LECT2‐KO mice were also given complementary LECT2 via hydrodynamic gene transfer after induction of arthritis. These mice all had improvement in hind paw thickness and decreased concentrations of IL‐1β, IL‐6, and other chemokines.
This work has been corroborated in cohort studies of Japanese and German populations. In these studies, certain LECT2 polymorphisms, specifically Val58Ile, were associated with worse cases of RA.19 Kameoka et al.19 obtained PCR studies of the LECT2 gene in 101 Japanese volunteers. In this study, there was a clear association between the genotypes 172AA (Ile/Ile) and 172GA (Val/Ile) and higher stage of RA (worsening disease severity) compared with 172GG (Val/Val; P = 0.017). These findings were confirmed by Graessler et al.,17 who found that in 204 patients with RA (using 81 patients with OA and 116 patients with gout as controls), those carrying the 172AA (Ile/Ile) genotype had worsening Larsen scores compared with those with 172GA (Val/Ile) and 172GG (Val/Val) genotypes (96.8 vs. 69.5 vs. 54.8; P = 0.001). However, these studies did not focus on the direct molecular causation of RA, but rather investigated the correlation of polymorphisms to disease presence or severity.
Hepatology
There is a developing body of evidence that LECT2 can either reflect or impact liver health, disease, and regeneration (also refer to below sections on metabolism, amyloidosis, and oncology) in mice and humans. For example, LECT2‐KO mice have significantly more severe liver injury following concanavalin A (Con A)‐induced hepatitis with an increased hepatic natural killer T cells in their livers.11 Saito et al.11 were able to generate these data by using targeting vectors to splice out LECT2 exons in mice. At baseline, LECT2‐KO mice had increased intrahepatic levels of CD3int NK1.1+ cells compared with the control group (26.1 ± 6.2% vs. 16.0 ± 4.6%; P < 0.01) as well as of CD4+ NK1.1+ cells (19.3 ± 4.8% vs. 11.1 ± 3.5%; P < 0.01). LECT2‐KO mice also had almost twice the levels of Va14 natural killer T cells at baseline. The group then induced hepatitis in WT and LECT2‐KO mice using 25 mg/kg Con A. At 5 h after Con A administration, LECT‐KO mice had elevated serum alanine aminotransferase (ALT) and IL‐4 as well as hepatic expression of FasL compared with WT mice. Livers from these mice were obtained at 5 h and examined histologically. Only the LECT2‐KO mice had focal degenerative change and cell clusters of apoptotic cells.
Segawa et al.2 were able to identify a reduction in LECT2 expression in mice with hepatic injury induced by Con A. The Con A (13 mg/kg) was administered i.v. to mice. At 8 h after infusion, there was a decrease in LECT2 seen on Western blot with a sharp increase in serum ALT and DNA fragmentation in the liver. This inverse relationship reversed at 24 h and then went back to baseline between 48 and 96 h (P < 0.01). LECT2 expression was also inversely related to tumor necrosis factor alpha and interferon‐γ.
Several groups have also begun to apply this knowledge to humans. A Japanese study by Sato et al.3 followed LECT2 serum levels in cases of living related donor liver transplantation. They trended LECT2 levels using ELISA in five recipients and their five donors and compared these values with other routine bloodwork. In both groups, LECT2 initially decreased with a nadir at 3–12 h for the donors and 12–48 h in the recipients, which is similar to the findings in mice by Segawa et al.2 Serum AST and ALT were inversely related to LECT2 with a peak between 12 and 24 h in both donors and recipients suggesting a role in liver regeneration. Interestingly, the serum LECT2 levels of donors were significantly higher than those of recipients on day 5 and 7 (9.5 +/‐ 5.9 ng/mL vs. 3.1 +/‐ 2.2 ng/mL; P = 0.04 and 9.3 +/‐ 3.8 ng/mL vs. 3.5 +/‐ 1.1 ng/mL; P = 0.04).
The same group also followed six adult patients with acute liver failure who were admitted to their hospital in 2002.6 The patients who died had significantly lower peak serum LECT2 levels than those who survived (0.96 +/‐ 0.8 ng/mL vs. 12.9 +/‐ 4.3 ng/mL). Although these studies need to be repeated with larger numbers of patients, they suggest a strong correlation with LECT2 and liver regeneration. These may be impacted by the Wnt/β‐catenin pathway, which is related to liver regeneration.
LECT2 expression is directly regulated by the Wnt/β‐catenin pathway and it is important to discuss hepatic functions of LECT2 in the context of the Wnt/β‐catenin pathway, which we will discuss.9 Unfortunately, β‐catenin is an intracellular protein and is unable to be measured without obtaining a liver biopsy. Because LECT2 is upregulated after Wnt/β‐catenin activation and secreted into the bloodstream, it can be theorized that LECT2 could be used as a reflection of Wnt/β‐catenin activation, liver regeneration, or even as a potential therapeutic target of the Wnt/β‐catenin pathway. There are ongoing investigations evaluating these possibilities and we will review some important features of the Wnt/β‐catenin pathway below as future areas of possible research for LECT2.
Mouse and human studies have described the correlation of liver regeneration and the Wnt/β‐catenin pathway. Mouse models dosed with nonlethal 300 mg/kg of acetaminophen showed extensive liver injury, but robust liver regeneration with several different pathways activated, as outlined in a study by Apte et al.4 and Bhushan et al.5 These pathways included IL‐6/STAT‐3, epidermal growth factor receptor / c‐Met / mitogen‐activated protein kinase regeneration, and the Wnt/β‐catenin pathway.5 However, when dosed with a lethal level of acetaminophen (600 mg/kg), mice had more extensive injury (higher serum ALT levels and percentage necrosis areas on histology), less regeneration, and decreased survival (P < 0.05).5 Interestingly, most regeneration pathways were still activated in these lethally dosed mice, except for the Wnt/β‐catenin pathway, suggesting a critical role in liver health. The study went further and bred mice with an overexpression of a more stable β‐catenin protein and compared lethal and nonlethal APAP dosing to WT mice. Overexpression of stable β‐catenin mice had improved serum ALT, percentage necrosis areas, and perinuclear neutrophil antibodies (pANCA) protein expression in hepatocytes suggesting regeneration.5
Knockout β‐catenin mice have also been studied for overall liver health. These mice were found to have increased levels of hepatic apoptosis and decreased serum ascorbic acid (vitamin C) levels by Nejak‐Bowen et al.7 This apoptosis was alleviated by ascorbic acid administration to cultured hepatocytes. Another study of β‐catenin knockout mice by Tan et al.8 showed an overall lower liver weight at 1 month of age that persisted throughout adulthood. When these mice were subjected to partial hepatectomy they had a slowed liver regeneration and increased apoptosis compared with WT mice.8 However, regeneration did eventually occur indicating several redundant pathways in the liver.
These animal studies have provided a foundation of knowledge that is slowly being applied to human studies as well. Retrospective liver samples were obtained by Apte et al.4 of adult acetaminophen‐induced acute liver failure to assess β‐catenin activation. Patients who survived had higher numbers of proliferating cell nuclear antigen staining cells indicating cell division / regeneration.4 These same samples also stained highly for intracellular β‐catenin.4 Samples from nonsurvivors showed decreased staining for proliferating cell nuclear antigen and β‐catenin.
Metabolic syndrome
LECT2 is also implicated in nutritional and metabolic syndrome pathways. A study of 200 Japanese individuals by Lan et al.22 presenting for their yearly physical found that there was a correlation between serum LECT2 levels and several aspects of metabolic syndrome. There was a positive correlation on linear regression with body mass index, waist circumference, homeostatic model assessment for insulin resistance, selenoprotein P, hemoglobin A1c, and systolic blood pressure with statistical significance in humans.
That same report then compared LECT2‐KO and WT mice and found that LECT2 had a positive correlation with markers of insulin resistance of skeletal muscle cells using models of heat production, running endurance, intraperitoneal glucose administration, and insulin tolerance tests. Interestingly, Lan et al.22 repeated a similar experiment using LECT2‐KO and WT mice after being fed a high‐fat diet (HFD) to create an obesity model. These obese mice still showed the same correlation with LECT2 and insulin resistance. LECT2‐KO mice had decreased overall weight gain at 14 weeks (P < 0.001), decreased blood glucose and insulin levels after meals (P < 0.05), and improved markers after intraperitoneal glucose and insulin tolerance tests (P < 0.01).
Using these mouse models, Lan et al.22 was then able to identify two molecular pathways that could lead to insulin resistance using Western blot and reverse transcribed RNA with real time PCR analysis. The first pathway involves the phosphorylation of the c‐JNK in C2C12 myocytes that has been implicated as a central player in obesity and insulin resistance.22, 25, 26 Additionally, LECT2 was also found to decrease Akt phosphorylation, which would then lead to the decreased translocation of glucose transporter 4.22, 27 The identification of these specific pathways could be an area of interest for future targeted therapies of type 2 diabetes mellitus, a known consequence of insulin resistance.
Insulin resistance is also a key factor in the development of nonalcoholic fatty liver disease (NAFLD). Okumura et al.41 described an increase in the serum LECT2 levels in patients with NAFLD compared with those patients without NAFLD in a cross‐sectional study of 231 adult Japanese subjects (48.7 ± 13.6 ng/mL vs. 40.5 ± 12.8 ng/mL; P < 0.001). Again, this study found positive correlations on linear regression with LECT2 and body mass index, waist circumference, waist to hip ratio, and waist to height ratio. They were also able to define cutoff values for LECT2 in the screening of obesity (41.8 ng/mL in men, 45.0 ng/mL in women) and NAFLD (43.3 ng/mL for men, 46.4 ng/mL for women) to help identify patients with these conditions. This suggests that LECT2 could be used as a biomarker in the setting of patients at risk for NAFLD.
Additional studies have identified molecular pathways for this process. Hwang et al.23 used HepG2 human HCC cell lines to study the effects of LECT2 on liver cells. They wanted to assess its effects on molecular markers of fatty liver disease. Through Western blot analysis, they found that LECT2 significantly increased phosphorylated mammalian target of rapamycin and SREBP‐1 cleavage as well as JNK phosphorylation via the CD209 receptor, which is similar to the activation of macrophages. In fact, after LECT2 administration, these human cell lines were found to have increased lipid accumulation on Oil Red O staining as well.
This may reflect the role of LECT2 in pathogenesis of NAFLD as LECT2 decreases insulin sensitivity and could worsen the metabolic syndrome that contributes to the development of NAFLD. However, as LECT2 plays a role in liver regeneration, it may be upregulated as the liver attempts to recover from NAFLD leading to worsening insulin resistance and further liver damage. This would need further clarified in future studies.
Recently, there have been studies examining LECT2 as a potential therapeutic target for insulin resistance and NAFLD. Dipeptidyl peptidase‐4 inhibitors are a class of medications used to treat insulin resistance via reducing cleavage of glucagon‐like peptide‐1 and increasing its insulinotropic action.52 Hwang et al.23 used gemigliptin, a dipeptidyl peptidase‐4 inhibitor, in mice fed an HFD that normally results in NAFLD and followed their outcomes compared with mice fed an HFD alone in addition to the HCC cell lines that were previously mentioned.23 They found that LECT2 expression was inhibited by gemigliptin via increased adenosine monophosphate‐activated protein kinase phosphorylation and JNK inhibitor‐dependent pathways.23 Additionally, mice fed an HFD treated with gemigliptin had improved markers of disease for NAFLD and insulin resistance compared with those fed an HFD alone. These included weight gain, blood tumor necrosis factor alpha levels, lipid accumulation in the liver on histology, and lower blood glucose levels after glucose tolerance testing.
Another aspect of metabolic syndrome that can lead to significant morbidity and mortality is atherosclerosis. LECT2 has also been implicated in the development of atherosclerotic blood vessels. This was again found to be regulated by the JNK phosphorylation and activation via the CD209 receptor, which shares the JNK pathway with insulin resistance.24, 25, 26 Various doses of LECT2 were given to human umbilical vein endothelial cells in this study by Hwang et al.23 Through Western blot and quantitative real‐time PCR, this group was able to demonstrate induction of pro‐inflammatory molecules, such as intercellular adhesion molecule 1, tumor necrosis factor alpha, monocyte chemo‐attractant protein‐1, and IL‐1β that are known to lead to atherosclerosis. Given that LECT2 is elevated in the setting of insulin resistance and is associated with NAFLD and atherosclerosis, LECT2 has been proposed as a potential target for future medical therapies.
Amyloidosis
Amyloidosis is a rare yet serious disease in which the abnormal folding of proteins lead to decreased proteolysis, the development of oligomers, and then deposition of these oligomers into various organs. This leads to a variety of phenotypes based on the specific proteins and organs involved. Within the last decade, a novel cause of amyloidosis has been linked to the abnormal folding of the LECT2 protein. This was first described in 2008 by Benson et al.34 in a patient with nephrotic syndrome of initially unknown etiology. The disease has since been expanded to include hepatic, splenic, adrenal glands, and pulmonary involvement and may represent as many as 25% of hepatic cases.35, 39 Hepatic leukocyte chemotactic factor‐associated amyloidosis most commonly presents as globular hepatic amyloid within the hepatic sinusoids and/or portal tracts.38 Little is known about the natural history of this disease especially how it is triggered and its prognosis. Data suggest that it is most common in patients of Mexican ancestry and autopsy studies have shown that these amyloid deposits can be found in the kidneys of up to 3.1% in patients of Hispanic descent.35, 36, 37 There is no single disease causing mutation currently discovered; however, many patients have been found to have a polymorphism of the G nucleotide in a nonsynonymous single nucleotide polymorphism at position 172 of the LECT2 gene, again most commonly found in patients from Latin America.36 This polymorphism, although common, is not sufficient for disease progression and it is suspected that there is some yet to be identified second hit to cause this disease.
Oncology
Given that uncontrolled inflammation and cell division can lead to malignancy, it should come as no surprise that LECT2 has become a protein of interest in cancer pathogenesis. Investigations using HCC and hepatoblastoma liver resections and RNA samples revealed that LECT2 was upregulated in almost all hepatoblastoma (13/14 samples) and only a subset of HCC (5/15 samples with and 6/33 samples without β‐catenin mutations).9 This correlates well with the knowledge that the deregulation of the Wnt / β‐catenin pathway is altered in >90% of hepatoblastoma and only 30–40% of HCC because LECT2 expression is regulated by the Wnt/β‐catenin pathway.9, 32 Other studies show that LECT2 expression can correlate with the stage of HCC. A study by Uchida et al.53 in 1999 used immunostaining to analyze the expression of LECT2 in atypical hyperplasia (a premalignant lesion), low‐grade malignant HCC, and advanced HCC.53 They found that as the HCC progressed from atypical hyperplasia to advanced HCC, hepatocytes went from generally positive for LECT2 in the cytoplasm of cells to generally negative staining and they provided sample images from the immunostaining.53 This fact likely contributes to the variable LECT2 expression in HCC seen in the Ovejero et al.9 study as lesions are found in different stages in patients. Okabe et al.29 was also able to demonstrate LECT2 expression by β‐catenin in an HCC mouse model and that mutations in β‐catenin led to increased serum LECT2. Indeed, their β‐catenin knockout mice had 117‐fold decreased expression of LECT2 compared with WT mice. When assessing tumor burden, their mice with histologic tumors had increased LECT2 compared with those without (55.9 ± 19.9 ng/mL vs. 24.9 ± 5.5 ng/mL; P < 0.01).
Interestingly, even though this upregulated gene expression is only present in a subset of patients with HCC, there is still evidence that elevated levels of LECT2 can be used as a biomarker of HCC in general regardless of β‐catenin mutation. Despite β‐catenin mutations, Okabe et al.29 was able to observe that serum levels >50 ng/mL of LECT2 were able to identify human patients with HCC with a specificity of 96.1% and positive predictive value of 97.0%. Given the potential role of LECT2 in the immune system, NAFLD, and liver regeneration, in addition to HCC, the clinical utility of LECT2 may be as a standalone test or as part of a battery of testing to personalize care. Mouse models also suggest a utility of LECT2 as a hepatic biomarker for cancer. Although β‐catenin is mutated and active in both colon and liver cancer and mice, only hepatic malignancies resulted in LECT2 upregulation suggesting that LECT2 is specific for hepatic malignancy in mice.9 Further mouse models have also demonstrated that LECT2 is a Wnt‐inhibitor and loss of LECT2 function may contribute to unregulated Wnt‐signaling, which is found in intestinal adenoma formation as was demonstrated by Phesse et al.31 using mouse models of Mbd2 deficiency. All of this suggests that increased LECT2 may be associated with hepatic malignancy but decreased or inhibited LECT2 may be associated with intestinal malignancy.
Additional studies have mapped a direct molecular pathway for the inhibition of vascular invasion in HCC by LECT2, which can correlate with survival. One study in particular by Chen et al.28 was able to assess the effect of LECT2 in both mice and human subjects as well as HCC cell lines. LECT2 was able to recruit protein tyrosine phosphatase 1B by binding to the HxGxD motif and decrease MET phosphorylation decreasing HCC's ability for vascular invasion and metastasis. This was correlated with decreased vascular invasion in human HCC (high LECT2 levels <20% vascular invasion, low LECT2 levels, and 100% vascular invasion; P < 0.001) and improved the Kaplan‐Meier analysis for survival. This may explain one of the changes that occurs as HCC progresses to its advanced stages, loses LECT2 expression, and results in increased vascular invasion and metastasis.
In mouse models of HCC development analyzed by Anson et al.,30 β‐catenin activation was associated with release of both pro‐inflammatory and anti‐inflammatory mediators, which creates a smoldering environment for HCC development. The main anti‐inflammatory mediators seen by real time PCR included LECT2 and it was associated with invariant natural killer T cells identified by flow cytometry and histology. The study group then developed LECT2 and invariant natural killer T single and double knockout mice, which developed β‐catenin‐activated HCC characterized by poorly controlled inflammation leading to significant lung metastases and more than double the number of tumor nodules seen in the controls.30 Interestingly, although β‐catenin activation generally seems to be pro‐inflammatory and oncogenic, it can also signal a negative feedback cascade using a somewhat anti‐inflammatory LECT2 signal that creates a more subtle inflammatory process for the creation of HCC.
Last, LECT2 may have a role in predicting the outcomes of patients with nonhepatic malignancies. LECT2 gene expression was found to be altered by smoking status.33 This, in combination with CYP1A1 and CETN1, was found to help predict breast carcinoma recurrence and survival among female smokers with median C‐index values of 0.8 and 0.73 for survival and recurrence, respectively. Conversely, nonsmokers only had a median C‐index value of 0.59.33
CONCLUSION AND FUTURE DIRECTIONS
These wide and varied research studies show how complex the role of LECT2 is in the human body. It has many important functions in almost every system in the human body from controlling and modulating the immune system, altering glucose metabolism, increasing bone growth, controlling cell cycle and division, and likely many others yet to be discovered. The clinical implications of changing levels of LECT2 in various disease states are just as varied (Table 1).6, 13, 17, 18, 22, 28, 29, 33, 41, 42 Given the novelty of this gene and protein, there are many areas that need further study and should be actively investigated. Additionally, many of these pathophysiologic functions interact with each other, and how they interplay with each other in the setting of different diseases needs to be addressed.
In the liver, LECT2 is implicated in the development of metabolic syndrome. Increased circulating levels of LECT2 are also associated with NAFLD. The development of insulin resistance and NAFLD could have a common mediator with LECT2. Additionally, LECT2 may also reflect liver regeneration and studies explaining the pathway of both NAFLD and liver regeneration through LECT2 should be clarified. Given the spectrum of liver diseases associated with LECT2, it may be beneficial to analyze LECT2 as part of an algorithm as opposed to an isolated test. For example, LECT2 could aid in NAFLD activity score assessments for which patients would progress to liver cirrhosis. LECT2 could also be used as a predictor for which patients with metabolic syndrome would develop NAFLD or atherosclerosis. Last, there are preliminary investigations of using LECT2 as a therapeutic target in mice, but human studies, including which drug, dosing, duration of therapy, and specific patient populations, still need to be performed.
It is also be important to note that hepatoblastoma and certain HCCs are associated with different levels of LECT2 and would need to be carefully considered in any screening guidelines. Further studies are needed in different hepatic malignancies to see how specific and sensitive LECT2 is for particular cancers. Additionally, LECT2 expression may have utility as a biomarker in breast cancer and could also be assessed as part of a battery of tests for various malignancies.
There is also increasing awareness of the abnormal folding and build of LECT2 in hepatic, renal, and pulmonary amyloidosis. Although a specific at‐risk polymorphism has been identified with some phenotypic description, there is significantly more work to be done in the fields of pathophysiology, genotype‐phenotype correlations, screening tests, environmental influences, prevention, and treatment. The same can be said regarding bone health and the role of LECT2 in arthritis syndrome.
Last, there may be several clinically applicable aspects of this protein in the setting of immunology and infectious disease. However, before LECT2 can likely be used clinically in humans, we need to understand how it modulates the immune system and through what mechanisms of action. For example, it is known to activate macrophages in mice, but there have been no conformational studies in human. In humans, levels of LECT2 are associated with severity of sepsis, but the mechanism of action and whether this is correlation or causation needs further study.
There are many areas of interest for the study of LECT2, but most research is still in the beginnings of understanding the basics of this protein. Further research to clarify the role of LECT2 across a multitude of diseases would allow LECT2 to have an important future as a biomarker and therapeutic target (Table 2).2, 3, 6, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 22, 23, 24, 28, 29, 30, 31, 33, 34, 35, 36, 37, 38, 39, 40, 42, 43
Acknowledgments
Voytek Slowik would like to acknowledge Udayan Apte for his mentorship on this project.
Author Contributions
U.A. designed the article design. V.S. drafted the manuscript. U.A. revised and approved the manuscript.
Conflict of Interest
The authors declared no conflict of interest.
References
- 1. Yamagoe, S. , Yamakawa, Y. , Matsuo, Y. , Minowada, J. , Mizuno, S. & Suzuki, K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol. Lett. 52, 9–13 (1996). [DOI] [PubMed] [Google Scholar]
- 2. Segawa, Y. , Itokazu, Y. , Inoue, N. , Saito, T. & Suzuki, K. Possible changes in expression of chemotaxin LECT2 mRNA in mouse liver after concanavalin A‐induced hepatic injury. Biol. Pharm. Bull. 24, 425–428 (2001). [DOI] [PubMed] [Google Scholar]
- 3. Sato, Y. et al Changes in serum LECT2 levels during the early period of liver regeneration after adult living related donor liver transplantation. Transplant. Proc. 36, 2357–2358 (2004). [DOI] [PubMed] [Google Scholar]
- 4. Apte, U . et al Beta‐catenin activation promotes liver regeneration after acetaminophen‐induced injury. Am. J. Pathol. 175, 1056–1065 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhushan, B . et al Pro‐regenerative signaling after acetaminophen‐induced acute liver injury in mice identified using a novel incremental dose model. Am. J. Pathol. 184, 3013–3025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato, Y . et al Serum LECT2 level as a prognostic indicator in acute liver failure. Transplant. Proc. 36, 2359–2361 (2004). [DOI] [PubMed] [Google Scholar]
- 7. Nejak‐Bowen, K.N. , Zeng, G. , Tan, X. , Cieply, B. & Monga, S.P. Beta‐catenin regulates vitamin C biosynthesis and cell survival in murine liver. J. Biol. Chem. 284, 28115–28127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan, X. , Behari, J. , Cieply, B. , Michalopoulos, G.K. & Monga, S.P . Conditional deletion of beta‐catenin reveals its role in liver growth and regeneration. Gastroenterology 131, 1561–1572 (2006). [DOI] [PubMed] [Google Scholar]
- 9. Ovejero, C . et al Identification of the leukocyte cell‐derived chemotaxin 2 as a direct target gene of beta‐catenin in the liver. Hepatology 40, 167–176 (2004). [DOI] [PubMed] [Google Scholar]
- 10. Lu, X.J. et al LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J. Exp. Med. 210, 5–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saito, T . et al Increase in hepatic NKT cells in leukocyte cell‐derived chemotaxin 2‐deficient mice contributes to severe concanavalin A‐induced hepatitis. J. Immunol. 173, 579–585 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Zhang, R.C. , Chen, J. , Li, C.H. , Lu, X.J. & Shi, Y.H . Prokaryotic expression, purification, and refolding of leukocyte cell‐derived chemotaxin 2 and its effect on gene expression of head kidney‐derived macrophages of a teleost fish, ayu (Plecoglossus altivelis). Fish Shellfish Immunol. 31, 911–918 (2011). [DOI] [PubMed] [Google Scholar]
- 13. Ando, K. , Kato, H. , Kotani, T. , Ozaki, M. , Arimura, Y. & Yagi, J . Plasma leukocyte cell‐derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol. Immunol. 56, 708–718 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Dang, M.H. et al Possible role of LECT2 as an intrinsic regulatory factor in SEA‐induced toxicity in d‐galactosamine‐sensitized mice. Clin. Immunol. 137, 311–321 (2010). [DOI] [PubMed] [Google Scholar]
- 15. Ma, H.L. , Shi, Y.H. , Zhang, X.H. , Li, M.Y. & Chen, J . A transmembrane C‐type lectin receptor mediates LECT2 effects on head kidney‐derived monocytes/macrophages in a teleost, Plecoglossus altivelis. Fish Shellfish Immunol. 51, 70–76 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Okumura, A. et al Suppressive role of leukocyte cell‐derived chemotaxin 2 in mouse anti‐type II collagen antibody‐induced arthritis. Arthritis Rheum. 58, 413–421 (2008). [DOI] [PubMed] [Google Scholar]
- 17. Graessler, J. et al Association of chondromodulin‐II Val58IIe polymorphism with radiographic joint destruction in rheumatoid arthritis. J. Rheumatol. 32, 1654–1661 (2005). [PubMed] [Google Scholar]
- 18. Ikeda, D. , Ageta, H. , Tsuchida, K. & Yamada, H . iTRAQ‐based proteomics reveals novel biomarkers of osteoarthritis. Biomarkers 18, 565–572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kameoka, Y. , Yamagoe, S. , Hatano, Y. , Kasama, T. & Suzuki, K . Val58IIe polymorphism of the neutrophil chemoattractant LECT2 and rheumatoid arthritis in the Japanese population. Arthritis Rheum. 43, 1419–1420 (2000). [DOI] [PubMed] [Google Scholar]
- 20. Wu, Q. , Zhu, M. , Rosier, R.N. , Zuscik, M.J. , O'Keefe, R.J. & Chen, D . Beta‐catenin, cartilage, and osteoarthritis. Ann. NY Acad. Sci. 1192, 344–350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koshimizu, Y. & Ohtomi, M . Regulation of neurite extension by expression of LECT2 and neurotrophins based on findings in LECT2‐knockout mice. Brain Res. 1311, 1–11 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Lan, F. et al LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 63, 1649–1664 (2014). [DOI] [PubMed] [Google Scholar]
- 23. Hwang, H.J. et al A dipeptidyl peptidase‐IV inhibitor improves hepatic steatosis and insulin resistance by AMPK‐dependent and JNK‐dependent inhibition of LECT2 expression. Biochem. Pharmacol. 98, 157–166 (2015). [DOI] [PubMed] [Google Scholar]
- 24. Hwang, H.J. et al LECT2 induces atherosclerotic inflammatory reaction via CD209 receptor‐mediated JNK phosphorylation in human endothelial cells. Metabolism 64, 1175–1182 (2015). [DOI] [PubMed] [Google Scholar]
- 25. Nakatani, Y. et al Modulation of the JNK pathway in liver affects insulin resistance status. J. Biol. Chem. 279, 45803–45809 (2004). [DOI] [PubMed] [Google Scholar]
- 26. Hirosumi, J . et al A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002). [DOI] [PubMed] [Google Scholar]
- 27. Cong, L.N. et al Physiological role of Akt in insulin‐stimulated translocation of GLUT4 in transfected rat adipose cells. Mol. Endocrinol. 11, 1881–1890 (1997). [DOI] [PubMed] [Google Scholar]
- 28. Chen, C.K. et al Leukocyte cell‐derived chemotaxin 2 antagonizes MET receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1B recruitment. Hepatology 59, 974–985 (2014). [DOI] [PubMed] [Google Scholar]
- 29. Okabe, H. et al Role of leukocyte cell‐derived chemotaxin 2 as a biomarker in hepatocellular carcinoma. PLoS One 9, e98817 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anson, M. et al Oncogenic β‐catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J. Clin. Invest. 122, 586–599 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phesse, T.J. et al Deficiency of Mbd2 attenuates Wnt signaling. Mol. Cell Biol. 28, 6094–6103 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Audard, V. et al Cholestasis is a marker for hepatocellular carcinomas displaying beta‐catenin mutations. J. Pathol. 212, 345–352 (2007). [DOI] [PubMed] [Google Scholar]
- 33. Andres, S.A. , Bickett, K.E. , Alatoum, M.A. , Kalbfleisch, T.S. , Brock, G.N. & Wittliff, J.L . Interaction between smoking history and gene expression levels impacts survival of breast cancer patients. Breast Cancer Res. Treat. 152, 545–556 (2015). [DOI] [PubMed] [Google Scholar]
- 34. Benson, M.D. , James, S. , Scott, K. , Liepnieks, J.J. & Kluve‐Beckerman, B . Leukocyte chemotactic factor 2: a novel renal amyloid protein. Kidney Int. 74, 218–222 (2008). [DOI] [PubMed] [Google Scholar]
- 35. Mereuta, O.M. et al Leukocyte cell‐derived chemotaxin 2 (LECT2)‐associated amyloidosis is a frequent cause of hepatic amyloidosis in the United States. Blood 123, 1479–1482 (2014). [DOI] [PubMed] [Google Scholar]
- 36. Larsen, C.P. , Kossmann, R.J. , Beggs, M.L. , Solomon, A. & Walker, P.D . Clinical, morphologic, and genetic features of renal leukocyte chemotactic factor 2 amyloidosis. Kidney Int. 86, 378–382 (2014). [DOI] [PubMed] [Google Scholar]
- 37. Larsen, C.P. , Beggs, M.L. , Wilson, J.D. & Lathrop, S.L. Prevalence and organ distribution of leukocyte chemotactic factor 2 amyloidosis (ALECT2) among decedents in New Mexico. Amyloid 23, 119–123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chandan, V.S. et al Globular hepatic amyloid is highly sensitive and specific for LECT2 amyloidosis. Am. J. Surg. Pathol. 39, 558–564 (2015). [DOI] [PubMed] [Google Scholar]
- 39. Khalighi, M.A. , Yue, A. , Hwang, M.T. & Wallace, W.D . Leukocyte chemotactic factor 2 (LECT2) amyloidosis presenting as pulmonary‐renal syndrome: a case report and review of the literature. Clin. Kidney J. 6, 618–621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagai, H. , Hamada, T. , Uchida, T. , Yamagoe, S. & Suzuki, K. Systemic expression of a newly recognized protein, LECT2, in the human body. Pathol. Int. 48, 882–886 (1998). [DOI] [PubMed] [Google Scholar]
- 41. MacDonald, B.T. , Tamai, K. & He, X . Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deacon, C.F. Dipeptidyl peptidase‐4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metab. 13, 7–18 (2011). [DOI] [PubMed] [Google Scholar]
- 43. Yamagoe, S. , Kameoka, Y. , Hashimoto, K. , Mizuno, S. & Suzuki, K. Molecular cloning, structural characterization, and chromosomal mapping of the human LECT2 gene. Genomics 48, 324–329 (1998). [DOI] [PubMed] [Google Scholar]
- 44. Okumura, A. et al Identification and assignment of three disulfide bonds in mammalian leukocyte cell‐derived chemotaxin 2 by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Biosci. Trends 3, 139–143 (2009). [PubMed] [Google Scholar]
- 45. Hiraki, Y. et al A novel growth‐promoting factor derived from fetal bovine cartilage, chondromodulin II. Purification and amino acid sequence. J. Biol. Chem. 271, 22657–22662 (1996). [DOI] [PubMed] [Google Scholar]
- 46. Yamagoe, S. , Watanabe, T. , Mizuno, S. & Suzuki, K . The mouse Lect2 gene: cloning of cDNA and genomic DNA, structural characterization and chromosomal localization. Gene 216, 171–178 (1998). [DOI] [PubMed] [Google Scholar]
- 47. Okumura, A. et al Leukocyte cell‐derived chemotaxin 2 is a zinc‐binding protein. FEBS Lett. 587, 404–409 (2013). [DOI] [PubMed] [Google Scholar]
- 48. Zheng, H. et al Crystal structure of human leukocyte cell‐derived chemotaxin 2 (LECT2) reveals a mechanistic basis of functional evolution in a mammalian protein with an M23 metalloendopeptidase fold. J. Biol. Chem. 291, 17133–17142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamagoe, S. et al Expression of a neutrophil chemotactic protein LECT2 in human hepatocytes revealed by immunochemical studies using polyclonal and monoclonal antibodies to a recombinant LECT2. Biochem. Biophys. Res. Commun. 237, 116–120 (1997). [DOI] [PubMed] [Google Scholar]
- 50. Shukunami, C. et al Molecular cloning of mouse and bovine chondromodulin‐II cDNAs and the growth‐promoting actions of bovine recombinant protein. J. Biochem. 125, 436–442 (1999). [DOI] [PubMed] [Google Scholar]
- 51. Yamagoe, S., Mizuno, S. & Suzuki, K. Molecular cloning of human and bovine LECT2 having a neutrophil chemotactic activity and its specific expression in the liver. Biochim. Biophys. Acta. 1396, 105–113 (1998). [DOI] [PubMed] [Google Scholar]
- 52. Okumura, A. et al Increased serum leukocyte cell‐derived chemotaxin 2 (LECT2) levels in obesity and fatty liver. Biosci. Trends 7, 276–283 (2013). [PubMed] [Google Scholar]
- 53. Uchida, T. et al Expression pattern of a newly recognized protein, LECT2, in hepatocellular carcinoma and its premalignant lesion. Pathol. Int. 49, 147–151 (1999). [DOI] [PubMed] [Google Scholar]


